Abstract
Background and aims
Cardiac ablation is a well-established method for treating atrial fibrillation (AF). Pulsed field ablation (PFA) is a non-thermal therapeutic alternative to radiofrequency ablation (RFA) and cryoballoon ablation (CRYO). PFA uses high-voltage electric pulses to target cells. The present analysis aims to quantify the costs, outcomes, and resources associated with these three ablation strategies for paroxysmal AF.
Methods
Real-world clinical data were prospectively collected during index hospitalization by three European medical centers (Belgium, Germany, the Netherlands) specialized in cardiac ablation. These data included procedure times (pre-procedural, skin-to-skin and post-procedural), resource use, and staff burden. Data regarding complications associated with each of the three treatment options and redo procedures were extracted from the literature. Costs were collected from hospital economic formularies and published cost databases. A cost-consequence model from the hospital perspective was built to estimate the impact of the three treatment options in terms of effectiveness and costs.
Results
Across the three centers, N = 91 patients were included over a period of 12 months. A significant difference was seen in pre-procedural time (mean ± SD, PFA: 13.6 ± 3.7 min, CRYO: 18.8 ± 6.6 min, RFA: 20.4 ± 6.4 min; p < 0.001). Procedural time (skin-to-skin) was also different across alternatives (PFA: 50.9 ± 22.4 min, CRYO: 74.5 ± 24.5 min, RFA: 140.2 ± 82.4 min; p < 0.0001). The model reported an overall cost of €216,535 per 100 patients treated with PFA, €301,510 per 100 patients treated with CRYO and €346,594 per 100 patients treated with RFA. Overall, the cumulative savings associated with PFA (excluding kit costs) were €850 and €1,301 per patient compared to CRYO and RFA, respectively.
Conclusion
PFA demonstrated shorter procedure time compared to CRYO and RFA. Model estimates indicate that these time savings result in cost savings for hospitals and reduce outlay on redo procedures. Clinical practice in individual hospitals varies and may impact the ability to transfer results of this analysis to other settings.
Disclaimer
As a service to authors and researchers we are providing this version of an accepted manuscript (AM). Copyediting, typesetting, and review of the resulting proofs will be undertaken on this manuscript before final publication of the Version of Record (VoR). During production and pre-press, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal relate to these versions also.Introduction
Atrial fibrillation (AF) is the most prevalent cardiac arrhythmia observed in clinical practice, characterized by rapid and disorganized electrical signals in the atria, and resulting in an irregular and fast heartbeat [1,2]. Its prevalence ranges from 2% in the general population up to 10–12% in people aged 80 years and older as the risk of AF increases with age [1,3]. AF prevalence is projected to rise due to extended longevity and an increase of other well-known AF risk factors such as hypertension, heart failure, and diabetes mellitus [3]. This means an increasing burden of AF from a patient, organizational, and economic perspective is expected [4].
Due to the growing number of patients needing treatment, healthcare systems and individual hospitals face scheduling challenges and long waiting lists. In a recent Canadian article, researchers analyzed the waiting times from referral to treatment for adults with AF requiring catheter ablation. The study revealed a concerning trend of increasing waiting times, coinciding with elevated health hazards and heightened healthcare utilization [5]. Moreover, a report by the British Heart Foundation disclosed a 49% increase in the number of people waiting to receive cardiac treatment from February 2020 to August 2022 [6]. This strains already limited resources, highlighting the urgent need for efficient treatment strategies for AF.
Catheter ablation has become a well-established treatment method for preventing AF recurrence in paroxysmal and persistent AF [7,8]. The cornerstone of AF catheter ablation is complete pulmonary vein isolation through point-by-point lesions around their antrum, or by single shot ablation [7]. The current European Society of Cardiology guidelines recommend catheter ablation as a first-line therapy for patients with symptomatic paroxysmal AF, persistent AF without major risk factors, and heart failure with reduced ejection fraction [8]. Moreover, catheter ablation for pulmonary vein isolation is recommended after a class I or II anti-arrhythmic drug treatment failed or was not tolerated [8].
Radiofrequency energy has been the most commonly used method for AF catheter ablation [9]. Cryoenergy is an alternative energy source used in catheter ablation. Both are thermal energy sources that potentially cause damage to adjacent organs, such as the esophagus and phrenic nerve [10]. To improve the effectiveness and safety of AF catheter ablation, new technologies are being explored. Pulsed field ablation (PFA) is a non-thermal energy approach that utilizes high-voltage, ultrarapid (<1 s) electric pulses to destroy target cells [11]. PFA has shown promising clinical outcomes, with clinical trials showing this approach to be feasible, effective, and safe, with low rates of complications [12,13].
In considering different options for cardiac ablation, there have been calls for an assessment of the economic impact of newer technologies such as PFA [14]. In May 2024, two studies reported on the cost impact of PFA in the English National Health Service (NHS), finding that PFA reduced costs by 3% (£343) per patient over 12 months compared with cryoablation but was associated with higher index procedure costs [15,16].
In this paper, we present a cost-consequence analysis using real-world procedural data from Belgium, Germany, and the Netherlands. The analysis compares three cardiac ablation options for paroxysmal AF: PFA, radiofrequency ablation (RFA) and, cryoablation (CRYO). The specific objectives were (i) to quantify the short-term hospital costs and resource use related to the three treatment options during the index hospital procedure, and (ii) to compare the medium-term (1 year) economic implications of adopting the three options in terms of redo procedures (effectiveness) and complications (safety).
Methods
Economic model
A cost-consequence model was designed to compare the resource use and associated costs of each of the three therapeutic options to treat paroxysmal AF. The model was constructed from the hospital perspective, acquiring primary data from three European centers with extensive experience in the field of cardiac ablation. Therefore, it only considers the costs that would be accrued by the hospital. The target population comprised patients with paroxysmal AF requiring an intervention with RFA, CRYO, or PFA. The time horizon was 1 year, considering the costs from index hospitalization until discharge as well as subsequent costs due to complications and reinterventions that may present within the first year. The study findings are reported according to the Consolidated Health Economic Evaluation Reporting Standards (CHEERS 2022) checklist [17].
Model structure
A decision-tree model was developed using Microsoft Excel, based on procedural real-world data. Figure 1 represents the movement of a hypothetical cohort of 100 patients with paroxysmal AF that require treatment, from index hospitalization to 1-year follow up. The clinical setting is a hospital in either Belgium, Germany, or the Netherlands.
The patients enter the model requiring an intervention for paroxysmal AF, which is performed using either RFA, CRYO, or PFA. Patients could either be discharged with or without complications post-procedure. Following that, patients are split into a subgroup in need of a repeat procedure due to recurrent paroxysmal AF, and a subgroup that does not require reintervention. Patients that have reintervention (redo procedures) are subject to additional risk of complications.
[Figure 1 near here]
Data Sources
Index hospitalization
Three European medical centers provided real-world data (RWD) regarding procedural times and resource consumption of patients with paroxysmal AF undergoing pulmonary vein isolation with PFA (FaraWave; Farapulse - Boston Scientific Inc, Marlborough, MA.), CRYO (no specific product), and RFA (no specific product). The participating centers were the Catharina Hospital, (Eindhoven, the Netherlands), CCB – Medizinisches Versorgungszentrum Frankfurt und Main-Taunus GbR, (Frankfurt, Germany), and Universitair Ziekenhuis Brussel – UZB (Jette, Belgium). The local ethics committees approved the study.
