Abstract
Aims
This study aimed to assess and compare the health care resource utilization (HCRU) and medical cost of metabolic dysfunction-associated steatohepatitis (MASH) by disease severity based on Fibrosis-4 Index (FIB-4) score among US adults in a real-world setting.
Materials and methods
This observational cohort study used claims data from the Healthcare Integrated Research Database (HIRD) to compare all-cause, cardiovascular (CV)-related, and liver-related HCRU, including hospitalization, and medical costs stratified by FIB-4 score among patients with MASH (identified by International Classification of Diseases, Tenth Revision, Clinical Modification [ICD-10-CM] code K75.81). Hospitalization and medical costs were compared by FIB-4 score using generalized linear regression with negative binomial and gamma distribution models, respectively, while controlling for confounders.
Results
The cohort included a total of 5,104 patients with MASH and comprised 3,162, 1,343, and 599 patients with low, indeterminate, and high FIB-4 scores, respectively. All-cause hospitalization was significantly higher in the high FIB-4 cohort when compared with the low FIB-4 reference after covariate adjustment (rate ratio, 1.63; 95% CI, 1.32–2.02; p < .0001). CV-related hospitalization was similar across all cohorts; however, CV-related costs were 1.26 times higher (95% CI, 1.11–1.45; p < .001) in the indeterminate cohort and 2.15 times higher (95% CI, 1.77–2.62; p < .0001) in the high FIB-4 cohort when compared with the low FIB-4 cohort. Patients with indeterminate and high FIB-4 scores had 2.97 (95% CI, 1.78-4.95) and 12.08 (95% CI, 7.35–19.88) times the rate of liver-related hospitalization and were 3.68 (95% CI, 3.11–4.34) and 33.73 (95% CI, 27.39–41.55) times more likely to incur liver-related costs, respectively (p < .0001 for all).
Limitations
This claims-based analysis relied on diagnostic coding accuracy, which may not capture the presence of all diseases or all care received.
Conclusions
High and indeterminate FIB-4 scores were associated with significantly higher liver-related clinical and economic burdens than low FIB-4 scores among patients with MASH.
PLAIN LANGUAGE SUMMARY
MASH is a serious liver disease that can lead to fibrosis, cirrhosis, and other complications. There is a need to understand the impact of disease severity on the burden of MASH. Health care claims data were used to assess the use of medical resources, including hospitalization, and medical costs among patients with 3 different levels of severity of MASH, as assessed via FIB-4 score. FIB-4 is a widely available non-invasive marker of severity. Rates of all-cause, cardiovascular-related and liver-related hospitalization and medical costs were several-fold higher in patients with high disease severity of MASH than those with low disease severity of MASH.
Introduction
Metabolic dysfunction-associated steatotic liver disease (MASLD) is characterized by excessive accumulation of triglycerides in the liver and encompasses a spectrum of conditions, ranging from hepatic steatosis to metabolic dysfunction-associated steatohepatitis (MASH) with advanced stages of fibrosisCitation1. MASLD is associated with obesity and other components of the metabolic syndrome, such as insulin resistance, type 2 diabetes (T2D), hypertension, and dyslipidemiaCitation2,Citation3. An estimated 30% of adults in the USA are affected by MASLD, making it the most common chronic liver disease. The prevalence is expected to increase concurrently with complications of the metabolic syndromeCitation4,Citation5.
MASH is a more serious and clinically progressive form of MASLD defined by steatosis, lobular inflammation, and hepatocyte ballooning, with or without fibrosis. Patients with MASH are at an increased risk of progressing to hepatic complications, including cirrhosis, decompensated cirrhosis, hepatocellular carcinoma, and liver failure, and ultimately, may require liver transplantationCitation6. MASH is projected to become the leading indication for liver transplantation as its prevalence continues to increaseCitation2. MASH is also associated with increased risk of extra-hepatic consequences, including cardiovascular disease, diabetes, and non-liver malignancies, that contribute to increased morbidity and mortalityCitation7.
