Abstract
Aims
Patients with obstructive hypertrophic cardiomyopathy (oHCM) experience significant clinical burden which is associated with a high economic burden. Peak oxygen uptake (pVO2), measured by cardiopulmonary exercise testing, is used to quantify functional capacity, and has been studied as a primary endpoint in recent clinical trials. This study aimed to gather evidence to consolidate the prognostic value of pVO2 in oHCM and to assess whether it is feasible to predict health outcomes in an economic model based on changes in pVO2.
Methods
A targeted literature review was conducted in MEDLINE (via PubMed) and Embase databases to identify evidence on the prognostic value of pVO2 as a surrogate health outcome to support future oHCM economic model development. Following screening, study characteristics, population characteristics, and pVO2 prognostic association data were extracted.
Results
A total of 4,687 studies were identified. In total, 3,531 and 538 studies underwent title/abstract and full-text screening, respectively, of which 151 were included and nine of these were in hypertrophic cardiomyopathy (HCM); only three studies focused on oHCM. The nine HCM studies consisted of one systematic literature review and eight primary studies reporting on 27 potentially predictive relationships from a pVO2-based metric with clinical outcomes including all-cause mortality, cardiovascular mortality, sudden cardiac death, transplant, paroxysmal, and permanent atrial fibrillation. pVO2 was described as a predictor of single and composite endpoints, in three and six studies, respectively, with one study reporting on both.
Limitations
This study primarily uses systemic literature review methods but does not qualify as one due to not entailing parallel reviewers during title–abstract and full-text stages of review.
Conclusion
The findings of this study suggest pVO2 is predictive of multiple health outcomes, providing a rationale to use pVO2 in the development of an economic model.
PLAIN LANGUAGE SUMMARY
Obstructive hypertrophy cardiomyopathy (oHCM) is a condition where the heart muscle thickens, obstructing blood flow and potentially impacting health. Peak oxygen uptake (pVO2) measures the highest amount of oxygen consumption during peak exercise and serves as an indicator of fitness. pVO2 can be used to assess heart health and predict severe conditions and death, acting as a surrogate endpoint. Surrogate endpoints are valuable in drug investigations since they allow earlier decisions on drug approval and funding before longer-term patient follow-up is available.
This study reviewed evidence on the relationship between pVO2 values in patients with heart disease and the risk of becoming sicker or dying. Our goal was to assess if these relationships had been established and whether it is feasible to use them to predict future treatment benefits and support economic evaluations of new treatments. Our review found that most studies reported on patients with heart failure, with only nine focusing on HCM. Evidence indicates that low pVO2 values in patients with heart disease are linked to an increased risk of developing other heart conditions, needing a heart transplant, or dying.
Introduction
Hypertrophic cardiomyopathy (HCM) is a genetic disorder of the heart muscle that can cause chest discomfort and shortness of breath, particularly with exertion. Additional symptoms can include palpitations, dizziness, and syncope. Patients with HCM are at risk of sudden cardiac deathCitation1. HCM is an underdiagnosed disease with estimated average prevalence rates around the world varying from one in 3,195 people in the United States (US) to a range of one in 2,013 to one in 2,500 people in European countriesCitation2–5. Data gathered between 2013 and 2019 from the HealthCore Integrated Research Database (HIRD) suggest that the number of clinically diagnosed patients with HCM in the US increased 1.5-fold to an estimated 262,591, in part due to enhanced clinical-diagnosis and contemporary diagnostic-testing strategies (e.g. advanced cardiovascular imaging, genetic testing of family members, increased recognition of HCM in the general practicing cardiology community)Citation6.
