Abstract
Aim
The current study compared preparation time, errors, satisfaction, and preference for a prefilled syringe (PFS) versus two RSV vaccines requiring reconstitution (VRR1 and VRR2) in a randomized, single-blinded time and motion study.
Methods
Pharmacists, nurses, and pharmacy technicians were randomized to a preparation sequence of the three vaccines. Participants read instructions, then consecutively prepared the three vaccines with a 3–5-min washout period in between. Preparations were video recorded and reviewed by a trained pharmacist for preparation time and errors using predefined, vaccine-specific checklists. Participant demographics, satisfaction with vaccine preparation, and vaccine preference were recorded. Within-subjects analysis of variance was used to compare preparation time. Mixed-effects Poisson and ordered logistic regression models were used to compare the number of preparation errors and satisfaction scores, respectively.
Results
Sixty-three pharmacists (60%), nurses (35%), and pharmacy technicians (5%) participated at four sites in the United States. The least squares mean preparation time per dose for PFS was 141.8 s (95% CI = 156.8–126.7; p <.0001) faster than for VRR1, 103.6 s (95% CI = 118.7–88.5; p <.0001) faster than for VRR2, and 122.7 s (95% CI = 134.2–111.2; p <.0001) faster than the pooled VRRs. Overall satisfaction (combined “Very” and “Extremely”) was 87.3% for PFS, 28.6% for VRR1, and 47.6% for VRR2. Most participants (81.0%) preferred the PFS vaccine.
Limitations
The study is limited by the inability to completely blind observers. To minimize the effects of order, we utilized a 3-sequence block design; however, the order in which the vaccines were prepared may have affected outcomes. Participants were assessed once, whereas if repeated preparations were performed there may have been trained efficiencies gained for each vaccine.
Conclusion
PFS vaccines can greatly simplify the vaccine preparation process, allowing administrators to prepare almost four times more doses per hour than with vial and syringe systems.
Introduction
Respiratory syncytial virus (RSV) is a common respiratory virus, causing approximately 159,000 hospitalizations, 119,000 emergency department admissions, and 1.4 million outpatient visits annually in United States (US) adults aged 65 years and olderCitation1. In Europe, RSV causes an estimated 250,000 hospitalizations and 17,000 in-hospital deaths every year in people aged 65 years and olderCitation2. Even when figures are adjusted for under-detection due to imperfect sensitivity of reverse transcription polymerase chain reaction testing of nasopharyngeal or nasal swabs, they are likely still underestimated for several reasons, including, but not limited to, the lack of routine RSV testing in clinical practiceCitation1.
RSV usually causes mild cold-like symptoms, but it can cause serious illness in adults (≥65 years) and infantsCitation3. Compared to coronavirus disease (COVID-19) or influenza, older adults hospitalized with RSV often have more severe disease, are more likely to be admitted to the intensive care unit, and receive standard flow oxygen, high-flow nasal cannula, or noninvasive ventilationCitation4. After hospital discharge, up to 15% of these individuals require a higher level of care than needed prior to admissionCitation5,Citation6. Infection with RSV is costly, with an estimated annual economic burden in US adults (≥60 years) of $6.6 billion, with $2.9 billion in direct medical costs, $1.1 billion in indirect costs due to losses in productivity from RSV-related morbidity, and $2.5 billion in indirect costs due to RSV mortalityCitation7.
The Advisory Committee on Immunization Practices (ACIP) currently recommends that adults over 60 years may receive a single dose of RSV vaccine, using shared clinical decision-making with their healthcare provider (HCP)Citation8. Two RSV vaccines are currently approved by the US Food and Drug Administration (FDA) for adults aged 60 years and older – AREXVY (RSVPreF3, GSK) and ABRYSVO (RSVpreF, Pfizer), both of which are vaccines requiring reconstitution (VRR). AREXVY (from here on addressed as VRR1) includes two vials, one with a lyophilized vaccine (powder) and one with an adjuvant suspension. Preparation steps for VRR1 include cleansing the vial stoppers, transferring the diluent into the vial of lyophilized vaccine using a separate syringe and needle, swirling to dissolve the powder, and withdrawal of the reconstituted vaccine into the syringe for administrationCitation9. ABRYSVO (addressed as VRR2) is available in two presentations: (i) two vials with one vial of lyophilized vaccine and one vial of liquid diluent, and (ii) one vial with lyophilized vaccine and a prefilled syringe (PFS) with sterile water diluent. Preparation of VRR2 in the second format, as utilized in the current study, includes cleansing the vial stopper, attachment of the provided adapter to the vial, connection of the PFS to the vial adapter, transferring the diluent from the PFS into the vial of lyophilized vaccine, swirling to dissolve the powder, withdrawal of the reconstituted vaccine for administration into the PFS, and locking the needleCitation10.
