?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
BACKGROUND
Human papillomavirus (HPV) causes several cancers such as cervical cancer and some head and neck (oral cavity, pharynx, and larynx), vulval, vaginal, anal, and penile cancers. As HPV vaccination is available, there is potential to prevent these cancers attributed to HPV and consequently the burden associated with them. The aim of this analysis was to estimate the number of HPV-related cancer deaths and the productivity costs due to years of life lost (YLL) in the United Kingdom (UK).
METHOD
A model was developed utilizing UK 2019 mortality data sourced from country-specific databases for England, Scotland, Wales, and Northern Ireland for the following HPV-related cancers: head and neck (ICD-10 C00-14 and C32), cervix uteri (C53), vaginal (C51), vulval (C52), anal (C21), and penile (C60). The proportion of deaths and years of life lost (YLL) due to HPV were estimated using HPV attributable fractions for each anatomic locations from the published literature. Labor force participation, retirement ages, and mean annual earnings, discounted at 3.5% annually, were applied to YLL to calculate the present value of future lost productivity (PVFLP).
RESULTS
A total of 1,817 deaths due to HPV-related cancers were reported in the UK in 2019 resulting in 31,804 YLL. Restricting to only YLL that occurred prior to retirement age yielded a total YPLL of 11,765 and a total PVFLP of £187,764,978.
CONCLUSIONS
There is a high disease burden in the UK for HPV-related cancers, with a large economic impact on the wider economy due to productivity losses. Implementing and reinforcing public health measures to maintain high HPV vaccination coverage in both males and females may further facilitate reduction of this burden.
Disclaimer
As a service to authors and researchers we are providing this version of an accepted manuscript (AM). Copyediting, typesetting, and review of the resulting proofs will be undertaken on this manuscript before final publication of the Version of Record (VoR). During production and pre-press, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal relate to these versions also.Introduction
Cancer is one of the leading causes of death globally, with over 180,000 deaths reported in the United Kingdom (UK) in 2022.Citation1 Cancer also has a vast economic burden in patients, societies and public health care systems. In the UK, the total cost of cancer was estimated at £19,994 (€23,002) million in 2018, with €6,633 million (28.8%) of this being productivity loss due to premature mortality.Citation2 Whilst there are varying causes of cancers, infections are responsible for approximately 13% of cancer worldwide, yielding a substantial number of cancer deaths that may be preventable.Citation3
Human papillomavirus (HPV) causes several cancers such as cervical cancer and some head and neck (oral cavity, pharynx, and larynx), vulval, vaginal, anal, and penile cancers.Citation4 The associated burden of the majority of cervical cancers and a certain proportion of vulvar, vaginal, and anal cancers can be prevented by available HPV vaccines. In the UK, HPV vaccination is recommended and available for free on the National Health Service (NHS) for children aged 12 to 13 years old and people at high risk from HPV.Citation5 The HPV national vaccination programme was introduced for adolescent females in 2008.Citation6 This was expanded to include adolescent males in 2019. In 2023, routine vaccination of all children in year 8 (12 to 13 years), as well as high-risk groups such as eligible gay, bisexual, and other men who have sex with men (GBMSM) under 25 years have moved from a two-dose schedule to a one-dose schedule.Citation7 In the academic year 2021/2022, coverage for people with at least one-dose HPV vaccine ranged from 62.4% in year 8 (12 to 13 year old) males to 86.5% in year 10 (14 to 15 year old) females. Notably, the COVID-19 pandemic led to some disruption of school-based immunisation, and whilst HPV vaccination coverage in 2021/2022 improved significantly, it had not yet returned to pre-pandemic levels (88.0% in year 8 females in 2018/2019 vs 76.6% in 2020/2021, with at least one dose). The shift to the one-dose schedule in higher risk groups aims to improve adherence and coverage and it is important to monitor the impact of this change.
Additionally, cancer screening is a key component in reducing cancer burden in the UK. The NHS provides cervical screening to women aged 25 to 64, aiming to detect abnormalities early and prevent cancer development.Citation8 In England, over a 3.5-year period, 68.6% and 75.0% of eligible women aged 25-49 years and 50-64 years, respectively, were screened adequately for cervical cancer.Citation9 However, this coverage rate is still under the acceptable threshold of 80% in 2022. Regular screening can identify precancerous changes, allowing for timely intervention and treatment, ultimately reducing the incidence and mortality rates associated with cervical cancer.