Approximately two-thirds of patients with HCM have left ventricular outflow tract obstruction, termed obstructive HCM (oHCM), which results in significant additional clinical burden including hypertension, heart failure, and atrial fibrillationCitation7. The burden of oHCM symptoms on patient quality-of-life (QoL) is variable, ranging from very little effect to significantly impaired functionality and reduced QoLCitation8,Citation9. In addition to the disease burden to the patient, oHCM presents a considerable economic burden to the health care system. A large US claims database study among 11,401 eligible patients with oHCM estimated that the total all-cause annual healthcare costs of symptomatic oHCM were $43,586 per patient (2019 US dollars) compared to $6,768 in matched healthy controlsCitation10. In addition, symptomatic oHCM patients experienced 8-times as many hospitalizations (38% vs 6.9%, respectively) and incurred ∼$35,000 more costs annually than matched controls without cardiomyopathy. Current treatment options for oHCM aim to minimize or prevent symptoms and reduce the risk of complications, such as heart failure and sudden deathCitation11, include β-blockers, calcium channel blockers, and disopyramide. Invasive surgeries are an alternative option for non-responsive patientsCitation12.
Contemporary management sees generally low rates of hospitalization and mortalityCitation6,Citation13,Citation14. This makes it difficult to perform randomized controlled trials using these endpoints, as caused by heart failure, within a reasonable timeframe. In 2022, the Heart Failure Collaboratory (HFC) and the Academic Research Consortium (ARC) Scientific Expert Panel recommended the use of functional and symptomatic endpoints to promote patient-centered clinical trial efficiency in heart failureCitation15. Traditionally, left ventricular outflow tract gradient (LVOT-G) has been used as a clinical endpoint in oHCM studies. LVOT-G has been shown to be the primary driver of exertional dyspnea, chest pain, fatigue, and exercise intolerance, which are heart failure symptoms that severely impact functional capacityCitation16. However, despite similar hemodynamic loading conditions at the time of LVOT-G measurement, daily variations have been reported which may lead to misclassification of disease severity and have implications on determining optimal treatmentCitation17. Peak oxygen uptake (pVO2), measured by cardiopulmonary exercise testing (CPET), is also used to quantify functional capacityCitation16. There is literature suggesting that patients with oHCM have compromised pVO2Citation13 and that there is a correlation between pV̇O2 valueCitation13, the New York Heart Association (NYHA) class and patients QoLCitation15.
There have been treatment advances, namely small-molecule cardiac myosin inhibitors, including aficamten and mavacamtenCitation18–20. Aficamten is an investigational, novel, oral, small-molecule cardiac myosin inhibitor designed to reduce the hypercontractility (the excessively increased systolic function and a high ejection fraction) associated with oHCMCitation21. Aficamten was investigated in the Phase 3 SEQUOIA-HCM study, for which the primary endpoint was the change from baseline to week 24 in pVO2 measured by CPETCitation20. The choice of pVO2 was motivated by its unique positioning both as an objective measure of functional capacity and as an expected prognosticator of adverse clinical events in oHCMCitation16. Topline positive results from the SEQUOIA-HCM trial demonstrated treatment with aficamten significantly improved exercise capacity compared to placebo, increasing pVO2 by a least square mean difference of 1.74 mL/kg/min (95% confidence interval [CI] = 1.04–2.44; p < 0.0001)Citation22. The efficacy and safety of mavacamten has been assessed in a phase 3, randomized, double-blind, placebo-controlled trial (EXPLORER-HCM), in which it demonstrated a 1.5 mL/kg per min or greater increase in pVO2Citation18.
Health technology assessment (HTA) agencies often rely on economic models that compare the available treatment options to inform their decision-making related to reimbursement. Health economic models can be used to extrapolate clinical and economic outcomes beyond short clinical trial durations and to combine the best available evidence for each treatment. There is a limited number of previously published economic models in oHCM evaluating pharmacotherapiesCitation23–28. Of those identified, no prior studies brought change in pVO2 into economic modeling. Typically, HTA guidelines recommend a lifetime horizon for models in chronic conditions and when interventions have differential effects on mortalityCitation29–31. Given the short-term nature of the available clinical evidence, and the need to estimate long-term cost-effectiveness, it is of interest to understand whether pVO2 could robustly act as a surrogate endpoint for final outcomes.
