Abstract
Aims
We aimed to describe the clinical, economic, and societal burdens of cystic fibrosis (CF) and impact of CF transmembrane conductance regulator modulator (CFTRm) treatment on people with CF, caregivers, and healthcare systems.
Material and methods
This retrospective study used linked real-world data from Swedish national population-based registries and the Swedish CF Quality Registry to assess clinical, economic, and societal burden and CFTR impact in CF. Records from people with CF and a ten-fold control population without CF matched by sex, birth year, and location were compared during 2019. Outcomes for a subset aged >6 years initiating lumacaftor/ivacaftor (LUM/IVA) in 2018 were compared 12 months pre- and post-treatment initiation.
Results
People with CF (n = 743) had >10 times more inpatient and outpatient specialist visits annually vs controls (n = 7406). Those aged >18 had an additional 77·7 (95% CI: 70·3, 85·1) days of work absence, at a societal cost of €11,563 (95% CI: 10,463, 12,662), while caregivers of those aged <18 missed an additional 6.1 (5.0, 7.2) workdays. With LUM/IVA treatment, people with CF (n = 100) had significantly increased lung function (mean change in ppFEV1 [3·8 points; 95% CI: 1·1, 6·6]), on average 0·5 (95% CI: −0·8, −0·2) fewer pulmonary exacerbations and 45·2 (95% CI: 13·3, 77·2) fewer days of antibiotics. Days of work lost by caregivers of people with CF aged <18 decreased by 5·4 days (95% CI: 2·9, 7·9).
Conclusion
CF is associated with a high clinical economic and societal burden in Sweden. Improvements in clinical status observed in people with CF treated with LUM/IVA were reflected in reduced caregiver and societal burden.
PLAIN LANGUAGE SUMMARY
Cystic fibrosis (CF) is a disease caused by a single faulty gene called CFTR, which affects the lungs, pancreas, and other organs. Medications known as CFTR modulators help improve the function of this faulty gene and have shown benefits for people with CF. In Sweden, two such medicines, lumacaftor and ivacaftor (LUM/IVA), have been available since July 2018 for treating CF. This study looks at the impact of CF on patients, caregivers, and the healthcare system, as well as the benefits of CFTR modulators. Using data from Swedish national healthcare and social insurance registries, the study compared 743 people with CF in 2019 to about 7400 people without CF, matched by sex, birth year, and location. The findings show that people with CF had 24 times higher direct healthcare costs, including outpatient visits, hospitalizations, and CF-related medications, totaling 23,233 Euros. Indirect costs, such as work absences for those over 18 with CF anssd caregivers’ absences to care for sick children, were 9,629 Euros, which is five times higher than the general population. Those over 6 years old treated with LUM/IVA showed improved lung health, reduced hospitalizations (though not significantly), and needed fewer antibiotics. Caregivers’ work absences decreased, but there was no change in work absences for adults with CF. Overall, treatment with LUM/IVA improved clinical outcomes and reduced the burden on caregivers and society.
Introduction
Cystic fibrosis (CF) is a rare genetic disease that leads to significant morbidity and reduction in life expectancy. The symptoms and complications of CF result from reduced or absent cystic fibrosis transmembrane conductance regulator (CFTR) functionCitation1. As an epithelial cell-surface anion channel, CFTR regulates exocrine functions across multiple organ systems and its dysfunction leads to serious clinical consequences in the lungs, pancreas, intestines, sinuses, and reproductive systemCitation2,Citation3.
Disease presentation and course vary among people with CF, and their care requires multifaceted management of the symptoms and complications of primary diseaseCitation1,Citation4. Clinical symptoms of CF usually appear in early childhood, with onset of pulmonary involvement seen in over 80% of children with CF who survive the neonatal periodCitation5. A spectrum of disease is often seen, whereby the development of CF-related symptoms varies among people with CF in terms of age of onset and severityCitation1,Citation4. Although variations in the CFTR gene contribute to the disease, specific variants and other genetic and environmental factors result in phenotypic differences that drive the need for complex healthcare support of the people with CFCitation1,Citation6,Citation7.
Noteworthy improvements in health outcomes and life expectancy in people with CF have been seen over the last few decades due to advances in respiratory and nutritional managementCitation8,Citation9. The therapeutic class of CFTR modulators (CFTRm), which address the underlying cause of CF by restoring CFTR function, has demonstrated clinical benefits in people with CF who have CFTRm-responsive mutationsCitation2,Citation10–17. Currently, four CFTRm treatments are approved in the European Union: ivacaftor, lumacaftor/ivacaftor (LUM/IVA), tezacaftor/ivacaftor (TEZ/IVA), and elexacaftor/tezacaftor/ivacaftor (ELX/TEZ/IVA)Citation18. In Sweden, LUM/IVA was the first CFTRm reimbursed; reimbursement was approved on 1 July 2018 for people with CF aged ≥6 yearsCitation19 and on 22 March 2019 for those aged ≥2 years. These decisions were followed more recently in December 2022 by reimbursement for TEZ/IVA and ELX/TEZ/IVA in patients aged ≥6 years.