Despite various public health measures such as cervical screening, HPV vaccination and disease awareness programs in the UK, the burden of these cancers remain high. Over 3,200 cervical cancer cases were reported in the UK in 2022.Citation1 Around 12,600 head and neck cancer cases, including 2,900 oropharyngeal cancer cases, and 1,500 vulval, 790 penile, 1,900 anal, and 230 vaginal cancer cases were reported, though not all of these cases will have been HPV-related. However, the presence of HPV is not systematically tested for in these cancers in the UK. There exists a clear opportunity to decrease the cancer burden by enhancing public health measures such as screening and disease awareness along with management and high HPV vaccination coverage in key populations. However, cervical is currently the only cancer caused by HPV where a national screening program exists,Citation8 as screening programmes will only be implemented under certain circumstances.Citation10
With regards to economic burden, the direct cost of HPV-related cancers has been explored,Citation11 but there is a gap in evidence for the economic impact due productivity losses as a result of premature mortality. The aim of this analysis was to estimate the number of HPV-related cancer deaths and productivity costs due to years of life lost (YLL) in the UK.
Materials and Methods
Model structure
An economic model was adapted from a previously published model which used the human capital approach to estimate productivity losses due to cancer-related deaths.Citation12–15 The adapted model estimated productivity losses resulting from premature death from HPV-related cancers in the UK. The model adopted a lifetime horizon, and estimated three key outcomes: YLL, years of productive life lost (YPLL), and present value of future lost productivity (PVFLP). Patients entered the model at the point of death and the YLL and cost of productivity losses were accumulated over the remaining lifetime had death been avoided from HPV-related cancers in that year. Hence, direct treatment costs which occurred while the patient was alive were not considered in the model.
The model population included patients who died in 2019 from six HPV-related cancers: head and neck cancers (ICD-10 C00-14 and C32), cervical (ICD-10 C53), vulval (ICD-10 C51), vaginal (ICD-10 C52), anal (ICD-10 C21), and penile (ICD-10 C60). Population attributable fractions (PAFs) were applied to calculate the number of cancer deaths attributable to HPV infection for each anatomic location. Deaths across all age categories were included in YLL calculations, however, only YLL before retirement age were included in YPLL and PVFLP calculations as productivity losses are not incurred after retirement. In the base-case, PVFLP took into account country-, age-, and gender-specific annual wages, as well as a country-specific labor force participation rate. The most recent available data prior to 2020 (i.e. 2019) were used to avoid confounding from the impact of COVID-19 on these parameters.
Model calculations
To calculate YLL, YPLL, and PVFLP, the model first estimated the expected life years remaining and expected productive life years remaining as follows:
where i= 1,2,3…n are population age groups used in the model and n is the number of age categories. These outputs were capped at zero to ensure there were no negative values.
Years of life lost
YLL calculations considered both the frequency of deaths, the number of those attributable to HPV, and the age at which they occurred, using the following formula:where i= 1,2,3…n are population age groups used in the model.
Years of productive life lost
YPLL is an estimate of the average number of years a person would have been in productive employment (defined in the model as earning a wage) had they not died prematurely due to cancer, calculated as follows:where i= 1,2,3…n are population age groups used in the model.
Present value of future lost productivity
PVFLP was calculated in two steps:
1) The PVFLP per person was calculated by multiplying the productive life years remaining by country-, age-, and gender-specific annual wages.
where i= 1,2,3…n are population age groups used in the model.
2) The PVLFP per person values were then multiplied by the age-specific mortality data to calculate the PVFLP for the UK as a whole.
where i= 1,2,3…n are population age groups used in the model.
PVFLP was adjusted to account for UK unemployment rates, using labor force participation as a measure of unemployment in the base case, to reflect the actual labor force characteristics. Annual earnings were discounted at a rate of 3.5% annually to obtain the present value of future earnings.Citation16
Inputs and assumptions
Epidemiological inputs
Epidemiological inputs included mortality data and life expectancies. Mortality data from 2019, stratified by cancer type and by age were sourced from country specific databases.Citation17–20 Retirement age (66 years) and gender-specific life expectancies in 2019 were sourced from The World Bank and The Office for National Statistics, respectively.Citation21, Citation22 Data from 2019 were used to avoid confounding from COVID-19.