A surrogate endpoint is a biomarker, physiological measure, or other indicator that captures the causal pathway through which a disease affects hard endpoints such as mortalityCitation32. Surrogates have become more accepted by HTA agencies, in recognition that capturing patient centered outcomes is not always feasible in certain disease areas or during the limited clinical trials period. This study aimed to gather evidence to consolidate the prognostic value of pVO2 in oHCM and to assess whether it is feasible to predict health outcomes, such as QoL and NYHA class, in an economic model based on changes in pVO2.
Methods
Search strategy
The TLR was performed in accordance with the methodological principles of conduct for literature reviews as detailed in the University of York Centre for Reviews and Dissemination’s “Guidance for Undertaking Reviews in Health Care”Citation33. This TLR was a structured comprehensive review with explicit methodology that was narrowed toward finding key evidence needed to answer the research questions without the use of two reviewers at each screening stage.
Data sources
Searches were conducted in MEDLINE (via PubMed) and Embase databases on 11 May 2023. A search strategy was developed with minimal limits criteria (English language, human studies) to avoid missing potentially relevant documents (Supplementary Appendix). The strategy was validated by comparing the search results to a sample set of published studies and review papers that were expected to be identified.
Screening
Each title and abstract were independently reviewed against the eligibility criteria (). Decisions at this phase were classified into one of two categories: included and excluded. Citations with uncertainty were included at this stage to avoid excluding potentially relevant full texts. Each citation was reviewed by one of the three reviewers.
Table 1. Inclusion and exclusion criteria.
All studies included in the first phase of screening proceeded to full-text review. The full publication was independently reviewed against the eligibility criteria by one reviewer. The bibliographies of relevant SLRs identified through the electronic database searches were hand-searched to identify any additional studies of relevance. Studies not providing sufficient information for the evaluation of the eligibility criteria were excluded at this stage to ensure that only relevant publications were captured.
Data extraction
Study characteristics, population characteristics, and pVO2 prognostic association data were extracted to an Excel® workbook ().
Table 2. Data extracted from selected studies.
The most recent publication presenting follow-up data was extracted when two or more full-text, peer-reviewed publications were identified for the same population (e.g. 5-year follow-up to clinical trial, a follow-up to interim trial data).
Ten percent of the extracted studies were randomly selected for data extraction to be double-checked by a second reviewer. Any discrepancies or missing information identified by the second individual was discussed by both individuals until a consensus was reached. Any unresolved conflicts were decided by a third individual.
Results
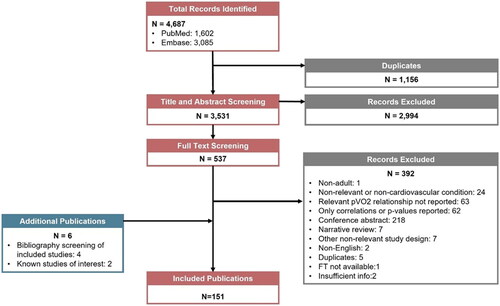
PRISMA diagram
The TLR identified 4,687 studies (PubMed: 1,602; Embase: 3,085). After removing 1,156 duplicates, 3,531 studies underwent title/abstract screening. Full-text screening occurred in 537 studies, of which 145 were included at the final stage. Manual search of included studies bibliography identified an additional four studies and there were two known studies of interest for a final total of 151 included studies. The PRISMA flow diagram is described in .
Predictors of clinical outcomes in HCM
The 151 included articles reported on various cardiovascular conditions including: heart failure (n = 90), pulmonary hypertension (n = 9), coronary heart disease (n = 8), cardiovascular disease (general term; n = 7), HCM (n = 9), other cardiomyopathies (n = 5), congenital heart disease (n = 4), cardiac amyloidosis (n = 1), and other cardiovascular conditions (n = 18). The nine HCM studies included eight primary studies and one SLR (); only three studies focused on oHCM. The eight primary studies reported 27 potentially predictive relationships for pVO2, all with clinical outcomes. A relationship was reported between clinical outcomes and pVO2 or the percent of predicted pVO2 in 70% and 32% of studies, respectively. Associations were commonly reported by hazard ratios (HRs; 59%), followed by Kaplan-Meier data (15%), relative risks (RRs; 11%), and odds ratios (ORs; 15%). Predictions of single (41%) and composite (59%) endpoints were investigated.
