?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background and objectives
Nearly one in ten individuals in South-East Asia are estimated to be affected by chronic kidney disease (CKD). The burden of end-stage kidney disease is significant and can be heavy on the healthcare system. The recent EMPA-KIDNEY trial demonstrated a significant reduction in the risk of kidney disease progression or cardiovascular death in patients with CKD with a broad range of kidney function using add-on empagliflozin versus standard of care (SoC) alone. The objective of this study was to estimate the economic benefit of empagliflozin for patients with CKD in Malaysia, Thailand and Vietnam.
Methods
An individual patient level simulation model with an annual cycle that estimates the progression of kidney function and associated risk-factors was employed. Local costs and mortality rates were estimated from a wide range of published literature. A healthcare perspective was used over a 50-year time horizon.
Results
The use of add-on empagliflozin versus SoC alone was found to be cost-saving in Malaysia and Thailand and cost-effective (ICER: 77,838,407 Vietnam Dong/QALY vs. a willingness to pay threshold of 96,890,026/QALY) in Vietnam. The bulk of the costs avoided over a lifetime is derived from the prevention or delay of dialysis initiation or kidney transplant – the cost offsets were nearly twice the additional treatment cost. The results were similar in patients with and without diabetes and across broad range of albuminuria.
Conclusions
The use of add-on empagliflozin in a broad population of patients with CKD is expected to be cost-saving in Malaysia and Thailand and cost-effective in Vietnam and will help alleviate the increasing burden of CKD in the region.
Introduction
Chronic kidney disease (CKD) is a progressive clinical condition characterized by a gradually declining kidney function until end-stage renal disease or even death. There were an estimated 697.5 million cases of CKD worldwide in 2017 with an incidence rate increasing by 29.3% compared with that in 1990Citation1.
The estimated prevalence of CKD stages 3–5 in South-East Asia is approximately 12% and the risk-factors associated with developing CKD such as older age, obesity, hypertension, diabetes, and dyslipidaemia are on the rise in the region - implying an increased burden on society in the futureCitation2. In Thailand, in 2009, the Thai Screening and Early Evaluation of Kidney Disease (SEEK) study group reported that the prevalence of CKD in Thailand was 17.5%Citation3 and the total annual cost (including ambulatory costs and hospitalization costs) for patients with CKD stage G3a, G3b, or G4 was 5,701.34 Thai Baht (equivalent to USD 189.92)Citation4. Similarly, in Vietnam, the average annual costs of treatment per patient for CKD stage 4 or 5 was nearly USD 315–775 in 2018Citation5. In Malaysia, the estimated costs of treating end-stage kidney disease (ESKD) alone in 2016 was nearly USD 1 billion adjusted for purchasing power parity (PPP), with 94% of those being driven by dialysis costsCitation6.
The cardiovascular (CV) outcome trials of Sodium-Glucose Transport Protein 2 Inhibitors (SGLT2i) not only showed the efficacy of those agents in reducing CV mortality and hospitalization for heart failure in patients with type 2 diabetes mellitus (T2DM), but the secondary outcomes from these early trials also revealed up to a 40% reduction in the risk of progression of kidney diseaseCitation7. More recently, the efficacy of SGLT2is in CKD, irrespective of the presence of T2DM, was reported. The DAPA-CKD trial investigated the benefits of dapagliflozin on kidney outcomes in participants with CKD within an estimated glomerular filtration rate (eGFR) range of 25 to <75 mL/min/1.73 m2 and urine albumin-creatinine ratio (uACR) range of 200–5,000 mg/g)Citation8. EMPA-KIDNEY evaluated the effects of empagliflozin on the progression of CKD in patients with or without T2DM with an even lower eGFR of 20–45 mL/min/1.73 m2 regardless of albuminuria or an eGFR 45–90 mL/min/1.73 m2 with uACR ≥200 mg/g (22 mg/mmol). This trial demonstrated a 28% reduction in the progression of kidney disease for patients on empagliflozin on top of standard of care (SoC) versus SoC aloneCitation9. The benefits of empagliflozin were similar among patients with or without diabetes and irrespective of eGFRCitation9.
In light of the current socio-economic burden of CKD in South-East Asian countries and the benefits demonstrated in terms of kidney disease progression in a broad range of patients studied in the EMPA-KIDNEY trial, the aim of this study was to assess the economic value of empagliflozin in patients with CKD in Malaysia, Thailand and Vietnam.
Methods
Model structure
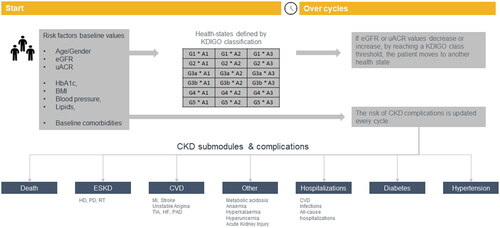
The model employed an individual patient level micro-simulation that utilizes several risk equations as well as data from published studies to predict the progression of CKD and the risk of acute events. The model structure is based on CKD health states defined by Kidney Disease: Improving Global Outcomes (KDIGO) classification and one absorbing state “all cause death”. Further details on the model and data sources used to inform the risk equations can be found in Uster et al.Citation10 In summary, the model tracks the evolution of key risk factors for CKD progression such as estimated eGFR, uACR, as well as total cholesterol, high-density lipoproteins, systolic blood pressure (SBP), body mass index (BMI) and glycated haemoglobin (HbA1c) based on risk prediction engines and clinical data were derived from CKD registries, such as CKD-Prognosis Consortium (CKD-PC) and Chronic Renal Insufficiency Cohort Study (CRIC) and other long-term cohort studies. According to the estimated eGFR and uACR levels at each time cycle, the individual patient is allocated to one of the 18 KDIGO health states. Based on the cycle-values of the eGFR, uACR and other risk factors, the model estimates the risk of clinical outcomes, including ESKD, acute kidney injury, CV disease including CV death, hypertension, and diabetes among others
A graphical summary of the model mechanics is presented in .