The stage of liver fibrosis is the strongest predictor of disease progression of MASH and of liver-related morbidity and mortality; therefore, it is important to identify high-risk patients who may develop fibrosis and cirrhosisCitation8,Citation9. While liver biopsy is considered the reference standard for diagnosing and differentiating MASLD and MASH, biopsies are not required for the diagnosis of MASLD or MASH and can be prohibitively expensive and invasiveCitation10. Recent professional society guidanceCitation1 recommends noninvasive initial screening for assessing the risk of more severe disease (MASH with advanced fibrosis) with the Fibrosis-4 Index (FIB-4). The FIB-4 uses patient age and quantifiable biomarkers, including aspartate transaminase, alanine transaminase, and platelet levels, to estimate liver fibrosis in patients at risk for chronic liver disease, and has been extensively studied and validated in different populations with liver diseaseCitation11,Citation12.
Real-world evidence on the medical costs and health care resource utilization (HCRU) associated with MASH is limited despite its significant disease burden. Further, the impact of the fibrosis stage on the clinical and economic burdens of MASH is not well understood. These data are critical for decision-makers and help guide resource planning from a public health perspectiveCitation13,Citation14. This study sought to address this evidence gap by comparing all-cause, cardiovascular (CV)-related, and liver-related medical costs and HCRU by FIB-4 score among patients with MASH in the real-world setting.
Methods
Study design and data source
This observational retrospective study used the Healthcare Integrated Research Database (HIRD; Carelon Research) to evaluate the clinical and economic burdens of MASH stratified by disease severity. The HIRD is a longitudinal, fully identifiable database which contains approved health care claims data for commercially insured and Medicare Advantage health plan members from 15 geographically diverse plans across the US. Administrative claims data in the HIRD are integrated across data sources and types (i.e. professional claims, facility claims, outpatient pharmacy claims, outpatient laboratory results, and enrollment information) for >80 million unique patients, representing members from each of the 50 states. Laboratory results are available in the HIRD for approximately 30% to 40% of tests that are conducted. Claims data and enrollment information were collected from 1 October 2015, to 31 May 2022 (study period), and patients were identified between 1 October 2016, and 30 April 2022 (). The date of first observed MASH diagnosis was termed the index date. The baseline period was the 12 months before the index date. The follow-up period was at least 1 month after the index date and extended from 1 day after the index date to the end of the study period, the end of health plan enrollment, or patient censored death, whichever came first.
Study population
Patient attrition is presented in . Patients aged ≥18 years with ≥1 inpatient or ≥2 outpatient medical claims for MASH (formerly nonalcoholic steatohepatitis [NASH]; based on International Classification of Diseases, Tenth Revision, Clinical Modification [ICD-10-CM] code K75.81 for NASH) during the patient identification period and ≥12 months pre-index and ≥1 month post-index continuous medical and pharmacy enrollment were included in the cohort. At the time of this study, the term MASH had not yet been adopted in ICD-10 coding; patients with claims for NASH were considered to have MASH at the time of writing to reflect the change in nomenclature. In addition to these criteria, patients were required to have laboratory results of aspartate aminotransferase, alanine aminotransferase, and platelet count within 3 months pre- and post-index to determine FIB-4 scores. FIB-4 scores were calculated using the laboratory results reported on the date closest to the index date and subsequently grouped as low (FIB-4 < 1.30), indeterminate (FIB-4 1.30 to 2.67), or high (FIB-4 > 2.67), following current evidenceCitation15.
Figure 2. Patient attrition. Patient attrition for the low FIB-4 score, indeterminate FIB-4 score, and high-FIB-4 score cohorts is shown. Percentages show the proportion of patients retained from the previous step in the diagram. FIB-4, fibosis-4 index; HIV, human immunodeficiency virus; ICD-10-CM, International Classification of Diseases, Tenth Revision, Clinical Modification; LAL-D, lysosomal acid lipase deficiency; MASH, metabolic dysfunction–associated steatohepatitis; PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis.

Exclusion criteria were ≥1 diagnosis of the following conditions during the study period (in order of descending prevalence): hepatitis B, hepatitis C, other viral hepatitis, alcoholic liver diseases, alcohol dependence, other and unspecified alcohol dependence, nondependent alcohol abuse, human immunodeficiency virus (HIV), toxic liver disease, hepatoptosis, Wilson disease, disorder of iron metabolism, autoimmune hepatitis, Gaucher disease, lysosomal acid lipase deficiency, primary biliary cholangitis, and primary sclerosing cholangitis.
Primary outcomes
Baseline demographics and clinical characteristics
Baseline demographics including age, race/ethnicity, health plan type, and geographic region of residence were documented on the index date. Clinical characteristics were recorded in the pre-index baseline period and include Quan-Charlson Comorbidity Index (QCI)Citation16,Citation17 chronic comorbidities of interest, and chronic medication use.