Table 3. HCM study characteristics.
One SLR reported that exercise test results are useful to determine prognosis in HCM among 7,525 patients from 18 included publications (2010 to 2018)Citation34. The major predictors of clinical outcomes identified included: abnormal heart rate recovery, abnormal blood pressure response exercise induced wall motion abnormalities, lower peak VO2, higher minute ventilation relative to CO2 production (VE/VCO2), and pulmonary hypertension/exercise-induced pulmonary hypertension.
pVO2 as a predictor of single and composite endpoints
Of the nine HCM studies identified, pVO2 was described as a predictor of single and composite endpoints in three and six studies, respectively, with one study reporting on both types of endpoints. Potentially predictive relationships based on pVO2 were defined using a continuous threshold (per ml/kg/min) in six studiesCitation35–40. Further, one study assessed the impact of “non-response” (< 0% change in pVO2) on the risk of deathCitation41, and another study investigated changes in risk of cardiac-related death, heart transplant, and functional deterioration leading to septal reduction therapy per 5 ml/kg/min increments in pVO2Citation42. The baseline pVO2 ranged from 18.8 to 26 ml/kg/min in the eight studies. All studies reported statistically significant relationships between pVO2 values and single or composite endpoints with two exceptions; Coats et al. reported that pVO2 did not significantly predict the composite endpoint of SCD or equivalentCitation35 and Smith et al. did not find the potential relationship between atrial fibrillation and pVO2 non-responsiveness to be statistically significantCitation41. The relationships with pVO2 in HCM are summarized in .
Table 4. pVO2 as a predictor of single and composite endpoints.
Discussion
A wealth of evidence across multiple cardiovascular conditions was identified supporting a link between higher peak oxygen consumption and fewer clinical events. This is the first study focusing on oHCM and describing the development of an economic model based on current evidence providing rationale to using proxy outcomes, specifically pVO2. Our results show pVO2 may be used as a surrogate endpoint to assess health outcomes of treatment for patients with oHCM. pVO2 was a predictor of single outcomes in HCM studies, including all-cause mortality, cardiovascular mortality, sudden cardiac death, transplant, paroxysmal atrial fibrillation (AF), and permanent AF and was also linked to multiple composite endpointsCitation35,Citation36. A model conceptualized around pVO2 may be of high clinical relevance.
Implications for model development
There is a limited number of previously published economic models in HCM evaluating pharmacotherapies; most published models compared defibrillators and other devicesCitation23–28. These models were either Markov models or a combination of decision tree with Markov model, mostly with long-term time-horizons, capturing HCM-related mortality and reporting outcomes in terms of cost per QALY gained. There was heterogeneity in the morbidities captured, but these included neurologic damage, atrial fibrillation stroke, infection, and heart failure. The cost-effectiveness model built for the assessment of mavacamten by the US Institute of Clinical and Economic Review (ICER) compared mavacamten with standard of care based on patient pathways through NYHA classes over their lifetime. No prior studies brought change in pVO2 into economic modeling.
The NYHA functional classification system was developed to evaluate cardiac symptoms on a patient’s daily activities. Although frequently used, there is no consistent method of assessing NYHA classification, as determined by a survey of 30 cardiologistsCitation43. A literature survey reported 99% of research papers did not reference or describe their methods of assigning NYHA classesCitation43. Thus, the reproducibility of the NYHA system is not well established; an interoperator variability study among two cardiologists assessing a series of 50 patients in classes II and III using NYHA classification showed only 54% concordanceCitation43. Furthermore, NYHA classification is not a reliable measure of disease status and progressionCitation44. Researchers have found the NYHA classification systems should not be used as the sole outcome measure of change in function in cardiac studiesCitation44. A more reliable and objective proxy endpoint is warranted.
