Abstract
Aims
Suboptimal treatment indicators, including treatment switch, are common among patients with Crohn’s disease (CD), but little is known about their associated healthcare resource utilization (HRU) and costs. This study assessed the impact of suboptimal treatment indicators on HRU and costs among adults with CD newly treated with a first-line biologic.
Methods
Adult patients with CD were identified in the IBM MarketScan Commercial Subset (10/01/2015–03/31/2020). The index date was defined as initiation of the first-line biologic, and the study period was defined as the 12 months following the index date. Patients were classified into Suboptimal Treatment and Optimal Treatment cohorts based on observed indicators of suboptimal treatment during the study period. Patients in the Suboptimal Treatment Cohort with a treatment switch were classified into the Treatment Switch Cohort and compared to patients with no treatment switch. All-cause HRU and costs were measured during the study period and assessed for patients with suboptimal vs optimal treatment and patients with vs without a treatment switch.
Results
The study included 4,006 patients (Suboptimal Treatment: 2,091, Optimal Treatment: 1,915). Treatment switch was a common indicator of suboptimal treatment (Treatment Switch: 640, No Treatment Switch: 3,366). HRU and costs were significantly higher among patients with suboptimal treatment than those with optimal treatment (annual costs: $92,043 vs $73,764; p < 0.01), and among those with a treatment switch than those with no treatment switch (annual costs: $95,689 vs $81,027; p < 0.01). Increases in the number of suboptimal treatment indicators were associated with increased costs.
Limitations
Claims data were used to identify suboptimal treatment indicators based on observed treatment patterns; reasons for treatment decisions could not be assessed.
Conclusion
This study demonstrates that patients with suboptimal treatment indicators, including treatment switch, incur substantially higher HRU and costs compared to patients receiving optimal treatment and those that do not switch treatments.
Introduction
Crohn’s disease (CD) is an inflammatory bowel disease (IBD) characterized by chronic inflammation that can occur throughout the gastrointestinal tractCitation1. CD manifests through persistent symptoms, including chronic abdominal pain, diarrhea, anemia, fatigue, and weight lossCitation1,Citation2. As the disease progresses, patients will often experience more severe intestinal complications, such as fistulas, abscesses, and stricturesCitation2. Globally, the prevalence of CD is risingCitation1,Citation3, with the incidence increasing with ageCitation4. In 2018, 0.40% of Medicare fee-for-service beneficiaries had received a diagnosis of Crohn’s diseaseCitation5. CD is associated with a substantial humanistic and economic burden, with one study estimating the total lifetime cost for a patient with CD in the United States (US) is $622,056 resulting in an estimated societal cost of $498 billionCitation3,Citation6–8. Additionally, patients with CD report significantly worse Physical Component Summary scores on the Short-Form-12 Survey compared to those without IBD, highlighting the adverse impact on health-related quality of life experienced by patients with CDCitation6.
The treatment approach for CD depends on several factors such as disease severity, location, and prognosisCitation2,Citation9. For patients with moderate-to-severe CD, standard therapies include 5-aminosalycylate (5-ASA), systemic corticosteroids, or immunosuppressive agents (e.g. azathioprine)Citation2,Citation9. However, for patients who experience steroid-dependent or refractory disease, are at risk of disease progression, or do not respond to standard therapies, current guidelines recommend treatment with biologic therapyCitation2,Citation9. After initial biologic treatment, options for patients who experience insufficient response, loss of response, intolerance, or primary non-response include dose escalation, switch to a different biologic class, or surgeryCitation2,Citation9.
Over the past two decades, the approval of numerous biologic agents for the treatment of patients with CD has greatly expanded the treatment landscape and improved clinical outcomes and patient quality of lifeCitation10,Citation11. Approved biologics include tumor necrosis factor inhibitors (adalimumab, infliximab, and certolizumab), anti-integrin agents (natalizumab and vedolizumab), and anti-interleukin agents (ustekinumab and risankizumab)Citation10. However, some patients may still experience non-response, loss of response, or intolerance to a given biologic agent. Accordingly, dose adjustments, augmentations, or other treatment changes may be necessary during the induction and/or maintenance phase of treatmentCitation10,Citation12. Despite these various treatment approaches, there remains an unmet need to optimize treatment pathways in this patient populationCitation9,Citation11.