Owing to its progressive, multiorgan impact, CF continues to limit survival and quality of life, resulting in a large burden of disease for people with CF, their families, and society at large in Europe. Therefore, it is important to evaluate not only the clinical but also the economic and societal burden associated with CF and the potential benefit of CFTRm in the real-world setting. Studies on the impact of ivacaftor (licensed already in 2012) on class 3 mutations (gating mutations) on the cost and burden of care like hospitalization have been described before but did not include the impact on sick leave and parental absence from workCitation20,Citation21. Ivacaftor is known to have a more profound (better) effect on sweat test values, ppFEV1 and hospitalization indicating a better pharmacological effect on CFTR. Instead LUM/IVA can be used in a larger population of patients (40%-50% who are homozygous for F508del in a country) but the clinical impact and impact on sweat test values are smaller. Although prior studies have examined the real-world burden of CF and the impact of CFTRm such as LUM/IVA in Europe, outcomes examined have been generally limited to clinical or utilization measures available in clinical settings, registries, or administrative databasesCitation9,Citation22–28. Through linkage of data from both disease-specific and national registries using personal identity numbers, we benefited from the unique opportunity of the Swedish setting to provide a holistic view of the real-world impact of CF and the potential benefit of CFTRm, in this case LUM/IVA.
Methods
Study design overview
This was a retrospective, population‐based study conducted using data from Swedish national population-based registries and the Swedish Cystic Fibrosis Quality Registry (SCFR). The main objective was to describe the clinical, economic, and societal burdens of CF in 2019 compared with general population controls. The second objective was to describe the clinical, economic, and societal impact of treatment with the CFTRm LUM/IVA in people with CF aged >6 years compared to the pre-LUM/IVA period. This study was approved by the Ethical Review Authority on 1 April 2020 (reference number 2019-06502).
Data sources
The SCFR is a disease-specific registry that includes >95% of people with CF in Sweden by gathering clinical data from all four Swedish CF centers since 2012Citation9,Citation23. Individual-level data in the SCFR and several national population-based registries in Sweden can be linked via the personal identifier given to each Swedish citizen. The Registry for the Total Population (RTB) includes all Swedish residents registered with the Swedish Tax Agency and contains information on date of birth, sex, and address. The Swedish Multi-Generation Registry (MGR) is part of the RTB and contains data linking caregivers to their children (both biological and adoptive).
For this study, three other national registries provided individual-level healthcare data across all people in Sweden: the National Patient Registry (NPR; capturing all hospital care), the Prescribed Drugs Registry (PDR), and the Cause of Death Registry (CDR). Socioeconomic data were provided by the Swedish National Longitudinal Integration Database for Health Insurance and Labor Market Studies (LISA). The Swedish Social Insurance Registry (SIR) provided information on sick leave (after day 14, day 1-13 is paid for by the employer and is not registered in SIR or any other centralized register), disability pension, and vård av barn (VAB) leave (i.e. a caregiver’s absence from work to care for a child). A further description of each registry can be found in Table S1.
Study design and populations
To analyze the burden of CF, demographics and clinical characteristics were documented for all people enrolled in the SCFR who had data available between 1 January and 31 December in 2019, the CF cohort. Healthcare resource utilization, missed work, and costs associated with CF in 2019 were analyzed by comparing the CF cohort to a general population control cohort, which was created by matching individuals from a random sample extracted from the RTB based on sex, birth year, and location at a 10:1 ratio to people in the CF cohort. Corresponding caregiver cohorts included parents and caregivers of people aged <18 years who were identified through linking Swedish national identifiers in the MGR. All study measures were examined over the calendar year 2019 for both the CF and matched cohorts.
To analyze the impact of CFTRm on outcomes, a LUM/IVA cohort was identified from the SCFR that included all people with CF in Sweden with a LUM/IVA index date (date of initiation of LUM/IVA treatment) between 1 January 2018 and 31 December 2018 and who had ≥12 months of follow-up available. Clinical measures, healthcare resource utilization (HCRU), missed work, and associated costs for people in this analysis were examined for 12 months prior to their index date (preindex) and for 12 months after index (postindex) with an additional 2-month window for capturing outcomes at month 12. Similarly to the burden of CF analysis, a caregiver cohort for people in the LUM/IVA cohort aged <18 years was identified through linking Swedish national identifiers in the MGR.
Study measures
Demographics (sex and age) and clinical measures (lung function, nutritional status, exacerbations) were described for the CF cohort. Demographics for the general population cohort would have matched the CF cohort, by definition, so were not analyzed. Sex and age were measured for the CF cohort as of 31 December 2019. Lung function among people with CF was measured using the best (highest) measure in 2019 for percent predicted forced expiratory volume (ppFEV1). The spirometry prediction equation used for ppFEV1 was that of the Global Lung Function Initiative (GLI)Citation25. Body mass index (BMI) in people aged ≥18 years and BMI z-score in children aged <18 years was calculated as of 31 December 2019. Pulmonary exacerbations (PEx) were identified based on dispensation of intravenous (IV) antibiotics, as captured in the PDR. Because records for IV antibiotics do not include days supplied, all courses of IV antibiotics were assumed to last 10 days, consistent with standard practice; thus, the end date of a course of antibiotics was defined as the dispensing date plus 10 days. Overlapping or consecutive antibiotic prescriptions were considered part of the same PEx event if ≤7 days had elapsed between the end date of the first course and dispensing date of the next course. If >7 days elapsed between two courses of IV antibiotics, they were considered to be indicators of separate PEx events.
