Abstract
Background
Bioprostheses with RESILIA tissue demonstrate a reduction in calcification and improve health outcomes in pre-clinical and clinical studies. Prior economic analyses which relied on 5 years of evidence from the COMMENCE trial demonstrate financial savings for RESILIA tissue valves relative to mechanical valves after surgical aortic valve replacement (SAVR). Given the recent release of 7-year COMMENCE data, this economic evaluation updates the estimate for long-run savings of bioprosthetic valves with RESILIA.
Methods
Simulation models estimated disease progression across two hypothetical SAVR cohorts (tissue vs. mechanical) of 10,000 patients each in the US. The primary comparison calculated the SAVR-related expenditures associated with each valve type ($US, 2023). Health outcome probabilities were based on the COMMENCE trial though year 7 and projected for an additional 8 years based on prior studies of tissue and mechanical SAVR. Costs for key outcomes (mortality, reoperation, bleeding, thromboembolism, endocarditis) and anticoagulant monitoring were sourced from the literature. Incidence rates of health outcomes associated with mechanical valves relied on relative risks of tissue valve versus mechanical valve patients.
Results
Seven-year savings are $13,415 (95% CI = $10,472–$17,321) per patient when comparing RESILIA versus mechanical SAVR. Projected 15-year savings were $23,001 ($US, 2023; 95% CI = $17,802–$30,421). Most of the 15-year savings are primarily attributed to lower anti-coagulation monitoring costs ($21,073 in ACM savings over 15 years), but lower bleeding cost (savings: $2,294) and thromboembolism-related expenditures (savings: $852) also contribute. Reoperation and endocarditis expenditures were slightly larger in the RESILIA cohort. If reoperation relative risk reverts from 1.1 to 2.2 (the level in legacy tissue valves) after year 7, savings are $18,064. RESILIA SAVR also reduce costs relative to legacy tissue valves.
Conclusion
Patients receiving RESILIA tissue valves are projected to have lower SAVR-related health expenditures relative to mechanical and legacy tissue valves.
Introduction
Surgical Aortic Valve Replacement (SAVR) is a frequently administered therapeutic intervention for the treatment of moderate-to-severe Aortic Stenosis (AS) – a commonly encountered diagnosis in the United States and globallyCitation1,Citation2. Valve choice has traditionally been split between mechanical and tissue valves although the tissue valve share has increased over the last 15 years and represents the majority of SAVR operations in the US currentlyCitation3–6. Key clinical tradeoffs include anticipated device durability and reduced propensity for reoperation – generally a factor favoring mechanical valves – relative to considerations that typically favor tissue valves such as the lower probability of sequelae (bleeding or thrombosis), reduced time and economic costs linked to anticoagulation monitoring (ACM) and improved quality-of-life related to healthier diet options and improved capacity to exerciseCitation1.
Prior meta-analyses and comparative studies have delineated health outcomes associated with tissue and mechanical SAVRCitation4,Citation7–16. Subsequently, economic evaluations have utilized health outcomes data from long-run cohort follow-up studies to compare the expected trajectory of SAVR-related health system costs over periods of up to 25 years in patients receiving either tissue and mechanic valvesCitation17–21. These incipient economic analyses advocated the utilization of tissue valves, notwithstanding the fact that the costs associated with reoperation were generally larger in the tissue valve cohorts that employed older “legacy” technology. Generally, this study defines legacy (older) tissue aortic valves as those that do not have RESILIA treatment. Specifically, incidence rates of legacy valves are derived from cohort studies that used the Carpentier-Edwards Perimount pericardial bioprosthesisCitation17–19.
However, subsequent innovations in the realm of material science have bolstered the durability of tissue valves. A salient advance between “legacy” tissue valves and contemporary tissue valve technology is the incorporation of RESILIA, a preservation technology that obstructs residual aldehyde groups that bind to calcium, thereby limiting calcification. Clinical data from the COMMENCE trial indicate lower reoperation rates and structural valve deterioration (SVD) for aortic valves that incorporate the RESILIA anti-calcification technology relative to rates from earlier studies of legacy aortic tissue valvesCitation17–19,Citation22–24. After the 5-year clinical data from COMMENCE was released, an economic model comparing RESILIA-treated tissue valves with mechanical valves updated the anticipated 5 year savings and projected savings out to 15 years. Generally, both savings were larger than levels intimated in the earlier economic analyses, in part, due to the lower reoperation rates measured in the COMMENCE trial for tissue SAVR patients relative to legacy tissue valve patientsCitation25. This economic model aims to account for the recently released 7-year COMMENCE data and thereby extend the window of time over which direct clinical data can be input into the model from 5 to 7 years. The longer “clinical data period” reduces the uncertainty of the long-run 15-year projected savings.