Indeed, suboptimal treatment frequently occurs with biologic use among patients with CD and may affect clinical outcomesCitation12–14. A systematic literature review of 41 real-world studies (2012–2017) of biologic treatment patterns in patients with IBD in the US found high rates of discontinuation (7–65%), dose escalation (8–35%), non-adherence (23–62%), and switch to another biologic (5–20%), supporting suboptimal biologic use in this patient populationCitation13. A more recent US retrospective claims-based study of patients with CD found that 79.4% of patients treated with biologics and 72.5% of patients treated with conventional therapies presented ≥1 indicator of suboptimal treatment within 12 months of treatment initiationCitation15. Furthermore, total costs increased as the number of suboptimal treatment indicators increased, suggesting that there is a potential economic burden associated with suboptimal treatment, although these findings were descriptive and statistical significance was not assessedCitation15.
These previous studies highlight the challenges faced by patients with CD in achieving optimal response to treatment, as well as the potential economic burden associated with suboptimal treatment. As the biologic treatment landscape continues to evolve for patients with CD, it is critical to further investigate the extent of the economic burden specifically associated with suboptimal biologic treatment among patients with CD, given the limited literature on the topic and the wider implications for healthcare systems. Therefore, the primary objective of this study was to assess the impact of suboptimal treatment indicators on HRU and costs among commercially insured adults in the US with CD treated with a first-line biologic. As a secondary objective, this study assessed HRU and costs among patients with a treatment switch, as this is a frequent and important indicator of suboptimal treatment in this population.
Methods
Data source
This study used data from the IBM MarketScan Commercial Subset (10/01/2015–03/31/2020)Citation16, which contains medical and pharmacy claims data for beneficiaries (including employees, spouses, and dependents) covered by employer-sponsored private health insurance across all US census regions. The data include standard demographic variables (race information is not available) as well as employer-paid portion of payments and any out-of-pocket expenses incurred by patients. Data are de-identified and comply with the Health Insurance Portability and Accountability Act regulations. Institutional Review Board exemption was not needed.
Study design
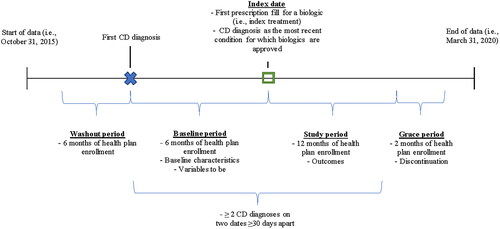
This was a retrospective longitudinal cohort study of commercially insured adult patients with CD who were newly initiated on a first-line biologic treatment. The index date was defined as the date of the first observed biologic prescription fill or injection after the first observed CD diagnosis (). The baseline period was defined as the 6-month period prior to the index date and was used to describe baseline characteristics. The study period was defined as the 12-month period following the index date and was used to identify indicators of suboptimal treatment and to describe HRU and costs. A 6-month period prior to the first CD diagnosis with no observed treatments or CD diagnoses (i.e. washout period) was used to identify newly diagnosed patients. In addition, a 2-month period following the study period (i.e. grace period) was used to allow observation of discontinuation up to and on the last day of the study period.
Study population
Selection criteria
This study included patients who had ≥2 distinct medical claims with a diagnosis of CD (International Classification of Diseases, Tenth Revision, Clinical Modification [ICD-10-CM] codes K50.x) on 2 dates ≥30 days apart; ≥1 biologic (vedolizumab, adalimumab, infliximab, certolizumab, natalizumab, or ustekinumab) prescription fill or injection on or after their first diagnosis of CD; CD as their most recent condition for which biologics are approved on or before the index date; continuous health plan enrollment during the 6-month washout period, 6-month baseline period, and 12-month study period; and who were ≥18 years old as of the index date. Patients were excluded if they had ≥2 medical claims with a diagnosis of ulcerative colitis at any time or if they did not have continuous health plan enrollment during the grace period.
Study cohorts
Patients were stratified into the following cohorts based on observed indicators of treatment response:
Suboptimal and optimal treatment cohorts
Patients with ≥1 observed indicator of suboptimal treatment during the study period were classified in the Suboptimal Treatment Cohort. Patients with no indicator of suboptimal treatment during the study period were classified in the Optimal Treatment Cohort.