HCRU and medical costs
Post-index all-cause, CV-related, and liver-related HCRU and medical costs, excluding medication costs, were summarized for each FIB-4 category. CV-related and liver-related HCRU and medical costs were identified based on the primary diagnostic ICD-10-CM code associated with the claim. Measures of HCRU obtained during the follow-up period included: the proportion of patients with ≥1 visit or hospitalization and the number of visits or hospitalizations per 100 patients per month for inpatient hospitalizations, emergency department (ED) visits, outpatient visits, and physician office visits; the proportion of patients with ≥1 laboratory test and the number of laboratory tests per 100 patients per month; the proportion of patients with ≥1 prescription fill and the number of fills per patient per month (PPPM) among patients with >1 fill; and the length of stay per 100 patients per month for inpatient hospitalization.
Measures of total medical costs, including inpatient, ED, and outpatient medical costs, were obtained during the follow-up period, and reported PPPM. Patient- and plan-paid medical costs were included and adjusted for inflation to 2021 US dollars. Hospitalization rate ratios, medical cost ratios, and mean medical costs were calculated to evaluate incremental clinical and economic burdens associated with different levels of disease severity among patients with MASH, respectively.
Statistical analyses
Descriptive statistics were performed for the low, indeterminate, and high FIB-4 score cohorts. Continuous variables were summarized using means and standard deviations, and categorical variables were summarized using counts and percentages. Differences across FIB-4 score cohorts were described using chi-square test for categorical variables and analysis of variance (ANOVA) or Kruskal-Wallis test for continuous variables.
Generalized linear models were used to compare inpatient hospitalization and medical costs by disease severity while controlling for confounders. Variable selection was performed using bi-directional step-wise regression with a significance level for entry = 0.20. Generalized linear regression with a negative binomial distribution model was used to evaluate the association between post-index hospitalization, and FIB-4 score category and baseline patient characteristics as covariates. The association between PPPM medical costs and FIB-4 score category and covariates was evaluated using a generalized linear regression with a gamma distribution and log-link modeling. The low FIB-4 cohort served as the reference for comparison with the high and indeterminate FIB-4 cohorts in both methods. Statistical analyses were performed with SAS, version 9.3 (SAS Institute Inc., Cary, NC, USA).
Additional analyses
Analyses assessing the annual prevalence of MASLD and MASH from 2016 to 2020 as well as the rate of progression to complications among patients with MASH were performed in addition to the analyses outlined above. The study design for these analyses is reported in Supplemental Figure 1.
Annual prevalence of MASLD and MASH
Patient attrition for the cohort used to calculate annual prevalence of MASLD and MASH from 2016 to 2020 is reported in Supplemental Table 1. Cohort demographics are reported in Supplemental Table 2 and the calculated annual prevalence rates are reported in Supplemental Figure 2.
Rate of progression to complications among patients with MASH
Patient attrition for the cohort used to evaluate the rate of progression to complications among those with MASH is reported in Supplemental Table 3. Baseline cohort demographics and clinical characteristics are reported in Supplemental Table 4. Supplemental Table 5 provides the proportion of patients with MASH who progressed to cirrhosis with complications, hepatocellular carcinoma, colorectal cancer, liver transplantation, and major adverse cardiac events (MACE) as well as the average time to the first occurrence of each of the listed complications.
ICD-10-CM codes used for all analyses are provided in Supplemental Table 6 and Supplemental Table 7.
Results
Patient characteristics
The cohort included a total of 5,104 patients with MASH and was composed of 3,162, 1,343, and 599 patients with low, indeterminate, and high FIB-4 scores, respectively ( and Supplemental Table 8). The cohort was predominantly White (low FIB-4, 53.3%; indeterminate FIB-4, 62.0%; high FIB-4, 71.3%) with a higher proportion of female patients (low FIB-4, 57.0%; indeterminate FIB-4, 57.2%; high FIB-4, 53.4%). Mean (standard deviation [SD]) age was highest in the high FIB-4 cohort at 62 (9.7) years and lowest in the low FIB-4 cohort at 47 (11.6) years. At baseline, the group with high FIB-4 scores appeared to include patients with higher QCI values, higher percentages of comorbidity, and higher use of medication ( and Supplemental Table 9). The follow-up period for all patients in the low, indeterminate, and high FIB-4 cohorts was ≥1 year. Notably, 55.9% and 58.4% of the high FIB-4 cohort had cirrhosis and cirrhosis with complications, respectively, at baseline; cirrhosis was present in 3.3% of patients in the low FIB-4 cohort and 13.8% of patients in the indeterminate FIB-4 cohort at baseline.