There are potentially multiple different ways to include pVO2 data in an economic model for oHCM. A key consideration is around whether pVO2 is leveraged using absolute continuous changes from baseline, or whether values at follow-up are categorized against threshold values (such as low vs high). The latter carries the risk of oversimplifying oHCM by ignoring clinically relevant nuances in the outcomes of patients close to and further from the given threshold in the same category. One approach could be a state-transition Markov model, using hazard ratios that account for changes in pVO2 to adjust long-term risks of mortality and other complications. Another approach could be a partitioned survival model, making use of Kaplan-Meier data for different levels of pVO2. Each model should be considered against data requirements and its ability to reflect the natural history of oHCM. The hazard ratios, odds ratios, and relative risks outlined in may be helpful in future economic models in HCM, depending on how the model has been conceptualized.
Surrogates
The only prior cost-effectiveness model of a pharmaceutical in oHCM was conducted by ICER in the US and based health states on NYHA classes, in line with National Institute for Health and Care Excellence (NICE) precedent in analogue conditionsCitation45. However, ICER did not describe the conceptualization process for the HCM model. Based on the findings of this TLR, we found there is clinical evidence to support using pVO2 as a surrogate in these areas: heart failure, pulmonary hypertension, coronary heart disease, cardiovascular disease, congenital heart disease, cardiac amyloidosis, and other cardiomyopathies/cardiovascular conditions. As new clinical evidence becomes available from novel therapies, the association between pVO2 and patient centered outcomes is anticipated to become even more robust. Until then, combining approaches that have previously been accepted by HTAs, such as modeling based on NYHA classes, with data on changes in pVO2, should be considered. Recently, pVO2 was used as the primary endpoint in the SEQUOIA-HCM trial which demonstrated aficamten added to standard of care therapy has a positive impact on exercise capacity as well as rapid and sustained effects on symptoms and functional class in patients with oHCMCitation22. These results and the use of pVO2 can potentially be used to inform the future economic model of SEQUOIA-HCM. Furthermore, these study results can be informative not only for conventional pharmacoeconomic evaluations but also for outcome-based contracting for pharmaceuticals that adopt short-term outcomesCitation46.
Evidence from clinical trials is usually of short duration, oftentimes too short to observe all potential clinical events of interest. Most HTA agencies recommend that economic evaluations extrapolate beyond the trial period to estimate lifetime benefitsCitation29,Citation47. This underlines the importance for finding appropriate surrogate evidence, where possible. However, this will inevitably be associated with uncertainty in long-term extrapolations, which can make HTA decision-making more difficult.
Limitations
The current review was not an SLR which would entail two reviewers screening all studies at both title-abstract and full-text stages. Apart from not using two reviewers, the current TLR used rigorous SLR methods including preplanned methodology with predefined outcome measures, a set search strategy, and comprehensive systematic searching with a quality check at the extraction phase of 10% of studies from another reviewer.
No other reviews for the prognostic value of pVO2 in HCM were identified that had an economic focus. This is a rapidly growing therapeutic area and there is urgency in gathering evidence to support HTAs. Given the scarcity of data in the HCM field and our methods, a scoping TLR was deemed more efficient in assessing the feasibility of using pVO2 in models. The current TLR provided evidence that pVO2 could be used to support modeling efforts. This work will help assess the need for a future SLR in this area.
Conclusions
In this study focusing on oHCM, we describe the current evidence that may support use of pVO2 to predict long-term outcomes in an economic model. A thoughtful approach needs to be taken when creating economic models in oHCM. Our study suggests pVO2 is predictive of multiple relevant health outcomes. pVO2 changes may be leveraged as a surrogate for clinical outcomes in future oHCM economic studies.
Transparency
Declaration of financial/other interests
CKM, ND, MA are paid employees of Maple Health Group, which was contracted by Cytokinetics Incorporated to work on this study. MB and SS are paid employees of Cytokinetics Incorporated, which is the manufacturer of Aficamten. AM reports research grants from Pfizer, Ionis, Attralus, and Cytokinetics and fees from Cytokinetics, BMS, Eidos, Pfizer, Ionis, Lexicon, Haya, BioMarin, Prothena, Alexion, AstraZeneca, and Tenaya.