Moderna has recently licensed mRESVIACitation11, an mRNA-based RSV vaccine which employs the same ready-to-use PFS delivery system used to administer the FDA-approved SPIKEVAX (mRNA-1273, Moderna) (addressed as PFS). Preparation of PFS includes removing the PFS tip cap and locking the needle (needle not included in the vaccine kit)Citation12. Preparation of mRNA-1273 PFS was utilized in the current study as a proxy for mRESVIA.
Different vaccine presentations involve varying time and risks for errors during preparation, both factors contributing to overall cost. In comparison with VRR, PFS are generally easier to handle and administer, and are associated with reductions in both time and errors in preparationCitation13,Citation14. De Coster et al. conducted a cross-over, randomized, open-label time and motion (T&M) study, and recorded HCPs as they prepared one PFS and one VRR vaccine consecutively with a 3–5-min washout period in between the preparationsCitation15. Overall, PFS preparation resulted in a mean time saving of 34.5 s [95% CI = 28.4–40.6] compared to VRR, and out of 96 preparations of each presentation, there were 10 (10.4%) errors reported during PFS preparation compared to 47 (48.9%) for VRRsCitation15. Another study indicated an average time saving of 1.1 min (66 s) for each PFS prepared and administered vaccine dose compared to VRRCitation13.
In the selection of vaccine presentations, HCP preference may play a role. Several studies have reported higher HCP preference for PFS presentations, with the most common reasons being reduced number of immunization errors, ease of administration, and work efficiencyCitation13,Citation16–19. HCPs also expressed that using PFS lowers the risk of needle contamination and needle stick injuries and reduces the possibility of dosage errorsCitation17,Citation19.
Up to now, studies have not addressed preparation time and errors along with satisfaction and preference of HCPs, nor have they been conducted with vaccines specific to RSV. T&M studies include independent and continuous observation and are more precise than self-reporting or work sampling techniques, which collect data at intervals of time. This study design has been used in healthcare to determine the timing and duration of procedures or tasks, and to calculate vaccine preparation time and errorsCitation15,Citation20. Video recording of HCPs has been shown to reduce the observer effect and has the potential to improve the quality of data collected by providing the opportunity to replay each task for review and analysis.
Study objectives
The primary objective of the study was to assess and compare the vaccine preparation times of PFS, VRR1, and VRR2 prior to administration. Secondary objectives of the study were to assess and compare the rate of vaccine preparation errors, participant satisfaction, and preference for PFS, VRR1, and VRR2.
We hypothesized that, compared to VRR1 and VRR2, PFS would result in reduced preparation time, fewer errors, and higher HCP satisfaction and preference.
Methods
Study design
A randomized, 3-period, 3-sequence cross-over, single-blinded T&M study was conducted at four sites in the United States (US) (). To maintain single-blind for the study, the PFS presentation was labeled “Vaccine A”, VRR1 was labeled “Vaccine B”, and VRR2 was labeled “Vaccine C”. The recruited participants were randomly assigned a vaccine preparation sequence (ABC, BCA, or CAB) blocked by site for balance. On arrival at the site, participants were asked to read blinded vaccine preparation instructions for each of the three study vaccines (generated from package inserts for each vaccineCitation9,Citation10,Citation12) and to complete an electronic survey on site (Supplementary Material S1) to gather information on demographic and previous experience with and training for vaccine preparation. All questions were mandatory.
Figure 1. Study design.
Abbreviations: HCPs, healthcare providers; PFS, prefilled syringe; Vaccine A, ready-to-use PFS; Vaccine B, VRR1 (vial with a lyophilized vaccine and vial with an adjuvant suspension); Vaccine C, VRR2 (vial with lyophilized vaccine and a prefilled syringe with sterile water diluent); VRR, vaccine requiring reconstitution; VRR1, VRR with vial with a lyophilized vaccine and vial with an adjuvant suspension; VRR2, VRR with vial with lyophilized vaccine and a prefilled syringe with sterile water diluent.
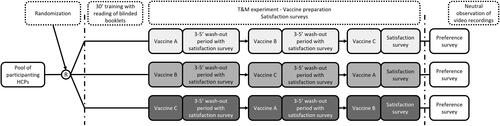
After a period of approximately 30 min, participants were asked to consecutively prepare one dose of each vaccine in the assigned randomized sequence with a 3–5-min washout period in between each preparation. Vaccines labels were blinded, and vaccines were laid out on the counter for participants, along with required materials to facilitate aseptic technique as per usual practice. During each washout period, the participants rated their satisfaction with the vaccine ease of preparation, preparation time, and overall satisfaction. After preparing all three vaccines, participants provided their overall preference between vaccine A, B, or C.
