Abstract
Objectives: Biosimilars improve patient access by providing cost-effective treatment options. This study assessed the potential savings and expanded patient access with increased use of two biosimilar disease modifying anti-rheumatic drugs (DMARDs): a) approved adalimumab biosimilars and b) the first tocilizumab biosimilar, representing an established biosimilar field and a recent biosimilar entrant in France, Germany, Italy, Spain, and the United Kingdom (UK).
Methods: Separate ex-ante analyses were conducted for each country, parameterized using country-specific list prices, unit volumes annually, and market shares for each therapy. Discounting scenarios of 10%, 20%, and 30% were tested for tocilizumab. Outputs included direct cost-savings associated with drug acquisition or the incremental number of patients that could be treated if savings were redirected. Two biosimilar conversion scenarios were tested.
Results: Savings associated with a 100% conversion to adalimumab biosimilar ranged from €10.5 to €187 million (UK and Germany, respectively), or an additional 1,096 to 19,454 patients that could be treated using the cost-savings. Introduction of a tocilizumab biosimilar provided savings up to €29.3 million in the most conservative scenario. Exclusive use of tocilizumab biosimilars (at a 30% discount) could increase savings to €28.8 to €113 million or expand access to an additional 43% of existing tocilizumab users across countries.
Conclusion: This study demonstrates the benefits that can be realized through increased biosimilar adoption, not only in an untapped tocilizumab market, but also through incremental increases in well-established markets such as adalimumab. As healthcare budgets continue to face downwards pressure globally, strategies to increase biosimilar market share could prove useful to help manage financial constraints.
Disclaimer
As a service to authors and researchers we are providing this version of an accepted manuscript (AM). Copyediting, typesetting, and review of the resulting proofs will be undertaken on this manuscript before final publication of the Version of Record (VoR). During production and pre-press, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal relate to these versions also.Introduction
The introduction of biologic disease-modifying anti-rheumatic drugs (bDMARDs) was a key advancement in the autoimmune treatment space [1]. Several bDMARDs are clinically proven to significantly reduce disease activity and improve quality of life; these include adalimumab, a TNFα inhibitor and tocilizumab, an IL-6 inhibitor [1-3]. Adalimumab and tocilizumab help to overcome the limitations of classic autoimmune therapies including conventional DMARDs (eg, methotrexate and leflunomide) and glucocorticoids. Several studies have reported methotrexate intolerance among patients with rheumatoid arthritis, including nausea and vomiting, abdominal pain, and behavioral symptoms such as restlessness and irritability [4,5]. Moreover, the long-term use of glucocorticoids has been associated with a high rate of comorbidities such as osteoporosis, osteonecrosis, increased risk of hypertension, arrhythmia, hyperglycemia, and metabolic disorders [6,7]. Although bDMARDs have been shown to be effective treatments, their overall use may not be optimal as the price of reference products may result in a considerable budget impact or a cost-effectiveness ratio higher than willingness-to-pay thresholds, ultimately restricting patient access. Complex economic analyses such as cost-effectiveness analyses or budget-impact assessments are used by health technology assessment bodies across the world to ensure health gains are maximised from available resources [8]. For example, analyses from payer perspectives that included adalimumab and tocilizumab reference products have demonstrated that despite the clinical benefits associated with their use, the high cost of treatment renders them non-cost effective. These cases of negative or restricted reimbursement for high-cost biologics mean physicians lose the opportunity to treat patients with a highly efficacious therapy [9-11]. Moreover, as healthcare spending increases in Europe [12], efficient use of healthcare budgets while maintaining funding for effective treatments is crucial. This is especially true for high-grossing drug therapies, such as adalimumab’s reference product, that has reported a net global revenue of $20.7 billion in 2021 [13]. Therefore, the budget impact of a particular health resource is also important from a payer’s perspective and helps to complement cost-effectiveness analyses by assessing the affordability of resources [14].
Biosimilars can help to improve patient access to bDMARDs by providing a drug with comparable efficacy at a more cost-effective price. The European Medicines Agency (EMA) defines a biosimilar as “a biological medicine highly similar to another already approved biological medicine” [15]. Biosimilars are not synonymous with generics due to their complex manufacturing and the variability of biologics, thus exact replicas of molecular microheterogeneity are not possible [15]. Nevertheless, biosimilars undergo a strict regulatory approval process where manufacturers must provide a body of evidence that demonstrate its comparable efficacy and safety to the reference product [16]. Ten adalimumab biosimilars are currently approved and used throughout Europe (Table 1); whereas, one tocilizumab biosimilar was recently authorised for use by the EMA [17-19]. In addition to demonstrating similar efficacy and safety as the reference product [20,21], several pharmacoeconomic studies also highlight the economic advantages of biosimilar bDMARDs [22,23]. Hesitancy surrounding the use of biosimilars may stem from immunogenicity concerns between biosimilars and reference products, extrapolation of indications for biosimilars, and real-world safety and efficacy [24-26]. However, approved adalimumab and tocilizumab biosimilars have demonstrated similar immunogenicity to reference biologics and the extrapolation of indications is an approved process by the EMA and FDA [25,27]. Moreover, the EMA reports that safety data from 1 million patient-treatment years, demonstrates the safety of biosimilars and supports the use of biosimilars [15]. As such, biosimilars can help to drive competition and improve patient access, thereby addressing several important unmet needs within the autoimmune therapeutic space.