Indicators of suboptimal treatment were selected based on a review of relevant literatureCitation12,Citation17,Citation18 and expert consensus and included treatment switch, prolonged corticosteroid use (>3 months; a gap of ≤7 days between corticosteroid fills was allowed to maintain the corticosteroid course), intravenous (IV) corticosteroid use, treatment augmentation, and dose escalation (i.e. doubling of maintenance dose) during the study period. Treatment switch was defined as switching to another biologic (≥1 prescription fill or injection for a non-index biologic treatment for CD) during the study period or ≥1 prescription fill for a 5-ASA or an immunomodulator during or after a discontinuation period. A discontinuation period was defined as a period >60 days with no index biologic treatment during the 14-month period following the index date (i.e. study period and grace period). Patients could have more than one observed indicator of suboptimal treatment. Refer to Supplemental Table S1 for a detailed list of indicators of suboptimal treatment.
Treatment switch and no treatment switch cohorts
Patients in the Suboptimal Treatment Cohort with ≥1 observed treatment switch were classified in the Treatment Switch Cohort. Patients in the Suboptimal Treatment Cohort with no observed treatment switch and those in the Optimal Treatment Cohort were classified in the No Treatment Switch Cohort.
Outcomes and measures
All results were reported separately for the Suboptimal and Optimal Treatment cohorts and the Treatment Switch and No Treatment Switch cohorts.
Patient characteristics
Patient characteristics were described and included demographic characteristics (i.e. age, gender, region, health plan type) on the index date and clinical characteristics (i.e. CD severity index score, most frequent comorbidities, and medications) during the baseline period.
HRU and costs
HRU and costs were described for each cohort during the study period. HRU components included all-cause inpatient (IP) admissions, outpatient (OP) visits, and emergency department (ED) visits. Cost components were evaluated from a payer’s perspective and included total costs (i.e. medical and pharmacy), medical costs (i.e. IP, OP, and ED costs), and pharmacy costs. Costs were adjusted for inflation using the medical care component of the US Consumer Price Index and reported in 2020 US dollars.
Statistical analysis
Entropy balancing was used to balance the characteristics of patients included in the Suboptimal Treatment Cohort and the Optimal Treatment Cohort. Patients included in the Optimal Treatment Cohort were reweighted such that specified characteristics had the exact same mean and standard deviation (SD) as those in the Suboptimal Treatment Cohort. Characteristics used to balance cohorts included demographics (i.e. age, gender, region, and health plan type) and clinical characteristics (i.e. number of months from first CD diagnosis to index date, Charlson Comorbidity Index [CCI] score, CD severity index scoreCitation19, CD location, CD severity-related comorbidities, use of selected medications).
Descriptive statistics were presented, consisting of means, SD, and medians for continuous variables and frequency counts and percentages for categorical variables. Absolute standardized differences (aSD) were reported for differences in characteristics between each cohort. All measures were reported before and after balancing for the Suboptimal Treatment Cohort and the Optimal Treatment Cohort. To address the secondary objective, all measures were reported for the Treatment Switch Cohort (i.e. subgroup of the Suboptimal Treatment Cohort) and the No Treatment Switch Cohort using the aforementioned weights derived using entropy balancing.
Unadjusted weighted generalized linear models (GLMs) were used to assess differences in HRU and costs between cohorts. GLMs with negative binomial family and log link were used to compare the rate of HRU visits/days between cohorts. GLMs with binomial family and logit link were used to compare the odds of incurring any HRU visits between cohorts. GLMs with gamma family and log link were used to compare the mean differences in costs between cohorts. P-values were presented alongside descriptive statistics.
All analyses were conducted using the Statistical Analysis System (SAS) Enterprise Guide, Version 7.1 (SAS, Cary, North Carolina, USA) and Stata, Version 15.1 (StataCorp LLC, College Station, Texas, USA).
Results
Demographic and clinical characteristics
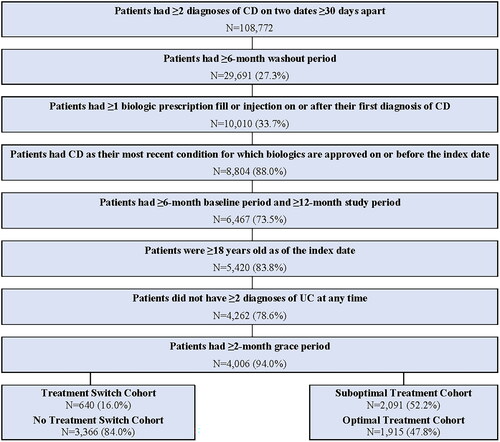
A total of 4,006 patients with CD met all eligibility criteria and were included in the study (). This included 2,091 (52.2%) patients in the Suboptimal Treatment Cohort and 1,915 (47.8%) patients in the Optimal Treatment Cohort. Among all patients included in the study, 640 (16.0%) were included in the Treatment Switch Cohort and 3,366 (84.0%) were included in the No Treatment Switch Cohort. Patient characteristics for all cohorts are presented in .