Figure 1. Underlying model structure. BMI, body mass index; CKD, chronic kidney disease; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; ESKD, end stage kidney disease; HbA1c, glycated haemoglobin; HF, heart failure; KDIGO, kidney disease: improving global outcomes; PAD, peripheral arterial disease; uACR, urine albumin-creatinine ratio; TIA, transient ischaemic stroke.
A cycle length of one year is adopted in the modelled economic evaluation and costs and utilities were discounted at 3% for all three countries.
The base case analysis has adopted a life-time horizon of 50 years to evaluate the full clinical and economic impact of empagliflozin in CKD patients that met the inclusion criteria of the EMPA-KIDNEY Intention to Treat (ITT) population using a healthcare perspective.
The primary outcome of this modelling study is the Incremental Cost Effectiveness Ratio (ICER) which is estimated as the incremental costs divided by the incremental Quality-Adjusted Life-Years (QALYs) gained between the two treatment options.
Treatment effects
Treatment effects on eGFR and uACR were implemented separately for the intervention (empagliflozin + SoC) and the comparator (SoC alone) based on values observed in the trial. The treatment effect was assumed to persist while patients remain on treatment; following treatment discontinuation, CKD progression was projected based on observations from large cohort studies. The annual changes in absolute eGFR and the ratios of uACR change as per KDIGO stage are presented in and Citation2, respectively.
Table 1. Annual absolute changes in eGFR based on KDIGO categories used in the model.
Table 2. Annual fold change in uACR based on KDIGO categories used in the model.
The values for health-states G2A1 and G3A1 were not estimated as these categories fall outside of the inclusion criteria for the EMPA-KIDNEY trial. Consequently, a conservative assumption of no treatment effect was applied for the purpose of this study.
Treatment effects on other risk factors were obtained from the EMPA-KIDNEY trial and are presented in .
Table 3. Treatment effects on other risk-factors used in the model.
Additionally, treatment effects for acute kidney injury (AKI), and hospitalization for heart failure (hHF) for empagliflozin plus SoC over SoC alone were assumed to be 0.22 (HR of 0.78) and 0.2 (HR of 0.80) based on findings from the EMPA-KIDNEY trialCitation9.
Annual discontinuation rates for empagliflozin + SoC and SoC were set to 12.56 and 14.16 per 100-patient-year, respectively, as informed from the EMPA-KIDNEY trialCitation9.
Costs and resource use
All costs are presented in the country’s local currency units – Malaysian Ringgit (MYR), Thai Baht (THB) and Vietnamese Dong (VND) – and were inflated to 2022 values based on the medical services sub-index reported in the official national statistics data sourcesCitation11–13.
For comparison purposes, key incremental costs are presented in United States Dollars (1 USD = 35.28 THB = 4.66 MYR = 24,224 VND)
Monitoring costs
In all countries, costs were only available for KDIGO eGFR states and not stratified by albuminuria stages. Consequently, it was assumed that the eGFR-specific costs presented in literature were valid across A1 to A3 levels within the same eGFR stage.
Annual monitoring costs for Malaysia were taken from a cross-sectional, observational study of Malaysian T2DM patients visiting 20 government health clinicsCitation14. The costs reported by Azmi et al. may be overestimated for the overall CKD population as those studied were specifically T2DM patients.
For Thailand, the costs were estimated from Vareesangthip et al. who reported the costs of CKD treatment obtained from the Chronic Kidney Disease Prevention in the Northeast Thailand (CKDNET) studyCitation15.
Nguyen et al. (2018) conducted a cross-sectional study to estimate the total cost associated with CKD; the study included 327 patients who received CKD treatment from the Kien Giang General Hospital in southwest Vietnam in 2017Citation16. To estimate the monitoring costs of CKD for Vietnam, the direct medical costs reported in this study were used.
Cardiovascular complications
Malaysia
Costs of myocardial infarction and stroke (acute first-year and subsequent years) in Malaysia were obtained from Shafie AA et al. (2020), who used a bottom-up approach to estimate the costs of managing diabetes complicationsCitation17. As with the monitoring costs for CKD, these costs may be overestimated in the overall population as the costs of CV events with T2DM are understood to be higher than those without T2DM.
The acute cost of heart failure was obtained from Ong et al. (2022), who estimated the cost of treating chronic adult patients with HF in three HF clinics between 2016 and 2018)Citation18. The annual subsequent costs were obtained from Shafie AA et al. (2020)Citation17.
The local costs of unstable angina (UA) and peripheral artery disease (PAD) were not identified and hence it was assumed to the be proportional to the relative Thai costs of either of the two with respect to the cost of myocardial infarction in Thailand.
Thailand
The (acute first-year and subsequent years) costs of CV complications for Thailand were obtained from a cost-effectiveness study of Dipeptidyl peptidase-4 inhibitor (DPP4i) versus sulphonylurea by Permsuwan et al. (2016)Citation19.
Vietnam
The annual costs for myocardial infarction and stroke (acute and follow-up) were obtained from Nguyen et al. (2021), who estimated the direct medical costs using a retrospective analysis of data from five large representative hospitals for acute coronary syndrome treatment in Vietnam in 2017Citation20. The cost of a hospitalization for a heart-failure event was based on an estimate by Reyes et al. (2016)Citation21. As with the case of Malaysia, the missing costs for UA and PAD (acute and follow-up) were assumed to the be proportional to the relative costs of either of the two with respect to myocardial infarction in Thailand.
ESKD and kidney replacement therapy (KRT) modalities
In the model, patients entering the ESKD health state could receive one of three modalities of KRT – haemodialysis (HD), peritoneal dialysis (PD), or kidney transplant (KT), or remain on conservative therapy.
Malaysia
The annual costs of PD and HD were estimated from a study by Surendra et al. (2019)Citation22. The costs of kidney transplants (first year and subsequent years) and the proportion of living versus deceased donors were informed from Bavanandan et al. (2015)Citation23.