Despite the demonstrated clinical efficacy and cost savings, the uptake of biosimilars has remained low in several markets. On average, biosimilar uptake reaches 40% after its first year of introduction and 60% after the first two years; however, not all biosimilars share the same experience (eg, infliximab biosimilars experienced lower market shares in the EU, only achieving a 40% uptake at 3-years) [28]. For adalimumab biosimilars in Europe, between-country variability also exists. In the EU4 (France, Germany, Italy, and Spain) adalimumab biosimilar market shares ranged from 52% to 84% as of Dec 2022 [29]. In other countries, uptake of biosimilars may be lower for several reasons, including a lack of awareness and educational initiatives for clinicians or healthcare decision makers, and a lack of policies that motivate biosimilar use. Without these initiatives healthcare decision makers may not be aware of the full economic benefits from biosimilars, which can ultimately limit access or use [30,31]. Given that the savings realized from the adoption of biosimilars can be used to treat more patients or redirected to other areas of healthcare [32], strategies aimed at increasing the uptake of biosimilars would also provide societal benefits. Moreover, a tocilizumab biosimilar can provide patients with improved access to a bDMARD that has demonstrated better efficacy than several existing bDMARDs,[33] and/or the opportunity to use tocilizumab as an earlier line of therapy.
The savings provided by biosimilars can be used by various ways in healthcare systems. For example, in one hospital in the UK, the savings associated with the use of biosimilars lead to the funding of an additional nurse specialist time, two administration support roles, and a biological pharmacist [34]. The switching program also provided savings that helped improve patient access to biologics with non-funded indications or who may not have meet reimbursement eligibility criteria [34].
This article performed an ex-ante analysis for five European markets: France, Germany, Italy, Spain, and the United Kingdom (UK) to demonstrate the hypothetical cost-savings that could be realized with an incremental increase in adalimumab biosimilar use and the introduction of a tocilizumab biosimilar. To put into perspective the potential impact of these savings, a second analysis considered the number of patients that could be treated with biosimilars, if the healthcare system chose to redirect economic savings towards biosimilar reimbursement.
Methods
This ex-ante analysis was performed separately for adalimumab and tocilizumab biosimilars in each country. All analyses were conducted from a payer perspective for a cohort of patients currently in need of treatment with adalimumab or tocilizumab. The analyses included a direct cost-savings model and a second model to assess total patients treated. The outputs for the direct-cost savings model provided the simulated direct savings from drug costs based on two biosimilar conversion scenarios (in 2022 Euros). The current analyses use the direct cost-savings along with the annual cost to treat one patient with adalimumab or tocilizumab biosimilars to provide the incremental (ie, additional) number of patients that could be treated with 1-year of adalimumab or tocilizumab with the redirected funds. The savings per patient was calculated from dividing the overall savings of each scenario by the panel size for the country. Similar ex-ante analyses have been used to estimate the forward-looking cost-effectiveness of biosimilars in other therapeutic areas such as pegfilgrastim for the treatment of febrile neutropenia in the United States [35,36].
Model Inputs
The population sizes for each analysis were calculated based on the total volume of adalimumab and tocilizumab unit sales in 2022 [37]. The EMA has approved the reference product for adalimumab for the treatment of rheumatoid arthritis, plaque psoriasis, psoriatic arthritis, axial spondylarthritis, Chron’s disease, ulcerative colitis, polyarticular juvenile idiopathic arthritis, enthesitis-related arthritis, hidradenitis suppurativa and non-infectious uveitis [38]. The reference product for tocilizumab is approved to treat rheumatoid arthritis, systematic and polyarticular juvenile idiopathic arthritis, giant cell arteritis, cytokine release syndrome, and COVID-19 [39]. Total subcutaneous (SC) unit sales were divided by 26 for the adalimumab model (based on biweekly dosing), and 52 for the tocilizumab model (assumption of weekly dosing), which aligns with the dosing guidelines for the majority of the adalimumab and tocilizumab indications, respectively [4,5]. Total intravenous (IV) unit sales were also incorporated into the tocilizumab panel size calculations. An average weight of 80kg across all countries was applied to estimate annual drug costs. Based on an 8 mg/kg dose, 640 mg of tocilizumab would be required for treatment. Two percent of unit sales were assumed to be ‘one time’ use for patients with COVID-19 and/or cytokine release syndrome, with the remaining unit sales divided by 13 to approximate a 4-week dosing schedule (based on dosing recommendations for rheumatoid arthritis, the most common indication for tocilizumab use) [39]. Calculated panel sizes are shown in Tables 1 and 2 for adalimumab and tocilizumab, respectively.
The adalimumab direct cost-saving model utilized the published list price for the reference product [40], with weighted-average list prices for biosimilar options [40], calculated based on market share data specific to each country. Base case market shares were informed by IQVIA 2022-year-end adalimumab biosimilar market share data. Two biosimilar conversion scenarios were tested. The more conservative scenario assumed biosimilar market share increased from current-day values to the mid-point between current day and 100%. For example, in France the difference in current-day market share and 100% was 48% (100% - 52%), this was divided by 2 (24%) and added to the current day-value which resulted in a market share of 76%. The less conservative scenario tested the complete conversion of reference product adalimumab to biosimilars. Table 2 summarizes the adalimumab model inputs.