Vaccine preparations were video recorded on-site by trained nurses. An independent, experienced, trained pharmacist reviewed each video to assess the time taken for each preparation, and documented any preparation errors using predefined, vaccine-specific checklists. A second pharmacist independently reviewed 24% (first nine participants and six randomly selected participants) preparation videos as quality control. Agreement between reviewers of ≥80% was considered acceptable, and no changes were needed based on quality control. The vaccine-specific error checklist was developed by reviewing all steps listed in the label of approved vaccines and cross-referencing with published literatureCitation15,Citation18.
On-site study personnel were trained to avoid any influence on the participant during vaccine preparation. Vaccine preparation was conducted in a research setting mimicking that of a busy retail pharmacy/clinic (i.e. open area with background noise from clinic daily activities). Study processes, including the completeness of error checklists, underwent internal validation with the first three study participants.
Sample size
De Coster et al.Citation15 reported average preparation time for VRR of 70.5 s and for PFS of 36.0 s, with a mean difference [SD] of 34.5 [30] seconds, i.e. roughly half the time. This suggests a large effect size (>0.5)Citation21. For the sample size calculations, we used a 0.5 effect size and a paired t-test, resulting in a samples size of 54 (18 for each cohort) with a statistical power of 90% at α = 0.025. Applying a non-parametric correction of 15% and assuming a 10% drop-out rate, the sample size increased to 63 (21 for each of the three sequence cohorts)Citation22.
Participants
Participants were eligible if they were at least 21 years of age, had a minimum 1.5 years of experience administering vaccines, had administered at least one vaccine in the month prior, and were either one of the following: US-licensed and practicing pharmacists, US-registered practicing nurse, or a practicing pharmacy technician certified by the Pharmacy Technician Certification Board. Participants with any current business relationship with Moderna, Pfizer, and/or GSK were excluded, including, but not limited to, employment, consultancy agreement, and/or holding individually managed stocks. The participants were screened into the study based on self-report of the inclusion and exclusion criteria.
Participant recruitment
Recruiters were trained to conduct recruitment in accordance with study criteria. The recruitment process involved outreach to an expert network of pharmacists, pharmacist technicians, and nurses. Further potential participants were also identified via email, LinkedIn, cold calls, and referrals. The largest source of participants came via LinkedIn and the second largest through referrals.
Outcomes
The outcome for the primary objectives was vaccine preparation time, defined as the time in seconds from when the participant touched any material on the experimental field (e.g. syringe, vial, needles) to the time the ready for administration syringe was laid down on the table.
Secondary outcomes included the total number of vaccine preparation errors and participants’ vaccine satisfaction and preference. The total number of errors was defined as the sum of specific errors for each participant and vaccine recorded by the observer in the vaccine-specific checklists. Observer comments made in the “other” error category were reviewed by the study team. Comments were not considered “other” errors if they were not mistakes made by the participant during preparation (i.e. preparation was out of view of the camera for a moment), were errors that were already accounted for by another error indicated by the observer in the checklist, or if they could reasonably be incorporated into an existing error category. Further, repetitive errors identified in the others category were pulled out into a new category (e.g. used more than one syringe). See Supplementary Materials S2 and S3 for more details on errors.
Satisfaction for ease-of-preparation, time taken for preparation, and overall vaccine preparation procedure were assessed through a self-administered online survey using a 7-point Likert scale. Satisfaction for each outcome was measured on a 7-point Likert scale (Extremely Dissatisfied; Very Dissatisfied; Dissatisfied; Neither Satisfied nor Dissatisfied; Satisfied; Very Satisfied; Extremely Satisfied). Vaccine preference was determined through a single item where participants selected the vaccine they preferred most.
Ethics, data privacy, and pharmacovigilance
This study was conducted in compliance with the principles of the Declaration of Helsinki. This study received Institutional Review Board (IRB) approval from Advarra Central IRB (MOD01999449). Written informed consent was obtained from each participant prior to starting the study. In this study no vaccine was administered. Participants who completed the study received fair market value (FMV) compensation for their time. Their travel time was compensated for half the hourly FMV rate for a maximum of 1.5 h.
Statistical analysis
To estimate mean preparation time (seconds) for each vaccine and to test the difference in vaccine preparation times, a within-subjects analysis of variance (ANOVA) approach was implemented through a linear mixed model (LMM). For the pairwise comparison of individual vaccines, the Dunnett multiple comparison adjustment was used. Normality of residuals was confirmed using quantile-quantile (q-q) plots. Comparisons of the number of preparation errors made for each vaccine was performed using a Poisson generalized linear mixed model (GLMM). The potential presence of over- (or under-) dispersion by fitting a negative binomial GLMM and assessing the significance of the dispersion parameter k. Comparisons of the satisfaction scores between vaccines were performed using mixed-effects ordered logistic regression models.
All models included preparation sequence and vaccine (i.e. PFS, VRR1, and VRR2) as fixed effects, and random effects (random intercepts) for subject and site. For endpoints related to preparation time, supplementary models were run including participant career experience (years), experience administering vaccines (years), and occupation as fixed effects.