The breakdown KRT modalities were taken from the 2021 report of the Malaysian dialysis and transplant registryCitation24.
The costs of inpatient and outpatient AKI events were taken from Ong et al. (2023)Citation25.
Thailand
The annual costs of PD and HD were taken from Permsuwan et al. (2016)Citation19 and the annual and follow-up costs of kidney transplants were taken from Phongphithakchai et al. (2022)Citation26. The proportion of deceased donors for kidney transplants was informed from Larpparisuth et al. (2021)Citation27.
The breakdown of KRT modalities was taken from the Thai Renal Replacement Therapy 2020 report of the Nephrology Society of ThailandCitation28.
The cost of an inpatient AKI event was taken from Chatmongkolchart et al. (2016)Citation29 and the outpatient costs were estimated based on the relative proportional costs of the two AKI events from Malaysia.
Vietnam
The cost of dialysis in Vietnam was taken from Karopadi et al. (2013) and this source estimated the costs of HD and PD to be similarCitation30. No peer-reviewed sources for transplantation costs were identified for Vietnam and hence the average relative costs of a living donor transplant and HD from Malaysia and Thailand were used to estimate those for Vietnam. Similarly, the average relative costs of deceased donor and living donor transplants for the two former countries were used to estimate the deceased donor costs for Vietnam. The proportion of the two in Vietnam was informed by Tran (2018)Citation31.
The breakdown of KRT modalities were taken from Alexander et al. (2021)Citation32.
The costs of an outpatient and inpatient AKI event was assumed to be similar to the corresponding CKD stage 4 costs as estimated by Nguyen-Thi et al. (2021)Citation5.
Other events
The annual costs of anaemia for Malaysia, Thailand and Vietnam were taken from Azmi et al. (2018) (estimated as the difference between the mean annual costs of the T2DM patients with and without anaemia), Thaweethamcharoen et al. (2014)Citation33, and Nguyen-Thi et al. (2021)Citation5, respectively. The annual costs for CKD stage G5 estimated from Azmi et al. included dialysis costs; to prevent double counting, a ratio of the G1:G5 costs from Thailand were used to adjust the Malaysian estimates. For Thailand, the treatment costs of anaemia were estimated using the formula for the erythropoietin dose required, assuming a steady state target Hb level of 12 g/dl and a pre-treatment level of 10.2 g/dl.
Reliable estimates of the costs of metabolic and mineral disorders were metabolic acidosis, hyperkalaemia, and hyperuricemia were not identified for all three countries and these were set to zero for the analysis.
Among adverse events, only lower limb amputation costs were considered; the costs for Malaysia, Thailand and Vietnam were taken from Shafie AA et al. (2020)Citation17, Permsuwan et al. (2016)Citation19 (sum of the costs of gangrene treatment and amputation prosthesis), and Patikorn et al. (2022)Citation34.
A summary of the costs is presented in .
Table 4. Summary of the cost inputs used in the model.
Treatment acquisition costs
Standard of care
Angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARB) were assumed to be the standard of care for CKD patients irrespective of their KDIGO classification. Within each class of drugs, the breakdown by active ingredient was informed based on the usage (at baseline) reported in the EMPA-KIDNEY trial.
The drugs representing approximately 90% of within-class usage in the trial were considered to simplify the costing process. Publicly available pricing data sources (Pharmacy Services Program Pricing Guide for Malaysia, Drug and Medical Supply Information Center for Thailand, and Drug Administration of Vietnam tender prices for Vietnam) were used to inform the price per tablet and subsequently the cost per year of each class of drug. If a particular price was not listed for a particular drug, then it was excluded from the analysis. The costing summary is presented in .
Table 5. Cost estimation for SoC.
Mortality estimates
The CKD-PM model enables separate projection of CV death and ESKD-related mortality. These causes of death are exempted from the country-specific all-cause mortality estimates. The latter were extracted from the WHO Global Health Observatory Life Tables by country dataset for 2019 for Thailand and VietnamCitation35 and from Department of Statistics Malaysia’s estimates for 2021 for MalaysiaCitation36.
The age-specific numbers of deaths for any cause, CV and renal-related causes were taken from the Global Burden of Disease Study 2019Citation1. These estimates were used to calculate the risk of death due to other causes.
Utilities
Utilities and disutilities are assigned to a specific health state, complication or event in an annual cycle. Health state utilities are applied to the KDIGO heath states, while separate utility values are applied to patients who initiate KRT and enter the ESKD submodule.
Disutilities are applied to complications and events, other than those in the ESKD submodule. QALYs for empagliflozin plus SoC and SoC alone are estimated by applying the health state utility to the simulated patient in each model cycle while a patient remains alive.
For the ESKD management options, a utility weight is applied for as long as a patient receives a particular treatment. Disutilities for acute disease related complications and adverse events are applied in the cycle that the event occurs. The same value is used for the first and recurrent myocardial infarction, stroke and transient ischaemic attack events. No disutility is applied for patients with diabetes or hypertension, as these are highly prevalent comorbidities and it is assumed that the respective impact on utility is captured in the overall health state utility.
Utility values specific to KDIGO health states were informed based on eGFR class-based estimates as reported by Jesky et al.Citation37 Disutilities for HD and PD were based in Liem at al.Citation38 while that of kidney transplant were taken from Lee et al.Citation39
Disutility estimates for other acute events are presented in the Supplementary Appendix.
KRT flow
The estimates for the flow of patients across the KRT modalities and survival were estimated based on the 2021 report of the Malaysian dialysis and transplant registryCitation24; these were assumed to hold true for the Thailand and Vietnam, too.
The 1-year survival rates for HD and PD were based on the 12-month estimate of the patient survival by type of dialysis modality − 0.88 for both HD and PD.
The percentages of PD and HD patients moving onto RT were taken for the year 2019 to avoid any impact of the COVID pandemic. The formula used is presented below.
No patients were assumed to move between HD and PD. A summary of the 1-year probabilities is presented in .