Number of dispensations of oral antibiotics and days of supply of oral antibiotics included all dispensations of oral antibiotics based on the PDR, with the exception of records of >4 weeks of treatment with flucloxacillin (Anatomical Therapeutic Chemical [ATC] code, J01CF05). Use of >4 weeks of flucloxacillin was excluded from the oral antibiotics analysis in order to limit the count to acute infections; however, these longer courses were included in the reporting of courses of “antibiotics- any formulation” to accurately reflect the burden of antibiotic use, which may include impact on the body and gut flora. Receipt of CF-related dispensations recorded in the CF registry (e.g. including adrenergic inhalators/bronchodilators, pancreatic enzymes, antidiabetics, and transplant immunosuppressants) were measured (see full list of CF treatment related medications in Table S2).
HCRU measures derived from the National registries included specialist physician visits conducted in a hospital outpatient setting, hospitalizations, hospitalization days, and CF-related prescription dispensations. Primary care visits are not aggregated in a national registry so were not included in the analysis. The same selected list of medications typically associated with CF was used for both the CF and general control cohorts; note that for simplicity the same term (CF-related dispensations) has been used to describe these dispensations. Societal burden was assessed as annual number of work absence days for people with CF, and VAB leave days for their caregivers.
Cost analyses included those associated with outpatient specialist visits, hospitalizations, CF-related drug dispensations, and indirect costs of patient or caregiver work absence. Cost of outpatient specialist visits was assigned based on diagnosis-related group (DRG) codes multiplied by the 2019 cost per physician visit summed over the one-year periodCitation29. Total cost of hospitalizations was computed using the DRG costing codes multiplied by the 2019 cost per hospitalization and summed over the one-year period. Total cost of filled prescriptions was calculated by summing the pharmacy retail price of all filled prescriptions over the year. ATC codes were used to identify treatments in the registry data. A list of ATC codes for CF-related dispensations can be found in Table S2. Indirect costs associated with days of VAB leave, sick leave, or disability pension were calculated by multiplying the number of days by an assumed value of lost productivity. In accordance with the human capital approach, this value was assumed to be equal to mean wage in Sweden plus payroll taxes (based on census statistics from Statistics Sweden)Citation26,Citation27. Wages were sex adjusted. Cost in euros was computed using the 2020 exchange rate of 1 euro to 10·49 Swedish kronaCitation28.
For the impact of CFTRm analysis, in addition to ppFEV1, BMI, and BMI z-score, lung clearance index (LCI), sweat chloride, and health-related quality of life in individuals aged ≥14 years measured by the respiratory domain of the Cystic Fibrosis Questionnaire Revised (CFQ-R14) were evaluated preindex and postindex. For all clinical outcomes, the measurement during the preindex period that was closest to the initiation of treatment and the measurement during the postindex period closest to the end of 12 months of follow-up +/− 2 months were used. PEx were counted over the 1-year preindex and postindex periods as described for the burden objective. HCRU (outpatient specialist visits, hospitalizations, hospital days, CF-related dispensations, dispensed antibiotics, dispensed oral antibiotics, supply of oral antibiotic in days) and societal burden (work absence days, VAB leave days) preindex and postindex were also calculated as described for the burden analysis, as were direct (outpatient specialist visits, hospitalizations, all drug dispensations) and indirect (work absence and VAB leave) costs.
Data analysis
For the analysis of clinical burden, only the people with measurements available (ppFEV1, BMI, and BMI z-score) in 2019 were included in the mean calculations. A comparison of HCRU and cost in 2019 was conducted between the CF and control cohorts. For the analysis of the impact of LUM/IVA, ppFEV1 was reported as the change from preindex to 12 months post index. HCRU and costs were analyzed during the 12-month preindex and postindex periods; missing data were not imputed. A within-group repeated measures analysis was used to compare key clinical parameters, HCRU, and economic burden before and after initiation of LUM/IVA. For both analyses, HCRU and cost analyses were stratified by age group (<18 years and ≥18 years); people were included in the <18-year group until the calendar year in which they turned 18.
All analyses calculated means with standard deviation (SD) for continuous variables and frequencies with percentages (%) for categorical variables. Comparisons among groups and time periods were reported as differences in the group means. All analyses of treatment burden were descriptive. For the analysis of impact of LUM/IVA, statistical significance was established using 95% CI. All data management and statistical analyses were performed using Stata 16·0 (StataCorp LLC, College Station, TX, USA).