Author contributions
All authors contributed to the study concept. CKM, ND, and MA carried out screening and data extraction. All authors contributed to the interpretation of the findings and definition of conclusions. CKM, ND, and MA took the lead in the composing this manuscript with critical input from MB, SS, and AM.
Reviewer comments
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Previous presentations
No previous presentations to declare.
Supplemental Material
Download MS Word (23.2 KB)Acknowledgements
The authors thank Izabela Aleksanderek, from Maple Health Group for providing medical writing support.
Additional information
Funding
References
- Wasfy J, Walton S, Beinfeld M, et al. Mavacamten for hypertrophic cardiomyopathy: effectiveness and value; evidence report. Institute for Clinical and Economic Review; 2021.
- Cytokinetics. Data on file. South San Francisco, CA: Prevalence of Target Population; 2021.
- Maron MS, Hellawell JL, Lucove JC, et al. Occurrence of Clinically Diagnosed Hypertrophic Cardiomyopathy in the United States. Am J Cardiol. 2016;117(10):1651–1654. doi: 10.1016/j.amjcard.2016.02.044.
- Magnusson P, Palm A, Branden E, et al. Misclassification of hypertrophic cardiomyopathy: validation of diagnostic codes. Clin Epidemiol. 2017;9:403–410. doi: 10.2147/clep.S139300.
- Husser D, Ueberham L, Jacob J, et al. Prevalence of clinically apparent hypertrophic cardiomyopathy in Germany-An analysis of over 5 million patients. PLoS One. 2018;13(5):e0196612. doi: 10.1371/journal.pone.0196612.
- Butzner M, Maron M, Sarocco P, et al. Clinical diagnosis of hypertrophic cardiomyopathy over time in the United States (A Population-Based Claims Analysis). Am J Cardiol. 2021;159:107–112. doi: 10.1016/j.amjcard.2021.08.024.
- Lu DY, Pozios I, Haileselassie B, et al. Clinical outcomes in patients with nonobstructive, labile, and obstructive hypertrophic cardiomyopathy. J Am Heart Assoc. 2018;7(5):e006657. doi: 10.1161/jaha.117.006657.
- Zytnick D, Heard D, Ahmad F, et al. Exploring experiences of hypertrophic cardiomyopathy diagnosis, treatment, and impacts on quality of life among middle-aged and older adults: an interview study. Heart Lung. 2021;50(6):788–793. doi: 10.1016/j.hrtlng.2021.06.004.
- Zaiser E, Sehnert AJ, Duenas A, et al. Patient experiences with hypertrophic cardiomyopathy: a conceptual model of symptoms and impacts on quality of life. J Patient Rep Outcomes. 2020;4(1):102. doi: 10.1186/s41687-020-00269-8.
- Jain SS, Li SS, Xie J, et al. Clinical and economic burden of obstructive hypertrophic cardiomyopathy in the United States. J Med Econ. 2021;24(1):1115–1123. doi: 10.1080/13696998.2021.1978242.
- Sharma S, Firoozi S, McKenna WJ. Value of Exercise Testing in Assessing Clinical State and Prognosis in Hypertrophic Cardiomyopathy. Cardiol Rev. 2001;9(2):70–76. doi: 10.1097/00045415-200103000-00005.
- Palandri C, Santini L, Argirò A, et al. Pharmacological management of hypertrophic cardiomyopathy: from bench to bedside. Drugs. 2022;82(8):889–912. doi: 10.1007/s40265-022-01728-w.
- Ciabatti M, Fumagalli C, Beltrami M, et al. Prevalence, causes and predictors of cardiovascular hospitalization in patients with hypertrophic cardiomyopathy. Int J Cardiol. 2020;318:94–100. doi: 10.1016/j.ijcard.2020.07.036.
- Minhas AMK, Wyand RA, Ariss RW, et al. Demographic and regional trends of hypertrophic cardiomyopathy-related mortality in the United States, 1999 to 2019. Circ Heart Fail. 2022;15(9):e009292. doi: 10.1161/circheartfailure.121.009292.