Post-hoc analyses were run comparing PFS to data from pooled VRRs (pVRR: data from VRR1 and VRR2 combined into a single cohort) for all endpoints. A p-value lower than .05 was considered statistically significant. All analyses were conducted using SAS v9.4.
Results
Participant recruitment and characteristics
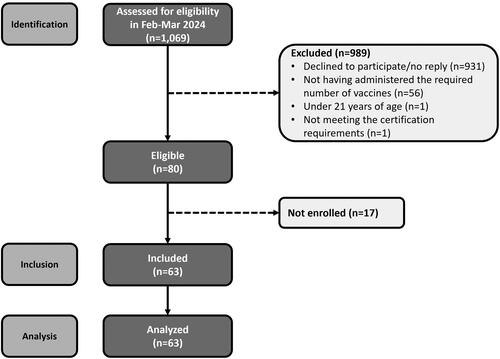
Recruitment involved outreach to 1,069 individuals, of which 80 were deemed eligible, and a total of 63 participants were enrolled and completed the study ().
Demographics of the 63 participants who completed the study (21 randomized to each of the three vaccine preparation sequences) are presented in . The mean (SD) age of participants was 41.9 years (10.7) and 31.7% were male (n = 20). The majority were either pharmacists (n = 38, 60.3%) or nurses (n = 22, 34.9%). Participants were largely from urban practices (n = 47, 74.6%), and primarily worked in a retail pharmacy setting (n = 36, 57.1%). Most participants had less than 20 years of experience in their current occupation, with 39.7% (n = 25) having 1–10 years of experience, and 33.3% (n = 21) having 11–20 years of experience. Over half had more than 10 years of experience administering vaccines (n = 32, 50.8%). Almost all indicated general training on vaccine preparation (n = 61, 96.8%) and specific training for VRR preparations (n = 60, 95.2%), while specific training for PFS preparations was less common (n = 53, 84.1%).
Table 1. Participant demographics, overall and by randomization sequence.
Vaccine preparation time
Complete vaccine preparation time were available for all participants for PFS and VRR1, and for 62 participants for VRR2, due to the malfunctioning of the vaccine kit vial adapter for one participant who was unable to complete the preparation (). Correlation of preparation time between observers was high (Pearson’s r = 0.99, n = 15). The observed mean time (SD) for vaccine preparation was 43.5 s (23.8) for PFS, 185.3 s (57.1) for VRR1, 147.2 s (56.9) for VRR2, and 166.4 s (59.9) for the pooled VRR cohort (pVRR).
Table 2. Vaccine preparation time, by vaccine and for pooled VRRs, with comparison.
Adjusting for randomization sequence, vaccine type, site, and individual subject, least square (LS) mean preparation times did not differ from observed means. The LS mean preparation time for PFS was 141.8 s (95% CI = 156.8–126.7; p <.0001) faster than for VRR1 and 103.6 s (118.7–88.5; p <.0001) faster than for VRR2 ().
When the two VRRs were pooled together, the mean (95% CI) preparation time was 166.2 s (95% CI = 155.9–176.5). The LS mean preparation time for PFS was 122.7 s (95% CI: 134.2–111.2; p <.0001) faster than the preparation time for pVRR.
Irrespective of a participant’s prior experience, experience administering vaccines, or occupation, the mean preparation time for PFS was consistently and significantly lower than each individual VRR or pVRR cohort (Supplementary Tables S1–S4).
Vaccine preparation errors
Vaccine preparation errors were assessed by reviewing the video recordings of each preparation. Agreement on errors between the two observers was 90.5% (n = 15). The mean total number of errors (SD) for PFS was 3.5 (1.1), VRR1 was 4.0 (1.5), VRR2 was 3.8 (1.5), and for combined VRRs it was 3.9 (1.5). However, those differences did not reach statistical significance (). For all three vaccines, QC errors were the most frequently made error type, while only one participant made an asepsis fault error throughout the experiment (for VRR1).
Table 3. Vaccine preparation errors, by vaccine and for pooled VRRs.