The patient population included adult patients (aged ≥18 years) affected by paroxysmal AF and undergoing pulmonary vein isolation. Inclusion and exclusion criteria for patient selection are reported in Supplementary Table S1. For each procedure, the centers retrospectively accessed and provided the data related to resource consumption (listed in Supplementary Table S2). Data for at least N = 30 patients (N = 10 per procedure type) were collected at each study site. The sample size of N = 90 patients was considered sufficient to ensure robust statistical analysis and meaningful interpretation of the results while remaining feasible within the study's scope. The choice of sample size aimed to balance the need for adequate statistical power with the constraints of available resources.
The data provided by the three centers were collected retrospectively on patients treated over a period from January 2022 to January 2023. This timeframe spans a total period of 12 months. Since RFA is no longer used in the Dutch center, the data collected refer to the most recent prior of use (i.e., 2013). This historical data was included to ensure a comprehensive analysis despite the current procedural practices.
Patients included were a convenience sample selected systematically starting from the most recent patients and working backwards until 10 patients per device type were included. The protocols of the three ablation methods are described in Supplementary Table S3. Collected data were not patient-identifiable and were focused on hospital resource-use indicators available in each hospital’s electronic health records, in addition to unit costs for drugs provided by each hospital’s pharmacy. As no patient identifiers were collected, follow-up beyond the index hospitalization for complications and repeat procedures was not possible. The collected data included:
Demographics/patient characteristics (age, gender, comorbidities, etc.).
Procedural timing, including:
Pre-procedure time, defined as the time from the beginning of patient preparation and the first sheath insertion.
Skin-to-skin time, defined as the time from the first sheath insertion to the last sheath removal.
Post-procedure time, defined as the time from the last sheath removal to the transfer of the patient to the ward.
Staff workload (physician, nurse, technician, etc.).
Drugs used for anesthesia (general anesthesia or sedation). General anesthesia was defined as complete loss of consciousness, absence of feeling and inability to hear or remember anything. With sedation, medications are given to make the patients asleep, comfortable, and relaxed. Performed exams (ECG, x-ray, etc.).Length of stay (LoS).
One-year outcomes
From a clinical standpoint, effectiveness outside the index hospitalization was evaluated as the proportion of patients undergoing a repeat procedure (redo), and incidence of major adverse events over the year following the index procedure. As these outcomes were not available from the hospital electronic health records accessed as part of this analysis, the clinical data considered in the model were extracted from published literature, a retrospective propensity score matched analysis of patients undergoing PFA, CRYO, and RFA [18]. PFA was reported to have a lower redo rate (13.8%) compared to CRYO and RFA (15.2% and 19.5%, respectively; Supplementary Table S4). Similarly, PFA was associated with a lower rate of certain complications, such as cardiac tamponade (0% for PFA, vs. 0.3% for both CRYO and RFA) or phrenic nerve injury (0% for PFA and RFA, vs. 0.3% for CRYO) (Supplementary Table S4). There were fewer cases of pericarditis in PFA compared to RFA and CRYO (0% vs. 0.9% and 3.2%, respectively, categorized in Supplementary Table S4 as part of Other).[18] In the CRYO group, transient phrenic nerve injury was the most frequent complication (4.8%; categorized in Supplementary Table S4 as part of Other).[18]
Costs
All costs were estimated from the hospital payer perspective; therefore, only direct hospital costs associated with the treatment of paroxysmal AF as well as related complications and redo procedures were considered.
To determine the procedure’s costs, a micro-costing approach was adopted. Costs collected and used to calculate the procedure cost include the procedure duration, human resource use of healthcare professionals (e.g., time incurred by physicians, nurses, etc.), length of hospital stay, administered drugs (e.g., anesthetics), and any diagnostic examinations performed during the procedure.
In addition to the procedural costs, patients may encounter supplementary expenses stemming from redo procedures and/or complications, which were extracted from published studies. The unit costs considered in the analysis and their sources are shown in Supplementary Table S4. Staff hourly costs were provided by the centers. The inpatient costs were calculated by multiplying the daily cost of hospital stay with the length of hospitalization. The cost of drugs and exams were extracted from the tariffs issued by the Healthcare Service by country. All costs within the model were in or inflated to 2024 EUR, discounting of costs was not applied due to the 1-year time horizon. It was assumed that a redo procedure would require an additional hospital admission.
Statistical analysis
To estimate the number of patient data points required in the analysis, the procedural time was used as the primary comparison. Initial data for PFA have demonstrated procedural times of 81 ± 20 minutes [19]. Time for CRYO and RFA vary in the literature, and an assumption of 100 minutes per procedure was taken as comparator time. The number of patients per group required was calculated using the online calculator: https://www.sealedenvelope.com/power/continuous-superiority/ (Sealed Envelope Ltd. 2012) and provided a sample size of 24 per arm. This was rounded up to 30 (10 per site) to ensure adequate coverage.
The analysis of RWD included descriptive statistics such as means, medians, quartiles, and standard deviations (SD) of the variables, shown in Supplementary Table S2. The statistical analysis aimed to assess potential differences in patient demographics and characteristics. One-way analysis of variance (ANOVA) was employed for continuous variables, followed by Bonferroni correction for any post-hoc comparisons. The chi-square test was utilized for categorical variables. Additionally, the median test was used to calculate the frequency of PFA, RFA and CRYO patients whose outcome value was either below or above the median. In all tests, the significance level considered for statistically significant difference was 0.05 (95% confidence interval), with adjustment for the Bonferroni correction where applicable.
Sensitivity analysis
One-way sensitivity analyses (OWSA) were performed to explore the uncertainty around model outcomes. The aim of the OWSA was to identify the model inputs that have the greatest impact on the model outputs; results are presented as a tornado diagram. In the probabilistic sensitivity analyses, a distribution was assigned to each parameter based on the mean and their corresponding variance: gamma distribution for cost data and beta distribution for proportions. In the analysis, the distributions of each parameter were randomly sampled over 1,000 Monte Carlo simulations. The results of the sensitivity analysis were reported as a 95% credible interval (95% CrI) next to the base-case results. An assumed variance of 25% was used when the variance of the cost input was unavailable; this was informed by the differences in procedure and treatment costs between the three countries.
The data provided by the three centers were collected retrospectively on patients treated over a period from January 2022 to January 2023. This timeframe spans a total period of 12 months. Since RFA is no longer used in the Dutch center, the data collected refer to the most recent prior of use (i.e., 2013). This historical data was included to ensure a comprehensive analysis despite the current procedural practices.
Results
Baseline characteristics
Data from three centers were collected for a total of N = 91 patients (N = 31 PFA, N = 30 RFA, and N = 30 CRYO). Each study site equally provided data for at least N = 30 patients (N = 10 per procedure type). The cohort had a mean age of 64 ± 10 years, with 51 patients (56%) being male. A summary of baseline characteristics is reported in Table 1.
Overall, the characteristics of the patients at baseline were well balanced among the three groups, except for the treatment with antiarrhythmic drugs (AAD). The majority of patients (79%) received treatment with AAD, primarily class Ic (33%) and class II beta-blockers (32%). In the RFA group, a higher percentage of patients received AAD treatment compared to the PFA and CRYO groups. Across all cardiac ablation strategies, more than 60% of patients received sedation.