Methods
The financial model adheres to established guidelines for economic evaluations within the public health domain, specifically the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) methodologyCitation26–29. The study adopts a health system perspective for the United States, focusing on expenditures associated with sequelae following initial SAVR procedures. The model structure mirrors a previously published approach, whereby the researchers assumed equally-sized synthetic cohorts for both mechanical SAVR and tissue SAVR patients (n = 10,000 each)Citation21,Citation25. The model estimates sequelae for 15 years following the initial SAVR procedure, accounting for mortality and five other clinical events linked to SAVR within each period, conditional on survival. These events are identical to those used in the prior economic analysis of tissue versus mechanical SAVR, including endocarditis, bleeding or hemorrhagic events, thrombosis, reoperation, and anti-coagulant monitoring (ACM). The study excluded stroke due to the possibility of double counting certain events as both a stroke and either a bleeding event (hemorrhagic stroke) or a thrombotic event (ischemic stroke caused by thromboembolism). The total SAVR-related expenditures were determined for each time period by multiplying the anticipated number of events occurring in a hypothetical cohort of patients by the unit cost per event (after considering discounting and medical inflation).
Incidence rates with novel tissue valves (RESILIA)
The study employed a mathematical model to estimate the probability of various clinical events following implantation of a novel tissue valve in patients undergoing surgical aortic valve replacement (SAVR). The model assessed the likelihood of these events occurring within the first 30 days post-surgery, and annually thereafter over a 15-year follow-up period (Supplementary Figure S3). For the initial 7 years, which the authors refer to as the “clinical data period”, the model’s estimates regarding event incidence were based on the clinical results observed within the COMMENCE trialCitation24,Citation30. Event definitions used in this trial were consistent with previously published standardsCitation21,Citation31. The model accounted for patient survival when estimating event probability within each time period, and relied on the initial sample size, the number of observed events, and the total life-years documented in the COMMENCE trial (Supplementary Table S1).
Beyond the 7-year mark, termed the “projection period”, the model extrapolates to predict event incidence over the remaining 8 years. Due to a lack of clinical data specific to the novel tissue valve during this extended timeframe, the model incorporates weighted data derived from three primary long-term studies that investigated legacy tissue valve cohorts (Supplementary Table S5)Citation17–19. This approach offers a conservative estimate of event incidence during years eight through fifteen following SAVR with the novel tissue valve. The 7-year data is likely the most reliable; however, incorporating the projection period for an additional 8 years is justified due to the significant impact reoperation and anticoagulant monitoring costs have on overall economic assessments. These costs are considered to rise progressively over time following surgery. By encompassing a longer follow-up period, the model generates a more conservative “long-run” estimate of potential cost savings relative to a linear extrapolation based solely on 7 years of data.
The model operates under the assumption that patients undergoing tissue surgical aortic valve replacement (SAVR) experience all-cause mortality (ACM) for a duration of 6 months following the surgery. In contrast, patients receiving mechanical SAVR are expected to require ACM intervention for the entirety of their lives. Additionally, in its conservative approach, the base case version of the model assumes an equivalent mortality rate between tissue and mechanical valves, although this is likely to result in comparatively higher costs associated with the tissue valve group.
Incidence rates with mechanical valves
The occurrence rates of various health outcomes within the mechanical valve group are determined by multiplying the relative risks specific to each event with the incidence rates of corresponding clinical events observed in the tissue valve group. Given that the COMMENCE trial exclusively focused on tissue valves without a mechanical valve comparator, we derive the relative risk for each event by dividing the annual incidence rate obtained from a similar study involving mechanical valves by the estimated rate observed in the COMMENCE trial for tissue valves (refer to Supplementary Table S2).
To summarize, there are three hypothetical cohorts tracked in the study: (A) a legacy tissue valve cohort; (B) a RESILIA cohort; and (C) a mechanical valve cohort. In the legacy tissue valve cohort the model uses the weighted (by sample size) incidence rates across the three Bourguignon studies (Equations 5 and 6 in the section entitled “Formulas for Total Expenditures, Costs and Probabilities” in the Appendix). In the RESILIA cohort incidence rates for outcomes are based on the COMMENCE trial results (through year 7). After year 7, the model assumes the RESILIA cohort has the same incidence rates as the legacy tissue valve cohort (assuming the RESILIA valve reverts to the performance of the legacy tissue valves after year 7). In the mechanical valve cohort incidence rates were estimated based on the studies cited in Supplementary Table S2 (the reported relative risks of in row D of Table S2 equals the COMMENCE absolute rate divided by the mechanical absolute rate (for each outcome)). The incidence rate for each outcome in the mechanical valve cohort is equal to the RESILIA cohort estimate divided by the relative risk estimate.
In certain scenario analyses, adjustments are made to the relative risks for each outcome to reflect more conservative or aggressive estimations (see Supplementary Table S3). The base case values generally align with the median of the three estimates presented in Supplementary Table S3A, excluding thromboembolism where a relatively conservative estimate is maintained for RESILIA tissue valves compared to mechanical valves. In the conservative scenario, the highest of the three relative risk estimates is utilized, consequently elevating the anticipated costs associated with RESILIA valve usage. However, the reoperation relative risk remains unchanged at 1.11, as the highest relative risk of 2.19 is separately assessed in a sensitivity analysis. Conversely, in the most aggressive scenario, the lowest of the three estimated relative risks is employed for each outcome, with the exception of a slightly higher estimate for thromboembolism.