Table 1. Select baseline demographic and clinical characteristics.
Post-index HCRU and medical costs
Post-index all-cause, CV-related, and liver-related HCRU generally increased with increasing FIB-4 score. The proportion of patients in the high FIB-4 cohort with ≥1 all-cause hospitalization was 36.1% compared with 21.7% of those in the indeterminate FIB-4 cohort and 16.3% of those in the low FIB-4 cohort (p < .0001; ). Additionally, the mean length of stay for all-cause hospitalization was highest in the high FIB-4 cohort at 160 total bed days per 100 patients per month vs 77 total bed days per 100 patients per month in the indeterminate FIB-4 cohort and 39 total bed days per 100 patients per month in the low FIB-4 cohort (p < .0001). This was also observed with CV-related hospitalization; 19.7% of patients in the high FIB-4 cohort had ≥1 CV-related hospitalization compared with those in the indeterminate (11.5%) and low (6.4%) FIB-4 cohorts (p < .0001). Liver-related resource utilization was significantly higher among patients with high FIB-4 scores. The greatest difference was observed with liver-related hospitalization; 18.2% of patients in the high FIB-4 cohort had liver-related hospitalization vs 3.1% of those in the indeterminate FIB-4 cohort and 0.8% in the low FIB-4 cohort (p < .0001).
Table 2. Post-index HCRU among patients with MASH stratified by FIB-4 score.
Table 3. Post-index medical costs among patients with MASH by FIB-4 score.
Similar to the observed HCRU, post-index medical costs generally increased with increasing FIB-4 score. Mean all-cause medical costs were $4,795 PPPM in the high FIB-4 cohort, $2,003 PPPM in the indeterminate FIB-4 cohort, and $1,627 in the low FIB-4 cohort (p < .0001; ). At follow-up, CV-related total medical costs were $1,303 PPPM, $568 PPPM, and $310 PPPM in the high, indeterminate, and low FIB-4 cohorts, respectively (p < .0001). Total liver-related medical costs were nearly 10 times higher in the high FIB-4 cohort compared with the indeterminate FIB-4 cohort ($1,860 PPPM vs $199 PPPM, respectively; p < .0001). For those in the high FIB-4 cohort, higher total medical costs were primarily driven by inpatient costs.
Covariate-adjusted hospitalizations and medical costs
After covariate adjustment, high FIB-4 scores were associated with significantly higher rates of hospitalization. The rate of all-cause hospitalization was 63% higher in the high FIB-4 group when compared with the low FIB-4 group reference (rate ratio [RR], 1.63; 95% CI, 1.32–2.02; p < .0001; and ). No significant differences in CV-related hospitalization were observed across FIB-4 score categories. Rates of liver-related hospitalization were significantly higher among those with high and indeterminate FIB-4 scores vs low FIB-4 scores. High FIB-4 scores were associated with 1,108% higher rates of liver-related hospitalization (RR, 12.08; 95% CI, 7.35–19.88; p < .0001). Patients with indeterminate FIB-4 scores had 197% higher rates of liver-related hospitalization (RR, 2.97; 95% CI, 1.78–4.95; p < .0001).
Figure 3. Adjusted post-index hospitalization rates and medical costs among patients with MASH by FIB-4 score. Covariate adjusted all-cause (a), CV-related (B), and liver-related (C) hospitalization rate ratios and cost ratios associated with high FIB-4 scores (close circles) and indeterminate FIB-4 scores (open circles) compared with low FIB-4 scores are shown. The low FIB-4 reference is depicted by a horizontal, dotted line at y = 1. Points are labeled with the corresponding ratio, 95% CI, and p value.
Abbreviations. CI, Confidence interval; CV, Cardiovascular; FIB-4, Fibosis-4 Index; MASH, Metabolic dysfunction–associated steatohepatitis
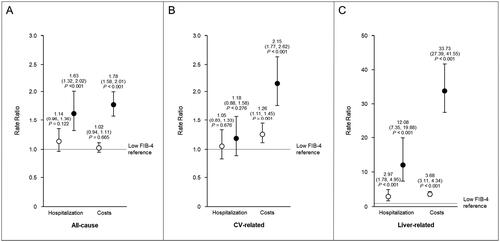
Table 4. Covariate-adjusted post-index hospitalizations among patients with MASH by FIB-4 score.