[Table 1 near here]
Index hospitalization
Resource consumption for index hospitalization
The mean pre-procedural time was 17.6 min. PFA had significantly shorter pre-procedural time compared to the other treatments (Table 2). Moreover, a significantly higher proportion of patients in the PFA group (93.5%) experienced pre-procedural time that were shorter than the median compared to CRYO and RFA (36.7% and 23.3%; median test: p < 0.001, Supplementary Table S5).
The mean procedure time (skin-to-skin) was significantly shorter in the PFA group compared to the other two treatments (mean ± SD: 50.9 ± 22.4 min for PFA, 74.5 ± 24.5 min for CRYO, and 140.2 ± 82.4 min for RFA; p < 0.0001; Table 2). The Bonferroni test (1:1 comparison) confirmed that both PFA and CRYO had significantly lower skin-to-skin times than RFA (p ≤ 0.001). No significant difference was observed between PFA and CRYO (p = 0.222). However, a higher proportion of patients in the PFA group (87.1%) had shorter than median skin-to-skin time compared to CRYO (43.4%) and RFA (26.7%; median test: p < 0.001, Supplementary Table S5).
Similarly, a significant difference among the three techniques concerning post-procedural time was seen, with PFA group having shorter time compared to the two other techniques (p = 0.003; Table 2). Moreover, a significantly higher proportion of patients in the PFA group (90.3%) had shorter than median post-procedural time, compared to CRYO (50.0%) and RFA (26.7%; median test: p = 0.001, Supplementary Table S5).
Table 2 also reports the cumulative staff time required to perform the cardiac ablation procedure in the three groups. The time of all healthcare professionals was significantly shorter in the PFA group compared to CRYO and RFA (p < 0.01). Applying the Bonferroni test (1:1 comparison), physician, nurse and anesthetist times were significantly lower in both PFA and CRYO groups compared to RFA (p < 0.05). Technician time was significantly lower in PFA vs. RFA (p < 0.05); no significant difference was reported between CRYO and RFA (p = 0.146). Comparing PFA vs. CRYO, a significant reduction was observed only for physician time. Nonetheless, a significantly higher proportion of patients in the PFA group had shorter than median time compared to CRYO and RFA for all the healthcare providers (median test: p < 0.05; Supplementary Table S5).
The significant difference observed in the median test analyses (by procedure phase and staff; Supplementary Table S5) suggests that PFA could be more predictable and reproducible compared to other procedures.
[Table 2 near here]
The mean LoS was 1.7 days. No differences emerged among techniques in terms of LoS (mean ± SD: 1.5 ± 0.6 days for PFA, 1.8 ± 1.3 days for CRYO and 1.8 ± 0.46 days for RFA; p = 0.346).
Costs for index hospitalization
Using a micro-costing approach for the resource consumption outputs of the analysis, PFA was associated with 22% lower procedure costs compared to CRYO and 35% lower procedure costs compared to RFA (Figure 2). The notable cost difference between the groups might have been driven by the difference in the procedural time, which was significantly lower in PFA. Other cost items, such as costs of medical therapy (including anesthesia), exams, and hospital stay were similar across treatment options. All cardiac ablation procedures required one standard set plus an ablation kit, regardless of the treatment option.
[Figure 2 near here]
Model results
Time for index hospitalization and redo procedures
The total procedure time per patient, including index hospitalization and redo procedures, were estimated as 91 min (95% CrI 58; 122) for PFA, 127 min (95% CrI 89; 164) for CRYO, and 217 min (95% CrI 107; 326) for RFA. The index procedure accounted for 79.58 min, 110.20 min, and 181.20 min for PFA, CRYO, and RFA respectively. Assuming an operating room running for 8 hours per day, the number of procedures that could be completed in a day is 6 for PFA, 4 for CRYO, and 2 for RFA. Allowing for 20 minutes of buffer time between procedures would limit the procedures per day to 5 for PFA, 3 for CRYO, and 2 for RFA.
Costs for index hospitalization, redo procedures, and complications
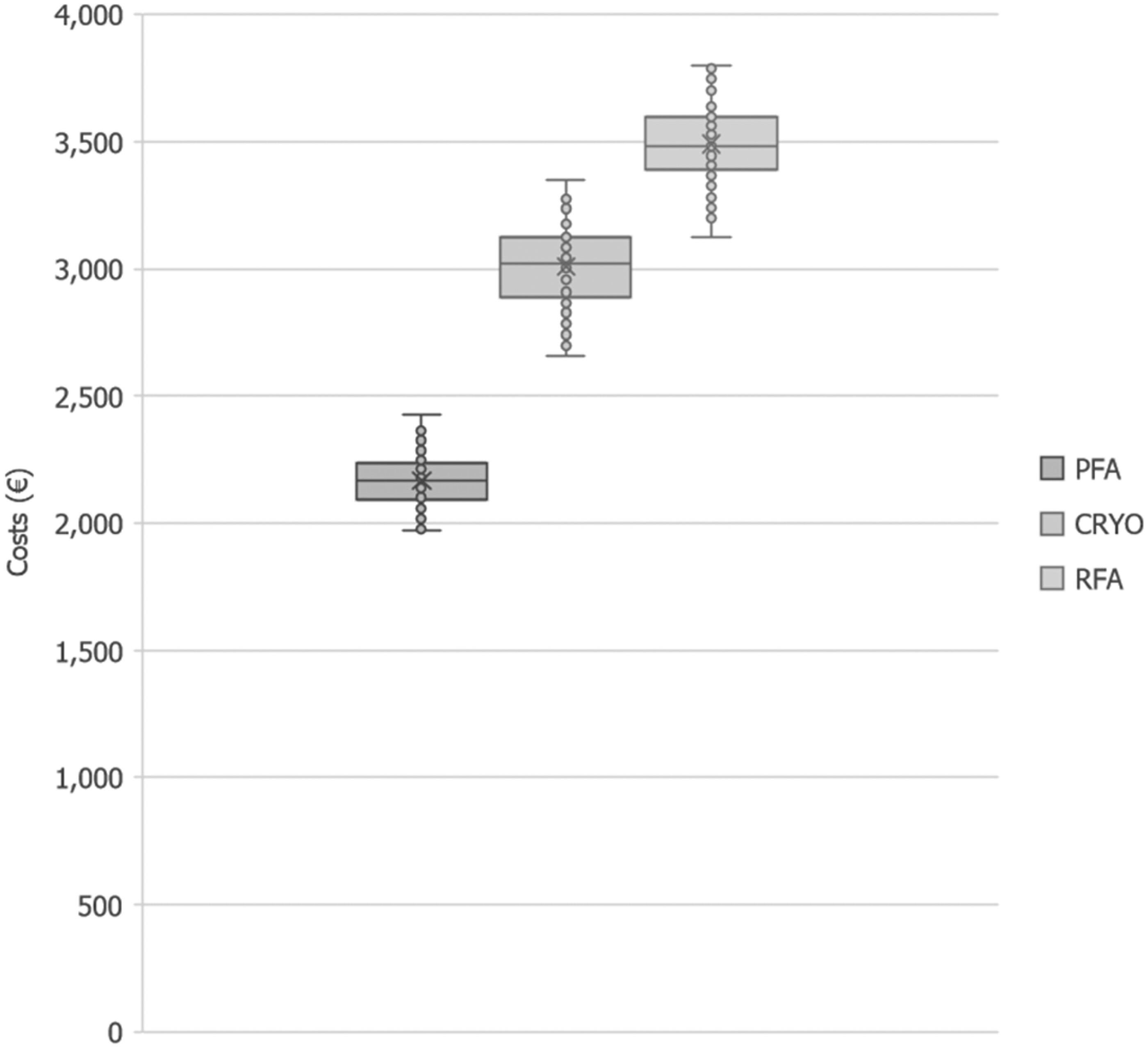
The overall costs for index hospitalization, redo procedures, and associated complications for the three ablation techniques are reported in Table 3. In general, PFA was shown to reduce costs by 28% compared to CRYO and 38% compared to RFA. In sensitivity analyses, the 95% CrI of cost reduction per patient with PFA ranged between 511€and 1,040€versus CRYO and between 939€and 1,497€versus RFA. The boxplot (Figure 3) shows the distribution of total costs per patient per ablation technique generated during the sensitivity analyses. On a country level, the cost savings per patient of PFA versus CRYO and PFA versus RFA were respectively 816€(95% CrI 551€; 1,123€) and 1,402€(95% CrI 1,111€; 1,713€) for Belgium; 624€(95% CrI 417€; 874€) and 963€(95% CrI 732€; 1,219€) for Germany; and 816 €(95% CrI 562€; 1,115€) and 1,260€(95% CrI 973€; 1,563€) for the Netherlands.