Events and costs
For each cohort, the total annual occurrences of various health events are projected by multiplying the cohort size at the beginning of the period by the incidence rate specific to that period. These occurrences are then multiplied by the associated cost of each event to estimate the overall costs across the entire cohort for each period. The model focuses solely on costs and does not incorporate measures of quality-of-life (QoL) nor calculate incremental cost-effectiveness ratios (ICERs). Cost data (refer to Supplementary Table S4) were sourced from relevant literature and adjusted to reflect 2023 US dollars based on historical medical inflation estimates for inpatient careCitation32–35. These estimates are most relevant to the US healthcare system and may not accurately represent costs in other countries.
In the “base case” scenario, the model assumes a medical inflation rate of 3% and discounts future costs by 3%, effectively offsetting each otherCitation28. The simulation model includes both mean estimates of cost inputs and measures of variation such as standard deviation. If standard deviations for specific cost inputs were unavailable, estimates of the coefficient of variation (CoV), calculated as the standard deviation divided by the mean, from prior analyses of US hospital inpatient costs were appliedCitation36.
Costs associated with hospitalizations for tissue and mechanical surgical aortic valve replacement (SAVR) procedures were based on Medicare reimbursement rates. Historical Medicare payment data slightly favored tissue SAVR over mechanical SAVR. These costs may include a Diagnosis-Related Group (DRG) payment from the payer to the hospital, which encompasses the cost of the device used. Therefore, the analysis offers insight into the potential cost savings associated with tissue SAVR over time. Any additional costs incurred for novel devices, absorbed by financial stakeholders such as payers, hospitals, or patients, could be subtracted from the estimated savings but are not specifically accounted for in the model due to variability and confidentiality.
Costs related to thromboembolism, endocarditis, and bleeding events were derived from literature sources and internal databases (Truven Marketscan). Bleeding event costs encompassed both the initial hospitalization and subsequent readmission costs and were comparable to literature-based costs of hospitalized strokesCitation35.
The model utilized a prior analysis based on time-dependent activity-based costing (TDABC) to factor in costs associated with anticoagulation medication and monitoring (ACM). TDABC considers labor time, fixed costs, and variable unit costs associated with prescribing anticoagulation medications and ensuring medication adherence through services and diagnosticsCitation37. Annually, the model estimated ACM costs at $1,823. This estimate was derived through a weighted calculation, encompassing different patient stability levels: stable patients accounted for 48% of the estimate ($1,136), average patients for 33% ($1,726), and unstable patients for 19% ($3,723). These proportions were determined based on international normalized ratio (INR) measurements and drawn from the experiences of patients at the Mayo Clinic who were undergoing warfarin treatment and attending ACM clinics.
Model types
The base case deterministic model calculates the total discounted costs associated with surgical aortic valve replacement (SAVR) for both tissue and mechanical valve patient cohorts over specified time intervals (initial 30 days, annually for the subsequent 15 years). These costs are determined by multiplying the expected number of clinical events during each period by the respective cost for each type of event. The cumulative costs over time are then aggregated across different durations ranging from 30 days to 15 years for both tissue and mechanical SAVR groups.
The deterministic model aids in computing the average expected outcome, whereas Monte Carlo simulations incorporate both the mean and distribution of key inputs. In the simulation model, probabilities of health events follow a beta distribution, and standard deviations are calculated based on absolute numbers of cases relative to the total number at risk in relevant studies. Cost inputs assume a gamma distribution and, given that these costs are based on US hospital data, standard deviations are estimated using previously reported coefficient of variation data for US hospitals.
When applying hazard rates or relative risks, a normal distribution is assumed with standard deviations obtained from the source studies. The simulation approach may yield slightly different averages compared to the deterministic model, and simulations also provide credibility intervals. Each simulation runs 10,000 trials based on random draws of each input, estimating mean and median output values along with and interval of the central 9,500 values (the 95% credibility interval or CI). This credibility interval is similar to a confidence interval, but is a function of the model input parameters which impact the outcome distribution. If this interval does not include $0, it is considered statistically significant. Sensitivity and scenario analyses are conducted to identify key determinants influencing the central outcome: the difference in cumulative SAVR-related healthcare costs per person between tissue and mechanical patient groups. The models are programmed using @Risk v8.2 within Microsoft Excel.
Results
Novel tissue vs. mechanical valves: base case results
The results indicate that tissue valves with RESILIA reduce expected health system expenditures relative to mechanical valves. These savings occur both in the first 7 years of the model and during the “projection period” (years 7–15) when the incidence “base case” rates for the tissue valve cohort generally revert to the weighted estimates from older tissue devices long-run registries (with the exception of reoperation rates; see and ). At year 7 the discounted cumulative savings in the deterministic (simulation) model are $13,088 (Median = $13,415; 95% CI = $10,472–$17,321). Net savings increase to $23,001 (Median = $23,315; 95% CI = $17,802–$30,421) at 15 years. In the base case model, RESILIA valves achieve statistically significant cost savings (the 95% CI exceeds $0) by the end of year 2 relative to mechanical valves and statistical significance is maintained through year 15.