PAFs were sourced from de Martel et al., and applied to calculate the number of cancer deaths attributable to HPV infection ().Citation23 The authors reviewed selected case series and included recent published literature to determine the proportion of DNA attribution in cancers associated with HPV.
Table 1. Population attributable fractions applied for HPV-related cancers
Economic inputs
To determine productivity costs through YLL, annual earnings were sourced from the Eurostat database which takes into account work hours which are both part-time and full-time.Citation24 The earnings data for 2018 were used as this was the most recent information available at the time of analysis. A labor force participation rate of 63.2% for 2019 was sourced from the World Bank.Citation25
Assumptions
The model used real world data to inform the inputs wherever possible, however, a number of assumptions were made to account for the granularity of data available (). Assumptions around age groups followed existing model approaches.Citation12, Citation15
Table 2. Parameter assumptions made in the model
Sensitivity and scenario analyses
A deterministic sensitivity analysis (DSA) was conducted. This involved varying the input parameters such as PAFs, mortality, retirement age, labor force participation, and average wage. All inputs were varied by a relative +/- 10%, including PAFs, as confidence intervals were not reported in de Martel et al.
Various PAFs are reported in the literature so the scenario used PAFs reported in Hartwig et al.Citation26 The PAF for oral cavity and pharynx cancer was calculated by weighting the Hartwig et al PAFs by the proportion of incidence of the following cancer types: oral cavity cancer (ICD-10 C02-06), nasopharynx cancer (ICD-10 C11), oropharynx cancer (ICD-10 C01, C09, C10), hypopharynx (ICD-10 C12-13), and pharynx cancer (ICD-10 C14). The incidence cases were sourced from England Cancer Data 2019 (Supplementary Table 1)Citation17.
Results
HPV-related number of cancer deaths and YLL
After applying the PAFs, a total of 1,817 deaths due to HPV-related cancers were reported in the UK in 2019. Of these, 27.5% (n = 499) occurred in males and 72.5% (n = 1,318) in females (). The cancer responsible for the greatest number of deaths was cervical cancer (n = 844; 46.4%), whilst penile cancer was responsible for the fewest deaths (n = 76; 4.2%).
Table 3. Number of cancer deaths related to HPV, by cancer type and by sex, in 2019
Deaths in England made up 81.5% of the overall deaths (n = 1,482), while 11.8% (n = 214) occurred in Scotland, 5.0% in Wales (n = 92), and 1.6% in Northern Ireland (n = 30) ().
Table 4. YLL and YPLL related to HPV, by cancer type and by sex, in 2019
Table 5. PVFLP related to HPV by cancer type across UK countries in 2019
When considering the expected life years remaining, the total YLL calculated due to HPV-related cancers in 2019 was 31,804 years (). Of the total YLL, 62.3% (n = 19,823) were due to cervical cancer and 14.9% (n = 4,733) due to anal cancer. As for number of deaths, penile cancer caused the fewest YLL, at 797 (2.5%). Restricting to only YLL that occurred prior to retirement age yielded a total YPLL of 11,765 (63% decrease relative to YLL) (). The distribution of YPLL by cancer and sex followed the same trend as YLL.
The majority of YLL and YPLL occurred in females (82.3% and 86.3%, respectively), primarily driven by YLL and YPLL due to cervical cancer, however, in non-sex specific cancers, a greater number of YLL and YPLL occurred in males (). In head and neck cancer, 63.1% of YLL and 68.1% of YPLL occurred in males. In anal cancer, the split was more even, with 48.2% of YLL and 54.0% of YPLL occurring in males ().
Across all HPV-related cancers, England accounted for the majority of YLL (80.8%) and YPLL (81.0%), whilst Northern Ireland had the fewest (1.9% and 2.2% for YLL and YPLL respectively; ). This aligned with the population sizes as England has the largest population (56.5 million), whereas Northern Ireland has lowest (1.9 million),Citation27 the same trend was observed for each cancer type.
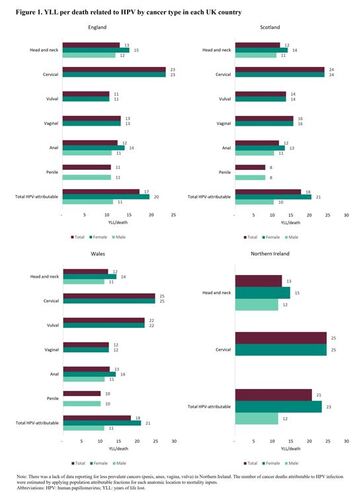
The overall YLL per death for HPV-related cancers was 18 years; 20 years in females and 11 years in males (). The YLL per death was highest for cervical cancer (23 years) and lowest for vulvar and penile cancer (11 years). The total YLL per death was 17 years in England, 18 years in Scotland, and Wales, and 21 years in Northern Ireland ().