Table 1. Patient characteristics.
Suboptimal and optimal treatment cohorts
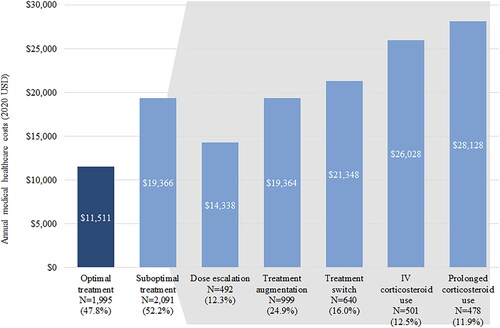
The most commonly observed indicators of suboptimal treatment were treatment augmentation (24.9%) followed by treatment switch (16.0%), IV corticosteroid use (12.5%), dose escalation (12.3%), and prolonged corticosteroid use (11.9%) ().
Figure 3. Annual medical costs by indicator of suboptimal treatment, 2020 USD.
Abbreviations: IV, Intravenous; USD, United States dollar.
Note: All suboptimal treatment costs are significantly higher (p < 0.01) than optimal treatment costs.
In both the Suboptimal and Optimal Treatment cohorts, after balancing, the mean age was 38.9 years, 48.4% of patients were female, and the mean time from first CD diagnosis to the index date was 4.6 months. Among both cohorts, the most common (>15%) CD severity-related comorbidities were pain (40.8%), diarrhea (25.3%), and cardiovascular disease (18.0%). Corticosteroids were the most common medication among patients in both cohorts (39.8%), followed by antibiotics (18.9%), 5-ASA (11.8%), and immunomodulators (7.8%).
Treatment switch and no treatment switch cohorts
In the Treatment Switch Cohort compared to the No Treatment Switch Cohort, mean age was similar (38.9 years vs 39.6 years, respectively), and a numerically higher proportion of patients were female (58.4% vs 49.5%, respectively). The mean time from first CD diagnosis to the index date was longer for patients in the Treatment Switch Cohort (5.7 months) than for patients in the No Treatment Switch Cohort (4.7 months). The most common (>15%) CD severity-related comorbidities were pain (Treatment Switch Cohort: 50.5%; No Treatment Switch Cohort: 44.4%), diarrhea (Treatment Switch Cohort: 35.9%; No Treatment Switch Cohort: 28.3%), and cardiovascular disease (Treatment Switch Cohort: 21.4%; No Treatment Switch Cohort: 21.3%). Among patients in the Treatment Switch Cohort, corticosteroids were the most common medication, (62.5%), followed by antibiotics (26.9%), 5-ASA (25.9%), and immunomodulators (15.8%). A similar but numerically lower pattern was observed among patients in the No Treatment Switch Cohort, with the most common medications being corticosteroids (47.0%), antibiotics (22.6%), 5-ASA (20.2%), and immunomodulators (17.4%).
Impact of suboptimal treatment on HRU and costs
Patients with an indicator of suboptimal treatment had significantly higher rates of HRU compared to patients with optimal treatment. Patients in the Suboptimal Treatment Cohort had higher rates of IP admissions (18.4% vs 10.7%; p < 0.01), ED visits (38.7% vs 33.3%; p < 0.01), and number of OP visits (19.2 vs 14.6; p < 0.01) compared with patients in the Optimal Treatment Cohort ().
Table 2. All-cause HRU and costs associated with suboptimal treatment incurred during the study period.