Figure 1. Cumulative net discounted savings over time per initial SAVR surgery associated with novel tissue vs. mechanical valves ($US 2023) base case model.
Table 1. Cumulative net discounted savings per initial SAVR surgery associated with novel tissue vs. mechanical valves ($US 2023) base case model.
Select sensitivity analyses
Probabilistic sensitivity analyses (PSAs) for the 7-year and 15-year models help identify which stochastic inputs influence the savings outcome the most (Supplementary Tables S7 and S8). In both the 7- and 15-year models which compare the RESILIA tissue and mechanical valves, inputs related to the probability of reoperation (5 year survival without reoperation for novel tissue valves patients based on the COMMENCE trial), probability of bleeding (5 years survival without a bleed based on the COMMENCE trial), and cost of tissue SAVR operation are the top three inputs that wield the largest impact on the savings associated with RESILIA tissue SAVR relative to mechanical SAVR. In both cases the 7-year and 15-year models mortality (overall survival) and cost of mechanical SAVR operation ranked fourth and fifth, respectively.
While the PSA helps identify reoperation as a central driver that influences savings, the exclusion of non-stochastic inputs, specifically anti-coagulant monitoring costs (which are not estimated with a distribution and variance in the simulation) prompted an alternative analysis aimed at highlighting the relative importance of each of the clinical sequelae and ACM costs. breaks down the contribution of each of the sequelae and ACM costs from the deterministic model. This analysis enables the joint accounting for multiple factors (including relative risk, probability of health outcome occurring in tissue valve cohort, cost per occurrence of each health outcome) that impact overall savings related to each health outcome.
Table 2. Disaggregated cumulative net discounted savings per initial SAVR surgery associated with novel tissue vs. mechanical valves ($US 2023) base case deterministic model.
Across almost all evaluated time periods, savings associated with ACM cost reduction in novel tissue valve patients constitute a substantial share of overall savings. By year 7, approximately 83% ($10,892 of $13,088) of the net savings are attributed to ACM cost reductions. This proportion increases to 92% by year 15. In scenarios where ACM costs were excluded from the analysis, savings persisted in patients receiving RESILIA valves compared to mechanical valves, albeit at substantially lower levels ($2,196 at year 7 and $1,928 at year 15).
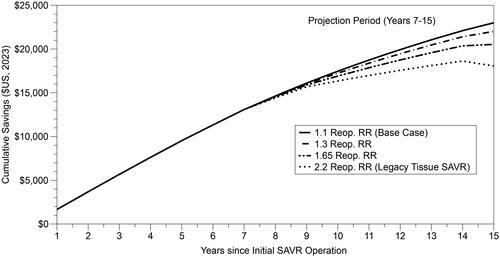
Given the potential importance of reoperation on costs, we also conducted a set of sensitivity analyses in which alternative reoperation relative risks are applied during the “projection period” between years 7 and 15 (the base case model assumes a reoperation rate relative risk of 1.1 which translates to 10% higher probability of reoperation in the RESILIA group relative to the mechanical valve group). As demonstrated in , if the relative risk increases to 2.2 for reoperation immediately upon year 7 (the assumption that was previously applied to legacy tissue valves) the net savings by year 15 drop to $18,064. Less conservative assumptions (lower relative risk values for the reoperation rate) naturally yield larger savings estimates compared to the most conservative approach. Net savings are $22,013 at 15 years if the relative risk is 1.3 (80% of the “benefit” level from year 0 to 5) and $20,532 if the relative risk is 1.65 (50% of the “benefit”).
Figure 2. Cumulative net discounted savings over time per initial SAVR surgery associated with novel tissue vs. mechanical valves ($US 2023), by reoperation relative risk estimate (projection period).
To account for expected savings in the younger cohorts (whom may have the initial replacement or follow on replacement over a period beyond 15 years), the model was extended to 25 years in one sub-analysis. If one assumes that the relative risk of reoperation in the “base case” scenario (relative risk of 1.1) is maintained in years 15–25 the savings increase from $23,001 at 15 years to $28,918 at 25 years. If the relative risk for reoperation increase to higher levels (e.g. 2.2 instead of 1.1) after year 7 through year 25, a much more conservative assumption, the savings are still positive, but lower ($14,386).
Relative risk scenario analyses
Given the frequent use of “single arm” studies to estimate incidence rates of important sequelae across different types of medical devices, our study applied three primary approaches to estimate relative risks between tissue and mechanical valves. First, clinical trial estimates from RESILIA valves were compared to mechanical valve studies with similar durations and endpoints. Second, existing literature including single one-off studies of legacy tissue valves and mechanical valves were evaluated. Lastly, robust and frequently cited meta-analyses were consulted. Each approach generally yielded different but generally comparable estimates. Supplementary Table S3 accounts for the dispersion in these relative risks and, in addition to the “base case” scenario discussed above, includes a “conservative” and “aggressive” scenario. Across all scenarios, the mortality relative risk is fixed at 1.0 (a conservative assumption for tissue valves). In the conservative scenario where both the bleeding and endocarditis relative risks increase to favor mechanical valves, the 7-year savings drop to $10,869 and the 15-year savings to $20,190 in the deterministic model. In the more aggressive model, 7-year and 15-year savings associated with novel tissue valves increase to $16,784 and $29,200, respectively ().