As tocilizumab biosimilars are not currently available in any market, the biosimilar price was estimated as a price discount relative to the reference product. The annual cost of the reference product was calculated as a weighted average based on the percentage of patients treated with SC and IV tocilizumab [37,40]. Three discount scenarios (20%, 30%, and 40%) were tested to approximate historical understandings of price reductions as multiple biosimilars enter the market. The base case scenario is in-line or slightly higher than published values for some countries, [41,42] but may be more representative of the real-world due to confidential agreements between manufacturers and healthcare decision makers. Furthermore, several of these markets are tender driven, which typically see deeper discount rates, as manufacturers seek to provide an optimal price to obtain the tender. The base case biosimilar market shares for the tocilizumab model were set to 0% to reflect the current real-world. Similar to the adalimumab model, two biosimilar conversion scenarios were tested; the first assumed biosimilar market share equal to the current-day market share values for adalimumab biosimilars, as it represents a mature biosimilar with established competition. The second scenario reported the savings achieved from a 100% conversion from reference tocilizumab to biosimilar tocilizumab. Table 3 summarizes the tocilizumab model inputs.
Assumptions
Both models assume clinically equivalent efficacy and similar safety and immunogenicity between the reference products and biosimilars, as demonstrated by published data on adalimumab and tocilizumab biosimilars [20,21]. Annual drug costs were calculated using published list prices, with a conservative dosing assumption of 40 mg biweekly SC adalimumab, 162 mg weekly SC tocilizumab, and 8mg/kg every four weeks for IV tocilizumab, with equivalent dosing for both the reference product and biosimilars. Moreover, only direct medication costs were included in the models, as healthcare resource utilization (eg, administration costs) and indirect costs (eg, lost productivity, travel) are expected to be the same between reference products and biosimilars.
Sensitivity Analysis
When biosimilars enter the market, manufacturers of the reference products may offer rebates to payers to maintain their current market share. Although information is proprietary and unavailable in the public domain, rebates offered may be as high as 50% for some markets and biologics [43,44]. Sensitivity analyses were conducted for the adalimumab and tocilizumab models to capture the direct cost savings following reference product rebating. The sensitivity analyses also assumed additional discounting for biosimilars, as manufacturers of biosimilars would need to remain competitive in the context of reference product rebating and discounting during confidential pricing negotiations. In the adalimumab model it is assumed that the reference product and biosimilar would be rebated/discounted by 50% to reflect the high competition between the reference product and biosimilars. In the tocilizumab model, where biosimilars are only recently entering the market and competition is low, the sensitivity analysis tested a price reflecting a 20% rebate for the reference product, and 15% for the biosimilar.
Results
Annual Drug Costs
The cost for one year of treatment with reference-product adalimumab ranged from €6,340 in France to €19,051 in Germany, according to list price. Comparatively, the cost for one-year of treatment with adalimumab biosimilars ranged from €5,404 in France to €11,815 in Spain, after weighting biosimilar prices by market share in each region. For tocilizumab, annual drug cost based on list price ranged from €10,513 in France to €19,801 in Germany. Calculated annual drug costs are presented in Tables 2 and 3.
Adalimumab Models
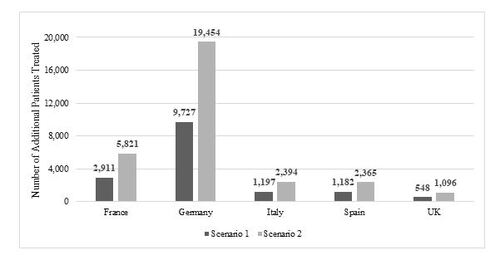
The cost savings associated with increased use of adalimumab biosimilars and the number of additional patients that can be treated using the redirected funds are presented in Table 4 and Figure 1, respectively. Looking at the region with the lowest drug cost and lowest current market share (France, 52%), increasing the biosimilar market share to 76% resulted in estimated savings of €15.7 million, or an additional 2,911 patients treated. Meanwhile, conversion to 100% biosimilar use provided drug cost savings as high as €31.5 million in France, or an additional 5,821 patients treated with adalimumab. In Germany, the region with the highest discounting, the low-conversion scenario (78% to 89%) resulted in a savings of €93.5 million, or an additional 9,727 patients treated. Exclusive use of biosimilars provided over €186 million in savings, or an additional 19,454 patients treated. The UK was the region with the highest adalimumab biosimilar utilization at baseline (90%). Nevertheless, an increase to 95% or 100% market share was still projected to provide an additional €5.3 to €10.5 million in savings annually, alternatively an additional 548 to 1,096 patients. Although adalimumab use in Italy and Spain was considerably lower adalimumab use (approximated population sizes of 40,000 and 60,000, respectively), full conversion to adalimumab biosimilars provided €24.2 million and €27.9 million, respectively, or an additional 2,394 and 2,365 patients. The savings per patient treated in scenario one ranged from €53 in the UK to €1,033 in Germany, while France, Italy, and Spain had similar savings per patient (€225, €303, and €233, respectively). Exclusive use of adalimumab biosimilars created €105 to €2,077 savings per patient (in the UK and Germany, respectively). In the sensitivity analysis, that includes a 50% rebate/discount to the adalimumab reference product and biosimilar price, the savings were €7.9, €46.7, €6.1, €7.0, and €2.6 million in France, Germany, Italy, Spain, and UK respectively.