[Figure 3 near here]
[Table 3 near here]
Scenario analysis
Redo rates were taken from literature as these data were not available in our RWD. It is possible that redo rates in the hospital settings explored here vary from those in the literature. In a scenario where the redo rate for PFA was set to 150% of that of CRYO (22.8% versus 15.2%), the findings of our analysis were not substantially changed. In this scenario PFA saved 29 min (95% CrI -28 min; 88 min) and 692€(95% CrI 341€; 869€) versus CRYO and 119 min (95% CrI -6 min; 245 min) and 1,143€(95% CrI 769€; 1,326€) versus RFA.
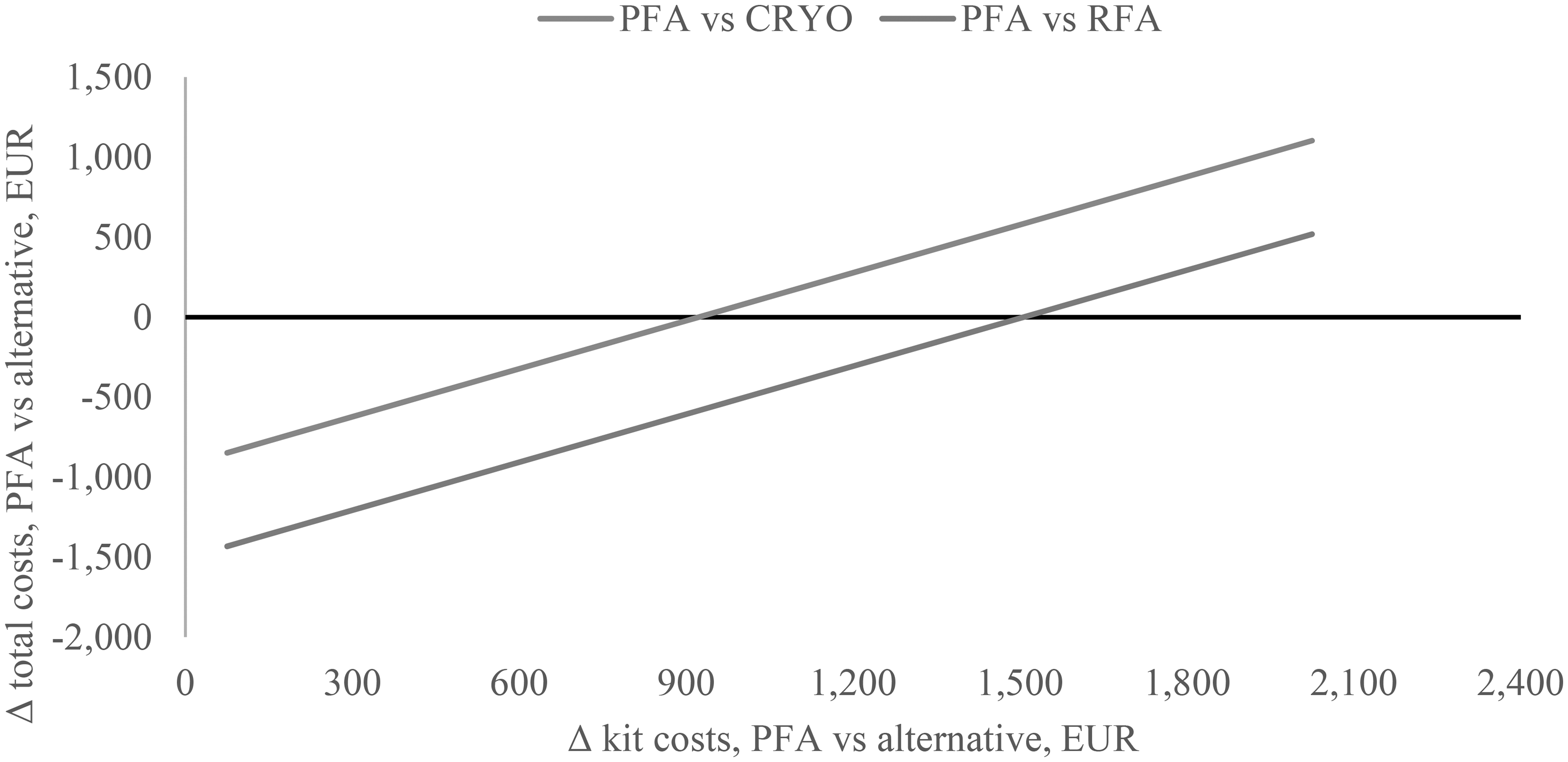
Since it was not possible to obtain pricing for all ablation devices used in the hospitals due to price variability across centers and confidentiality of kit costs (i.e., standard set plus ablation kit) from various suppliers, a scenario analysis was performed where the total cost difference was plotted against possible price differences between PFA, CRYO, and RFA kits (Figure 4). This analysis shows that PFA would be cost saving vs. CRYO (Δ total costs < 0) up to a price difference of +€850 (i.e., the PFA kit is more expensive than the CRYO kit). Similarly, PFA would be cost saving vs. RFA (Δ total costs < 0) up to a price difference of +€1,301 (i.e., the PFA kit is more expensive than the RFA kit).