Table 3. Cumulative net discounted savings per initial SAVR surgery associated with novel tissue vs. mechanical valves ($US 2023) scenario analyses for relative risk (deterministic models).
Legacy tissue valve savings
In the comparable model for legacy tissue valves (which have higher re-operation rates than novel tissue valves) the savings relative to mechanical valves are $9,653 at year 7, $3,435 less than the 7-year savings in the base case model for a novel tissue valve (Supplementary Table S6). After including the “projection window” (years 7–15) the savings are $14,528 at year 15, $8,473 less than the savings anticipated in the “base case” model for the novel tissue valve. A large share of the differential between the legacy tissue valve and the novel tissue valve is due to the substantially larger reoperation costs (and the balance of the difference primarily stems from event probabilities in the first 7 years). At 7 years expected per patient reoperation costs are $1,679 more than mechanical valves in the legacy model, but just $123 in the novel tissue valve model. Similarly at 15 years the differential in reoperation costs between legacy and RESILIA tissue valves increases to $5,594. In the case of the legacy tissue valves the savings generated from reduced ACM are substantially offset by the larger reoperation costs. This countervailing cost is substantially lower in the case of RESILIA tissue valves.
Discussion
This analysis builds on prior studies that compare tissue and mechanical SAVR valve expected costs over time by incorporating the most recent 7-year results from the COMMENCE clinical trialCitation21,Citation25. The deterministic and simulation models both demonstrate that RESILIA treated tissue valves are associated with long-run savings relative to mechanical valves. Prior economic analyses found that legacy tissue valves yield savings relative to mechanical valves and, based on 5 years of COMMENCE data, the savings appeared even larger for RESILIA treated valves, this analysis updates expectations given the 7-year clinical data for novel tissue valves and finds that expected savings are slightly larger than anticipated based on prior analyses. The expected enhancement stems from the anticipated improvement in reoperation rates while the ACM savings remain intact.
One limitation of the study is the 15-year duration. In contrast, the prior economic analysis for legacy tissue valves spanned a 25-year period, supported by data from long-term cohort studies. Due to the availability of only 7 years of clinical data for RESILIA treated tissue valves, alongside promising laboratory analyses, we deemed it reasonable to confine the model to 15 years (as was the case with the 5-year model previously published). During this period, we employed relatively conservative assumptions regarding incidence and relative risk for the “projection period” between years 7 and 15. However, reoperations can occur over extended periods, and it is not uncommon for them to happen beyond 15 years, likely at a higher rate over time. The 25 year sub-analysis on the model suggests that total net savings continue to accrue beyond 15 years (which may be more relevant for younger patients). Generally prior research, and our analysis, find countervailing impacts of reoperation costs and ACM savings for tissue valve patients (relative to mechanical patients). These two factors are also the dominant determinants of net savings. Essentially, if younger populations rely on SAVR over longer periods than those presented in the 15-year results, the model still demonstrates savings, but the magnitude depends on the expected relative risk of reoperation in years 15–25. As additional years of clinical follow-up transpire, further data will provide a more precise understanding of the benefits associated with advanced tissue valves.
Another limitation is the absence of randomized controlled trials (RCTs) directly comparing outcomes for surgical aortic valve replacement (SAVR) valve options. Consequently, our findings should be viewed as associations rather than indicative of causal evidence. Moving forward, comparative cohort studies tracking similar patients who undergo either tissue or mechanical SAVR procedures may help bridge the “causation gap” and enhance the robustness and validity of evidence for clinical and economic assessments. While selecting studies to inform the model inputs, we generally included large trials or cohort studies with extended follow-up periods. Furthermore, this analysis relies on large, historical studies that encompass various mechanical valve technologies. Therefore, the savings estimates for tissue valves in this analysis are best interpreted as a comparison based on the historical performance of mechanical valves as a collective rather than on a specific model. If certain mechanical valves perform better than the historical average in practice, cost savings may differ and potentially be reduced. The absence of an RCT design and the utilization of generalized mechanical SAVR data are key factors driving the need for sensitivity and scenario analyses. Also, with regard to generalizability, the application of US costs as inputs makes the savings results applicable to the US context. Additionally, this model updates costs to $US 2023 which, according to the US PCE Medical Costs indices, are approximately 7% larger than $US 2020 prices so some of the expenditure difference between the prior publication for 5-year data and this analysis is a statistical artifact of medical inflation.