Results
Clinical burden of CF
A total of 743 people were identified in the SCFR in 2019 (). Among the 625 people who had ppFEV1 measurement data in 2019, the mean (SD) ppFEV1 was 80·3 (23.2), which is close to the lower bound of the range considered normal (80-120). People with CF experienced a mean (SD) of 0·95 (1.64) PEx events in 2019. Of CF-related treatments dispensed during 2019, the most frequently administered were antibiotics (received by 93% of patients) followed by adrenergic inhalators/bronchodilators (89%) and pancreatic enzymes (81%). In 2019, 13% of people with CF were receiving transplant immunosuppressants (96/743).
Table 1. Demographics and clinical characteristics of people with CFTable Footnotea in 2019 (n = 743).
HCRU of people with CF compared to the general population
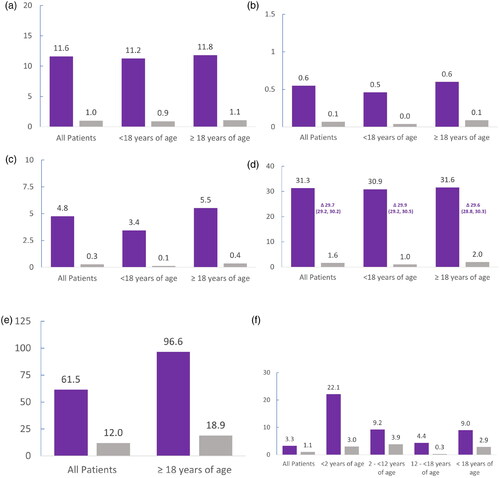
People with CF were matched to 7406 controls from the general population; available demographic data for the general population was limited to age and sex, which exactly matched the CF cohort (data not shown). Among people of all ages, HCRU was substantially higher in those with CF (). In 2019, people with CF had ten times as many outpatient specialist visits compared with the general population (11·6 vs 1·0, respectively), which was consistent across the aged <18 years and aged ³18 years sub-cohorts. In the people with CF compared with the general population, more than six times as many hospitalizations (0·6 per year vs 0·1 per year, respectively) and an average of 4·5 additional hospitalization days (4·8 days vs 0·3 days, respectively) were reported. Approximately, 30 more CF-related dispensations were recorded for the people with CF in comparison to the general population in 2019, with equal distribution between age subcohorts. Among people of working age (aged ³18 years), those with CF were observed to have 77·7 more days of missed work per year compared with the general population; when averaged over people of all ages, those with CF had 49·5 more days of missed work. In people of all ages, CF was associated with 2·2 more VAB leave days when a parent or caretaker stayed home from work to care for a child with CF. The greatest difference in VAB leave days between the CF and general population (19·1 additional days) was among caregivers of the youngest children (aged <2 years). Among caregivers of all children aged <18, a difference of 6·1 days was observed vs the general population.
Figure 1. Mean HCRU in 2019 for people with CF compared with the general population. (a) Number of outpatient specialist visits. (b) Number of hospitalizations. (c) Number of hospitalization days. (d) Number of CF-related dispensations. (e) Number of days’ work absence. (f) Number of VAB leave days. Values graphed are means. Deltas (Δ) indicate differences in the means, which are followed by the 95% confidence intervals. The number of people with CF included in the analyses were: 743 of any age, 270 children aged <18 years, 473 adults aged ≥18 years, 18 children aged <2 years, 159 children aged 2 to <12 years, and 93 children aged 12 to <18 years. The number of people in the control groups were: 7406 of any age, 2700 children aged <18 years, 4706 adults aged ≥18 years, 180 children aged <2 years, 1590 children aged 2 to <12 years, and 930 children aged 12 to <18 years. CF-related dispensations included adrenergic inhalators/bronchodilators, antibiotics, anticoagulants, anticonvulsants, antidepressants, antidiabetics, antifungals, antihistamines, anti-inflammatory agents, anxiolytics, corticosteroids, expectorant drugs, gastroesophageal reflux disease medications, growth hormones, opioids, osteoporosis drugs, oxygen, pancreatic enzymes, transplant immunosuppressants, and vitamins. CF, cystic fibrosis; HRCU, healthcare resource utilization; VAB, care of sick child (vård av barn).
Direct and indirect costs of CF compared to the general population
Direct costs related to HCRU for a person with CF in 2019 were on average an additional €4163 for outpatient specialist visits, €3814 for hospitalizations and €12,460 for CF treatment dispensations compared to the general population (). The total difference in indirect costs for lost productivity in 2019 averaged €7691. This represents an average difference of €11,563 in costs of work absence for adults with CF aged ³18 years and of €902 in VAB leave days for caregivers of all children with CF aged <18 years.
Table 2. Comparison of total costs (€ 2020) in people with CF and controls in 2019Table Footnotea.