Table 6. 1-Year transition probabilities across KRT modalities or death.
For RT, the proportion of individuals with a failed RT (death and/or graft failure) is taken to be 6.1% and individuals that had a failed RT, but survived were assumed to move to HD.
Scenario analyses
Patients with normo- and microalbuminuria
While the EMPA-KIDNEY trial was not powered to assess the primary endpoints based on albuminuria levels, an assessment of the value of empagliflozin on preventing or delaying time to ESKD in patients with normo- and microalbuminuria will be of interest for health policy or clinical decisions. These patients have a slower rate of kidney function decline compared to patients with macroalbuminuriaCitation40. While the primary endpoints were not significant for these two groups, the EMPA-KIDNEY trial had a median follow-up duration of only two years, and it is unlikely that a significant number of these patients experienced a progression of kidney disease in either arm for the analyses to reach statistical significance.
Nonetheless, the group treated with empagliflozin had a lower rate of annual decrease in eGFR when compared with placebo – −1.37 compared to −2.75 mL/min per 1.73 m2 per year, respectively, when looking at chronic slope. As described earlier, the progression model tracks change in eGFR (and uACR) to inform the risks of developing ESKD over a life-time horizon. This allows for the modelling the long-term benefits of Empagliflozin in these groups and so, the model was run for uACR A1 and A2 groups.
Presence of diabetes
A scenario analysis was conducted to assess cost-effectiveness in patients with and without diabetes, accounting for differences the baseline risk in these subgroups.
Sensitivity analyses
A deterministic one-way sensitivity analysis was conducted for all three countries. Net monetary benefit (NMB) was chosen as the outcome (rather than ICER) as this would allow the representation of positive and negative incremental costs simultaneously; NMB was estimated by multiplying the incremental discounted QALYs gained with the country-specific wiliness to pay thresholds and then subtracting the discounted incremental costs. The willingness to pay thresholds assumed for Malaysia, Thailand and Vietnam were MYR 52,689 (USD 11,307), THB 160,000 (USD 4,535), and VND 96,890,026 (USD 4,000), respectively – while no official Vietnamese guidelines are published, a 1× Gross Domestic Product (GDP) per capita value for 2022 was used as a threshold.
A probabilistic sensitivity analysis (PSA) was additionally conducted for all three countries. Due to computational requirements of the microsimulation model, 300 iterations were conducted for each country.
Results
The results of the analyses over a 50-year time-horizon for the three countries are presented below in .
Table 7. Summary of the cost-utility analysis results.
The majority of the costs avoided over a lifetime are driven by the avoidance or delay of KRT initiation; per patient, the costs avoided in Malaysia, Thailand and Vietnam come to MYR 15,569 (USD 3,340), THB 176,731 (USD 5,010), and VND 67,312,952 (USD 2,780), respectively. The cost offset for KRT is nearly 2 times the additional treatment cost of empagliflozin.
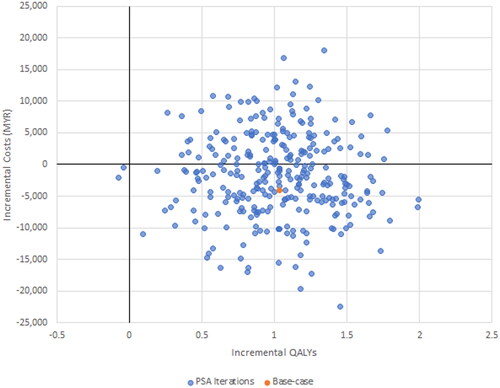
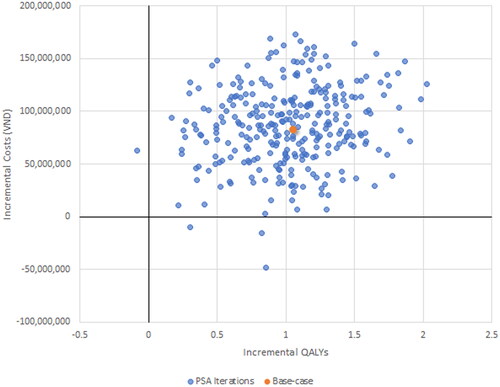
Consequently, the use of empagliflozin is estimated to be cost-saving in Malaysia and Thailand, and cost-effective (ICER of VND 77,838,407/QALY or USD 3,213/QALY against a willingness to pay threshold of 1GDP per capita of VND 96,890,026 or USD 4,000) in Vietnam.
The outcomes for each country by treatment arm were similar due to the common risk equations used to estimate them. The average event counts are presented below in .
Table 8. Average risk of CV and renal outcomes for empagliflozin (plus SoC) versus SoC alone over the modelled time horizon.
Across the three countries, on an average, the use of empagliflozin is estimated to reduce the risk of initiation of dialysis by nearly 10% and renal transplants by nearly 1% over a 50-year time horizon. The risk of CV events in patients on empagliflozin is increased due to the survival paradox, as these patients are projected to live longer (undiscounted LY gains is on an average 1.9 years) and experience higher risks of CV outcomes with increasing age. Additionally, the risk of ESKD related mortality is reduced by 8.2%, while the risks of CV and all-cause mortality in the empagliflozin group is increased by 4.3 and 3.9%, respectively.
Subgroup results
Diabetes status
Empagliflozin + SoC is the dominant option compared to SoC alone in both patients with diabetes and patients without diabetes in Malaysia and Thailand and is cost-effective in Vietnam compared to the earlier assumed WTP threshold ().
Table 9. Model outcomes based on T2DM status.
The finding that the use of empagliflozin in patients with non-diabetic CKD provides more value than in patients with diabetes could be attributed to the fact that modelled patients with diabetes are older (as per the EMPA-KIDNEY baseline-characteristics) with fewer projected remaining life years than patients with other causes of CKD, who tend to be younger (average mortality rate of 13.56 vs. 10.69 per 100 patient-years in the SoC arms, respectively). Thus, a larger proportion of patients without diabetes may require KRT in a lifetime and hence, incremental cost savings from the use of empagliflozin over SoC are more pronounced in this subgroup.