Tocilizumab Models
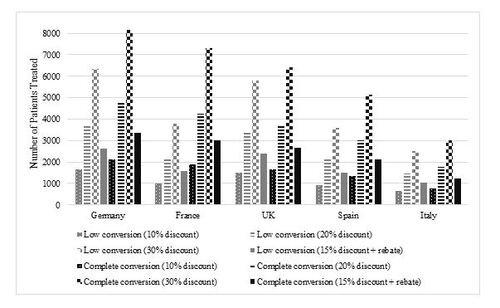
The cost savings associated with the introduction of tocilizumab biosimilars and the number of additional patients that can be treated using the redirected funds are presented in Table 5. Similar to the adalimumab model, France was the region with the lowest drug cost. The most conservative scenario of 52% market share and 10% discounting resulted in a savings of over €9.3 million, or 982 additional patients treated (6% increase in access). Exclusive use of adalimumab biosimilar, with a 30% discounting created €53.6 million in savings, or an additional 7,286 patients treated (43% increase). In Germany, the region with the largest panel size, a 10% discount led to a savings of €29.3 million or an additional 1,647 patients (9% increase) treated at a market share of 78%, savings were as high as €113 million (or 8,143 patients, representing a 43% increase over current day) in the 100% conversion, high-discount scenario. The UK had the highest biosimilar market share in the first conversion scenario, at 90%. A 10% discount relative to reference product yielded savings of €17.1 million or 1,500 additional patients treated (10% increase). Increasing the market shares to 100% at 30% discounting, provided €56.9 million in savings, or an additional 6,429 patients treated (43% increase in access). Spain and Italy had the smallest population sizes for the tocilizumab model (12,000 and 7,000 respectively), however, even here, the introduction of a biosimilar at 10% discounting and market share conversion of 70% and 84%, respectively, could allow an 8% and 9% increase in patient access to the therapy. In the most conservative scenario, the saving per patient ranged from €547 in France to €1,544 in Germany. In the UK, Spain, and Italy, the savings per patient were approximately €1,000 (€1,138, €964, and €1,152, respectively). Complete conversion to tocilizumab biosimilars at a 30% discount were estimated to yield €3,154 to €5,940 savings per patient in France and Germany, while savings per patient in UK, Spain, and Italy surpassed €3,000.
In the sensitivity analysis (Table 5), the rebates for the tocilizumab reference product and biosimilar were assumed to be 20% and 15%, respectively. In France, the direct savings from tocilizumab biosimilar use in scenario 1 (52% of market share) and scenario 2 (exclusive use of a biosimilar) created €11.1 million (€656 savings per patient) and €21.4 million (€1,262 savings per patient) in savings. In Germany, the savings associated with a 78% market share of tocilizumab biosimilar were €35 million with a savings per patient of €1,853. Exclusive use of a tocilizumab biosimilar yielded €45 million of savings with a savings per patient of €2,376. In the UK, a 90% of biosimilar market share provided €20.5 million (€1,366 savings per patient) and the exclusive use of a tocilizumab biosimilar provided €22.8 million (€1,518 savings per patient). In Spain and Italy, the savings per patient in scenario 1 (70% and 84% market share, respectively) was €1,157 and €1,382, respectively. Similar savings per patient were also observed when a tocilizumab biosimilar was used exclusively (€1,653 and €1,645, respectively).
Discussion
Healthcare budgets are facing constant downwards pressure due to rising expenses such as those associated with a growing, aging population or innovative medicines. Biosimilar medicines not only provide effective and safe treatment options, but also represent an important tool for managing healthcare budgets, through providing direct cost-savings and/or increased biologic use from the redirected funds. This study demonstrates the potential of incremental increases in adoption of more affordable adalimumab biosimilars, using the current real-world situation as a basis to project the economic benefit that has yet to be realized in various markets. This study also provides novel data on the projected economic benefit that could be realized from the introduction of a tocilizumab biosimilar. This is particularly important given that, despite considerable therapeutic advantages in effectiveness and tolerability, the reference product tocilizumab has faced reimbursement restrictions in some countries [45,46].
This analysis aligns with existing published economic analyses demonstrating the high cost associated with reference product bDMARDs, and the potential savings that can be realized through increased use of biosimilars [22,47]. A 2021 analysis of biosimilars from nine therapeutic areas found that the use of biosimilars led to €5.7 billion in list price savings across Europe [28]. In Canada the introduction of filgrastim, infliximab, and insulin glargine biosimilars provided a savings of $46.2 million (CAD) at a market share of 4.22%; in scenario analysis, an increase to market shares of 50% or 100% could have reached savings over $500 million and $1 billion, respectively [48]. For the United States, the projected savings from adalimumab biosimilars upon market entry (2023-2025) was $19.5 billion USD which corresponded to 50.8% of the total savings from new biosimilars in 2021 to 2025 [49].
The cost-saving model demonstrated that significant savings can be realized by the increased use of adalimumab biosimilars and the introduction of a tocilizumab biosimilar in France, Germany, Italy, Spain, and the UK. The total of savings for each country varied based on the patient population size which was influenced by the current number of therapy users in each country, as well as the current market share and extent of discounting by region. The largest savings in the adalimumab model were seen in Germany which was influenced by the considerable difference in list price between the reference product and weighted adalimumab biosimilars (∼50% lower). Comparatively, the smallest projected economic savings were seen in France under the low-conversion scenario, due to low drug costs overall and a low initial market share across a large panel of patients, however, these savings actually yielded an increase in the number of patients treated compared to other regions that had higher annual treatment costs and lower discounts, such as Spain. The analysis from the German perspective also demonstrated the largest direct savings in the tocilizumab model, for both the low and high scenarios, attributed to the large cohort size and high price of reference product tocilizumab.
The economic savings provided by the increased use of more affordable adalimumab and tocilizumab biosimilars can be used to increase the number of patients treated with these powerful disease-modifying therapies, increasing the total number of patients able to receive treatment and its associated benefits. In the adalimumab model, an additional 548 to 19,454 patients could be treated across both market share conversion scenarios. In the tocilizumab model, the number of additional patients ranged from 6%-10% in the most conservative scenario (France; UK; 10% discount), to a 43% increase in patients receiving therapy for all countries (at 100% conversion and 30% discounting). Moreover, the sensitivity analysis demonstrated that meaningful savings could be realized even with the additional rebates and discounting for reference products and biosimilars.