Clinical impact of treatment with LUM/IVA
A total of 231 people with CF were treated with LUM/IVA during the study period of whom 100 met the inclusion criterion of 12 months of follow-up available. Of these, 56% were male and the mean age at index was 24·1 years. A statistically significant improvement in all reported clinical outcomes was observed at 12 months of treatment vs pre-index values (). Mean ppFEV1 significantly increased by 3·8 points (95% CI: 1·1, 6·6), and mean lung clearance index (LCI) significantly decreased (improved) 1·0 (95% CI: −1·6, −0·3) points, demonstrating an improvement in pulmonary function at 12 months following treatment initiation with LUM/IVA. Sweat chloride tests showed a 20·9 mmol/L (95% CI: −25·1, −16·6) decrease from preindex (difference of the means: −20·9 mmol/L); and CFQ-R14 values increased by 6·7 points (95% CI: 1·1, 12·3), indicating a significant improvement in quality of life related to respiratory symptoms for people with CF with CFTRm treatment. Nutritional parameters, including BMI and BMI z-score showed significant improvement for both adults and children. The annual number of PEx events postindex (Mean:1·2; SD: 1.8) significantly decreased by 29% from the preindex mean of 1·7 events (SD: 2.1; a mean decrease of 0·5 events [95% CI: −0·8, −0·2). Similar improvements were seen in both the number of dispensations of oral antibiotics (−1·5; 95% CI: −2·27, −0·67) and the number of days’ supply of oral antibiotics (−45·2 days; 95% CI: −77·17, −13·27) ().
Table 3. Impact of treating with LUM/IVA on clinical outcomesTable Footnotea.
Table 4. Impact of treatment with LUM/IVA on resource use and costsTable Footnotea.
Impact of treatment with LUM/IVA on resource use
In the postindex period, the mean number of outpatient specialist visits increased by 1·09 (95% CI: 0·01, 2·17) vs preindex (). This difference reflects the additional monitoring (every 6 weeks) required for patients with CF after initiation of treatment in Sweden. The change in number of hospitalizations (−0·17; 95% CI: −0·40, 0·06) and duration of hospitalizations (−0·87 days; 95% CI: −2·25, 0·51) suggest a decreasing trend for the time period; however, these decreases were not statistically significant. The number of CF-related pharmacy dispensations significantly decreased by −8·6% (a difference of −3·23; 95% CI: −5·23, −1·23) in the postindex period; this change was largely driven by a significant 19·2% decrease in antibiotic use, which represented nearly three fewer antibiotics dispensations per year. The details of individual drugs dispensed for CF are presented in Table S3. While 9·2 (95% CI: −6·17, 24·51) more work absence days in adults were reported, this result was not statistically significant. The mean number of VAB days significantly decreased by 2·16 (1·05, 3·26) days in the total LUM/IVA cohort and by 5·39 (2·89, 7.90) days in caregivers of children with CF aged <18 years.
Impact of treatment with LUM/IVA on direct and indirect costs
Mirroring the increased frequency of visits, the mean annual cost for outpatient specialist visits increased by €459 (95% CI: 32, 886) during the postindex period (). Costs for all dispensations were significantly lower in the postindex period. Although the total number of hospitalizations decreased in the 12 months postindex, the mean cost for hospitalizations increased by €651 (95% CI: −2049, 3351); this discrepancy was largely driven by the costs of a single lung transplantation. Excluding that individual, costs for hospitalizations decreased non-significantly in the post-index period.
The cost of long-term work absence for the overall LUM/IVA cohort increased by €808(95% CI: −494, 2109) in the postindex period; which represented a mean difference of €1346 (95% CI: −838, 3530) among those aged ≥18 years. The cost of VAB leave days was reduced by −€316 (95% CI: 154, 477) in the overall LUM/IVA cohort, representing a reduction of −€789 (95% CI: 423, 1155) when analysis was limited to caregivers of people with CF aged <18 years.
Discussion
This retrospective study used linked data from the Swedish CF Registry and several nationwide Swedish administrative health registries to describe the clinical, economic, and societal burden in people with CF compared with the general population and to quantify the clinical and economic impact of treatment with the CFTRm LUM/IVA.
In 2019, people with CF in Sweden (n = 743) had expectedly higher HCRU and economic burden compared with the general population. Direct costs in 2019 were approximately €23,200 for an average person with CF, representing €22,300 greater direct costs than in matched controls in the general population. These costs reflect a higher number of outpatient specialist visits, hospitalizations, days of hospitalizations, and CF-related dispensations in the people with CF. The mean total costs of outpatient specialist visits were similar for children and adults. However, the cost of hospitalizations among adults was almost double that of children, which could be partly explained by increasing complications of CF with age and is consistent with results of previous studiesCitation22,Citation24. The findings from this study are consistent with those seen in other assessments and show that CF complications result in long and numerous hospital stays accompanied by elevated healthcare costsCitation24.
This study also showed that CF has a wide-ranging indirect economic impact on people with CF and their caregivers in line with prior research showing a strong correlation between health status and employmentCitation30 and a high caregiver burden in CFCitation31. In 2019, adults with CF had on average 78 more days of work absence than the general population due to long-term sick leave or early retirement. This is most probably an underestimation since there are no centralized registration of short-term sick leaves under 14 days. That means that a “normal” IV treatment at home for 10 days will not be seen in the SIR registry. VAB leave days were 6·1 days higher for caregivers of children with CF compared with those of controls, reflecting days home from work to provide necessary support; caregivers of infants aged <2 years with CF lost nearly an additional month (19·1 days) of work per year. This overall difference in work loss resulted in an increased annual indirect cost of approximately €7,700 in comparison to the general population. These results confirm and further quantify previous findings that have significant societal implications beyond direct costs such as hospitalizations, medications, and outpatient specialist visits, indirect costs related to informal care, VAB leave, and work absence due to sick leave or disability pensionCitation32,Citation33.