Thirdly, as a strictly economic evaluation, these analyses do not incorporate the value associated with improvements in QoL. It is advisable for stakeholders such as health insurers and payers to complement this analysis with considerations regarding the inherent value of enhancing QoL through improved health outcomes. Clinical events may inherently carry more weight in clinician decision-making and patient preference compared to mere financial savings, which may not directly benefit the patient. Moreover, the model assumes equivalence in mortality rates. If one perceives that the relative risk of mortality is higher (lower) than one in tissue valves compared to mechanical valves, the model would underestimate (overestimate) the savings associated with tissue valves.
Fourth, the model utilizes reoperation data regardless of whether the reoperation is attributed to structural valve deterioration (SVD). Since only two instances of SVD were identified within the first 7 years of the COMMENCE trial, the model may potentially overestimate the number of reoperations linked to RESILIA valves. Assuming there are very few reoperations related to SVD, the net savings associated with RESILIA tissue valves could be underestimated. Consequently, if fewer reoperations were indeed associated with novel tissue valves compared to the current base case model, the savings would be more substantial. While the definition of SVD used in the COMMENCE trial (Akins) is different than the VARC-3 definition, which accounts for hemodynamic compromise, it is reoperations rather than SVD itself that impacts the associated savings in the model. However, data on both SVD and reoperations for RESILIA treated tissue valves do not yet extend beyond 7 years.
Fifth, the findings should be interpreted as the anticipated savings, collectively, for a cohort of patients with an average age of 67, varying in age from younger to older individuals. Although the COMMENCE trial lacked sufficient sample size to draw conclusions about subgroups stratified by age, it enrolled patients from 32 centers with relatively broad inclusion and exclusion criteria. Future economic research utilizing larger sample sizes within younger or older cohorts will shed light on the relationship between age, health outcomes, and anticipated savings. Furthermore, as historical guidelines have recommended the use of mechanical valves in younger patients (<50 years old) and tissue valves in older patients (>65 years old), it’s important to recognize that our clinical comparisons may not fully address selection biases. If older and sicker patients historically tended to receive tissue valves, any bias in this direction might overstate clinical events and underestimate cost savings for tissue valve patients relative to those with mechanical valves. However, without complete patient information across studies, only future causal studies can precisely determine the clinical and economic value associated with tissue valves.
Sixth, strokes are not factored into either the “bleeding” or “thromboembolism” health outcomes, which could bias the results. It is probable that this omission would lead to an underestimation of savings associated with tissue valves if strokes, like bleeding and thromboembolism, are more frequent with mechanical valves. Additionally, the costs related to bleeding and thromboembolism events may not encompass certain long-term expenses associated with stroke, such as rehabilitation expenditures. A prior long-term study by Chiang finds a slight increase in stroke incidence in mechanical valves (8.6% 15-year cumulative incidence) relative to tissue valves (7.7% 15-year cumulative incidence), but the hazard ratio is not statistically significant (HR = 1.04; 95% CI = 0.75–1.43)Citation9. By excluding stroke we may be lowering the total absolute level of expected cost in both cohorts, but the relative difference is likely similar (or slightly conservative if one believes that stroke is more predominant after mechanical valve replacement).
Seventh, the base case result applies when comparing a tissue valve patient who does not require additional ACM after the initial 6 months. If a tissue SAVR patient requires ongoing ACM treatment for a condition such as atrial fibrillation, savings would be substantially less since a large share of the savings stem from ACM costs savings ().
Eighth, this analysis is focused on the patients (and/or physicians) that opt for an SAVR to treat their aortic stenosis. The guidelines and clinical practice are fluid and TAVR/TAVI share has certainly increased dramatically since its introduction. As longer-term studies emerge for TAVR/TAVI, it may be possible to compare the long run outcomes with patients that initiate treatment of AS with SAVR. We also do not examine treatment with conservative management in lieu of SAVR.
Lastly, while our analysis suggests there is an additional savings premium associated with the use of RESILIA valves, relative to legacy tissue valves, the comparison is indirect. Future clinical comparisons of different tissue valve technologies could improve the accuracy of the relative value associated with improved medical technology.
Conclusion
Payers, patients, physicians, and other stakeholders take into account various factors when aiming to optimize clinical and economic outcomes associated with treatments for aortic stenosis (AS). While financial considerations and costs represent just one aspect of the decision-making process, this assessment strongly indicates that tissue valves are likely to offer long-term economic advantages compared to mechanical valves for payers, insurers, and patients. Furthermore, advancements in tissue SAVR technology in recent years may have further widened the economic benefits relative to mechanical valves compared to the era of legacy tissue valves.
Transparency
Declaration of funding
This research was supported by Edwards Lifesciences.
Declaration of financial/other relationships
Two authors are employees of the funder, Edwards Lifesciences, and two authors are paid consultants to Edwards Lifesciences.
Author contributions
Concept and design: EK, MR, TN. Acquisition of data: EK. Analysis and interpretation of data: EK, MR, AP, TN. Drafting of manuscript: EK. Critical revision of paper for important intellectual content: EK, MR, AP, TN. Obtaining funding: EK, MR, AP. Administrative, technical and logistical support: EK, MR, AP. Statistical analysis: EK, MR, TN. Supervision: EK, MR, AP, TN.
Acknowledgements
None stated.