Patients with normo- or microalbuminuria
While the use of empagliflozin + SoC in patients without proteinuria is a cost-effective option in Malaysia and Thailand, the findings of a less prominent economic value in these patients as compared to the overall ITT population is as expected, considering that these patients have a slower rate of progression to ESKD. Thus, the costs offsets associated with KRT versus CVD are lower in this group when compared to the overall ITT population. Nonetheless, the average KRT costs saved versus SoC more than offset in the increased treatment costs across all three countries ().
Table 10. Model outcomes for patients with normo- or microalbuminuria.
One-way sensitivity analyses
The results of the OWSA show that the outcomes were largely consistent across a range of the parameters tested ().
Figure 2. One-way sensitivity analysis results – Malaysia. The horizontal axis is the Net Monetary Benefit (NMB) in Malaysian Ringgit (MYR); 1 USD = 4.66 MYR. A positive NMB indicates a cost-effective result (considering a willingness-to-pay threshold of MYR 52,689 (USD 11,307))
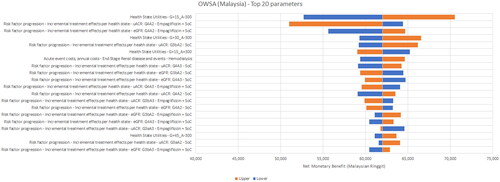
Figure 3. One-way sensitivity analysis results – Thailand. The horizontal axis is the Net Monetary Benefit (NMB) in Thai Baht (THB); 1 USD = 35.28 THB. A positive NMB indicates a cost-effective result (considering a willingness-to-pay threshold of THB 160,000 (USD 4,535))
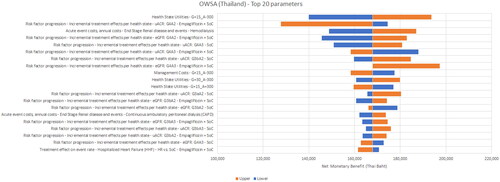
Figure 4. One-way sensitivity analysis results – Vietnam. The horizontal axis is the Net Monetary Benefit (NMB) in Vietnamese Dong (VND); 1 USD = 24,224 VND. A positive NMB indicates a cost-effective result (considering a willingness-to-pay threshold of VND 96,890,026 (USD 4,000))
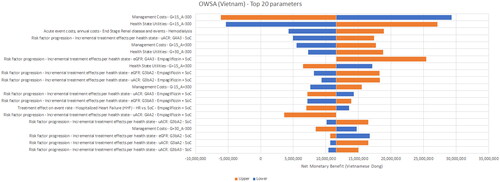
The most sensitive parameters were the treatment effects for empagliflozin + SoC with respect to the annual change in eGFR for the G4 health states and utilities for the same state. The only non-cost-effective scenarios were found for Vietnam, when the annual management costs were assumed to be higher and health-state utilities were assumed to be lower for the G4A3 KDIGO stage. The ratio of PD or HD costs to G4 costs in Vietnam was only around three while that for Malaysia and Thailand was about 10 – implying that preventing or delaying a transition to ESKD in Vietnam did not offset the pre-ESKD management costs sufficiently.
Probabilistic sensitivity analyses
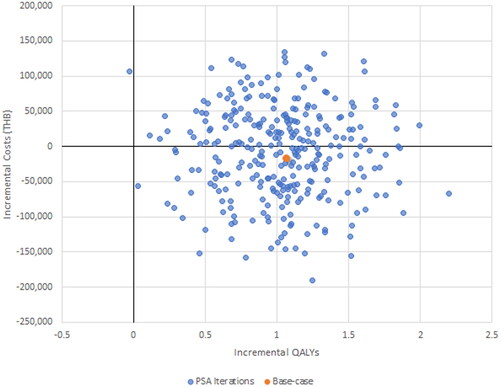
The results of the PSA demonstrate for Malaysia none of the 300 iterations exceeded the willingness to pay threshold, just 1.3% of iterations did so for Thailand and 40% for Vietnam ().
Discussion
The study results suggest that the use of Empagliflozin vs. SoC in three South-East Asian countries – namely Malaysia, Thailand and Vietnam – is expected to be a cost-saving option in Malaysia and Thailand and cost-effective in Vietnam. Scenario analyses also showed that these findings held true irrespective of T2DM presence.
Based on the broad eGFR and uACR criteria for the EMPA-KIDNEY ITT population, the average KRT cost-offset versus SoC alone in Malaysia, Thailand and Vietnam is estimated to be approximately MYR 15,500 (USD 3,340), THB 177,000 (USD 5,010), and VND 67.3 million (USD 2,780), respectively, over a lifetime horizon – with the average time on treatment of around 5.5 years. In all three cases, the cost savings from KRT exceeded the increased treatment costs from the use of empagliflozin.
EMPA-KIDNEY studied a broader range of patients with CKD compared to other SGLT2i kidney trials. When considering only patients with normo- or micro-albuminuria, the modelling estimates indicate that empagliflozin was still cost-saving in these lower-risk patients in Malaysia, cost-effective in Thailand and marginally over the willingness to pay threshold for cost effectiveness in Vietnam.
CKD is known to be a progressive disease and an earlier intervention in these patients should delay the progression to ESKD and thus alleviate the economic burden to patients, caregivers, and also to the healthcare system. The outcomes from this modelling study show that the incidence of ESKD could be reduced by almost 10% through early use of empagliflozin in patients with CKD across the broad range of albuminuria values.
Studies that assessed the cost-effectiveness of empagliflozin in the United StatesCitation41, United KingdomCitation42, ItalyCitation43, and the NetherlandsCitation44 reported similar results – the use of empagliflozin + SoC was a dominant option compared to SoC alone. In another study, performed a CEA in patients with T2DM, based on EMPAREG-OUTCOME trial, Reifsnider et al. estimated that the use of empagliflozin to be a cost-effective option in patients with T2D and CKD in the USCitation45.