Vaccine satisfaction and preference
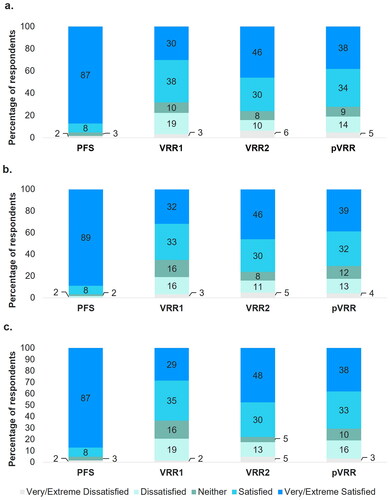
Participants frequently indicated that they were “Very Satisfied” or “Extremely Satisfied” with the PFS vaccine in terms of the ease-of preparation (n = 12 and 43, respectively; 87.3% total) and preparation time (n = 7 and 49, respectively; 88.9% total) ( and Supplementary Table S5). Satisfaction scores for VRR1 and VRR2 were more widely distributed, and the most selected satisfaction score for both vaccines was “Satisfied” for each satisfaction measure. Overall, only 1.6% (n = 1) of participants indicated any degree of dissatisfaction (combined “Dissatisfied”, “Very Dissatisfied”, and “Extremely Dissatisfied”) for PFS, while 20.6% and 17.5% said the same for VRR1 (n = 13) and VRR2 (n = 11), respectively. The overall satisfaction (combined “Very Satisfied” and “Extremely Satisfied”) was 87.3% (n = 55) for PFS, 28.6% (n = 18) for VRR1, and 47.6% (n = 30) for VRR2 ( and Supplementary Table S5).
Figure 3. Participant satisfaction ratings for (a) ease of preparation, (b) time of preparation, and (c) overall satisfaction with vaccine preparation.
Abbreviations: PFS, ready-to-use prefilled syringe; VRR, vaccine requiring reconstitution; VRR1, VRR with vial with a lyophilized vaccine and vial with an adjuvant suspension; VRR2, VRR with vial with lyophilized vaccine and a prefilled syringe with sterile water diluent.
Compared to VRR1 and VRR2, PFS had a 19.93 (95% CI = 8.32, 47.72; p <.0001) and 10.87 (95% CI = 4.72, 25.06; p <.0001) times increase in the odds of receiving a higher overall satisfaction score, respectively. Similarly, compared to VRRs combined, PFS had 14.72 (95% CI = 6.68–32.41; p <.0001) times greater odds of receiving a higher overall satisfaction score. This trend was consistent across “ease of use” and “preparation time” satisfaction measures ().
Table 4. Comparison of participant satisfaction with vaccine preparation procedures.
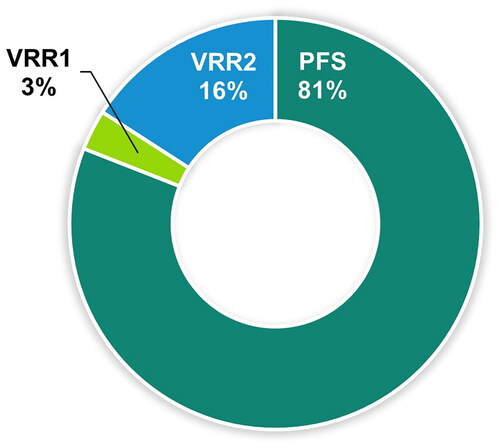
After all vaccine preparations were complete, participants selected their preferred vaccine. The majority of participants (81.0%) reported a preference for the ready-to-use prefilled syringe vaccine, PFS ( and Supplementary Table S6).
Figure 4. Participant preference for vaccine preparations.
Abbreviations: PFS, ready-to-use prefilled syringe; VRR, vaccine requiring reconstitution; VRR1, VRR with vial with a lyophilized vaccine and vial with an adjuvant suspension; VRR2, VRR with vial with lyophilized vaccine and a prefilled syringe with sterile water diluent.
Discussion
This is the first study to compare vaccine preparation time, errors, satisfaction, and preferences for RSV vaccines. We found that preparation time with PFS was significantly lower as compared to VRRs, both when testing each VRR separately and when combining them. Study participants were extremely satisfied and preferred a PFS over VRRs. We did not observe any significant differences in the number of errors between vaccine preparations.
Vaccine preparation time
Consistent with prior published literature,Citation13,Citation15 results from this study demonstrated that the preparation time with a PFS vaccine was significantly lower as compared to vaccines that require reconstitution. As mentioned previously, De Coster et al.Citation15 observed that the average preparation time for the ready-to-use PFS was 36 s versus 70.5 s for the VRR (powder and suspension for injection in a PFS presentation), a reduction of nearly half. In a survey by Cuesta-Esteve et al.Citation13, paediatric nurses reported spending 8.08 min (484.8 s) to prepare (from patient counseling to administration) a ready-to-use PFS versus 9.16 min (549.6 s) for a VRR with powder and suspension for injection in a PFS presentation. In our study the preparation time with PFS took on average 43.5 s and was nearly 4 times lower as compared to VRR1 and VRR2, both when testing each VRR separately and when combining them. The differences in the type of vaccine, study population, steps included in the calculation of time, and methods used to assess vaccine preparation time (self-reported vs measured) prevent a direct comparison of previously reported results with those from our study. Based on the vaccine preparation times observed in our study, we can infer that vaccine administrators can prepare 83 PFS vaccines per hour, 20 VRR1 vaccines per hour, and 24 VRR2 vaccines per hour consecutively. Considering the timing of pVRR, we can infer that vaccine administrators can prepare 22 reconstituted vaccines per hour.