[Figure 4 near here]
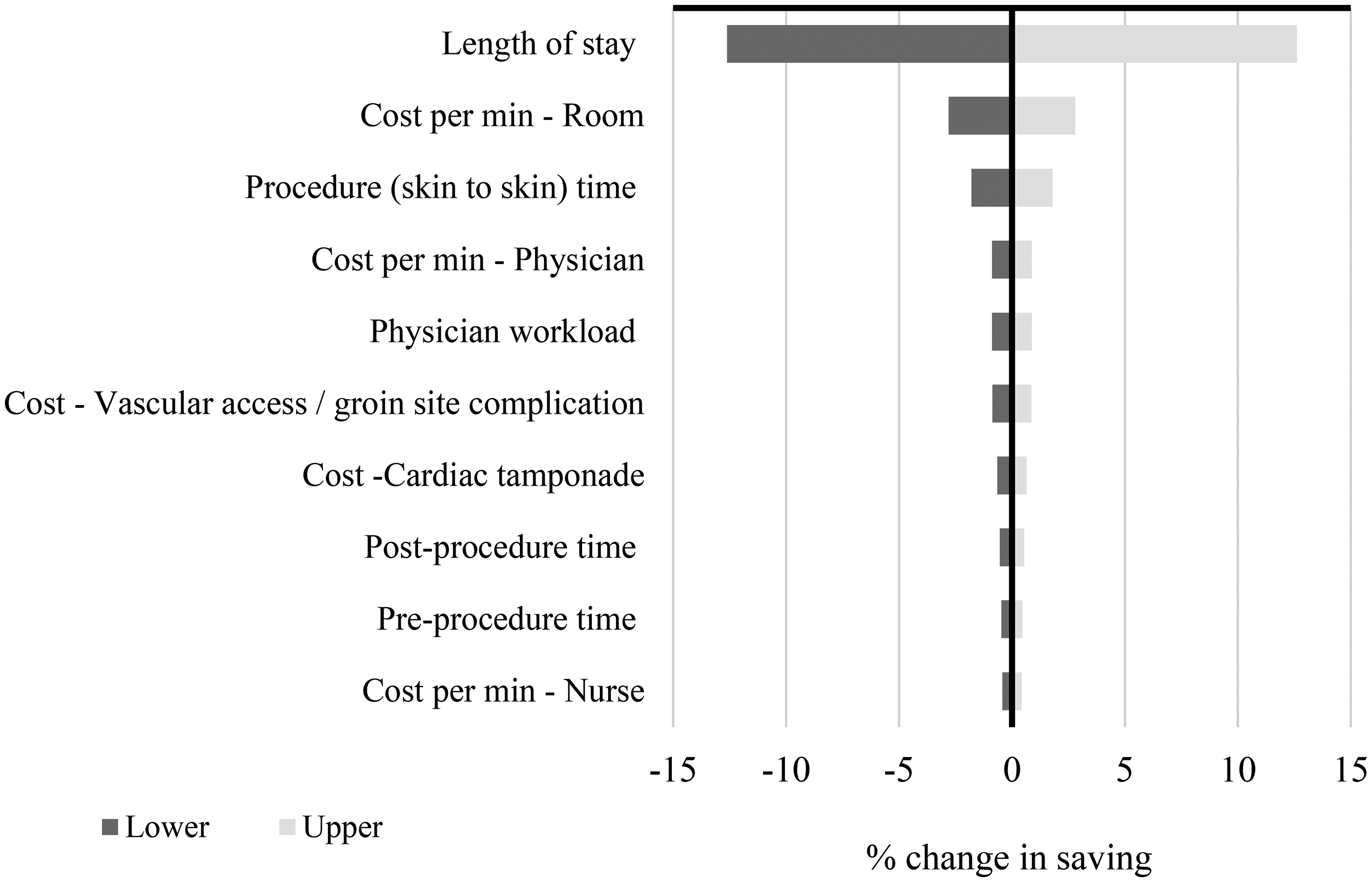
According to the OWSA shown by the tornado plot (Figure 5), out of all model inputs the LoS had the most substantial impact on the model result. Results of the probabilistic analysis are reported in Figure 3 and in the supplementary material (Supplementary Figure S1). The results of the sensitivity analyses enhance the reliability of the base case and confirm the conclusions drawn from the base case analysis are not overly reliant on specific assumptions or input values.
[Figure 5 near here]
Discussion
The results of the RWD analysis show that PFA is associated with a shorter procedural and staff time compared to RFA, while being similar between CRYO and PFA. Results from the cost-consequence model suggest that PFA is a cost-saving therapeutic option compared to both CRYO and RFA. These findings are in line with those of other resource use studies, that found shorter procedure time and costs (excluding device costs) with PFA versus CRYO and RFA [15,16], and aligned with the meta-analysis of de Campos et al., reporting a 21.68 minute mean reduction in procedure time with PFA versus thermal ablation (CRYO or RFA) [20].
The shorter pre-and post-procedure times associated with PFA compared to RFA and CRYO can be attributed to the streamlined setup process. Both RFA and CRYO require additional preparatory steps. RFA procedures entail the mapping system setup with multiple cables and electrode placement. CRYO procedures involve the management of gas tank and gas deduction capabilities, which adds logistical process steps. In contrast, PFA requires no additional procedural setup steps, thereby enhancing operational efficiency which translates into staff and cost savings due to improved resource utilization. Our analysis further demonstrated that use of PFA could increase the number of ablation procedures performed per day per operating/procedure room. This could further increase hospital efficiencies and potentially result in a reduction in waiting list times.
In the present analysis, data from a 2023 study [18] was considered for redo procedures and complication rates, with the study reporting PFA to be associated with similar rates of complications and a lower rate of redo procedures, which can have a significant impact on costs. The selected source represents the most recent published literature including a substantial sample size (more than 800 patients) undergoing PFA, CRYO, and RFA [18]. However, data on redo procedures are based on early experiences with PFA and results may differ over time as physicians get more familiar with the technology. Notably, there may be a potential underestimation of cardiac tamponade rates, ranging from 0% to 0.3%, as reported in the literature. In a recent analysis (2024) [21] assessing the efficacy and safety of the combined use of PFA and RFA, a higher incidence of cardiac tamponade/perforation (1.5%) was reported. This underscores the importance of considering evolving data and real-world experiences when evaluating the safety profile of PFA and other techniques.
The results from the ADVENT study showed a smaller difference in redo rates between treatments (4.6% for PFA vs. 6.6% for thermal ablation) and a slightly higher rate of serious complications for PFA than for thermal ablation (2.0% vs. 1.3%) [22]. Exploring these inputs in a scenario analysis of our cost-consequence model did not substantially impact the results or conclusions drawn, with PFA still being cost-saving in the range of 28%-38% compared to CRYO and RFA, respectively. Of note, ADVENT was conducted in US centers only, where market approval is still pending. In Europe, the use of PFA is more common, with more than 25,000 patients treated. When focusing on procedural time, other studies such as Della Rocca et al. [18] show similar times to the ones collected in the present RWD study for PFA and CRYO. However, the times for RFA are considerably higher in the present study compared to the literature, [18] which could be due to our relatively small sample size.
Although costs are key in the hospital setting, they are not the only concern. Hospitals face the challenge of resource planning and elective procedure scheduling in order to maximize utilization, while ensuring that procedures are performed on time as highlighted by Monnickendam et al. [23] Unpredictable procedure times are disadvantageous since a greater safety margin needs to be planned for to ensure that operating room (OR) schedules are met, while consistent and reliable results can increase hospital planning confidence in the anticipated duration of the procedure. The analysis of the real-world procedural data provided also showed that the SD associated with procedural time (skin-to-skin) was lower for PFA compared to RFA and CRYO. Lower SD can be interpreted as reduced variability of procedural time, which can lead to more predictability. As a result, it may be possible to plan more interventions per day and may indicate an increased probability that procedures will be completed on time, increasing hospital productivity. This could potentially reduce waiting lists and the risk of procedure cancellations associated to long waiting times. Nevertheless, we acknowledge that there are other constraints when it comes to scheduling and time in the hospital that might fall outside of using a specific technology. Therefore, it would be interesting to quantitatively analyze data regarding the real-world impact of these time savings in the OR.