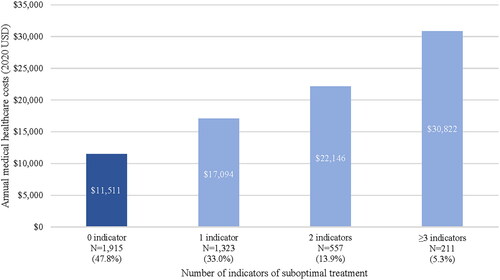
Similarly, costs were significantly higher among patients with indicators of suboptimal treatment compared to patients with optimal treatment. The Suboptimal Treatment Cohort incurred significantly higher total annual all-cause costs than the Optimal Treatment Cohort ($92,043 vs $73,764; p < 0.01; ), which was driven by both medical costs ($19,366 vs $11,511; p < 0.01) and pharmacy costs ($72,677 vs $62,253; p < 0.01). Medical costs varied by suboptimal indicator and were highest in patients who experienced prolonged corticosteroid use ($28,128), IV corticosteroid use ($26,028), and treatment switch ($21,348) (). Additionally, increases in the number of suboptimal treatment indicators were associated with increases in annual medical costs (1 indicator: $17,094; 2 indicators: $22,146; 3+ indicators: $30,822; ).
Impact of treatment switch on HRU and costs
Results among patients with a treatment switch were consistent with the findings above, highlighting the association between treatment switching and higher rates of HRU and costs. Patients in the Treatment Switch Cohort had higher rates of IP admissions (25.9% vs 12.6%; p < 0.01), ED visits (41.6% vs 35.4%; p < 0.01), and number of OP visits (22.8 vs 17.0; p < 0.01) compared with patients in the No Treatment Switch Cohort (). Additionally, total annual all-cause costs were higher among patients in the Treatment Switch Cohort than the No Treatment Switch Cohort ($95,689 vs $81,027; p < 0.01; ), which was driven by both medical costs ($24,135 vs $14,416; p < 0.01) and pharmacy costs ($71,554 vs $66,611; p < 0.01).
Table 3. All-cause HRU and costs associated with treatment switch incurred during the study period.
Discussion
In this real-world retrospective cohort study, indicators of suboptimal treatment were observed in over half of biologic-treated patients with CD. Rates of HRU, including IP admissions, ED visits, and OP visits, as well as total annual all-cause costs were significantly higher in patients with suboptimal treatment than those with optimal treatment. Furthermore, increases in the number of suboptimal treatment indicators were associated with increasing annual medical costs. A substantial proportion of patients with suboptimal treatment had a treatment switch in the 12 months following biologic initiation. These patients experienced significantly higher rates of HRU and incurred higher all-cause costs compared with those who did not switch treatment, suggesting that treatment switch may be an important contributor to the overall HRU and cost burden observed.
The finding that indicators of suboptimal treatment are common among patients with CD is consistent with prior studiesCitation12,Citation13,Citation15,Citation18. A US claims analysis found that the proportion of patients with CD who had ≥1 suboptimal treatment indicator within 6 months of biologic initiation was 54.3%, and this proportion increased to 91.4% in 36 monthsCitation12. Two other US claims analyses found approximately 80% of patients with CD had ≥1 suboptimal treatment indicator within 12 monthsCitation15,Citation18; this proportion is higher than the current observation of 52.2%, partially because of the additional indicators considered in those studies that included surgery, hospitalizations, and other HRU. Surgery was not considered as an indicator of suboptimal treatment in the current study as early surgical intervention has been shown to improve clinical outcomes for patients with CD and reduce HRU and costs in the long termCitation20.
The suboptimal treatment indicators considered in this study, including treatment switch, augmentation, dose escalation, and corticosteroid use, could be regarded as indicators of real-world treatment effectiveness and safety in CD, as treatment changes often occur to improve response or mitigate side effectsCitation12,Citation15,Citation21,Citation22. The current finding demonstrating higher rates of HRU among patients with suboptimal treatment indicators compared to those with optimal treatment may therefore be partially attributed to poorer clinical response and the need for more intensive care, consequently leading to increased costs. Previous research has shown that healthcare costs tend to be higher among patients with CD who had suboptimal treatment after biologic or conventional therapy initiationCitation15, which is consistent with the current observation. The current study adds to the literature by demonstrating a statistically significant increase in both HRU and costs specifically associated with suboptimal biologic treatment as well as a statistically significant increase in costs associated with an increasing number of suboptimal treatment indicators, providing additional context to the economic burden associated with suboptimal treatment in patients with CD in the US. Although this study evaluated direct costs from a payer’s perspective, indirect costs which consider the economic burden from a societal perspective may also be higher among patients with suboptimal biologic treatment and warrant additional investigation.