High FIB-4 scores were also associated with significantly higher medical costs across all categories, while significantly higher CV- and liver-related medical costs were observed with indeterminate FIB-4 scores ( and ). CV-related medical costs were 26% higher (RR, 1.26; 95% CI, 1.11–1.45; p < .001) in the indeterminate FIB-4 cohort and 115% higher (RR, 2.15; 95% CI, 1.77–2.62; p < .0001) in the high FIB-4 cohort. Similar to hospitalization rates, the greatest differences across FIB-4 cohorts were observed with liver-related medical costs. Patients with indeterminate and high FIB-4 scores had 268% higher (RR, 3.68; 95% CI, 3.11–4.34, p < .0001) and 3273% higher (RR, 33.73; 95% CI, 27.39–41.55; p < .0001) liver-related medical costs, respectively, than patients with low FIB-4 scores.
Table 5. Covariate-adjusted post-index medical costs among patients with MASH by FIB-4 score.
Discussion
Data on the HCRU and economic burden of MASH, particularly by disease severity, are limited, despite the high prevalence of this chronic liver disease in the US. This study expands our understanding of the burden of MASH by FIB-4 score, the primary means recommended for assessing risk of MASH with advanced fibrosis in patients with MASLD, by identifying the demographic and clinical characteristics, HCRU, and medical costs associated with different levels of disease severity in the real-world setting. With professional society guidance documents recommending utilization of FIB-4 scores for initial screening for patients at risk of more advanced fibrosis related to MASLD and MASH, knowledge of the relative clinical and economic burden associated with FIB-4 scores is critical. The most important observation of this study is that the clinical and economic burdens of MASLD and MASH are significant among patients with high and indeterminate FIB-4 scores, reinforcing the utility of FIB-4 for screening and stratification at a population level. There are several nuanced aspects of this study that merit detailed consideration.
One of the important findings of this analysis is that ICD-10 codes for MASH are assigned with a frequency that is much lower than the known prevalence of MASH in the US population. Of the ∼80 million unique patients in the HIRD database, 48,161 (<0.1%) were assigned an ICD-10 code for MASH for ≥1 inpatient or ≥2 outpatient encounters (). The prevalence of MASH in a recent prospective study was estimated to be ∼14% among middle-aged patients in the USCitation18. Approximately one in 80 patients with MASH is diagnosed to the extent of receiving an ICD-10 code for MASH, highlighting the importance of screening for MASH in at-risk populations. Cardiometabolic comorbidities were higher among patients at risk of more advanced fibrosis (indeterminate and high FIB-4 vs low FIB-4) and over half of the patients in each cohort had chronic use of CV disease medications and antihypertensives at baseline. More than two thirds of the patients in the high FIB-4 cohort had ≥3 high-risk comorbid conditions (T2D, hypertension, and hyperlipidemia) and more than half of those in the low and indeterminate cohorts had ≥2 of these conditions. This is consistent with other studies reporting high rates of cardiometabolic comorbidities with higher stages of MASLD or MASH fibrosis or cirrhosisCitation19,Citation20 High rates of high-risk comorbidities in the study population, especially among those with high FIB-4 scores, may have contributed to the high rates of all-cause utilization, and all-cause and CV-related medical costs.
In this real-world analysis, high FIB-4 scores were associated with higher rates of HCRU than indeterminate and low FIB-4 scores, with or without covariate adjustment. These findings are consistent with other studies reporting a substantial clinical burden of MASLD/MASH and significant impact of advanced fibrosis on HCRUCitation20,Citation21,Citation22 Gordon et al.Citation20 reported a 50% increase in post-index all-cause inpatient admissions and a 22% increase in post-index all-cause emergency visits among patients with FIB-4-based estimates of F3-F4 fibrosis (FIB-4 > 2.67); however, no comparisons with lower estimated stages of fibrosis (FIB-4 < 2.67) were performed. Significant increases in post-index all-cause inpatient admissions were also reported by Wong et al.Citation21, where patients with compensated cirrhosis and decompensated cirrhosis had 62% and 140% increases in inpatient hospitalizations, respectively. This trend was not exclusive to those with advanced liver disease, as the study reported a 103% increase in inpatient admissions among patients with MASLD/MASH without advanced liver disease. Romero-Gomez et al.Citation22 also reported higher numerical rates of all-cause admissions among those with MASLD/MASH and compensated and decompensated cirrhosis when compared with those without advanced liver disease; however, no significant changes in post-index rates were observed in that study. Importantly, these studies assessed changes in HCRU within each severity cohort, while our study compared HCRU across severity cohorts, highlighting the incremental burden associated with each FIB-4 score category.