Economic value of years of life lost from HPV-related cancers
The total PVFLP in 2019 due to HPV-related cancers was £187,764,978. Cervical cancer contributed the greatest amount to the overall PVFLP (£125,353,865; 66.8%), followed by anal (£25,670,143; 13.7%) (). This trend was observed across all UK countries except Northern Ireland, due to lack of reported data on vulval, vaginal, anal, and penile cancers in Northern Ireland. The lowest PVFLP in the UK was from vulval cancer deaths (£3,341,744; 1.8%) ().
The majority of PVFLP was in females (79.0%; £148,381,786), driven by productivity loss due to cervical cancer (£125,353,865; ). When excluding sex-specific cancers (cervical, vulval, vaginal, penile), and focusing on head and neck, and anal cancer, males suffered a greater PVFLP versus females (£34,315,270 vs £15,175,926, respectively) in which head and neck cancer was the bigger contributor.
Table 6. PVFLP related to HPV by cancer type and by sex in the UK in 2019
Sensitivity and scenario analysis
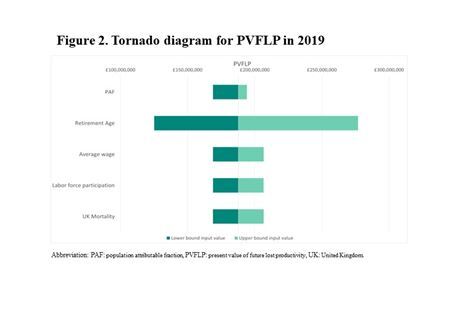
The DSA showed the results to be most sensitive to the retirement ages used in the model, with the upper and lower bound PVFLP ranging from £125,147,239 and £276,823,816 respectively (). This is because retirement age impacts the model’s measure of productivity, which considers the number of years a person remains productive. Varying the PAFs had the smallest impact on results (£168,988,480 to £194,006,089).
When the PAFs reported in Hartwig et al. 2017 were used, the number of deaths due to cancers related to HPV decreased by 1.37% to 1,792 from the base-case, a decrease of 25 deaths. PVFLP in the UK increased by 0.3% to £188,297,559, an increase of £532,581. This was mainly driven by head and neck cancer with a 16.2% increase in PVFLP as the PAF for head and neck cancer in Hartwig et al., was slightly higher than the base case. This trend was observed across all UK countries (Supplementary Table 6).
Discussion
Based on this analysis, there were 1,817 HPV-related cancer deaths in the UK in 2019, leading to 31,804 YLL. An approximately equal mortality burden occurred in males and females. Cervical cancer had the highest mortality, whilst vaginal and penile cancers caused the fewest deaths. The total productivity cost was £187,764,978, of which cervical cancer and anal cancer contributed the most. These trends were reflected across each of England, Scotland, and Wales. However, in Northern Ireland, only cervical cancer and head and neck cancer were included in the analysis due to underreporting of the other cancer types (Supplementary Table 5). A previous study by Bencina et al. (2024) reported the global productivity loss as per WHO region, of which the European (EU) region had a productivity loss of $32 billion (∼25 billion [USD:EUR 0.78]).Citation28 The UK had a higher productivity cost associated with HPV-related cancers compared to a previous analysis conducted in nine Central Eastern European countries where the highest productivity loss was seen in Romania (€44 million; ∼£37 million [EUR:GBP 0.85]) although Romania is a much smaller population the population of the UK (28.4%).Citation28 Similarly, another study conducted in Sweden found that premature mortality losses amounted to $36 million (∼£31 million [USD:EUR 0.78]).Citation29 Given that the number of cervical cancer cases are projected to increase by 3.5% in the UK by 2035, the productivity cost burden will only continue to rise.Citation30
The high mortality and economic burden estimated in this analysis highlights the need for widespread public health measures including education, screening, risk management, and vaccination against HPV. Whilst HPV vaccination is included in the national immunization program and HPV vaccination coverage rates are high in the UK,Citation6 there is still a significant burden, given that it will take a number of years for the impact of reduced HPV infection rates to be reflected in mortality rates. In the UK, the adolescent population, GBMSM under 45, and individuals at a higher risk from HPV routinely receive HPV vaccination,Citation5 and so mortality rates may remain high in older age groups where persons may already have contracted the infection, are not eligible for the national immunization efforts or have limited screening options. It is critical to maintain cervical cancer uptake to support in reducing the burden of HPV in the short- and long-term.Citation8
HPV vaccination was first introduced in 2008 for adolescent females (aged 12 to 13 years old) in the UK, due to the clear evidence for the role of HPV in causing cervical cancer cases.Citation6 This was alongside a catch-up program for girls aged 13 to 18 years to ensure broader coverage. In 2019, in a significant shift in cervical cancer screening, the UK moved from a cytology-based testing to HPV-first testing process.Citation31 Previously, cervical screening involved a cytology test first to detect abnormal cells, with HPV testing as a secondary measure to clarify ambiguous results. The new process relies on the fact that HPV primary screening is a more sensitive and accurate test. The test is better at eliminating individuals without infection and identifying those at a higher risk for cervical cancer who require further testing, further contributing to the reduction of mortality and productivity losses.