The real-world benefits of increased biosimilar use have been documented elsewhere [50]. For example, in Norway, the introduction of biosimilars increased competition within the national tender system for biologics. Based on data from the Norwegian Hospital Procurement Trust, access to biologics for patients has improved substantially (eg, increased 100% for infliximab and >200% for adalimumab) after introduction of biosimilars into the market [50]. The paper noted that even with additional patients using biologics, savings of $80 million was still observed [50]. This was also seen with the National Institute for Health and Care Excellence, which provides evidence-based reimbursement recommendations to the UK. Adalimumab along with other biologics, including tocilizumab, were originally recommended for reimbursement for patients with severe rheumatoid arthritis [45]. After biosimilar introduction, the recommendation was updated to include patients with moderate rheumatoid arthritis; further expanding patient access [51]. The introduction of a tocilizumab biosimilar could help to relax reimbursement restrictions, thereby providing patients with moderate rheumatoid arthritis additional treatment options.
Alternatively, if a country has already reached optimal prescription levels, providing adalimumab and tocilizumab to those in need, the savings associated with the use of biosimilars could instead be redirected to other areas within the healthcare system [52]. This is known as “benefit-sharing”, where the cost savings associated with the use of biosimilars are to be reinvested back into the healthcare system, specifically to the healthcare teams or clinical groups involved. Several examples can be seen currently throughout Europe [52]. Rheumatoid arthritis, the primary indication for both adalimumab and tocilizumab, is a well-documented example of a disease area where patients have benefited from the introduction of biosimilars. An example of the “benefit-sharing” model used for patients with rheumatoid arthritis was demonstrated in the UK [34] In 2016, the York Trust Rheumatology Service investigated the launch of an etanercept biosimilar in a switch cohort of 337 patients. Projections forecasted that a 50% gain in market share could yield a 2-year savings of ₤1.64 million [34]. After patients were switched to the biosimilar, the savings were used to fund additional nurse specialist time, two administration support roles and a biological pharmacist, [34] highlighting the benefit potential of biosimilars beyond patient access.
Several factors influence the uptake of biosimilar market share in Europe, including policies allowing pharmacists to substitute a biosimilar, and policies encouraging switching patients from a reference product to a biosimilar (seen more frequently in Europe compared to the United States due to the lack of an interchangeability designation in the EMA regulatory process) [53]. Moreover, physicians may also receive incentives for using biosimilars. For example, in France the savings from switching patients to the reference product are distributed such that a portion (∼30%) go to the prescribers and the remaining savings to the national Health Insurance [54]. Although numerous studies have demonstrated the economic benefits associated with increased uptake in biosimilars market share, reference product biologics maintain a strong foothold in the market, as evidenced by IQVIA data (Table 2). From a payer perspective, aggressive rebating from reference product manufacturers may also reduce the use of biosimilars as the reference product is prescribed at a lower price due to biosimilar competition. In clinical practice, hesitancy surrounding biosimilars can be caused by the nocebo effect which occurs when there is a loss of effectiveness or presence of adverse events due to a patient’s negative expectations of the treatment [55]. Physicians and pharmacists in certain regions may also have limited information regarding the long-term efficacy and safety of biosimilars, and knowledge gaps related to biosimilar development [56]. Therefore, clinicians or patients, if given the choice, may choose to not switch to biosimilars. Therefore, continued education by key stakeholders (eg, manufacturers and nationally recognized medical societies or health economic institutions) and the implementation of policies or targets by payers could help to facilitate the increased use of biosimilars.
Although drug acquisition costs and increased patient access are critical considerations for health-economic decision-makers, several other factors could influence the mandate to support a switch to biosimilars. These may include regionally-specific factors such as the set-up of the healthcare system (national versus regional/local), or criteria topical for the present day, such as sustainable manufacturing or more recently, due to the coronavirus pandemic, supply chain assurances and efficiencies [57,58]. Similarly, to reduce barriers to adoption, biosimilar manufacturers should ensure that sufficient support programs are made available to physicians and patients, particularly educational supports to communicate the comparable clinical and safety outcomes [31].
This study has several notable strengths. Firstly, these analyses were conducted from a payer perspective of five countries, with pricing, market share, and population size all varying according to the region, helping to improve the validity of the results in the specific country, and increasing generalizability to other European markets. Secondly, both models were informed by actual country-level unit volumes and market share data from 2022, to be as reflective of the current-world as possible prior to forecasting. Given the large differences in adalimumab use in the various regions, this approach builds on previous ex-ante analyses on biosimilars that used a set, hypothetical panel size, which in the current study would have over- or under-estimated the true patient population to larger degree. Thirdly, this study assessed multiple uptake scenarios to reflect historic and regional differences in biosimilar uptake), providing well-rounded results that demonstrate the as-of-yet unrealized economic potential of increased biosimilar uptake. Finally, this is the first study to demonstrates the potential impact of tocilizumab biosimilars, which is important given the current use restrictions on this highly effective therapy [45,46], and the recent launch of a tocilizumab biosimilar[59].