- Psotka MA, Abraham WT, Fiuzat M, et al. Functional and symptomatic clinical trial endpoints: the HFC-ARC scientific expert panel. JACC Heart Fail. 2022;10(12):889–901. doi: 10.1016/j.jchf.2022.09.012.
- Coats CJ, Maron MS, Abraham TP, et al. Exercise capacity in patients with obstructive hypertrophic cardiomyopathy: SEQUOIA-HCM baseline characteristics and study design. JACC Heart Fail. 2023;12(1):199–215. doi: 10.1016/j.jchf.2023.10.004.
- Geske JB, Sorajja P, Ommen SR, et al. Left ventricular outflow tract gradient variability in hypertrophic cardiomyopathy. Clin Cardiol. 2009;32(7):397–402. doi: 10.1002/clc.20594.
- Olivotto I, Oreziak A, Barriales-Villa R, et al. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2020;396(10253):759–769. doi: 10.1016/s0140-6736(20)31792-x.
- Maron MS, Masri A, Choudhury L, et al. Phase 2 study of aficamten in patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2023/01/03/. 2023;81(1):34–45. doi: 10.1016/j.jacc.2022.10.020.
- CY 6031 Study Will Evaluate the Effects of Treatment With Aficamten (CK-3773274) Over a 24-week Period on Cardiopulmonary Exercise Capacity and Health Status in Patients With Symptomatic oHCM (SEQUOIA-HCM)
- Aficamten [Internet]. Cytokinetics. [cited 2024 Jun 19]. Available from: https://cytokinetics.com/aficamten/
- Cytokinetics. Cytokinetics announces positive results from SEQUOIA-HCM, the pivotal phase 3 clinical trial of aficamten in patients with obstructive hypertrophic cardiomyopathy. 2023. https://ir.cytokinetics.com/news-releases/news-release-details/cytokinetics-announces-positive-results-sequoia-hcm-pivotal
- You JJ, Woo A, Ko DT, et al. Life expectancy gains and cost-effectiveness of implantable cardioverter/defibrillators for the primary prevention of sudden cardiac death in patients with hypertrophic cardiomyopathy. Am Heart J. 2007;154(5):899–907. doi: 10.1016/j.ahj.2007.06.026.
- Takura T, Kyo S, Ono M, et al. Preliminary report on the cost effectiveness of ventricular assist devices. J Artif Organs. 2016;19(1):37–43. doi: 10.1007/s10047-015-0858-5.
- Magnusson P, Wimo A. Health economic evaluation of implantable cardioverter defibrillators in hypertrophic cardiomyopathy in adults. Int J Cardiol. 2020;311:46–51. doi: 10.1016/j.ijcard.2020.02.055.
- Haag MB, Hersh AR, Toffey DE, et al. Cost-effectiveness of in-home automated external defibrillators for children with cardiac conditions associated with risk of sudden cardiac death. Heart Rhythm. 2020;17(8):1328–1334. doi: 10.1016/j.hrthm.2020.03.018.
- Haag MB, Hersh AR, Toffey DE, et al. Cost-effectiveness of implantable cardioverter-defibrillators in children with cardiac conditions associated with risk for sudden cardiac death. Pediatr Cardiol. 2020;41(7):1484–1491. doi: 10.1007/s00246-020-02395-y.
- Goldenberg I, Moss AJ, Maron BJ, et al. Cost-effectiveness of implanted defibrillators in young people with inherited cardiac arrhythmias. Ann Noninvasive Electrocardiol. 2005;10(4 Suppl):67–83. doi: 10.1111/j.1542-474X.2005.00070.x.
- CADTH. Guidelines for the economic evaluation of health technologies: Canada. 4th ed. CADTH; 2017.
- ICER’s. Reference Case for Economic Evaluations: principles and Rationale; 2020.
- NICE health technology evaluations: the manual. Process and methods. 2022.