In light of mounting evidence for the role of HPV in cancers other than cervical, HPV vaccination has been recommended in adolescent males in the UK since 2019.Citation32 This analysis highlighted the high burden of HPV-related cancer in males, with a total PVFLP of £39,383,192 arising from 499 deaths. Despite being recommended since 2019, HPV vaccination coverage in males remains slightly lower in 12 to 13 (year 9) year old males (62.4%) relative to their female counterparts (69.6%).Citation6 A similar trend is seen in catch up vaccination for (14 to 15 year olds), where vaccination is offered to those younger than 25.Citation6, Citation7 Previous studies of parental attitudes towards HPV vaccination have shown parents of girls to be more willing to vaccinate their child than parents of boys.Citation33 This highlights the need to educate and raise awareness about the benefits of HPV vaccination in both boys and girls. Vaccinating males not only helps protect males directly but also contributes to herd immunity, indirectly benefitting unvaccinated individuals and accelerating the control of HPV-related diseases across the population. The high burden of non-female-specific HPV-related cancers highlighted in this analysis strengthens the rationale for vaccination in adolescents of both sexes. The development and implementation of HPV vaccination programs requires a comprehensive approach with all stakeholders aligned e.g. healthcare providers, policymakers, politicians, parents/guardians, school nurses, general physicians (GPs) and academics to be successful.
This analysis used mortality data from 2019 to avoid skews in the data as a result of the COVID-19 pandemic. HPV vaccination coverage in the UK fell from 2019 to 2021 due to school closures during national lockdown, and lower attendance rates than normal until March 2021.Citation6 It is therefore also crucial to ensure that HPV vaccination coverage returns to pre-pandemic levels, and that vaccination rate can be tracked accurately in the population along with regular updates on incidence and mortality rates.Unsurprisingly given the respectively population sizes, the majority of deaths, YLL, and PVFLP in this analysis occurred in England, with the fewest in Northern Ireland. Nevertheless, the YLL per death was higher in Northern Ireland relative to the other countries, indicating that people died at a younger age. Notably there was a lack of reporting for some cancer types in Northern Ireland, contributing to the underestimation of the overall results as well as within Northern Ireland. Comprehensive and regular reporting is important to help strengthen the evidence base necessary for developing effective public health policies aimed at reducing mortality and productivity losses.
HPV is a significant risk factor for cervical cancer. In the UK, cervical cancer incidence rates have shown fluctuations over time, influenced by changes in screening programs and the uptake of HPV vaccination. Notably, since the early 1990s, age standardized incidence rates (ASIR) of cervical cancer have decreased by 25% across the UK.Citation34 On the other hand, ASIR of non-sex-specific cancers, such as anal cancer has risen by 37% in both genders.Citation35 Moreover, there has been an increase in head and neck cancers incidence rates in the UK. Since the early 1990s, ASIR for head and neck cancers have increased by 34% in the UK for both sexes combined.Citation36 Specifically, ASIR for head and neck cancers in females and males increased by 45% and 22%, respectively. Enhancing and expanding screening programs for these cancers coupled with increasing HPV vaccine uptake, could present an encouraging prospect for mitigating their incidence rates. However, screening programs can only exist under specific conditions. The consequences of false-positive or false-negative results coupled with overdiagnosis mean it is important for stakeholders to consider any potential screening programs in the context of their health care system.Citation10 Continued research into population subgroups which could benefit from screening and innovative technologies is required.Citation37
Key strengths and limitations of the approach have been reported previously.Citation12–15 This model provides quantitative evidence of the lost productivity burden of HPV-related cancers. The model used publicly available datasets from reliable sources, generating robust outputs. The use of country-specific inputs such as retirement age, life expectancy, and average wage, ensures that the results are reflective of the local setting. Given that a number of HPV-related cancers are sex-specific, the use of gender-specific inputs ensures that productivity losses are not over- or underestimated as a result.