Of the people with CF who were treated with LUM/IVA with 12 months follow-up available (n = 100), this study’s results using real-world data demonstrated consistent improvements in lung function in people with CF aged ≥6 years that are in line with published data from pivotal clinical trials of LUM/IVACitation34–36.
The analysis of economic and broader societal impacts of LUM/IVA shows that the number of outpatient specialist visits increased in the postindex period, which can be explained by the additional monitoring required after initiation of treatment. The number of CF-related drug dispensations decreased at 12 months postindex, with a significant decrease in the mean number of oral antibiotics prescriptions. The relatively modest, nonsignificant decreases in number of hospitalizations (−0·2) and duration of hospitalizations (−0·9 days) possibly reflect the fact that IV antibiotics are often administered in the home setting in Sweden and therefore may reflect other causes of hospitalization such as severe disease with low FEV1 or CF related complications. Despite the reduction in hospitalizations and improved clinical status of people with CF after treatment, the point estimate for work absence increased across the analysis period for people treated with LUM/IVA; however; the difference was not significant. This increase may be due to absences related to monitoring visits or due to respiratory symptoms related to LUM/IVA initiation that may have resolved with longer follow-up. Importantly, this study demonstrated a significant 5-day decrease in work loss for caregivers of children with CF. This reduction in societal burden related to caregiving is a novel finding of meaningful impact that warrants further study with ELX/TEZ/IVA, which is a more efficacious CFTRm combination available to a broader population.
Strengths and limitations
Although this study provided a comprehensive analysis of the societal impact of CF and the potential benefits of CFTRm treatment; this study is not without limitations. Because CF is a rare disease, the sample size was limited; however, due to the high coverage of the people with CF in the Swedish CF registry, this sample is likely to provide unbiased estimates of the burden of CF in Sweden and may be generalizable to other high-income countries with similar health systems.
Most likely, the true burden of the disease is somewhat underestimated in the present study because data for some relevant costs and resources were either missing or not captured in the registries, including short term sick-leave, over-the-counter medications, hospital-administered medications, and primary care visits. In particular, sick leave is only recorded in the sickness insurance registries when it lasts for ≥14 days because shorter leave periods are paid for by the employer and not documented in the registries. The inability to include these short-term leaves in the present study was likely a source of underestimation of the true burden of CF and the differences before and after LUM/IVA treatment. Moreover, only prescriptions filled in pharmacies were captured because drugs given in the hospital setting without a prescription are not covered in the PDR. However, the rate of self-administered medication usage may be overestimated in this study because utilization was based on filled prescriptions at the pharmacy and at-home administration could not be confirmed. The missing information of primary care visits is probably of minor importance since practically all CF care is delivered by the CF centers or shared care hospitals due to the frequent outpatient visits.
Of note, the COVID-19 pandemic would not have affected the results of this study because the entire index period predated the pandemic.
In conclusion, people with CF in Sweden have significantly higher indirect and direct costs compared with a matched non-CF control population. The study shows an improvement in clinical endpoints over the 12 months after initiation of LUM/IVA compared with the preindex period, with results very similar to those seen in pivotal clinical studies of LUM/IVA. This treatment had a significant positive effect (decrease) in the number of PEx and the number of courses of prescribed oral antibiotics and, furthermore, a significant decrease of VAB days for caregivers of children with CF. A direct comparison of the total cost for CF care with other high income countries can be problematic due to different health care systems (for example cost for sick leave, involvement of primary care physicians and different treatment policies). Since CFTRm were introduced at different time points in different countries, comparison over the same years may not be possible. This also applies to the use of other drugsCitation37. Despite differences, there are also many similarities that make this study of interest also for other countries. As data for ELX/TEZ/IVA in Sweden matures, similar analyses linking clinical, economic, and societal measures may show even greater impacts of CFTRm.
Transparency
Declaration of funding
Funding was received from Vertex Pharmaceuticals Incorporated.
Author contributions
AL, IM, MG, CK, and LM were involved in study conceptualization. AL, MA, and JB supervised the study, developed the methodology, conducted the investigation, and assessed and verified the data. MA and JB performed the formal analyses. All authors were involved in interpretation of the results. MA prepared the first draft of the manuscript and AL and MA worked on the visualization of data. All authors reviewed the manuscript critically throughout its development. Medical writers and editors contributed to writing and editing drafts of the manuscript under the direction of the authors. All authors approved the final manuscript and were responssible for the decision to submit.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download MS Word (78.2 KB)Acknowledgements
The authors would like to thank the Swedish national registries and the Swedish Cystic Fibrosis Quality Registry for the use of the data to conduct this study. Additionally, they would like to thank the patients, care providers, and clinic coordinators at CF centers throughout Sweden for their contributions to the Swedish Cystic Fibrosis Quality Registry. The authors wish to thank Zahra Bhaiwala for her contributions to the study management and coordination. Writing support was provided by Martin Hägglund, PhD and Tracy Bunting-Early, PhD, under the guidance of the authors. Zahra, Martin, and Tracy are employees of Vertex Pharmaceuticals Incorporated and may own stock or stock options in the company. Additional editorial and project management support were provided by Lucretia Ramnath, PhD and Harish Erra of Nucleus Group, which received funding from Vertex Pharmaceuticals Incorporated. Vertex Pharmaceuticals Incorporated funded this study, but was not involved in study design, data collection, or analysis.