Of note, recent updates have been made to the label for VRR2 (ABRYSVO), which now includes two presentations; a vial of lyophilized powder and a vial of liquid diluent, and a vial of lyophilized powder and a PFS diluentCitation10, the latter of which was used in this study. Although both require reconstitution, use of the vial and PFS presentation may explain the slightly lower preparation times compared to VRR1. The vaccine kit for VRR1 includes two vials, and preparation steps are therefore more like those of the VRR2 vial and vial presentation.
The majority of the population receives their vaccines in a pharmacy settingCitation23,Citation24. Due to the start of respiratory season and recommendations guiding the administration of RSV and influenza vaccines, these vaccines are administered during the fall season every yearCitation8, resulting in increased pressure on community pharmacies resources. A recent survey of community pharmacists in the US has demonstrated that 74.9% of the pharmacists were experiencing burnoutCitation25–28. Further, a systematic review identified 19 articles across eight countries and estimated a burnout prevalence of 51%Citation25. Some of the risk factors associated with burnout were high patient and prescription volumes and increased workload. The increased demand for vaccination during the fall season might result in higher patient volume and workload leading to pharmacists’ burnout and potential errorsCitation29,Citation30. Results from the study suggest that an alternative RSV vaccine in a ready-to-use PFS might alleviate heavy workloads.
Vaccine preparation errors
The total number of errors made during preparation did not differ significantly between vaccines. Likewise, the total number of vaccine-specific errors were similar across vaccines. The ability to detect statistical differences in error rates in our study was limited because the study was powered for the primary outcome detecting time differences and not for error rates. Further, this was a controlled experiment and error rates in a busy retail clinic may not be accurately reflected here.
For the PFS vaccine, user technique error related to tip cap removal was the main source of preparation errors. These errors did not result in any leaking of the vaccine and hence would not impact product quality or prevent vaccine administration. For the VRR1 and VRR2, there was a numerical trend for higher proportion of spillage or leakage. In addition, due to the requirement for reconstitution and additional associated steps, additional errors such as “Lack of cleansing of vials rubber stopper after removing the plastic flip off” or “Vial content (adjuvant or reconstituted vaccine) not fully aspired into syringe” were observed. This is in line with other published studiesCitation15,Citation18. A survey-based evaluation of vaccine related errors by Lee et al. found 76.4% of physicians and 41.5% of nurses experienced errors related to reconstitution, including “vial contents not aspired into syringe” (52.0% and 14.7%, respectively), “inadequate shaking” (51.6% and 19.1%, respectively), “spillage or leakage during reconstitution” (42.4% and 14.7%, respectively), “needle twisted when inserted in vial stopper” (29.6% and 11.8%, respectively), “same needle used for reconstitution and injection” (23.2% and 10.0%, respectively), “forgetting to reconstitute” (19.2% and 7.6%, respectively), and “other” (31.6% and 11.1%, respectively)Citation18.
Satisfaction and preferences
In line with previous published literature, the study results showed that study participants were more likely to be satisfied with PFS than VRRs, and overall preferred a PFS over VRRs. Though understanding the drivers of this satisfaction and preference were out of the study scope, the high satisfaction on ease and speed of preparation indicate these are contributing factors to the overall preference. In support, a recent targeted literature review found PFS was most often preferred by physicians and nurses with common reasons cited that included reduced immunization errors, errors typically occurring during reconstitution, followed by ease of administrationCitation14.
Study limitations
There are several limitations to the current study design, including the inability to completely blind observers to the three vaccines given the differences between preparations, and it is possible that the observers were familiar with the different vaccines. While the strength of the cross-over design allowed for subjects to act as their own controls, the order in which the vaccines were prepared may have affect outcomes including timing, errors, satisfaction, and preference. To minimize the effects of order, we utilized a 3-sequence block design to balance the order of vaccine preparation among participants. Lastly, participants performed a single assessment for each vaccine whereas, if repeated preparations were performed, there may have been trained efficiencies gained.
The strengths of the design lie in the direct time assessment and recorded review of preparations that allowed for repeated views to determine timing and errors. In addition, independent assessments between observers were performed to ensure agreement (>80%).
Conclusions
Given the workload in pharmacy settings, implementation of an RSV program using a PFS vaccine may provide significant advantages. PFS vaccines can greatly simplify the vaccine preparation process, allowing administrators to prepare almost four times more doses per hour than with vial and syringe systems.
Transparency
Declaration of funding
Sponsorship for this study and Rapid Service Fee were provided by Moderna Inc.
Declaration of financial/other interests
DM, NVdV, and BH are employed by Moderna, Inc. SK, SF, DC, MH, AP, and MM are employed by ICON Plc. and have worked with Moderna Inc., GlaxoSmithKline Pharmaceuticals, and Pfizer Inc.