This study also demonstrated substantial economic burden associated with higher FIB-4 scores. Post-index medical costs, with and without covariate adjustment, were significantly higher in the high FIB-4 cohort compared with the low FIB-4 cohort. The findings of the present study are consistent with reported trends of increased costs with advanced liver disease. Post-index total health care costs reported by Gordon et al.Citation20 increased by 48% among patients with FIB-4-based F4 (cirrhosis). Similarly, Romero-Gomez et al.Citation22 reported significantly higher medical costs among patients with MASLD/MASH with compensated and decompensated cirrhosis when compared with patients with MASLD/MASH without complications; adjusted post-index all-cause PPPM costs were 13% higher in patients with compensated cirrhosis and 40% higher in those with decompensated cirrhosisCitation22. Wong et al.Citation21 also reported significantly higher costs among patients with MASLD/MASH with advanced liver diseases compared with those without advanced liver disease; however, adjusted post-index all-cause annual costs were 22% higher among patients with compensated cirrhosis and 464% higher in those with decompensated cirrhosis when compared with patients without advanced liver disease. We observed 78% higher adjusted post-index all-cause PPPM medical costs in the high FIB-4 cohort compared with the low FIB-4 cohort. This demonstrates a similar trend of increased costs with increased disease severity; however, direct comparisons are limited by variation in methodology across studies. Additionally, the populations in these studies included both patients with MASLD and MASH, while our study only assessed costs in patients with MASH.
Notably, over half of the patients in the high FIB-4 cohort had a diagnosis of cirrhosisat baseline in the present study. This finding reinforces the impression that, not only is MASH diagnosed in a small fraction of patients with MASH, but that the prevalence of advanced disease is high at the time of diagnosis. The prevalence of advanced fibrosis in patients with MASH in cross-sectional studies is ∼5%Citation18. Canbay et al.Citation19 reported a significant increase in hospitalization rates from 40.9% to 66.9% in patients with MASLD/MASH after diagnosis of compensated cirrhosis, highlighting the importance of early identification of patients. Boursier et al.Citation23 similarly reported a 300% increase in annual hospitalization and 250% increase in annual hospitalization costs after diagnosis of compensated cirrhosis in patients with MASLD/MASH. The high prevalence of cirrhosis in the high FIB-4 cohort may have been a primary contributor to the elevated HCRU and costs observed in this study; however, additional studies are needed to evaluate the cirrhosis-specific impact on clinical and economic burdens.
The most striking differences in clinical and economic burden were observed when isolated to liver-related hospitalizations and medical costs. After adjusting for covariates, liver-related hospitalization rates were 197% and 1,108% higher in patients with indeterminate and high FIB-4 scores, respectively, when compared with those with low FIB-4 scores. Liver-related medical costs were 268% and 3,237% higher in the indeterminate and high FIB-4 cohorts than the low FIB-4 cohort, respectively. These trends were not captured in other studies only assessing all-cause utilization and costs. The findings of the present study describe the incremental increase in liver-related clinical and economic burden in moving from a low to indeterminate FIB-4 score and from indeterminate to high FIB-4 score. Additionally, these data highlight the magnitude of impact higher stages of fibrosis have on liver-related hospitalization and costs.
This study provides a current assessment of the impact of disease severity on HCRU and medical costs among patients with MASH in a large managed care population in the US. Our study complements other studies assessing outcomes based on FIB-4 scores and expands our understanding of the association between CV- and liver-related outcomes with FIB-4 scoreCitation24,Citation25. Given recent professional society guidance on the use of non-invasive assessments, such as FIB-4, in high-risk patients, the findings reported here advance our understanding of the clinical and economic burdens associated with FIB-4 score categories and highlight the need to identify patients in earlier stages of disease.