In this cost-consequence analysis, RWD were collected from three centers to calculate the index hospitalization resource use and costs, while published literature was used to anticipate redo procedures and complications. This methodological approach presents a limitation in our analysis, potentially introducing uncertainty to the estimates and impacting the robustness of our findings. The incorporation of long-term costs and consequences from the hospital, when available, could have enriched the depth of our analysis; however, such data were not accessible. Given these constraints, the use of a hybrid approach is a common practice in economic modelling and can be useful to estimate long-term outcomes. In future, longitudinal economic studies are required to assess the budgetary implications for hospitals over time. A second limitation of the analysis is the convenience sample size of N = 91 cases. Although the sample size may be considered relatively modest for drawing definitive conclusions, it is notable that the results demonstrated consistency across centers and aligned with findings from a study published in 2023 for PFA and CRYO, [18] indicating consistency.
We acknowledge that variations in procedure time may be influenced by factors such as the severity and location of the underlying disorder, potentially introducing selection bias. It is important to underline that centers considered consecutively enrolled patients according to specific inclusion and exclusion criteria. Patients were selected systematically, with the most recent patients meeting the inclusion/exclusion criteria in a retrospective manner. The considered approach aimed to reflect real-world clinical practice while ensuring comparable characteristics across patient groups. Moreover, the analysis of the baseline characteristics confirmed similarity of patient characteristics between groups, which supported the absence of selection bias. This suggests that the variations in procedure time were not driven by inherent differences in patient characteristics but rather by procedural intricacies or other factors not accounted for in the study. To address these limitations, sensitivity analysis has been conducted to test the robustness of our results under various assumptions and scenarios. Despite the retrospective design, these measures help to mitigate potential biases and strengthen the validity of our conclusions.
The cost of the technologies (ablation kit) was not included in the analysis, and we appreciate this cost item would be important to provide for a complete estimate of costs. However, it was not possible to collect this data due to purchasing contracts. Nevertheless, the present study could be useful for specialized centers to assess if using the PFA kits is cost-saving in their hospital, considering the pricing and reimbursements schemes in their setting. In our analysis, cumulative savings associated with PFA (excluding kit costs) were €850 and €1,301 compared to CRYO and RFA, respectively. This provides an initial estimate of the additional purchase cost PFA could incur per patient and still be considered cost neutral. With a switch to PFA, the hospital could expect to perform more procedures per day. In such a scenario, the relative cost of PFA could be higher before the breakeven point is reached. This is because fixed hospital costs would be spread over more procedures, resulting in a lower cost per procedure, and the hospital would have the opportunity to recoup more reimbursement per day. In the context of a complete economic analysis, the direct and indirect cost benefits should be evaluated against the possible difference in acquisition costs among PFA, CRYO, and RFA. In this vein, the analysis of Duxbury et al., found that PFA (including device costs) increased per procedure costs for the index event but reduced costs by £343 per patient over the course of 12 months [16].
We acknowledge the uncertainty of the data used in the economic analysis considering that comparative effectiveness and safety of the three alternatives does not come from large systematic literature reviews and/or meta-analyses. At the time of analysis, this level of clinical evidence had not been published. The first studies of this type are currently appearing in press and are in line with our data and findings. As more data become available, it may be worth returning to the analyses presented here to understand if findings remain valid.
Conclusions
In our study, we found pulse field ablation to have significantly shorter procedure times than radiofrequency ablation. Pulse field ablation appeared to have less time variability compared to cryoablation and radiofrequency ablation, which could potentially increase scheduling flexibility and hospital productivity. The cost-consequence model results suggested pulse field ablation may reduce hospital costs due to lower resource consumption during the procedure. Future research with larger cohorts to explore procedural time variability and scheduling is warranted.
Transparency
Declaration of funding
This analysis was financially supported by Boston Scientific.
Declaration of financial/other interests
S.U. and J.M. are employees of Boston Scientific. Catharina Hospital and CCB hospital received fees for data collection. G.B.C. received teaching compensation from Medtronic, Abbott, and Boston-Scientific.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
The Editor in Chief helped with adjudicating the final decision on this paper.
Author contributions
All authors were involved in the conception and design of the study. M.V.D.K., L.D., I.T., D.D.R., L.D.R.V., J.C. participated in the data collection. S.U. was involved in the analysis and interpretation of data. All authors contributed to the drafting and critical revision of the manuscript. All authors approved the final version of the manuscript.
Acknowledgements
The authors would like to thank all participants who contributed to the study.
Data availability statement
The data underlying this article will be shared on reasonable request to the corresponding author.
Table 1. Resource consumption real-world analysis: baseline characteristics of the cohort.
Table 2. Resource consumption real-world analysis: procedural time and healthcare professional workload by phase and treatment option.
Table 3. Total costs for a cohort of 100 patients
Figure 1. Decision tree structure for the cost-consequence analysis
*Includes costs on procedural time, administered drugs, exams, and length of stay.
CRYO, cryoablation; PFA, pulsed field ablation; RFA, radiofrequency ablation.
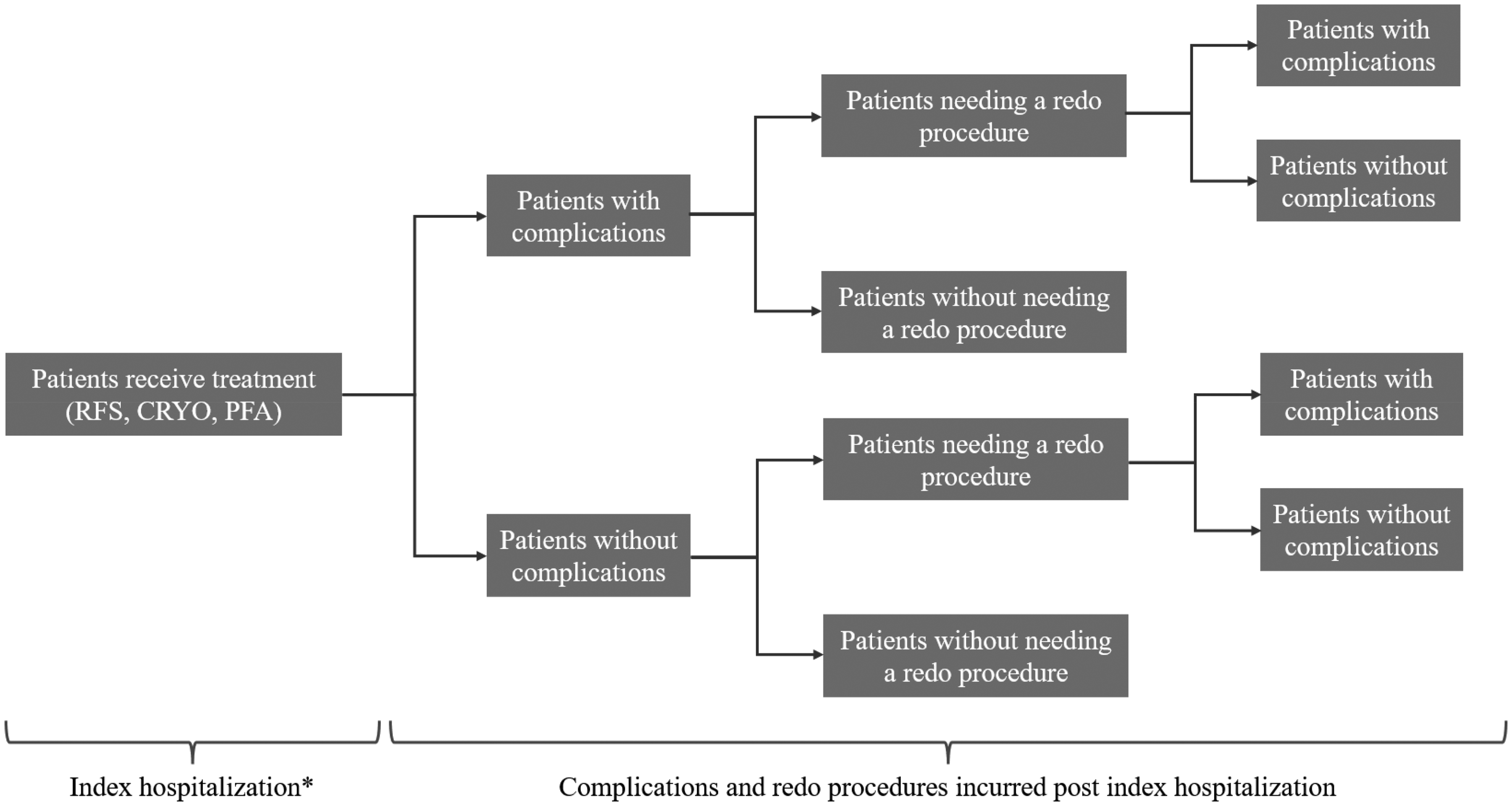
Figure 2. Total cost per patient of the cardiac ablation procedure by treatment option
CRYO, cryoablation; PFA, pulsed field ablation; RFA, radiofrequency ablation.