While prior studies have described optimal biologic treatment sequences and evaluated the real-world HRU and cost outcomes of treatment switching associated with specific therapies for CD, particularly among patients refractory to anti-tumor necrosis factor treatmentCitation23–27, data on HRU and costs related to treatment switch in general among patients with CD have been limited. The current study assessed treatment switch across a range of CD treatments, and findings support that switching from a first-line biologic treatment is associated with increases in HRU and costs. Further studies are warranted to better understand the HRU and costs associated with different treatment modalities, sequences, and timing among patients with CD. Notably, reasons for a treatment switch in CD may include medical reasons, such as lack of response, disease progression, or treatment side effectsCitation3, as well as non-medical reasons, such as formulary changes or preference for lower-cost therapiesCitation28,Citation29. Regarding non-medical treatment switching, a study conducted by the Institute for Patient Access that included patients with CD reported that this type of treatment switching disrupted patient care and resulted in higher downstream medical costs associated with IP, OP, and ED visitsCitation28,Citation30. Moreover, surveys have found that a majority of physicians and patients believe non-medical switching negatively affects some aspects of care, including reduced effectiveness, complications, and lowered adherence with the newly switched treatmentCitation28,Citation29,Citation31. More research is needed to evaluate the impact of demographics (e.g. age, sex, socioeconomic status) on non-medical switching and its effects on the long-term clinical and economic outcomes among patients with CD in the US.
Together, the current findings highlight that suboptimal treatment, including treatment switching from an initial biologic, poses a burden on healthcare systems. The availability of more effective and safe treatment options for patients with CD may potentially result in more optimal treatment responses and alleviate some of the HRU and cost burden associated with suboptimal treatment. Although recent data support a “top-down” approach for the treatment of CDCitation20,Citation32, our real-world study shows a low use of biologics and high use of corticosteroids, highlighting the potential to improve current disease management practices. In addition, the study findings may also help raise awareness about the negative consequences associated with suboptimal treatment, including treatment switching, on patient care and outcomes, which should be considered by stakeholders when formulating treatment decisions and reimbursement policies. This study assessed biologics as a treatment class; further research could evaluate whether there are differences between individual biologics with respect to the clinical and economic impact of suboptimal treatment.
The findings of this study should be interpreted with several limitations. First, information reported was limited to that available in the commercial claims database, which did not include clinical information. As such, treatment patterns could only be defined based on the timing of claims of CD-related agents. Second, this study is subject to inherent limitations of claims-based studies such as billing inaccuracies and missing data. Third, this study was conducted among patients with commercial insurance; hence, the results may not be representative of the general CD population in the US or patients who have public or no health insurance. Fourth, this study focused on adult patients with CD newly treated with a biologic; therefore, characteristics of treated and untreated adults with CD could not be compared. Fifth, reasons for treatment changes in this study could not be evaluated. As such, the indicators for suboptimal treatment may have been misidentified. For example, treatment augmentation or corticosteroid use can occur for several reasons and may not always be an indicator of suboptimal treatment. Future research evaluating the reasons underlying treatment decisions (e.g. switch, discontinuation, augmentation, prolonged use of corticosteroids) among patients with CD in the US is warranted. More research is also needed to better understand the economic impact of treatment switch vs other treatment changes as a result of suboptimal treatment response.
Conclusions
Among adult patients with CD newly treated with a biologic, patients who experienced suboptimal treatment, including treatment switch, in the 12 months following initiation of a first biologic agent had significantly higher rates of HRU and higher costs compared to patients with optimal treatment. Treatment switch was a frequently observed indicator of suboptimal treatment and was independently associated with significantly higher HRU and costs compared to patients without a treatment switch. These findings highlight that suboptimal treatment, including treatment switch, continue to occur frequently in patients with CD and are associated with a substantial burden on healthcare systems, underscoring the need to optimize treatment strategies and improve patient outcomes in CD. Randomized head-to-head studies are needed to determine whether optimized or more effective therapies can truly reduce costs, which would eliminate patient variables affecting treatment outcomes.
Transparency
Declaration of funding
This study was funded by Janssen Scientific Affairs, LLC.