The findings of the current study are in line with the results seen for other SGLT2 inhibitors like dapagliflozin. Specifically, Vareesangthip et al. found the use of dapagliflozin to be cost-saving in Thai patients with CKD with the cost offsets arising from the lower costs of KRTCitation15. In a Japanese study by Kodera et al. Dapagliflozin was also estimated to be cost-saving in CKD 3b patients and cost-effective in 3a patientsCitation46. Outside of Asian population, Nguyen et al. found dapagliflozin + SoC and canagliflozin + SoC to be cost-saving compared to SoC alone in patients with CKD and T2DM in a Canadian healthcare settingCitation47. McEwan et al. also estimated dapagliflozin to be a cost-effective option to standard therapy in the United Kingdom, Spain and GermanyCitation48.
The use of real-world CKD cohorts to inform the risk-equations and evolution of risk factors associated with CKD in this study enhance the generalizability of the results. The EMPA-KIDNEY trial was terminated early thus, an assessment of long-term economic implications can only be performed through modelling techniques.
Using long-term kidney cohorts data to inform the changes in eGFR and uACR over the modelled horizon enables the assessment of the long-term benefits of treatment with empagliflozin across various sub-groups.
Another advantage of using real-world cohorts to inform the additional risk equations in conjunction with an individual patient simulation model, is that the heterogeneity of disease progression in patients or sub-groups can be captured, in contrast to a simpler approach like a Markov cohort model, which assumes that the whole cohort is represented by the “average” patient. While the latter may have been more suitable to the population studied in DAPA-CKD, the broader range of patients with a diverse set of risk profiles enrolled in the EMPA-KIDNEY trial required the use of an individual patient simulation approach.
While the current analyses focused on three countries, an initial literature search for model inputs was conducted for a number of countries in the South-East Asian region. However, the search revealed major data gaps (in particular cost, associated with complications and comorbidities) in other countries in the region. As a result, the scope of this study is limited to three countries, that are representative of a divers socio-economic archetypes of countries in the region - with Malaysia and Thailand representing upper middle-income countries and Vietnam a lower-middle income country (according to the 2022 World Bank classification). Hence, the findings of this study could be generalizable to other countries in the region.
This study incorporated a healthcare perspective and as such did not factor in the broader societal perspective which would have captured the caregiver burden and productivity losses associated with CKDCitation49 in the region. For e.g. Kieu et al. estimated that the annual direct non-medical costs and indirect costs associated with T2DM in Vietnam was USD 52.5 and USD 185, respectivelyCitation50. Similarly, Chatterjee et al. estimated that in Thai patients with T2DM, 23% of the annual cost was direct medical cost, 40% was direct non-medical cost and 37% was indirect costCitation51. A broader societal perspective would have further increased the estimated value of empagliflozin in these countries.
The limitation of the study is that the majority of the real-world cohorts used to inform the risk-equations consist primarily of US or European populations. Outside of a couple of East-Asian cohorts, very few to no South-East Asian cohort data were included in the publications that developed these risk equations. However, one study observed that Asians appear to have faster CKD progression and lower mortality rates compared to CaucasiansCitation52 – implying the results from this study may be more conservative, as the time spent in KRT is key driver of the results.
Another limitation is the lack of a probabilistic sensitivity analyses performed, that was intentional given the number of countries assessed in the study and the computational resources required for the individual simulation model. However, except for the costs, the risk equations used in the model were informed by data from large cohorts followed over long-term periods, and thus can be considered more reliable than extrapolations of short-term trial data, reducing the uncertainty in the estimates from the study.
Lastly, with respect to costs, there is a limitation associated with the paucity of data pertaining to CKD in the region, and additional studies are required to obtain more reliable estimates.
Conclusion
The use of empagliflozin + SoC over SoC alone in a broad range of patients with CKD is expected to be cost-saving Malaysia and Thailand and cost-effective in Vietnam. The cost-offsets from avoidance or delay of KRT in these patients exceed the additional treatment cost and can help alleviate the increasing burden of CKD in the South-East Asian region.
Transparency
Author contributions
The author(s) meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE).
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download MS Word (28.9 KB)Acknowledgements
The authors would like to acknowledge Mafalda Ramos, Laetitia Gerlier and Mark Lamotte for their work in developing the CKD-PM model
Declaration of financial/other interests
All authors are employees of Boehringer Ingelheim group of companies. Boehringer Ingelheim was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations. The study was supported and funded by Boehringer Ingelheim & Eli Lilly and Company Diabetes Alliance. The EMPA-KIDNEY trial was initiated, designed, and conducted by the University of Oxford in collaboration with a Steering Committee of experts and Boehringer Ingelheim. The presented analyses were initiated and conducted by Boehringer Ingelheim independently from the EMPA-KIDNEY Collaborative Group.
Additional information
Funding
References
- Vos T, Lim SS, Abbafati C, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–1222. doi: 10.1016/S0140-6736(20)30925-9.
- Suriyong P, Ruengorn C, Shayakul C, et al. Prevalence of chronic kidney disease stages 3–5 in low- and middle-income countries in Asia: a systematic review and meta-analysis. PLOS One. 2022;17(2):e0264393. doi: 10.1371/journal.pone.0264393.
- Ingsathit A, Thakkinstian A, Chaiprasert A, et al. Prevalence and risk factors of chronic kidney disease in the Thai adult population: Thai SEEK Study. Nephrol Dial Transplant. 2010;25(5):1567–1575. doi: 10.1093/ndt/gfp669.
- Songsermlosakul S, Permsuwan U, Singhan W. Treatment costs for patients with chronic kidney disease who received multidisciplinary care in a district hospital in Thailand. CEOR. 2020;12:223–231. doi: 10.2147/CEOR.S253252.