Author contributions
DM, SK, and MM were involved in study conception. Material preparation was performed by SF, DC, MM, and SK. All authors were involved in the study design. Data analysis was performed by MH and AP. The manuscript was drafted by SF, DC, and SK. All authors critically reviewed drafts of the manuscript and reviewed and approved the final manuscript. All authors agreed to take responsibility and be accountable for the contents of the article.
Reviewer disclosures
Peer reviewers on this manuscript have received an honorarium from JME for their review work but have no other relevant financial relationships to disclose.
Previous presentations
An earlier version of this manuscript has been shared on the medRxiv preprint server prior to peer review: Mehta D, Kimball-Carroll S, Clark DR, Fossati S, Hunger M, Pahwa A, Malmenas M, Hille B, Van de Velde N. Vaccine preparation time, errors, satisfaction, and preference of prefilled syringes versus RSV vaccines requiring reconstitution: randomized, time and motion study. medRxiv. 2024:2024.2004.2016.24305921. Available from: https://www.medrxiv.org/content/10.1101/2024.04.16.24305921v1
Supplemental Material
Download MS Word (84.6 KB)Acknowledgements
We would like to thank the following people: Anna Krivelyova (ICON Plc) for overall project support; Mohana Giruparajah and Adeola Adejumo (ICON Plc) for their operational support to the project; Jaya Paranilam (ICON Plc) for the IRB submission; Ruchika Sharma and Shravan Kumar Adepu (ICON Plc) for running the statistical analysis; Natalia Olter and Sabine Grothues (ICON Plc) for reviewing the vaccine preparation video-recordings for time and errors; Kathleen Walker (AccellaCare) for coordinating the AccellaCare sites; Katy Klein, Erin Hackett, Emma Hughes, Devon Brown, Djemila Fields, Chernessa Campbell, Sarah Utech, and Alphonso Chambers (AccellaCare) for coordinating participants and data collection at site level; Erin Butler, Jennifer Haynes, Sydney Rabinowitz, and Tammy Joseph (GLG) for recruitment of participants; and all healthcare professional who participated to the study preparing the vaccines and completing the survey for their time.
Data availability statement
All data generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- McLaughlin JM, Khan F, Begier E, et al. Rates of medically attended RSV among US adults: a systematic review and meta-analysis. Open Forum Infect Dis. 2022;9(7):ofac300.
- European Medicines Agency. First vaccine to protect older adults from respiratory syncytial virus (RSV) infection; 2023 [updated 2023 Apr 26; 2024 Jun 12]. Available from: https://www.ema.europa.eu/en/news/first-vaccine-protect-older-adults-respiratory-syncytial-virus-rsv-infection
- World Health Organization. Respiratory Syncytial Virus (RSV) disease; 2024 [2024 Mar 07]. Available from: https://www.who.int/teams/health-product-policy-and-standards/standards-and-specifications/vaccine-standardization/respiratory-syncytial-virus-disease
- Surie D, Yuengling KA, DeCuir J, et al. Disease severity of respiratory syncytial virus compared with COVID-19 and influenza among hospitalized adults aged >/=60 years – IVY Network, 20 U.S. States, February 2022-May 2023. MMWR Morb Mortal Wkly Rep. 2023;72(40):1083–1088. doi: 10.15585/mmwr.mm7240a2.
- Branche AR, Saiman L, Walsh EE, et al. Change in functional status associated with respiratory syncytial virus infection in hospitalized older adults. Influenza Other Respir Viruses. 2022;16(6):1151–1160. doi: 10.1111/irv.13043.
- Goldman CR, Sieling WD, Alba LR, et al. Severe clinical outcomes among adults hospitalized with respiratory syncytial virus infections, New York City, 2017-2019. Public Health Rep. 2022;137(5):929–935. doi: 10.1177/00333549211041545.
- Carrico J, Hicks KA, Wilson E, et al. The annual economic burden of respiratory syncytial virus in adults in the United States. J Infect Dis. 2023;jiad559. doi: 10.1093/infdis/jiad559.
- Centers for Disease Control and Prevention. Respiratory Syncytial Virus (RSV) Vaccine VIS: Centers for Disease Control and Prevention; 2023 [updated 2023 Oct 19; cited 2024 Apr 08]. Available from: https://www.cdc.gov/vaccines/hcp/vis/vis-statements/rsv.html#:∼:text=CDC%20recommends%20a%20single%20dose,most%20of%20the%20United%20States
- AREXVY [package insert]. Durham (NC): GlaxoSmithKline; 2023.
- ABRYSVO™ [package insert]. New York (NY): Pfizer Inc; 2023.
- US Food and Drug Administration. BLA Approval Cambridge, MA; 2024 [cited 2024 Jun 12]. Available from: https://www.fda.gov/media/179015/download?attachment
- SPIKEVAX [package insert]. Cambridge (MA): ModernaTX, Inc; 2022.