Limitations of the model surround the assumptions made due to the complexity of real-world data. For example, mortality was assumed to be uniform within age groups, and average wage was used for the proportion of the population actively employed, which is a simplification of the true situation. If a greater number of deaths occurred in lower income groups, then the value of lost productivity would be overestimated. For example, cervical cancer incidence rates in England are 65% higher for women living in the more deprived areas compared to the least deprived and mortality rates are 148% higher.Citation34, Citation38
There are also limitations related to the use of PAFs. For instance, HPV vaccination status was not recorded in this dataset but it was assumed that these cancer patients were not vaccinated against HPV, as vaccinations were only recommended in 2008 in the UK in adolescents only.Citation6 Furthermore, HPV assessment is not recorded in cancers such as head and neck cancer and penile cancer. Additionally, it is important to note that the available data assumes equal PAFs across different age groups. However, given that HPV vaccination in the UK is predominantly in adolescents, and as the incidence of HPV infection varies across age groups due to changes in sexual activity, it is unlikely that the impact of HPV on cervical cancer incidence is uniform across all age groups.
For patient anonymity, mortality was not widely reported for cancer types of lower incidence and mortality in Northern Ireland due to the small population size. However, as these are small numbers, the results are only slightly underestimated.
The model accounts only for productivity costs from premature mortality and does not consider direct costs to the healthcare system, such as medication or hospital costs. These costs are not insignificant, with a recent study estimating life-time direct medical costs (inpatient and outpatient) per patient of £16,911 for oropharyngeal cancer, £12,539 for penile cancer, £12,676 for vaginal cancer, £13,773 for anal cancer, and £12,721 for cervical cancer in England.Citation11 Productivity losses due to absenteeism resulting from HPV-related morbidity are also not accounted for. These results may therefore be considered a conservative estimate of the true economic burden of HPV-related cancers in the UK.
Conclusion
There is a high mortality burden and associated productivity cost in the UK for HPV-related cancers, with a large economic impact on the wider economy due to productivity losses. It is critical to implement and reinforce public health measures with the aim to reduce the incidence of HPV-related diseases, and the subsequent premature cancer deaths. Maintaining high HPV vaccination coverage in both males and females along with widespread screening, disease awareness and treatment availability in key populations will support towards reducing this burden in future decades.
Transparency
Declaration of funding
Declaration of financial/other interests
KE, OO, EM, DN and GB are employees of MSD subsidiaries of Merck & Co., Inc., Rahway, NJ, USA and may own stocks and/or stock options in Merck & Co., Inc., Rahway, NJ, USA.
HS, AM, GW and RH are employees of Adelphi Values (PROVE), paid consultants to MSD.
Reviewer disclosures
Peer reviewers on this manuscript have received an honorarium from JME for their review work but have no other relevant financial relationships to disclose.
Author Contributions
GB and GW conceptualized and designed the study. AM and GW supervised the study. OO and KE conducted the data analysis. GW, RH, and AM visualized and interpreted the data, and reviewed the literature. HB drafted the manuscript with supervision from GW and AM. All authors contributed to the interpretation of the results and commented on the manuscript. All authors read and approved the final version of the manuscript. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Acknowledgements
None stated.
Previous presentations
The results of this analysis were previously presented at the 2023 ISPOR Annual European Congress (Kayla Engelbrecht, Olga Ovcinnikova, Dionysios Ntais, Robert Hughes, Georgie Weston and Goran Bencina). Indirect Cost of HPV-Attributable Cancer in the United Kingdom. Value in Health 2023, 26:12_suppl, S124).
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials where links are available to the publicly available datasets used.