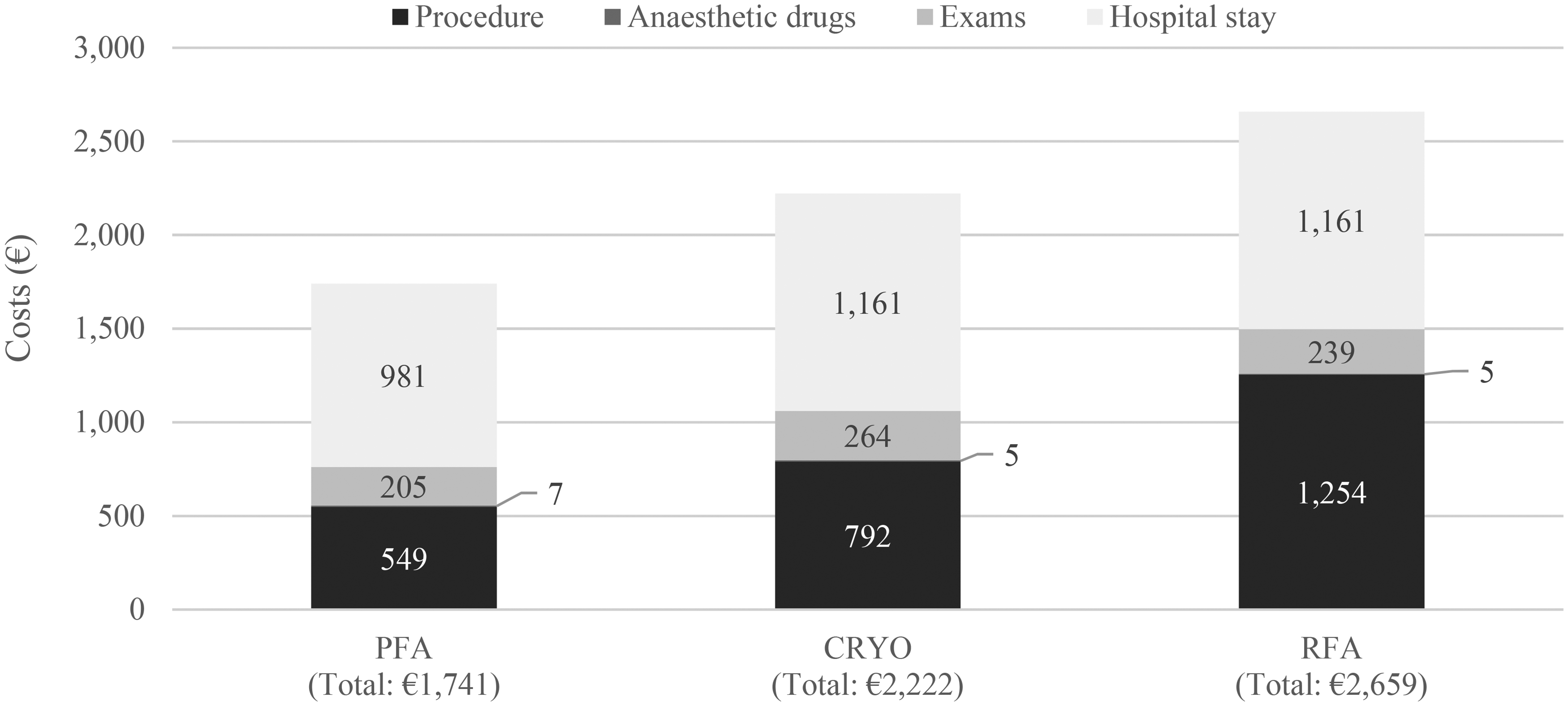
Figure 3. Boxplot of total cost per patient. Each box represents the interquartile range of the total patient cost. The median value is indicated by the line inside each box, with whiskers extending above and below the box. Outliers are shown as individual points
Supplemental Material
Download PNG Image (40.1 KB)References
- January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation. 2019;140: e125–e151. doi: 10.1161/CIR.0000000000000665/FORMAT/EPUB.
- Baman JR, Passman RS. Atrial Fibrillation. JAMA. 2021;325: 2218–2218. doi: 10.1001/JAMA.2020.23700.
- Sagris M, Vardas EP, Theofilis P, Antonopoulos AS, Oikonomou E, Tousoulis D. Atrial Fibrillation: Pathogenesis, Predisposing Factors, and Genetics. Int J Mol Sci. 2021;23. doi: 10.3390/IJMS23010006.
- Ohlrogge AH, Brederecke J SR. Global Burden of Atrial Fibrillation and Flutter by National Income: Results From the Global Burden of Disease 2019 Database. J Am Hear Assoc. 2023; 12(17):e030438. doi: 10.1161/JAHA.123.030438.
- Qeska D, Singh SM, Qiu F et al. Variation and clinical consequences of wait-times for atrial fibrillation ablation: population level study in Ontario, Canada. Europace. 2023; 25(5). doi: 10.1093/europace/euad074.
- British Heart Foundation. The Tipping Point. Why heart care must be prioritised now. BHF Rep. 2022; 1–35. Available: https://www.bhf.org.uk/-/media/files/what-we-do/influencing-change/tipping-point-bhf-report.pdf?rev=820040973e5d43a684e80bc784a86fe4&hash=D8A7033C7D8D798497455461C2679A07
- Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Hear Rhythm. 2017;14: e275–e444. doi: 10.1016/J.HRTHM.2017.05.012.
- Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the Europea. Eur Heart J. 2021;42: 373–498. doi: 10.1093/EURHEARTJ/EHAA612.
- Di Monaco A, Vitulano N, Troisi F, Quadrini F, Romanazzi I, Calvi V, et al. Pulsed Field Ablation to Treat Atrial Fibrillation: A Review of the Literature. J Cardiovasc Dev Dis. 2022;9. doi: 10.3390/JCDD9040094.