Although there are several strengths that support the robustness of the analyses, several limitations also exist. The current savings presented in the manuscript only apply to markets where the reference product is currently reimbursed and available to patients. The analysis does not forecast the changing biosimilar landscape or consider the effect of additional adalimumab or tocilizumab biosimilars entering the European market. It is possible that the increased competition from additional biosimilars could influence the price of reference products and previously prescribed biosimilars, as manufacturers compete for shares, however, this is also regionally specific varying by the type of market (eg, pull-through, France). The drug-acquisition costs used in both the adalimumab and tocilizumab models were based on published list prices in each region, and so do not capture local discounting or rebating practices. Moreover, the current manuscript does not describe potential savings from markets that may exclusively use a lower priced reference product following biosimilar competition. As such, the total savings presented in the current research may be overestimated. The use of manufacturer (list) price was used to reduce the number of assumptions in the model [60], and is recommended in the EU as the preferred and more transparent price type, as rebates and discounting (which can be as high as 50%) [43] differ by system (eg, tender versus retail) and are largely confidential. In addition, the percent difference on list price between reference products and biosimilars is likely to serve as a proxy for the net difference after discontinuing/rebates. In addition, a sensitivity analysis for the adalimumab and tocilizumab was performed to estimate the savings if rebates were applied to the reference product. The savings may also vary per region in countries such as Italy and Spain that have a decentralized healthcare system or have different treatment costs across regions [61,62]. In addition, to estimate the cohort size for each country, a 40 mg biweekly and 162 mg weekly dosing schedule was used for adalimumab and tocilizumab, respectively, as well as assuming an average patient weight of 80kg. This may have slightly over-estimated the panel sizes (eg, patients with hidradenitis suppurativa or pediatric Crohn’s [≥40 kg weight] would require more frequent dosing of adalimumab). The selected dosing assumptions are in line with other literature [9,11], are reasonable given they represent the vast majority of the population, and, as discussed above, are closer to the real-world situation than a hypothetical panel. Specific to the tocilizumab biosimilar model, assumptions were applied to the market share in the first year, and a variety of discounting scenarios were tested, as real-world data is not available given biosimilars have only recently launched [59,63]. Finally, since tocilizumab is typically prescribed as a later treatment after failure of one or more biologics [45], the introduction of a tocilizumab biosimilar is likely to expand the use of tocilizumab, with potential for patients to be treated earlier in the treatment cycle. As such, the economic impact from a tocilizumab biosimilar in the real world may be greater than the current projections.
Conclusion
In conclusion, this study adds to a growing body of evidence that demonstrates the economic benefit of biosimilar adoption. These findings may help to stimulate the uptake of biosimilars in the autoimmune space, or support efforts that promote incremental increases in conversion to biosimilars, such as policy development. Future studies assessing the savings associated with tocilizumab after launch, including more formalized economic analyses such as a budget-impact model capable of modelling more complex market dynamics between competitors and reference products, may be warranted to gain a better understanding of its cost-savings in these regions.
Transparency
Declaration of funding
This study was sponsored by Fresenius Kabi.
Declaration of financial/other relationships
KC, MAG, and NF are employees of EVERSANA, a company which received consulting fees from Fresenius Kabi. KS is an employee of Fresenius Kabi, a manufacturer of biosimilar medicines.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author Contributions
Fresenius Kabi SwissBioSim GmbH participated in the writing, review, and approval of the manuscript. All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Kerise Clarke and Margaret Ainslie-Garcia. The first draft of the manuscript was written by Kerise Clarke, and all authors commented on previous versions of the manuscript. All authors and Fresenius Kabi SwissBioSim GmbH read and approved the final manuscript.
Acknowledgments
None to declare.
Previous Presentations
This study was presented as a poster at the Annual Meeting of The International Society of Pharmacoeconomics and Outcomes Research Europe, 12 November- 15 November 2023 in Copenhagen, Denmark. However, since that time, the data has been updated with the most recently available inputs.
Table 1. Adalimumab biosimilar EMA approval
Table 2. Adalimumab model inputs
Table 3: Tocilizumab model inputs
Table 4: Projected direct drug savings associated with increasing biosimilar adalimumab market share, by countrya
Table 5: Projected direct drug savings associated with the introduction of biosimilar tocilizumab, by countrya
References
- Li P, Zheng Y, Chen X. Drugs for Autoimmune Inflammatory Diseases: From Small Molecule Compounds to Anti-TNF Biologics. Front Pharmacol. 2017;8:460.
- Townes SV, Furst DE, Thenkondar A. The impact of tocilizumab on physical function and quality of life in patients with rheumatoid arthritis: a systematic literature review and interpretation. Open Access Rheumatol. 2012;4:87-92.
- Smolen JS, Beaulieu A, Rubbert-Roth A, et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomized trial Lancet 2008;371(9617):987-997.
- Tekeoglu S. Ab0350 Rheumatoid Arthritis Management in Southeast Turkey, Experience from Rural Area. Abstracts Accepted for Publication2019. p. 1631.2-1632.
- Nowell WB, Karis E, Gavigan K, et al. Patient-Reported Nausea and Fatigue Related to Methotrexate: A Prospective, Self-Controlled Study in the ArthritisPower((R)) Registry. Rheumatol Ther. 2022 Feb;9(1):207-221.
- Hoes JN, Jacobs JW, Boers M, et al. EULAR evidence-based recommendations on the management of systemic glucocorticoid therapy in rheumatic diseases. Ann Rheum Dis. 2007 Dec;66(12):1560-7.
- Wang H, Zhou J, Guo X, et al. Use of glucocorticoids in the management of immunotherapy-related adverse effects. Thorac Cancer. 2020 Oct;11(10):3047-3052.
- National Institute for Health and Care Excellence. The guidelines manual [updated November 30, 2012;June 21, 2024]. Available from: https://www.nice.org.uk/process/pmg6/chapter/assessing-cost-effectiveness
- Huoponen S, Aaltonen KJ, Viikinkoski J, et al. Cost-effectiveness of abatacept, tocilizumab and TNF-inhibitors compared with rituximab as second-line biologic drug in rheumatoid arthritis. PLoS One. 2019;14(7):e0220142.