Supplemental Material
Download MS Word (47.8 KB)Additional information
Funding
References
- World Health Organization. Estimated number of new cases in 2022, United Kingdom, both sexes, all ages (excl. NMSC). Accessed 26th February, 2024. https://gco.iarc.fr/today/en/dataviz/tables?mode=cancer&populations=826&types=1&sort_by=value1
- Hofmarcher T, Lindgren P, Wilking N, Jönsson B. The cost of cancer in Europe 2018. Eur J Cancer. Apr 2020;129:41-49. doi: 10.1016/j.ejca.2020.01.011.
- de Martel C, Georges D, Bray F, Ferlay J, Clifford GM. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health. Feb 2020;8(2):e180-e190. doi: 10.1016/s2214-109x(19)30488-7.
- National Cancer Institute. HPV and Cancer. Accessed 30th October, 2023. https://www.cancer.gov/about-cancer/causes-prevention/risk/infectious-agents/hpv-and-cancer
- National Health Services (NHS). HPV vaccine. Updated 1 September 2023. Accessed Oct, 2023. https://www.nhs.uk/conditions/vaccinations/hpv-human-papillomavirus-vaccine/#:∼:text=All%20children%20aged%2012%20to,born%20after%201%20September%202006
- UK Health Security Agency. Human papillomavirus (HPV) vaccination coverage in adolescents in England: 2021 to 2022. Health Protection Report. Vol. 16. 20 December 2022. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1126762/hpr1322-HPV2.pdf
- HPV vaccination programme moves to single dose from September 2023. 18 December 2023, 2023. https://www.gov.uk/government/news/hpv-vaccination-programme-moves-to-single-dose-from-september-2023#:∼:text=If%20you%20missed%20your%20HPV,vaccine%20until%20your%2025th%20birthday.
- National Health Services (NHS). Screening and earlier diagnosis, cervical screening. Accessed 28 February, 2024. https://www.england.nhs.uk/cancer/early-diagnosis/screening-and-earlier-diagnosis/#cervical-screening
- UK Government. Cervical screening standards data report 2021 to 2022. Accessed 28 February, 2024. https://www.gov.uk/government/publications/cervical-screening-standards-data-report-2021-to-2022/cervical-screening-standards-data-report-2021-to-2022#contents
- World Health Organization. Regional office for Europe. Screening programmes: a short guide. 2020. Accessed 05th June 2024. chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://iris.who.int/bitstream/handle/10665/330829/9789289054782-eng.pdf
- Fabiano G, Marcellusi A, Mennini FS, Sciattella P, Favato G. Hospital resource utilisation from HPV-related diseases in England: a real-world cost analysis. The European Journal of Health Economics. 2023/02/01 2023;24(1):75-80. doi: 10.1007/s10198-022-01453-x.
- Bencina G, Chami N, Hughes R, et al. Breast cancer-related mortality in Central and Eastern Europe: years of life lost and productivity costs. J Med Econ. Jan-Dec 2023;26(1):254-261. doi: 10.1080/13696998.2023.2169497.
- Bencina G, Chami N, Hughes R, Weston G, Baxter C, Salomonsson S. Assessing the impact of kidney cancer-related premature mortality and productivity loss in Greece and Portugal. Expert Rev Pharmacoecon Outcomes Res. Apr 2023;23(4):391-398. doi: 10.1080/14737167.2023.2180356.
- Bencina G, Chami N, Hughes R, et al. Indirect Costs Due to Lung Cancer-Related Premature Mortality in Four European Countries. Adv Ther. May 17 2023; doi: 10.1007/s12325-023-02509-x.
- Bencina G, Chami N, Hughes R, Weston G, Golusiński PJ. Lost productivity due to head and neck cancer mortality in Hungary, Poland, and Romania. Journal of Cancer Policy. 2022/12/01/ 2022;34:100366. doi: 10.1016/j.jcpo.2022.100366.