- Muthalaly RG, John RM, Schaeffer B, Tanigawa S, Nakamura T, Kapur S, et al. Temporal trends in safety and complication rates of catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol. 2018;29: 854–860. doi: 10.1111/JCE.13484.
- Reddy VY, Neuzil P, Koruth JS, Petru J, Funosako M, Cochet H, et al. Pulsed Field Ablation for Pulmonary Vein Isolation in Atrial Fibrillation. J Am Coll Cardiol. 2019;74: 315–326. doi: 10.1016/J.JACC.2019.04.021.
- Reddy VY, Dukkipati SR, Neuzil P, Anic A, Petru J, Funasako M, et al. Pulsed Field Ablation of Paroxysmal Atrial Fibrillation: 1-Year Outcomes of IMPULSE, PEFCAT, and PEFCAT II. JACC Clin Electrophysiol. 2021;7: 614–627. doi: 10.1016/J.JACEP.2021.02.014.
- Ekanem E, Reddy VY, Schmidt B, Reichlin T, Neven K, Metzner A, et al. Multi-national survey on the methods, efficacy, and safety on the post-approval clinical use of pulsed field ablation (MANIFEST-PF). Europace. 2022;24: 1256–1266. doi: 10.1093/EUROPACE/EUAC050.
- Hijazi W, Vandenberk B, Rennert-May E, Quinn A, Sumner G, Chew DS. Economic evaluation in cardiac electrophysiology: Determining the value of emerging technologies. Front Cardiovasc Med. 2023;10: 1142429. doi: 10.3389/FCVM.2023.1142429/BIBTEX.
- Calvert P, Mills MT, Xydis P, Essa H, Ding WY, Koniari I, et al. Cost, Efficiency and Outcomes of Pulsed Field Ablation versus Thermal Ablation for Atrial Fibrillation: A Real World Study. Hear Rhythm. 2024;0. doi: 10.1016/j.hrthm.2024.05.032.
- Duxbury C, Begley D, Heck PM. Pulsed field ablation with the pentaspline catheter compared with cryoablation for the treatment of paroxysmal atrial fibrillation in the UK NHS: a cost-comparison analysis. BMJ Open. 2024;14: 79881. doi: 10.1136/BMJOPEN-2023-079881.
- Husereau D, Drummond M, Augustovski F, de Bekker-Grob E, Briggs AH, Carswell C, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) 2022 Explanation and Elaboration: A Report of the ISPOR CHEERS II Good Practices Task Force. Value Heal. 2022;25: 10–31. doi: 10.1016/J.JVAL.2021.10.008.
- Della Rocca DG, Marcon L, Magnocavallo M, Menè R, Pannone L, Mohanty S, et al. Pulsed electric field, cryoballoon, and radiofrequency for paroxysmal atrial fibrillation ablation: a propensity score-matched comparison. EP Eur. 2023;26. doi: 10.1093/EUROPACE/EUAE016.
- Kawamura I, Neuzil P, Shivamurthy P, Petru J, Funasako M, Minami K, et al. Does pulsed field ablation regress over time? A quantitative temporal analysis of pulmonary vein isolation. Hear Rhythm. 2021;18: 878–884. doi: 10.1016/j.hrthm.2021.02.020.
- de Campos MCAV, Moraes VRY, Daher RF, Micheleto JPC, de Campos LAV, Barros GFA, et al. Pulsed-field ablation versus thermal ablation for atrial fibrillation: A meta-analysis. Hear Rhythm O2. 2024. doi: 10.1016/J.HROO.2024.04.012.
- Duytschaever M, Račkauskas G, De Potter T, Hansen J, Knecht S, Phlips T, et al. Dual energy for pulmonary vein isolation using dual-energy focal ablation technology integrated with a three-dimensional mapping system: SmartfIRE 3-month results. EP Eur. 2024;26: 5–8. doi: 10.1093/EUROPACE/EUAE088.
- Reddy VY, Gerstenfeld EP, Natale A, Whang W, Cuoco FA, Patel C, et al. Pulsed Field or Conventional Thermal Ablation for Paroxysmal Atrial Fibrillation. N Engl J Med. 2023; 1–12. doi: 10.1056/NEJMoa2307291.
- Monnickendam G, de Asmundis C. Why the distribution matters: Using discrete event simulation to demonstrate the impact of the distribution of procedure times on hospital operating room utilisation and average procedure cost. Oper Res Heal Care. 2018;16: 20–28. doi: 10.1016/J.ORHC.2017.12.001.
- Andrade JG, Champagne J, Deyell MW, Essebag V, Lauck S, Morillo C, et al. A randomized clinical trial of early invasive intervention for atrial fibrillation (EARLY-AF) - methods and rationale. Am Heart J. 2018;206: 94–104. doi: 10.1016/J.AHJ.2018.05.020.
- Andrade JG, Deyell MW, Badra M, Champagne J, Dubuc M, Leong-Sit P, et al. Randomised clinical trial of cryoballoon versus irrigated radio frequency catheter ablation for atrial fibrillation-the effect of double short versus standard exposure cryoablation duration during pulmonary vein isolation (CIRCA-DOSE): methods and rationale. BMJ Open. 2017;7: 17970. doi: 10.1136/bmjopen-2017-017970.
- Turagam MK, Neuzil P, Schmidt B, Reichlin T, Neven K, Metzner A, et al. Safety and Effectiveness of Pulsed Field Ablation to Treat Atrial Fibrillation: One-Year Outcomes From the MANIFEST-PF Registry. Circulation. 2023 [cited 9 Jun 2023]. doi: 10.1161/CIRCULATIONAHA.123.064959.
- Taghji P, Haddad M El, Phlips T, Wolf M, Knecht S, Vandekerckhove Y, et al. Evaluation of a Strategy Aiming to Enclose the Pulmonary Veins With Contiguous and Optimized Radiofrequency Lesions in Paroxysmal Atrial Fibrillation A Pilot Study. 2018.
- Kanters TA, Wolff C, Boyson D, Kouakam C, Dinh T, Hakkaart L, et al. Cost comparison of two implantable cardiac monitors in two different settings: Reveal XT in a catheterization laboratory vs. Reveal LINQ in a procedure room. Europace. 2016;18: 919–924. doi: 10.1093/EUROPACE/EUV217.
- NAVLIN | Global Pricing & Market Access Database. [cited 7 May 2024]. Available: https://data.navlin.com/alspc/#!/
- Nomenclatuur : Zoekcriteria. [cited 22 Apr 2024]. Available: https://webappsa.riziv-inami.fgov.be/Nomen/nl/search
- InEK GmbH, www.g-drg.de. InEK GmbH www. g-drg. de. Flat rate case catalog 2023, InEK GmbH. [cited 17 Apr 2024]. Available: https://www.g-drg.de/ag-drg-system-2023/fallpauschalen-katalog/fallpauschalen-katalog-20232.
- Struijvé E. Standaardprijslijst overige zorgproducten 2023. [cited 17 Apr 2024]. Available: https://www.jeroenboschziekenhuis.nl/sites/default/files/documents/2022-12/2023-standaardprijslijst-overige-zorgproducten.pdf
- Financiële feedback per pathologie - ETCT. [cited 22 Apr 2024]. Available: https://tct.fgov.be/webetct/etct-web/national_data?lang=nl.