- Park SH, Han X, Lobo F, et al. A Cost per Responder Model for Abatacept versus Adalimumab Among Rheumatoid Arthritis Patients with Seropositivity. Clinicoecon Outcomes Res. 2020;12:589-594.
- Fellous S, Rkain H, Ahid S, et al. One-year direct costs of biological therapy in rheumatoid arthritis and its predictive factors: data from the Moroccan RBSMR registry. Rheumatol Int. 2021 Apr;41(4):787-793.
- OECD, Union E. Health at a Glance: Europe 2020. 2020.
- Gibbons JB, Laber M, Bennett CL. Humira: the first $20 billion drug. Am J Manag Care. 2023 Feb;29(2):78-80.
- Gov.UK. Budget impact analysis: health economic studies [updated January 28, 2021;June 21, 2024]. Available from: https://www.gov.uk/guidance/budget-impact-analysis-health-economic-studies
- European Medicines Agency. Biosimilars medicines: Overview. 2023.
- European Medicines Agency. Biosimilar medicines: marketing authorisation [April 2023]. Available from: https://www.ema.europa.eu/en/human-regulatory/marketing-authorisation/biosimilar-medicines-marketing-authorisation#3.–assessment-of-the-application-section
- Generics and Biosimilars Initiative. Biosimilars approved in Europe [updated May 12, 2023;Janauary 30, 2024]. Available from: https://www.gabionline.net/biosimilars/general/biosimilars-approved-in-europe
- European Medicines Agency. Tyenne [updated February 10, 2023;January 30, 2024]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/tyenne
- The center of biosimilars. European Union — Adalimumab Biosimilars [updated March 22, 2024;March 24, 2024]. Available from: https://www.centerforbiosimilars.com/biosimilar-approvals
- Schwabe C, Illes A, Ullmann M, et al. Pharmacokinetics and pharmacodynamics of a proposed tocilizumab biosimilar MSB11456 versus both the US-licensed and EU-approved products: a randomized, double-blind trial. Expert Rev Clin Immunol. 2022 May;18(5):533-543.
- Hercogova J, Papp KA, Chyrok V, et al. AURIEL-PsO: a randomized, double-blind phase III equivalence trial to demonstrate the clinical similarity of the proposed biosimilar MSB11022 to reference adalimumab in patients with moderate-to-severe chronic plaque-type psoriasis. Br J Dermatol. 2020 Feb;182(2):316-326.
- Sardesai A, Dignass A, Quon P, et al. Cost-effectiveness of tofacitinib compared with infliximab, adalimumab, golimumab, vedolizumab and ustekinumab for the treatment of moderate to severe ulcerative colitis in Germany. J Med Econ. 2021 Jan-Dec;24(1):279-290.
- Petryszyn P, Ekk-Cierniakowski P, Zurakowski G. Infliximab, adalimumab, golimumab, vedolizumab and tofacitinib in moderate to severe ulcerative colitis: comparative cost-effectiveness study in Poland. Therap Adv Gastroenterol. 2020;13:1756284820941179.
- Feagan B. Benefits, Concerns, and Future Directions of Biosimilars in Inflammatory Bowel Disease. Gastroenterol Hepatol (N Y). 2017 Dec;13(12):745-747.
- Tesser JR, Furst DE, Jacobs I. Biosimilars and the extrapolation of indications for inflammatory conditions. Biologics. 2017;11:5-11.
- Leonard E, Wascovich M, Oskouei S, et al. Factors Affecting Health Care Provider Knowledge and Acceptance of Biosimilar Medicines: A Systematic Review. J Manag Care Spec Pharm. 2019 Jan;25(1):102-112.
- Cheng CA, Jiang AL, Liu YR, et al. Investigation of Immunogenicity Assessment of Biosimilar Monoclonal Antibodies in the United States. Clin Pharmacol Ther. 2023 Dec;114(6):1274-1284.
- IQVIA. The Impact of Biosimilar Competition in Europe. 2021.
- IQVIA. Adalimumab Biosimilar Market Overview. 2022.
- Baumgart DC, Misery L, Naeyaert S, et al. Biological Therapies in Immune-Mediated Inflammatory Diseases: Can Biosimilars Reduce Access Inequities? Front Pharmacol. 2019;10:279.
- Oskouei ST, Kusmierczyk AR. Biosimilar Uptake: The Importance of Healthcare Provider Education. Pharmaceut Med. 2021 Jul;35(4):215-224.
- Medicines for Europe. Policy recommendations on uptake of biosimilar medicines in the retail market 2021.
- Stevenson M, Archer R, Tosh J, et al. Adalimumab, etanercept, infliximab, certolizumab pegol, golimumab, tocilizumab and abatacept for the treatment of rheumatoid arthritis not previously treated with disease-modifying antirheumatic drugs and after the failure of conventional disease-modifying antirheumatic drugs only: systematic review and economic evaluation. Health Technol Assess. 2016 Apr;20(35):1-610.
- Smolen JS, Goncalves J, Quinn M, et al. Era of biosimilars in rheumatology: reshaping the healthcare environment. RMD Open. 2019;5(1):e000900.
- McBride A, Wang W, Campbell K, et al. Economic modeling for the US of the cost-efficiency and associated expanded treatment access of conversion to biosimilar pegfilgrastim-bmez from reference pegfilgrastim. Journal of Medical Economics. 2020;23(8):856-863.