- Nguyen-Thi H-Y, Le-Phuoc T-N, Tri Phat N, et al. The economic burden of chronic kidney disease in Vietnam. Health Serv Insights. 2021;14:117863292110360. doi: 10.1177/11786329211036011.
- Ismail H, Manaf MRA, Gafor AHA, et al. Economic burden of ESRD to the Malaysian health care system. Kidney Int Rep. 2019;4(9):1261–1270. doi: 10.1016/j.ekir.2019.05.016.
- Krishnan A, Shankar M, Lerma EV, et al. Sodium glucose cotransporter 2 (SGLT2) inhibitors and CKD: are you a #Flozinator? Kidney Med. 2023;5(4):100608. doi: 10.1016/j.xkme.2023.100608.
- Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436–1446. doi: 10.1056/NEJMoa2024816.
- Herrington WG, Staplin N, Wanner C, et al. Empagliflozin in patients with chronic kidney disease. N Engl J Med. 2023;388(2):117–127. doi: 10.1056/NEJMoa2204233.
- Uster A, Ramos M, Gerlier L, et al. FR-PO940 – CKD progression model (CKD-PM): development and validation. J Am Soc Nephrol. 2023;34(11S):666–666. doi: 10.1681/ASN.20233411S1666b.
- Malaysia Department of Statistics. Consumer price index by main group, Malaysia, 2010–2022. Available from: https://newss.statistics.gov.my/newss-portalx/ep/epLogin.seam.
- Vietnam General Statistics Office. Monthly consumer price index. Available from: https://www.gso.gov.vn/en/px-web/?pxid=E0836&theme=Trade%2C%20Price%20and%20Tourist.
- Thailand Ministry of Commerce. Thailand: Bureau of Trade and Economic Indices. Medical care sub-index. Available from: http://www.price.moc.go.th/price/cpi/index_new_e.asp.
- Azmi S, Goh A, Muhammad NA, et al. The cost and quality of life of Malaysian type 2 diabetes mellitus patients with chronic kidney disease and anemia. Value Health Reg Issues. 2018;15:42–49. doi: 10.1016/j.vhri.2017.06.002.
- Vareesangthip K, Deerochanawong C, Thongsuk D, et al. Cost-utility analysis of dapagliflozin as an add-on to standard of care for patients with chronic kidney disease in Thailand. Adv Ther. 2022;39(3):1279–1292. doi: 10.1007/s12325-021-02037-6.
- Nguyen TQ, Vo TQ, Luu GH, et al. Socioeconomic costs of chronic kidney disease: evidence from Southwest Vietnam. JCDR. 2018;12:LC99. doi: 10.7860/JCDR/2018/36719.11716.
- Shafie AA, Ng CH. Estimating the costs of managing complications of type 2 diabetes mellitus in Malaysia. MJPS. 2020;18(2):15–32. doi: 10.21315/mjps2020.18.2.2.
- Ong SC, Low JZ, Yew WY, et al. Cost analysis of chronic heart failure management in Malaysia: a multi-centred retrospective study. Front Cardiovasc Med. 2022;9:971592. doi: 10.3389/fcvm.2022.971592.
- Permsuwan U, Dilokthornsakul P, Thavorn K, et al. Cost-effectiveness of dipeptidyl peptidase-4 inhibitor monotherapy versus sulfonylurea monotherapy for people with type 2 diabetes and chronic kidney disease in Thailand. J Med Econ. 2017;20(2):171–181. doi: 10.1080/13696998.2016.1238386.
- Thi Thu Nguyen T, Van Do D, Mellstrom C, et al. Cost-effectiveness of ticagrelor compared with clopidogrel in patients with acute coronary syndrome from Vietnamese healthcare payers’ perspective. Adv Ther. 2021;38(7):4026–4039. doi: 10.1007/s12325-021-01743-5.
- Reyes EB, Ha J-W, Firdaus I, et al. Heart failure across Asia: same healthcare burden but differences in organization of care. Int J Cardiol. 2016;223:163–167. doi: 10.1016/j.ijcard.2016.07.256.
- Surendra NK, Abdul Manaf MR, Hooi LS, et al. Cost utility analysis of end stage renal disease treatment in Ministry of Health dialysis centres, Malaysia: hemodialysis versus continuous ambulatory peritoneal dialysis. PLOS One. 2019;14(10):e0218422. doi: 10.1371/journal.pone.0218422.
- Bavanandan S, Yap Y-C, Ahmad G, et al. The cost and utility of renal transplantation in Malaysia. Transplant Direct. 2015;1(10):e45. doi: 10.1097/TXD.0000000000000553.
- Malaysian Society of Nephrology. 29th Report of the Malaysian Dialysis and Transplant Registry – 2021; 2021. Available from: https://www.msn.org.my/nrr/29th-report-of-the-malaysian-dialysis-and-transplant-registry-2021/.
- Ong SC, Low JZ, Linden S. Cost-effectiveness of adding empagliflozin to the standard of care for patients with heart failure with reduced ejection fraction from the perspective of healthcare system in Malaysia. Front Pharmacol. 2023;14:1195124. doi: 10.3389/fphar.2023.1195124.
- Phongphithakchai A, Phisalprapa P, Kositamongkol C, et al. Preemptive living-related kidney transplantation is a cost-saving strategy compared with post-dialysis kidney transplantation in Thailand. Front Med. 2022;9:869535. doi: 10.3389/fmed.2022.869535.
- Larpparisuth N, Cheungpasitporn W, Lumpaopong A. Global perspective on kidney transplantation: Thailand. Kidney360. 2021;2(7):1163–1165. doi: 10.34067/KID.0002102021.
- The Nephrology Society of Thailand. Thai renal replacement therapy report – 2020; 2020. Available from: https://www.nephrothai.org/wp-content/uploads/2022/06/Final-TRT-report-2020.pdf.
- Chatmongkolchart S, Chittawatanarat K, Akaraborworn O, et al. Cost of critically ill surgical patients in Thailand: a prospective analysis of a multicenter THAI-SICU Study. J Med Assoc Thai. 2016;99(Suppl 6):S31–S37.