- NICE. NICE health technology evaluations: the manual. Process and methods [PMG36]. https://www.nice.org.uk/process/pmg36/chapter/economic-evaluation
- NHS Digital. CancerData: Cancer incidence and mortality Updated 20 October 2022. https://www.cancerdata.nhs.uk/incidence_and_mortality
- Public Health Scotland. Cancer Mortality: Annual update to 2021 Updated 25 October 2022. https://publichealthscotland.scot/publications/cancer-mortality/cancer-mortality-in-scotland-annual-update-to-2021
- Public Health Wales. Cancer Mortality in Wales, 2002-2021 Updated 16 March 2022. https://phw.nhs.wales/services-and-teams/welsh-cancer-intelligence-and-surveillance-unit-wcisu/cancer-mortality-in-wales-2002-2021/
- Queen’s University Belfast. Northern Ireland Cancer Registry, Official Statistics. Updated 2020. https://www.qub.ac.uk/research-centres/nicr/CancerInformation/official-statistics
- World Bank. Women, Business, and the Law. Pension. https://wbl.worldbank.org/en/data/exploretopics/wbl_gpen
- Office for National Statistics. National life tables - life expectancy in the UK: 2018 to 2020. Updated 22 September 2021. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/bulletins/nationallifetablesunitedkingdom/2018to2020
- de Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. International Journal of Cancer. 2017;(1097-0215 (Electronic))
- Eurostat. Mean annual earnings by sex, age and economic activity (2018). https://ec.europa.eu/eurostat/databrowser/view/EARN_SES18_27__custom_1501377/default/table?lang=en
- World Bank. Labor force participation rate, total (% of total population ages 15-64) (modelled ILO estimate) - United Kingdom https://data.worldbank.org/indicator/SL.TLF.ACTI.ZS?locations=GB
- Hartwig S, St Guily JL, Dominiak-Felden G, Alemany L, de Sanjosé S. Estimation of the overall burden of cancers, precancerous lesions, and genital warts attributable to 9-valent HPV vaccine types in women and men in Europe. Infectious Agents and Cancer. 2017/04/11 2017;12(1):19. doi: 10.1186/s13027-017-0129-6.
- Office for National Statistics (ONS). Population estimates for the UK, England, Wales, Scotland and Northern Ireland: mid 2021. Updated 21 December 2022. Accessed 28 November, 2023. https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/bulletins/annualmidyearpopulationestimates/mid2021
- Bencina G, Oliver E, Meiwald A, et al. Global burden and economic impact of vaccine-preventable cancer mortality. Journal of Medical Economics. 2024/04/19 2024;27(sup2):9-19. doi: 10.1080/13696998.2024.2350877.
- Östensson EA-OX, Silfverschiöld M, Greiff L, et al. The economic burden of human papillomavirus-related precancers and cancers in Sweden. 2017;(1932-6203 (Electronic))
- World Health Organization. Cancer tomorrow. Estimated number of new cases from 2022 to 2035. Accessed 05th June, 2024. https://gco.iarc.fr/tomorrow/en/dataviz/bubbles?types=0&populations=8_40_56_70_100_112_191_196_203_208_233_246_250_276_300_348_352_372_380_428_440_442_470_498_499_528_578_616_620_642_643_688_703_705_724_752_756_804_807_826&cancers=23&group_populations=0&group_cancers=1&multiple_cancers=1&years=2035&sexes=0
- UK Government. Changes to cervical cancer screening, Department of Health and Social Care. Accessed 30th May, 2024. https://www.gov.uk/government/news/changes-to-cervical-cancer-screening
- JCVI. Statement on HPV vaccination. https://assets.publishing.service.gov.uk/media/5b4e0a34e5274a73119d7614/JCVI_Statement_on_HPV_vaccination_2018.pdf
- Waller J, Forster A, Ryan M, Richards R, Bedford H, Marlow L. Decision-making about HPV vaccination in parents of boys and girls: A population-based survey in England and Wales. Vaccine. Jan 29 2020;38(5):1040-1047. doi: 10.1016/j.vaccine.2019.11.046.
- Cancer Research UK. Cervical cancer incidence statistics. Accessed 08 January, 2024. https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/cervical-cancer
- Cancer Research UK. Anal cancer incidence statistics. Accessed 28 February, 2024. https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/anal-cancer/incidence#heading-Two
- Cancer Research UK. Head and neck cancer incidence statistics. Accessed 28 February, 2024. https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/head-and-neck-cancers/incidence#heading-Two
- Cancer Research UK. Screening for mouth and oropharyngeal cancer. Accessed 05th June, 2024. https://www.cancerresearchuk.org/about-cancer/mouth-cancer/getting-diagnosed/screening
- Cancer Research UK. Cervical cancer mortality statistics. Accessed 08 January, 2024. https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/cervical-cancer/mortality#heading-Four