- Ciani O, Grigore B, Blommestein H, et al. Validity of Surrogate Endpoints and Their Impact on Coverage Recommendations: a Retrospective Analysis across International Health Technology Assessment Agencies. Med Decis Making. 2021;41(4):439–452. doi: 10.1177/0272989x21994553.
- Centre for Reviews and Dissemination. Systematic Reviews: CRD's guidance for undertaking reviews in health care. York: Centre for Reviews and Dissemination, University of York; 2008.
- Rodrigues T, Raposo SC, Brito D, et al. Prognostic relevance of exercise testing in hypertrophic cardiomyopathy. A systematic review. Int J Cardiol. 2021;339:83–92. doi: 10.1016/j.ijcard.2021.06.051.
- Coats CJ, Rantell K, Bartnik A, et al. Cardiopulmonary Exercise Testing and Prognosis in Hypertrophic Cardiomyopathy. Circ Heart Fail. 2015;8(6):1022–1031. doi: 10.1161/circheartfailure.114.002248.
- Azarbal F, Singh M, Finocchiaro G, et al. Exercise capacity and paroxysmal atrial fibrillation in patients with hypertrophic cardiomyopathy. Heart. 2014;100(8):624–630. doi: 10.1136/heartjnl-2013-304908.
- Masri A, Pierson LM, Smedira NG, et al. Predictors of long-term outcomes in patients with hypertrophic cardiomyopathy undergoing cardiopulmonary stress testing and echocardiography. Am Heart J. 2015;169(5):684–692.e1. doi: 10.1016/j.ahj.2015.02.006.
- Magri D, Agostoni P, Sinagra G, et al. Clinical and prognostic impact of chronotropic incompetence in patients with hypertrophic cardiomyopathy. Int J Cardiol. 2018;271:125–131. doi: 10.1016/j.ijcard.2018.04.019.
- Moneghetti KJ, Stolfo D, Christle JW, et al. Value of Strain Imaging and Maximal Oxygen Consumption in Patients With Hypertrophic Cardiomyopathy. Am J Cardiol. 2017;120(7):1203–1208. doi: 10.1016/j.amjcard.2017.06.070.
- Sorajja P, Allison T, Hayes C, et al. Prognostic utility of metabolic exercise testing in minimally symptomatic patients with obstructive hypertrophic cardiomyopathy. Am J Cardiol. 2012;109(10):1494–1498. doi: 10.1016/j.amjcard.2012.01.363.
- Smith JR, Layrisse V, Medina-Inojosa JR, et al. Predictors of exercise capacity following septal myectomy in patients with hypertrophic cardiomyopathy. Eur J Prev Cardiol. 2020;27(10):1066–1073. doi: 10.1177/2047487319898106.
- Finocchiaro G, Haddad F, Knowles JW, et al. Cardiopulmonary responses and prognosis in hypertrophic cardiomyopathy: a potential role for comprehensive noninvasive hemodynamic assessment. JACC Heart Fail. 2015;3(5):408–418. doi: 10.1016/j.jchf.2014.11.011.
- Raphael C, Briscoe C, Davies J, et al. Limitations of the New York Heart Association functional classification system and self-reported walking distances in chronic heart failure. Heart. 2007;93(4):476–482. doi: 10.1136/hrt.2006.089656.
- Bennett JA, Riegel B, Bittner V, et al. Validity and reliability of the NYHA classes for measuring research outcomes in patients with cardiac disease. Heart Lung. 2002;31(4):262–270. doi: 10.1067/mhl.2002.124554.
- Single Technology Appraisal. Tafamidis for treating transthyretin amyloid cardiomyopathy [ID1531] Committee Papers. 2020.
- Alkhatib NS, Abraham I. The six Delta platform for outcome-based contracting for pharmaceuticals. J Med Econ. 2020;23(11):1209–1214. doi: 10.1080/13696998.2020.1824161.
- Excellence NIfC. Guide to the methods of technology appraisal; 2008. http://www nice org uk/aboutnice/howwework/devnicetech/technologyappraisalprocessguides/guidetothemethodsoftechnologyappraisal jsp