Declaration of competing interest
AL reports receiving consulting fees (e.g. honoraria, travel expenses from Vertex Pharmaceuticals for a presentation at the company. IM and MG report payments from Vertex Pharmaceuticals to her institution for lectures and is a principal investigator for several Vertex-sponsored clinical trials. CK reports receiving payments from Vertex Pharmaceuticals and Chiesi Farmaceutici for presentations. LM is an employee of Vertex Pharmaceuticals and owns its stock and stock options. JB and MA report that they are/were employees of Quantify Research who was paid by Vertex Pharmaceuticals to conduct this research.
Data sharing statement
Each registry holder collects and manages its own data and maintains processes for researchers to request data. Restrictions apply to the sharing of these data, which were accessed for the purposes of the study based on an ethics approval and individual agreements with each respective registry holder.
References
- Bell SC, Mall MA, Gutierrez H, et al. The future of cystic fibrosis care: a global perspective. Lancet Respir Med. 2020;8(1):65–124. doi: 10.1016/S2213-2600(19)30337-6.
- Lopes-Pacheco M. CFTR modulators: the changing face of cystic fibrosis in the era of precision medicine. Front Pharmacol. 2019;10:1662. doi: 10.3389/fphar.2019.01662.
- Saint-Criq V, Gray MA. Role of CFTR in epithelial physiology. Cell Mol Life Sci. 2017;74(1):93–115. doi: 10.1007/s00018-016-2391-y.
- O'Sullivan BP, Freedman SD. Cystic fibrosis. Lancet. 2009;373(9678):1891–1904. doi: 10.1016/S0140-6736(09)60327-5.
- McColley SA, Ren CL, Schechter MS, et al. Risk factors for onset of persistent respiratory symptoms in children with cystic fibrosis. Pediatr Pulmonol. 2012;47(10):966–972. doi: 10.1002/ppul.22519.
- Paranjapye A, Ruffin M, Harris A, et al. Genetic variation in CFTR and modifier loci may modulate cystic fibrosis disease severity. J Cyst Fibros. 2020;19 Suppl 1(Suppl 1):S10–S14. doi: 10.1016/j.jcf.2019.11.001.
- Sepahzad A, Morris-Rosendahl DJ, Davies JC. Cystic fibrosis lung disease modifiers and their relevance in the new era of precision medicine. Genes (Basel). 2021;12(4):562. doi: 10.3390/genes12040562.
- Cystic Fibrosis Foundation Patient Registry. 2021 annual data report. Bethesda (MD); 2022.
- Zolin A, Orenti A, Jung A, et al. European Cystic Fibrosis Society Patient Registry 2021 Annual Data Report. 2023.
- Barry PJ, Mall MA, Álvarez A, et al. Triple therapy for cystic fibrosis Phe508del-gating and -residual function genotypes. N Engl J Med. 2021;385(9):815–825. doi: 10.1056/NEJMoa2100665.
- De Boeck K, Munck A, Walker S, et al. Efficacy and safety of ivacaftor in patients with cystic fibrosis and a non-G551D gating mutation. J Cyst Fibros. 2014;13(6):674–680. doi: 10.1016/j.jcf.2014.09.005.
- Heijerman HGM, McKone EF, Downey DG, et al. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet. 2019;394(10212):1940–1948. doi: 10.1016/S0140-6736(19)32597-8.
- Keating D, Marigowda G, Burr L, et al. VX-445-tezacaftor-ivacaftor in patients with cystic fibrosis and one or two Phe508del alleles. N Engl J Med. 2018;379(17):1612–1620. doi: 10.1056/NEJMoa1807120.
- Middleton PG, Mall MA, Dřevínek P, et al. Elexacaftor-tezacaftor-ivacaftor for cystic fibrosis with a single Phe508del allele. N Engl J Med. 2019;381(19):1809–1819. doi: 10.1056/NEJMoa1908639.
- Ramsey BW, Davies J, McElvaney NG, et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365(18):1663–1672. doi: 10.1056/NEJMoa1105185.
- Rowe SM, Daines C, Ringshausen FC, et al. Tezacaftor-ivacaftor in residual-function heterozygotes with cystic fibrosis. N Engl J Med. 2017;377(21):2024–2035. doi: 10.1056/NEJMoa1709847.
- Taylor-Cousar JL, Munck A, McKone EF, et al. Tezacaftor-ivacaftor in patients with cystic fibrosis homozygous for Phe508del. N Engl J Med. 2017;377(21):2013–2023. doi: 10.1056/NEJMoa1709846.