Declaration of financial/other relationships
PGS, AG, and MC are employees of Analysis Group, Inc., a consulting company that provided paid consulting services to Janssen Global Services, LLC, for the conduct of this study. MD was an employee of Analysis Group, Inc. at the time of the study. MS, SK, and DN are employees of the Janssen Pharmaceutical Companies of Johnson & Johnson. TH was an employee of the Janssen Pharmaceutical Companies of Johnson & Johnson at the time this study was conducted. CW is a consultant for Goldfinch Biotech Inc. and Otsuka Pharmaceutical, and a scientific advisor or member of the Journal of Clinical Therapeutics, Editorial Board.
Author contributions
PGS, MD, AG, and MC contributed to the study conception and design, collection and assembly of data, and data analysis and interpretation. MS, CW, SK, TH, and DN contributed to the study conception and design, data analysis and interpretation. All authors reviewed and approved the final content of this manuscript.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Ethical approval
Data were de-identified and comply with the patient requirements of the Health Insurance Portability and Accountability Act (HIPAA) of 1996; therefore, no review by an institutional review board was required per Title 45 of CFR, Part 46.101(b)(4) (https://www.hhs.gov/ohrp/regulations-and-policy/regulations/45-cfr-46/#46.101).
Previous presentations
Results were presented as separate posters at the American College of Gastroenterology (ACG) 2022 meeting in Charlotte, NC, USA and at the Digestive Disease Week (DDW) 2023 conference in Chicago, IL, USA.
Supplemental Material
Download MS Word (17.3 KB)Acknowledgements
Medical writing support was provided by Cody Patton, BSc, an independent consultant working on behalf of Analysis Group, Inc., as well as Flora Chik, PhD, MWC, and Loraine Georgy, PhD, MWC, employees of Analysis Group, Inc., a consulting company that has received research grants from Janssen Scientific Affairs, LLC, to conduct this study.
Data availability statement
The data that support the findings of this study are available from MarketScan, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Any researchers interested in obtaining the data used in this study can access the database through MarketScan, under a license agreement, including the payment of appropriate license fee.
References
- Roda G, Chien Ng S, Kotze PG, et al. Crohn’s disease. Nat Rev Dis Primers. 2020;6(1):22.
- Lichtenstein GR, Loftus EV, Isaacs KL, et al. ACG Clinical Guideline: management of Crohn’s disease in adults. Am J Gastroenterol. 2018;113(4):481–517.
- GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5(1):17–30. doi: 10.1016/s2468-1253(19)30333-4.
- Xu F, Dahlhamer JM, Zammitti EP, et al. Health-risk behaviors and chronic conditions among adults with inflammatory bowel disease - United States, 2015 and 2016. MMWR Morb Mortal Wkly Rep. 2018;67(6):190–195.
- Xu F, Carlson S, Liu Y, et al. Prevalence of inflammatory bowel disease among medicare fee-for-service beneficiaries - United States, 2001–2018. MMWR Morb Mortal Wkly Rep. 2021; 70(19):698–701. doi: 10.15585/mmwr.mm7019a2.
- Ganz ML, Sugarman R, Wang R, et al. The economic and health-related impact of Crohn’s disease in the United States: evidence from a nationally representative survey. Inflamm Bowel Dis. 2016;22(5):1032–1041. doi: 10.1097/mib.0000000000000742.
- Lichtenstein GR, Shahabi A, Seabury SA, et al. Lifetime economic burden of Crohn’s disease and ulcerative colitis by age at diagnosis. Clin Gastroenterol Hepatol. 2020;18(4):889–897.e10.
- Manceur AM, Ding Z, Muser E, et al. Burden of Crohn’s DIsease in the United States: long-term healthcare and work-loss related costs. J Med Econ. 2020;23(10):1092–1101.
- Sulz MC, Burri E, Michetti P, et al. Treatment algorithms for Crohn’s Disease. Digestion. 2020;101(Suppl 1):43–57.
- Dunleavy KA, Pardi DS. Biologics: how far can they go in Crohn’s disease? Gastroenterol Rep . 2022;10:goac049.
- Samaan M, Campbell S, Cunningham G, et al. Biologic therapies for Crohn’s disease: optimising the old and maximising the new. F1000Res. 2019;8:8.
- Patel H, Lissoos T, Rubin DT. Indicators of suboptimal biologic therapy over time in patients with ulcerative colitis and Crohn’s disease in the United States. PLOS One. 2017;12(4):e0175099.