Conflict of interest disclosures
EK and TN receive consulting fees from Edwards Lifesciences. TN also receives fees as a speaker for Edwards Lifesciences. MR and AP are all employees of Edwards Lifesciences. TN did not receive any fees as a consequence of this project.
Reviewer disclosures
Peer reviewers on this manuscript have received an honorarium from JME for their review work but have no other relevant financial relationships to disclose.
Supplemental Material
Download MS Word (49 KB)References
- Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;70(2):252–289.
- Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368(9540):1005–1011. doi: 10.1016/S0140-6736(06)69208-8.
- Fujita B, Ensminger S, Bauer T, et al. Trends in practice and outcomes from 2011 to 2015 for surgical aortic valve replacement: an update from the German Aortic Valve Registry on 42 776 patients. Eur J Cardiothorac Surg. 2018;53(3):552–559. doi: 10.1093/ejcts/ezx408.
- Glaser N, Jackson V, Holzmann MJ, et al. Aortic valve replacement with mechanical vs. biological prostheses in patients aged 50–69 years. Eur Heart J. 2016;37(34):2658–2667. doi: 10.1093/eurheartj/ehv580.
- Kiyose AT, Suzumura EA, Laranjeira L, et al. Comparison of biological and mechanical prostheses for heart valve surgery: a systematic review of randomized controlled trials. Arq Bras Cardiol. 2019;112(3):292–301. doi: 10.5935/abc.20180272.
- Masuda M, Okumura M, Doki Y, et al. Thoracic and cardiovascular surgery in Japan during 2014: annual report by The Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg. 2016;64(11):665–697. doi: 10.1007/s11748-016-0695-3.
- Badhwar V, Ofenloch JC, Rovin JD, et al. Noninferiority of closely monitored mechanical valves to bioprostheses overshadowed by early mortality benefit in younger patients. Ann Thorac Surg. 2012;93(3):748–753. doi: 10.1016/j.athoracsur.2011.12.032.
- Brennan JM, Edwards FH, Zhao Y, et al. Long-term safety and effectiveness of mechanical versus biologic aortic valve prostheses in older patients: results from the Society of Thoracic Surgeons Adult Cardiac Surgery National Database. Circulation. 2013;127(16):1647–1655. doi: 10.1161/CIRCULATIONAHA.113.002003.
- Chiang YP, Chikwe J, Moskowitz AJ, et al. Survival and long-term outcomes following bioprosthetic vs mechanical aortic valve replacement in patients aged 50 to 69 years. JAMA. 2014;312(13):1323–1329. doi: 10.1001/jama.2014.12679.
- Etnel JR, Huygens SA, Grashuis P, et al. Bioprosthetic aortic valve replacement in nonelderly adults: a systematic review, meta-analysis, and microsimulation. Circ Cardiovasc Qual Outcomes. 2019;12(2):e005481. doi: 10.1161/CIRCOUTCOMES.118.005481.
- Goldstone AB, Chiu P, Baiocchi M, et al. Mechanical or biologic prostheses for aortic-valve and mitral-valve replacement. N Engl J Med. 2017;377(19):1847–1857. doi: 10.1056/NEJMoa1613792.
- Hirji SA, Kolkailah AA, Ramirez-Del Val F, et al. Mechanical versus bioprosthetic aortic valve replacement in patients aged 50 years and younger. Ann Thorac Surg. 2018;106(4):1113–1120. doi: 10.1016/j.athoracsur.2018.05.073.
- Isaacs AJ, Shuhaiber J, Salemi A, et al. National trends in utilization and in-hospital outcomes of mechanical versus bioprosthetic aortic valve replacements. J Thorac Cardiovasc Surg. 2015;149(5):1262–1269.e3. doi: 10.1016/j.jtcvs.2015.01.052.
- Kilic A, Bianco V, Gleason TG, et al. Hospital readmission rates are similar between patients with mechanical versus bioprosthetic aortic valves. J Card Surg. 2018;33(9):497–505. doi: 10.1111/jocs.13781.
- Son J, Cho YH, Jeong DS, et al. Mechanical versus tissue aortic prosthesis in sexagenarians: comparison of hemodynamic and clinical outcomes. Korean J Thorac Cardiovasc Surg. 2018;51(2):100–108. doi: 10.5090/kjtcs.2018.51.2.100.
- Zhao DF, Seco M, Wu JJ, et al. Mechanical versus bioprosthetic aortic valve replacement in middle-aged adults: a systematic review and meta-analysis. Ann Thorac Surg. 2016;102(1):315–327. doi: 10.1016/j.athoracsur.2015.10.092.
- Bourguignon T, Bouquiaux-Stablo A-L, Candolfi P, et al. Very long-term outcomes of the Carpentier-Edwards Perimount valve in aortic position. Ann Thorac Surg. 2015;99(3):831–837. doi: 10.1016/j.athoracsur.2014.09.030.
- Bourguignon T, El Khoury R, Candolfi P, et al. Very long-term outcomes of the Carpentier-Edwards Perimount aortic valve in patients aged 60 or younger. Ann Thorac Surg. 2015;100(3):853–859. doi: 10.1016/j.athoracsur.2015.03.105.