- Karopadi AN, Mason G, Rettore E, et al. Cost of peritoneal dialysis and haemodialysis across the world. Nephrol Dial Transplant. 2013;28(10):2553–2569. doi: 10.1093/ndt/gft214.
- Tran SN, Du TTN, Thai SM, et al. Current status of organ donation for transplantation in Vietnam. Transplantation. 2018;102(Supplement 7):S382. doi: 10.1097/01.tp.0000543140.64985.00.
- Alexander S, Jasuja S, Gallieni M, et al. Impact of national economy and policies on end-stage kidney care in South Asia and Southeast Asia. Int J Nephrol. 2021;2021:1–11. doi: 10.1155/2021/6665901.
- Thaweethamcharoen T, Sakulbumrungsil R, Nopmaneejumruslers C, et al. Cost-utility analysis of erythropoietin for anemia treatment in Thai end-stage renal disease patients with hemodialysis. Value Health Reg Issues. 2014;3:44–49. doi: 10.1016/j.vhri.2014.01.001.
- Patikorn C, Blessmann J, Nwe MT, et al. Estimating economic and disease burden of snakebite in ASEAN countries using a decision analytic model. PLOS Negl Trop Dis. 2022;16(9):e0010775. doi: 10.1371/journal.pntd.0010775.
- World Health Organization. Life tables by country (GHE: Life tables). Available from: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/gho-ghe-life-tables-by-country.
- Department of Statistics Malaysia. Abridged life tables, Malaysia, 2021–2023. Available from: https://www.dosm.gov.my/portal-main/release-content/abridged-life-tables-malaysia.
- Jesky MD, Dutton M, Dasgupta I, et al. Health-related quality of life impacts mortality but not progression to end-stage renal disease in pre-dialysis chronic kidney disease: a prospective observational study. PLOS One. 2016;11(11):e0165675. doi: 10.1371/journal.pone.0165675.
- Liem YS, Bosch JL, Hunink MGM. Preference-based quality of life of patients on renal replacement therapy: a systematic review and meta-analysis. Value Health. 2008;11(4):733–741. doi: 10.1111/j.1524-4733.2007.00308.x.
- Lee AJ, Morgan CL, Conway P, et al. Characterisation and comparison of health-related quality of life for patients with renal failure. Curr Med Res Opin. 2005;21(11):1777–1783. doi: 10.1185/030079905X65277.
- Koye DN, Magliano DJ, Reid CM, et al. Risk of progression of nonalbuminuric CKD to end-stage kidney disease in people with diabetes: the CRIC (chronic renal insufficiency cohort) study. Am J Kidney Dis. 2018;72(5):653–661. doi: 10.1053/j.ajkd.2018.02.364.
- Chatterjee S, et al. N3 Cost-effectiveness analysis of empagliflozin vs standard of care in patients with chronic kidney disease in the United States. J Manage Care Spec Pharm. 2023;29:S1–S138.
- Ramos M, Gerlier L, Uster A, et al. EE420 cost-effectiveness of empagliflozin in chronic kidney disease (CKD) management in the UK. Value in Health. 2023;26(12):S131–S132. doi: 10.1016/j.jval.2023.09.686.
- Costanzo AD, Uster A, Vassallo C, et al. EE117 cost-effectiveness of empagliflozin in patients affected by chronic kidney disease in Italy. Value in Health. 2023;26(12):S73. doi: 10.1016/j.jval.2023.09.387.
- Fens T, Weersma M, Postma M, et al. EE421 cost-effectiveness of empagliflozin in adult patients with chronic kidney disease in the Netherlands. Value in Health. 2023;26(12):S132. doi: 10.1016/j.jval.2023.09.687.
- Reifsnider OS, Kansal AR, Wanner C, et al. Cost-Effectiveness of empagliflozin in patients with diabetic kidney disease in the United States: findings based on the EMPA-REG outcome trial. Am J Kidney Dis. 2022;79(6):796–806. doi: 10.1053/j.ajkd.2021.09.014.
- Kodera S, Morita H, Nishi H, et al. Cost-effectiveness of dapagliflozin for chronic kidney disease in Japan. Circ J. 2022;86(12):2021–2028. doi: 10.1253/circj.CJ-22-0086.
- Nguyen B-N, Mital S, Bugden S, et al. Cost-effectiveness of canagliflozin and dapagliflozin for treatment of patients with chronic kidney disease and type 2 diabetes. Diabet Obesity Metab. 2023;25(10):3030–3039. doi: 10.1111/dom.15201.
- McEwan P, Darlington O, Miller R, et al. Cost-effectiveness of dapagliflozin as a treatment for chronic kidney disease: a health-economic analysis of DAPA-CKD. CJASN. 2022;17(12):1730–1741. doi: 10.2215/CJN.03790322.
- Michalopoulos SN, Gauthier-Loiselle M, Aigbogun MS, et al. Patient and care partner burden in CKD patients with and without anemia: a US-based survey. Kidney Med. 2022;4(4):100439. doi: 10.1016/j.xkme.2022.100439.
- Kieu TTM, Trinh HN, Pham HTK, et al. Direct non-medical and indirect costs of diabetes and its associated complications in Vietnam: an estimation using national health insurance claims from a cross-sectional survey. BMJ Open. 2020;10(3):e032303. doi: 10.1136/bmjopen-2019-032303.
- Chatterjee S, Riewpaiboon A, Piyauthakit P, et al. Cost of diabetes and its complications in Thailand: a complete picture of economic burden. Health Soc Care Community. 2011;19(3):289–298. doi: 10.1111/j.1365-2524.2010.00981.x.
- Barbour SJ, Er L, Djurdjev O, et al. Differences in progression of CKD and mortality amongst Caucasian, Oriental Asian and South Asian CKD patients. Nephrol Dial Transplant. 2010;25(11):3663–3672. doi: 10.1093/ndt/gfq189.