- Khan S, Rupniewska E, Neighbors M, et al. Real-world evidence on adherence, persistence, switching and dose escalation with biologics in adult inflammatory bowel disease in the United States: a systematic review. J Clin Pharm Ther. 2019;44(4):495–507.
- Perry J, Chen A, Kariyawasam V, et al. Medication non-adherence in inflammatory bowel diseases is associated with disability. Intest Res. 2018;16(4):571–578.
- Pilon D, Ding Z, Muser E, et al. Indicators of suboptimal treatment and associated healthcare costs among patients with Crohn’s disease initiated on biologic or conventional agents. Crohns Colitis 360. 2022;4(3):otac021.
- Merative™ MarketScan® Research Databases. 2023. Available from: https://www.merative.com/documents/brief/marketscan-explainer-general.
- Perera S, Yang S, Stott-Miller M, et al. Analysis of healthcare resource utilization and costs after the initiation of biologic treatment in patients with ulcerative colitis and Crohn’s disease. J Health Econ Outcomes Res. 2018;6(1):96–112.
- Rubin DT, Mody R, Davis KL, et al. Real-world assessment of therapy changes, suboptimal treatment and associated costs in patients with ulcerative colitis or Crohn’s disease. Aliment Pharmacol Ther. 2014;39(10):1143–1155.
- Chen G, Lissoos T, Dieyi C, et al. Development and validation of an inflammatory bowel disease severity index using US administrative claims data: a retrospective cohort study. Inflamm Bowel Dis. 2021;27(8):1177–1183.
- Lee KE, Tu VY, Faye AS. Optimal management of refractory Crohn’s disease: current landscape and future direction. Clin Exp Gastroenterol. 2024;17:75–86.
- Billioud V, Sandborn WJ, Peyrin-Biroulet L. Loss of response and need for adalimumab dose intensification in Crohn’s disease: a systematic review. Am J Gastroenterol. 2011; 106(4):674–684.
- Chen C, Hartzema AG, Xiao H, et al. Real-world pattern of biologic use in patients with inflammatory bowel disease: treatment persistence, switching, and importance of concurrent immunosuppressive therapy. Inflamm Bowel Dis. 2019;25(8):1417–1427.
- Chiorean M, Afzali A, Cross RK, et al. Economic outcomes of inflammatory bowel disease patients switching to a second anti-tumor necrosis factor or vedolizumab. Crohns Colitis 360. 2020;2(2):otaa031.
- Kawalec P, Moćko P. An indirect comparison of ustekinumab and vedolizumab in the therapy of TNF-failure Crohn’s disease patients. J Comp Eff Res. 2018; 7(2):101–111.
- Manlay L, Boschetti G, Pereira B, et al. Comparison of short- and long-term effectiveness between ustekinumab and vedolizumab in patients with Crohn’s disease refractory to anti-tumour necrosis factor therapy. Aliment Pharmacol Ther. 2021;53(12):1289–1299.
- Volkers A, Straatmijer T, Duijvestein M, et al. Real-world experience of switching from intravenous to subcutaneous vedolizumab maintenance treatment for inflammatory bowel diseases. Aliment Pharmacol Ther. 2022;56(6):1044–1054.
- Bressler B. Is there an optimal sequence of biologic therapies for inflammatory bowel disease? Therap Adv Gastroenterol. 2023;16:17562848231159452.
- Alliance for Patient Access. A study of the qualitative impact of non-medical switching. Washington DC: Alliance for Patient Access; 2019.
- Salam T, Duhig A, Patel AA, et al. Physicians’ perspectives regarding non-medical switching of prescription medications: results of an internet e-survey. PLOS One. 2020;15(1):e0225867.
- Institute for Patient Access. Cost-motivated treatment changes & non-medical switching - commercial health plans analysis. Washington DC: Alliance for Patient Access; 2017.
- Costa OS, Salam T, Duhig A, et al. Specialist physician perspectives on non-medical switching of prescription medications. J Mark Access Health Policy. 2020;8(1):1738637.
- Noor NM, Lee JC, Bond S, et al. A biomarker-stratified comparison of top-down versus accelerated step-up treatment strategies for patients with newly diagnosed Crohn’s disease (PROFILE): a multicentre, open-label randomised controlled trial. Lancet Gastroenterol Hepatol. 2024;9(5):415–427. doi: 10.1016/S2468-1253(24)00034-7.