- Cuesta Esteve I, Fernández Fernández P, López Palacios S, et al. Health care professionals’ preference for a fully liquid, ready-to-use hexavalent vaccine in Spain. Prev Med Rep. 2021;22:101376. doi: 10.1016/j.pmedr.2021.101376.
- Mehta DK-CS, Krivelyova A, Yu WW, et al. Vaccine time, cost and preference comparison between pre-filled syringe formulations and vaccines that require reconstitution: a targeted literature review. Poster session presented at: ISPOR EU 2023 Nov 12–15; Copenhagen, Denmark.
- De Coster I, Fournie X, Faure C, et al. Assessment of preparation time with fully-liquid versus non-fully liquid paediatric hexavalent vaccines. A time and motion study. Vaccine. 2015;33(32):3976–3982. doi: 10.1016/j.vaccine.2015.06.030.
- Aljunid SM, Al Bashir L, Ismail AB, et al. Economic impact of switching from partially combined vaccine "Pentaxim(R) and hepatitis B" to fully combined vaccine "Hexaxim(R)" in the Malaysian National Immunization Program. BMC Health Serv Res. 2022;22(1):34.
- Icardi G, Orsi A, Vitali Rosati G, et al. Preferences of healthcare professionals regarding hexavalent pediatric vaccines in Italy: a survey of attitudes and expectations. J Prev Med Hyg. 2020;61(3):E424–E444.
- Lee YH, Harris RC, Oh HW, et al. Vaccine-related errors in reconstitution in South Korea: a national physicians’ and nurses’ survey. Vaccines (Basel). 2021;9(2):117. doi: 10.3390/vaccines9020117.
- Lloyd AJ, Nafees B, Ziani E, et al. What are the preferences of health care professionals in Germany regarding fully liquid, ready-to-use hexavalent pediatric vaccine versus hexavalent pediatric vaccine that needs reconstitution? Patient Prefer Adherence. 2015;9:1517–1524. doi: 10.2147/PPA.S87229.
- Finkler SA, Knickman JR, Hendrickson G, et al. A comparison of work-sampling and time-and-motion techniques for studies in health services research. Health Serv Res. 1993;28(5):577–597.
- Cohen JE. Statistical power analysis for the behavioral sciences. Hillsdale (NJ): Lawrence Erlbaum Associates, Inc; 1988.
- Lehmann EL. Nonparametrics: statistical methods based on ranks. Saddle River, (NJ): Prentice Hall; 1998.
- IQVIA Institute. Trends in Vaccine Administration in the United States: IQVIA Inc.; 2023 [updated 2023 Jan 13; cited 2024 Apr 08]. Available from: https://www.iqvia.com/insights/the-iqvia-institute/reports-and-publications/reports/trends-in-vaccine-administration-in-the-united-states
- IQVIA Institute. Trends in global adult vaccination: impact of COVID-19: IQVIA, Inc.; 2023 [updated 2023 Jul 18; cited 2024 Apr 08]. Available from: https://www.iqvia.com/insights/the-iqvia-institute/reports-and-publications/reports/trends-in-global-adult-vaccination
- Patel SK, Kelm MJ, Bush PW, et al. Prevalence and risk factors of burnout in community pharmacists. J Am Pharm Assoc. 2021;61(2):145–150. doi: 10.1016/j.japh.2020.09.022.
- Centers for Disease Control and Prevention. RSV vaccination coverage, adults 65 years and older, United States: Centers for Disease Control and Prevention; 2024 [updated 2024 Mar 5; cited 2024 Apr 10]. Available from: https://www.cdc.gov/vaccines/imz-managers/coverage/rsvvaxview/adults-65yrs-older-coverage.html
- Centers for Disease Control and Prevention. Seasonal flu vaccines: Centers for Disease Control and Prevention; 2024 [updated 2024 Mar 12; cited 2024 Apr 10]. Available from: https://www.cdc.gov/flu/prevent/flushot.htm
- Centers for Disease Control and Prevention. Influenza vaccinations administered in pharmacies and physician medical offices, adults, United States: Centers for Disease Control and Prevention; 2024 [cited 2024 Apr 10]. Available from: https://www.cdc.gov/flu/fluvaxview/dashboard/vaccination-administered.html
- Golbach AP, McCullough KB, Soefje SA, et al. Evaluation of burnout in a national sample of hematology-oncology pharmacists. J Clin Oncol Oncol Pract. 2022;18(8):e1278–e1288. doi: 10.1200/OP.21.00471.
- Skrupky LP, West CP, Shanafelt T, et al. Ability of the Well-Being Index to identify pharmacists in distress. J Am Pharm Assoc. 2020;60(6):906–914.e2. doi: 10.1016/j.japh.2020.06.015.