- European Medicines Agency. Medicines: Cystic Fibrosis [cited 2024 February 9]. Available from: https://www.ema.europa.eu/en/medicines
- Vertex Announces Long-Term Access Agreement in Sweden for Cystic Fibrosis Medicine ORKAMBI® (lumacaftor/ivacaftor). [Internet]. London; June 18. 2018. Available from: https://investors.vrtx.com/news-releases/news-release-details/vertex-announces-long-term-access-agreement-sweden-cystic
- Suthoff ED, Bonafede M, Limone B, et al. Healthcare resource utilization associated with ivacaftor use in patients with cystic fibrosis. J Med Econ. 2016;19(9):845–851. doi: 10.1080/13696998.2016.1178125.
- Thorat T, McGarry LJ, Jariwala-Parikh K, et al. Long-term impact of ivacaftor on healthcare resource utilization among people with cystic fibrosis in the united states. Pulm Ther. 2021;7(1):281–293. doi: 10.1007/s41030-021-00154-9.
- Bresnick K, Arteaga-Solis E, Millar SJ, et al. Burden of cystic fibrosis in children <12 years of age prior to the introduction of CFTR modulator therapies. BMJ Open Resp Res. 2021;8(1):e000998. doi: 10.1136/bmjresp-2021-000998.
- de Monestrol I, Lindblad A. CF-registret Årsrapport 2019. CF-registret: ett svenskt kvalitetsregister. 2019.
- Hassan M, Bonafede MM, Limone BL, et al. The burden of cystic fibrosis in the Medicaid population. Clinicoecon Outcomes Res. 2018;10:423–431. doi: 10.2147/CEOR.S162021.
- Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–1343. doi: 10.1183/09031936.00080312.
- Skatteverket. 2019 Arbetsgivaravgifter (General payroll tax) [cited 2024 Feb 9]. Available from: https://skatteverket.se/foretagochorganisationer/arbetsgivare/arbetsgivaravgifterochskatteavdrag/arbetsgivaravgifter.4.233f91f71260075abe8800020817.html
- Statistics Sweden. Statistikdatabasen - Genomsnittlig månadslön, kronor efter utbildningsgrupp SUN 2000, kön och år (Average monthly salary); 2019 [cited 2024 Feb 9]. Available from: https://www.statistikdatabasen.scb.se/pxweb/sv/ssd/START__AM__AM0110__AM0110C/LonUtbKon/table/tableViewLayout1/
- Sveriges Riksbank. Årsgenomsnitt valutakurser (ackumulerat) (Annual average exchange rates); 2020. Available from: https://www.riksbank.se/sv/statistik/sok-rantor–valutakurser/arsgenomsnitt-valutakurser/?y=2020&m=12&s=Comma&f=y
- Sveriges Kommuner och Regioner. Kostnad per patient (KPP): Retrospektiva DRG-vikter Somatisk vård 2012-2022; 2023 [cited 2024 Feb 9]. Available from: https://skr.se/download/18.737db4ca18beb8381802ef8/1700486868583/Retrospektiva%20DRGvikter%20Somatisk%20v%C3%A5rd%202012-2022.xlsx
- Böckerman P, Ilmakunnas P. Unemployment and self-assessed health: evidence from panel data. Health Econ. 2009;18(2):161–179. doi: 10.1002/hec.1361.
- Daly C, Ruane P, O'Reilly K, et al. Caregiver burden in cystic fibrosis: a systematic literature review. Ther Adv Respir Dis. 2022;16:17534666221086416. doi: 10.1177/17534666221086416.
- Angelis A, Kanavos P, López-Bastida J, et al. Social and economic costs and health-related quality of life in non-institutionalised patients with cystic fibrosis in the United Kingdom. BMC Health Serv Res. 2015;15(1):428. doi: 10.1186/s12913-015-1061-3.
- Frey S, Stargardt T, Schneider U, et al. The economic burden of cystic fibrosis in germany from a payer perspective. Pharmacoeconomics. 2019;37(8):1029–1039. doi: 10.1007/s40273-019-00797-2.
- Chilvers MA, Davies JC, Milla C, et al. Long-term safety and efficacy of lumacaftor-ivacaftor therapy in children aged 6-11 years with cystic fibrosis homozygous for the F508del-CFTR mutation: a phase 3, open-label, extension study. Lancet Respir Med. 2021;9(7):721–732. doi: 10.1016/S2213-2600(20)30517-8.
- Ratjen F, Hug C, Marigowda G, et al. Efficacy and safety of lumacaftor and ivacaftor in patients aged 6-11 years with cystic fibrosis homozygous for F508del-CFTR: a randomised, placebo-controlled phase 3 trial. Lancet Respir Med. 2017;5(7):557–567. doi: 10.1016/S2213-2600(17)30215-1.
- Wainwright CE, Elborn JS, Ramsey BW, et al. Lumacaftor-ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. N Engl J Med. 2015;373(3):220–231. doi: 10.1056/NEJMoa1409547.
- Orenti Zolin Jung AAA. 2022. ECFSPR Annual Report.