- Bourguignon T, Lhommet P, El Khoury R, et al. Very long-term outcomes of the Carpentier-Edwards Perimount aortic valve in patients aged 50–65 years. Eur J Cardiothorac Surg. 2015;49(5):1462–1468. doi: 10.1093/ejcts/ezv384.
- Kittayarak C, Reifenberger M, Chan S, et al. Reimbursement savings associated with tissue versus mechanical surgical aortic valve replacement in Thailand. Value Health Reg Issues. 2022;32:23–30. doi: 10.1016/j.vhri.2022.06.003.
- Nguyen TC, Walker T, Gunnarsson C, et al. Long-term healthcare expenditures over time for tissue and mechanical aortic valve replacement. Ann Thorac Surg. 2021;112(2):526–531. doi: 10.1016/j.athoracsur.2020.07.106.
- Bartus K, Litwinowicz R, Bilewska A, et al. Final 5-year outcomes following aortic valve replacement with a RESILIA™ tissue bioprosthesis. Eur J Cardiothorac Surg. 2021;59(2):434–441. doi: 10.1093/ejcts/ezaa311.
- Bavaria JE, Griffith B, Heimansohn DA, et al. Five-year outcomes of the COMMENCE trial investigating aortic valve replacement with RESILIA tissue. Ann Thorac Surg. 2022;115(6):1429–1436. doi: 10.1016/j.athoracsur.2021.12.058.
- Beaver T, Bavaria JE, Griffith B, et al. Seven-year outcomes following aortic valve replacement with a novel tissue bioprosthesis. J Thorac Cardiovasc Surg. 2023; In Press. doi: 10.1016/j.jtcvs.2023.09.047.
- Keuffel EL, Reifenberger M, Marfo G, et al. Long run savings associated with surgical aortic valve replacement using a RESILIA tissue bioprosthetic valve versus a mechanical valve. J Med Econ. 2023;26(1):120–127. doi: 10.1080/13696998.2022.2159662.
- Drummond M, Barbieri M, Cook J, et al. Transferability of economic evaluations across jurisdictions: ISPOR Good Research Practices Task Force report. Value Health. 2009;12(4):409–418. doi: 10.1111/j.1524-4733.2008.00489.x.
- Husereau D, Drummond M, Petrou S, et al. Consolidated health economic evaluation reporting standards (CHEERS)—explanation and elaboration: a report of the ISPOR health economic evaluation publication guidelines good reporting practices task force. Value Health. 2013;16(2):231–250. doi: 10.1016/j.jval.2013.02.002.
- Neumann PJ, Sanders GD, Russell LB, et al. Cost-effectiveness in health and medicine. New York, NY: Oxford University Press; 2016.
- Husereau D, Drummond M, Augustovski F, et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. Int J Technol Assess Health Care. 2022;38(1):e13. doi: 10.1017/S0266462321001732.
- Bavaria JE, Tommaso CL, Brindis RG, et al. 2018 AATS/ACC/SCAI/STS expert consensus systems of care document: operator and institutional recommendations and requirements for transcatheter aortic valve replacement: a joint report of the American Association for Thoracic Surgery, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2019;73(3):340–374. doi: 10.1016/j.jacc.2018.07.002.
- Akins CW, Miller DC, Turina MI, et al. Guidelines for reporting mortality and morbidity after cardiac valve interventions. Eur J Cardiothorac Surg. 2008;33(4):523–528. doi: 10.1016/j.ejcts.2007.12.055.
- Dunn A, Grosse SD, Zuvekas SH. Adjusting health expenditures for inflation: a review of measures for health services research in the United States. Health Serv Res. 2018;53(1):175–196. doi: 10.1111/1475-6773.12612.
- Grosse SD, Nelson RE, Nyarko KA, et al. The economic burden of incident venous thromboembolism in the United States: a review of estimated attributable healthcare costs. Thromb Res. 2016;137:3–10. doi: 10.1016/j.thromres.2015.11.033.
- Morita Y, Haruna T, Haruna Y, et al. Thirty‐day readmission after infective endocarditis: analysis from a nationwide readmission database. J Am Heart Assoc. 2019;8(9):e011598. doi: 10.1161/JAHA.118.011598.
- Simon AW, Kugelmass A, Brown P, et al. 90-day cost and clinical outcomes comparing TAVR to SAVR: do the economics work? Paper presented at: Session aortic valve disease: optimizing TAVR. American College of Cardiology; 2018; Orlando, FL.
- Cooper Z, Craig SV, Gaynor M, et al. The price ain’t right? Hospital prices and health spending on the privately insured. Q J Econ. 2019;134(1):51–107. doi: 10.1093/qje/qjy020.
- Bobade RA, Helmers RA, Jaeger TM, et al. Time-driven activity-based cost analysis for outpatient anticoagulation therapy: direct costs in a primary care setting with optimal performance. J Med Econ. 2019;22(5):471–477. doi: 10.1080/13696998.2019.1582058.