Limitations
This study was confined to members continuously enrolled in commercial or Medicare health plans, therefore, its findings may not be applicable to those with government-sponsored health insurance or those who are uninsured or underinsured and lack access to care. Claims data rely on the accuracy of diagnostic coding; therefore, the ability to capture the presence of all diseases or all care received may be impacted by potential coding errors and missing data. Additionally, the identification of patients with MASH was solely based on ICD-10-CM codes. At the time of this study, there were no approved therapies for MASH; therefore, the presence of MASH in diagnostic coding may be underestimated.
This study relied on FIB-4 score as a surrogate marker for the stages of liver fibrosis. FIB-4 cut-off values help differentiate between advanced fibrosis vs the absence of fibrosis; the range of values in between the cut-off values is termed the indeterminate zone. Additionally, the accuracy of the FIB-4 scores calculated in this study is difficult to ascertain as the biomarker data in the database may not have been obtained at the same time. Laboratory information used to determine FIB-4 score were available for <30% of the population after all other inclusion and exclusion criteria were implemented. It is possible that the requirement for laboratory data resulted in potential selection bias favoring patients who were more likely to receive routine laboratory analyses due to other comorbid conditions or complications. Medical costs among this cohort may be higher than those of the general population with MASH due to their potential increased engagement with health care.
Conclusion
This study demonstrates the significant impact of fibrosis stage on the clinical and economic burdens of MASH among US adults in the real-world setting. Compared with patients with low FIB-4 scores, those with high and indeterminate FIB-4 scores utilized more health care resources, had higher rates of hospitalization, and incurred higher all-cause, CV-related, and liver-related medical costs. A substantial increase in the clinical and economic burdens of MASH, especially with advanced fibrosis, is likely given the rising prevalence of this progressive disease and associated comorbidities. The findings reported here underscore the importance of increased awareness, early detection, diagnosis, and management of MASH to mitigate the risk of complications and limit economic burden.
Transparency
Declaration of financial/other interests
ITM, RL, AH, and CU are employees of Novo Nordisk Inc. ITM was not an employee of Novo Nordisk Inc. at the time of this study.
MC has consulted for Novo Nordisk, Pfizer, Madrigal, Sagimet, Ocelot, Novartis, Merck, Bristol Myers Squibb, 89Bio, and Intercept, and received research support from NorthSea, Pfizer, and Madrigal.
ITM and ZZ were employees of Carelon Research Inc. at the time of this study. CCT is an employee of Carelon Research Inc., a wholly-owned subsidiary of Elevance Health Inc., and they received funding from Novo Nordisk to perform this research.
FA is an employee of Panalgo and a contractor for Carelon Research Inc.
Author contributions
All authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article. All authors contributed to data analysis and interpretation, drafting of the manuscript, and critical review of the paper for important intellectual content.
Reviewer disclosures
Peer reviewers on this manuscript have received an honorarium from JME for their review work but have no other relevant financial relationships to disclose.
Previous presentations
A poster of this work was presented at the American Association for the Study of Liver Diseases The Liver Meeting®, held on 10–14 November 2023 in Boston, Massachusetts. Data reported in the supplemental materials were presented at the Academy of Managed Care Pharmacy Nexus 2023 held on 16–19 October 2023 in Orlando, FL and the 18th annual Cardiometabolic Health Congress held on 18–21October 2023 in Boston, Massachusetts as poster presentations.
Supplemental Material
Download MS Word (129.8 KB)Acknowledgements
Writing assistance was provided by Victoria Jeter, of PRECISIONscientia, Yardley, PA, and was supported financially by Novo Nordisk Inc., in compliance with international Good Publication Practice guidelines.
Data availability statement
Data were provided by Carelon Research Inc. and used under license for this study; therefore, the data are not publicly available.
Additional information
Funding
References
- Rinella ME, Neuschwander-Tetri BA, Siddiqui MS, et al. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023;77(5):1797–1835. doi: 10.1097/HEP.0000000000000323.
- Flisiak-Jackiewicz M, Bobrus-Chociej A, Wasilewska N, et al. From nonalcoholic fatty liver disease (NAFLD) to metabolic dysfunction-associated fatty liver disease (MAFLD)-new terminology in pediatric patients as a step in good scientific direction? J Clin Med. 2021;10(5):924.
- Younossi ZM, Stepanova M, Younossi Y, et al. Epidemiology of chronic liver diseases in the USA in the past three decades. Gut. 2020;69(3):564–568. doi: 10.1136/gutjnl-2019-318813.
- Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313(22):2263–2273. doi: 10.1001/jama.2015.5370.