- McBride A, MacDonald K, Fuentes-Alburo A, et al. Conversion from pegfilgrastim with on-body injector to pegfilgrastim-jmdb: cost-efficiency analysis and budget-neutral expanded access to prophylaxis and treatment. J Med Econ. 2021 Jan-Dec;24(1):598-606.
- MIDAS Quarterly Sales Audit from Q1 2018 to Q4 2022: Adalimumab, Tocilizumab [Internet]. 2022 [cited March 22 2023].
- European Medicines Agency. Humira - Summary of Product Characteristics. 2022.
- European Medicines Agency. RoActemra- Summary of Product Characteristics. 2023.
- Data on file. 2023.
- Moorkens E, Vulto AG, Huys I, et al. Policies for biosimilar uptake in Europe: An overview. PLoS One. 2017;12(12):e0190147.
- Skulski S. Biosimilar Pricing in Europe: A look at Infliximab 2019.
- Vogler S, Zimmermann N, Habl C, et al. Discounts and rebates granted to public payers for medicines in European countries. South Med Rev. 2012 Jul;5(1):38-46.
- Navitus Health Solutions. Navitus to Remove Humira® from Formularies Effective June 1 [updated February 28, 2024;May 27, 2024]. Available from: https://blog.navitus.com/navitus-removes-humira-from-formularies#:∼:text = Since%20January%201%2C%202023%2C%20the,cost%20declines%20in%20the%20category.
- National Institute for Health and Care Excellence. Adalimumab, etanercept, infliximab, certolizumab pegol, golimumab, tocilizumab and abatacept for rheumatoid arthritis not previously treated with DMARDs or after conventional DMARDs only have failed. 2016.
- Mackie SL, Brouwer E, Conway R, et al. Clinical pathways for patients with giant cell arteritis during the COVID-19 pandemic: an international perspective. Lancet Rheumatol. 2021 Jan;3(1):e71-e82.
- Ghabri S, Binard A, Pers YM, et al. Economic Evaluation of Sequences of Biological Treatments for Patients With Moderate-to-Severe Rheumatoid Arthritis and Inadequate Response or Intolerance to Methotrexate in France. Value Health. 2020 Apr;23(4):461-470.
- Mansell K, Bhimji H, Eurich D, et al. Potential cost-savings from the use of the biosimilars filgrastim, infliximab and insulin glargine in Canada: a retrospective analysis. BMC Health Serv Res. 2019 Nov 12;19(1):827.
- Mulcahy A, Buttorff C, Finegold K, et al. Projected US savings from biosimilars, 2021-2025. American Journal of Managed Care. 2022;28(7).
- Kvien TK, Patel K, Strand V. The cost savings of biosimilars can help increase patient access and lift the financial burden of health care systems. Semin Arthritis Rheum. 2021 Dec 30:151939.
- National Institute for Health and Care Excellence. Adalimumab, etanercept, infliximab and abatacept for treating moderate rheumatoid arthritis after conventional DMARDs have failed 2021.
- Barcina Lacosta T, Vulto AG, Turcu-Stiolica A, et al. Qualitative Analysis of the Design and Implementation of Benefit-Sharing Programs for Biologics Across Europe. BioDrugs. 2022 Mar;36(2):217-229.
- Bennett CL, Schoen MW, Hoque S, et al. Improving oncology biosimilar launches in the EU, the USA, and Japan: an updated Policy Review from the Southern Network on Adverse Reactions. Lancet Oncol. 2020 Dec;21(12):e575-e588.
- IQVIA. Incentives For Using Biosimilars In France And Europe. 2023. p. 1-28.
- Sarzi-Puttini P, Marotto D, Caporali R, et al. Biosimilars vs originators: Are they the same? Autoimmun Rev. 2019 Dec;18(12):102404.
- Schmidt KJ, Konstanski M, Martinez JS, et al. Navigating the road to successful biosimilar uptake in Europe – Still some way to go? [updated February 16, 2023;May 27, 2024]. Available from: https://www.xcenda.com/insights/htaq-spring-2023-the-road-to-successful-biosimilar-uptake-in-europe#:∼:text = A%20survey%20among%20physicians%20and,to%20switch%20from%20an%20originator
- Toolan M, Walpole S, Shah K, et al. Environmental impact assessment in health technology assessment: principles, approaches, and challenges. Int J Technol Assess Health Care. 2023 Feb 23;39(1):e13.
- Smeeding J, Malone DC, Ramchandani M, et al. Biosimilars: Considerations for Payers. P T. 2019 Jan;44(2):54-63.
- Biosimilar Development. Fresenius Kabi Launches Tyenne*, The First Approved Tocilizumab Biosimilar In The European Union [updated November 1, 2023;November 13, 2023]. Available from: https://www.biosimilardevelopment.com/doc/fresenius-kabi-launches-tyenne-the-first-approved-tocilizumab-european-union-0001
- IQVIA. Understanding Net Pharmaceutical Expenditure Dynamics in Europe. 2022. p. 26.
- Benucci M, Ravasio R, Damiani A. Mean cost per number needed to treat with tocilizumab plus methotrexate versus abatacept plus methotrexate in the treatment of rheumatoid arthritis in patients previously treated with methotrexate. Clinicoecon Outcomes Res. 2017;9:403-410.
- Jimenez-Morales A, Caliz R, Aceituno S, et al. A cost-consequence analysis of the preferential use of secukinumab versus adalimumab for the treatment of psoriatic arthritis. Reumatol Clin (Engl Ed). 2021 Nov;17(9):536-542.
- Coghlan J, He H, Schwendeman AS. Overview of Humira® Biosimilars: current European landscape and future implications. Journal of pharmaceutical sciences. 2021;110(4):1572-1582.