- Estes C, Razavi H, Loomba R, et al. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018; 67(1):123–133. doi: 10.1002/hep.29466.
- Alexander M, Loomis AK, van der Lei J, et al. Risks and clinical predictors of cirrhosis and hepatocellular carcinoma diagnoses in adults with diagnosed NAFLD: real-world study of 18 million patients in four European cohorts. BMC Med. 2019;17(1):95. doi: 10.1186/s12916-019-1321-x.
- Niederseer D, Wernly B, Aigner E, et al. NAFLD and cardiovascular diseases: epidemiological, mechanistic and therapeutic considerations. J Clin Med. 2021;10(3):467.
- Dulai PS, Singh S, Patel J, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology. 2017;65(5):1557–1565. doi: 10.1002/hep.29085.
- Ekstedt M, Hagström H, Nasr P, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61(5):1547–1554. doi: 10.1002/hep.27368.
- Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012; 55(6):2005–2023. doi: 10.1002/hep.25762.
- European Association for the Study of the Liver, Clinical Practice Guideline P, Chair, et al. EASL clinical practice guidelines on non-invasive tests for evaluation of liver disease severity and prognosis - 2021 update. J Hepatol. 2021;75(3):659–689.
- Castera L, Friedrich-Rust M, Loomba R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019; 156(5):1264–1281 e4. doi: 10.1053/j.gastro.2018.12.036.
- Rabarison KM, Bish CL, Massoudi MS, et al. Economic evaluation enhances public health decision making. Front Public Health. 2015;3:164. doi: 10.3389/fpubh.2015.00164.
- Durand-Zaleski I. Why cost-of-illness studies are important and inform policy. Vasc Med. 2008;13(3):251–253. doi: 10.1177/1358863X08091738.
- Anstee QM, Lawitz EJ, Alkhouri N, et al. Noninvasive tests accurately identify advanced fibrosis due to NASH: baseline data from the STELLAR trials. Hepatology. 2019;70(5):1521–1530. doi: 10.1002/hep.30842.
- Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005; 43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83.
- Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–682. doi: 10.1093/aje/kwq433.
- Harrison SA, Gawrieh S, Roberts K, et al. Prospective evaluation of the prevalence of non-alcoholic fatty liver disease and steatohepatitis in a large middle-aged US cohort. J Hepatol. 2021;75(2):284–291. doi: 10.1016/j.jhep.2021.02.034.
- Canbay A, Meise D, Haas JS. Substantial comorbidities and rising economic burden in real world non‐alchoholic fatty liver disease (NAFLD)/non‐alcoholic steatohepatitis (NASH) patients with compensated cirrhosis (CC): a large German claims database study [Abstract]. J Hepatol. 2018;68(supp. 1):S32. doi: 10.1016/S0168-8278(18)30282-4.
- Gordon SC, Kachru N, Parker E, et al. Health care use and costs among patients with nonalcoholic steatohepatitis with advanced fibrosis using the fibrosis-4 score. Hepatol Commun. 2020;4(7):998–1011. doi: 10.1002/hep4.1524.
- Wong RJ, Kachru N, Martinez DJ, et al. Real-world comorbidity burden, health care utilization, and costs of nonalcoholic steatohepatitis patients with advanced liver diseases. J Clin Gastroenterol. 2021;55(10):891–902. doi: 10.1097/MCG.0000000000001409.
- Romero-Gomez M, Kachru N, Zamorano MA, et al. Disease severity predicts higher healthcare costs among hospitalized nonalcoholic fatty liver disease/nonalcoholic steatohepatitis (NAFLD/NASH) patients in Spain. Medicine. 2020;99(50):e23506. doi: 10.1097/MD.0000000000023506.
- Boursier J, Shreay S, Fabron C, et al. Hospitalization costs and risk of mortality in adults with nonalcoholic steatohepatitis: analysis of a French national hospital database. EClinicalMedicine. 2020;25:100445. doi: 10.1016/j.eclinm.2020.100445.
- Tapper EB, Krieger N, Przybysz R, et al. The burden of nonalcoholic steatohepatitis (NASH) in the United States. BMC Gastroenterol. 2023;23(1):109. doi: 10.1186/s12876-023-02726-2.
- Vieira Barbosa J, Milligan S, Frick A, et al. Fibrosis-4 index as an independent predictor of mortality and liver-related outcomes in NAFLD. Hepatol Commun. 2022;6(4):765–779. doi: 10.1002/hep4.1841.

