 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Aim
Insufficient adherence to colorectal cancer (CRC) screening impedes individual and population health benefits, with about one-third of individuals non-adherent to available screening options. The impact of poor adherence is inadequately considered in most health economics models, limiting the evaluation of real-world population-level screening outcomes. This study introduces the CAN-SCREEN (Colorectal cANcer SCReening Economics and adherENce) model, utilizing real-world adherence scenarios to assess the effectiveness of a blood-based test (BBT) compared to existing strategies.
Materials and methods
The CAN-SCREEN model evaluates various CRC screening strategies per 1,000 screened individuals for ages 45-75. Adherence is modeled in two ways: 1) full adherence and 2) longitudinally declining adherence. BBT performance is based on recent pivotal trial data, while existing strategies are informed using literature. The full adherence model is calibrated using previously published Cancer Intervention and Surveillance Modeling Network (CISNET) models. Outcomes, including life-years gained (LYG), CRC cases averted, CRC deaths averted, and colonoscopies, are compared to no screening.
Results
Longitudinal adherence modeling reveals differences in the relative ordering of health outcomes and resource utilization, as measured by the number of colonoscopies performed per 1,000, between screening modalities. BBT outperforms fecal immunochemical test (FIT) and the multitarget stool DNA (mtsDNA) test with more CRC deaths averted (13) compared to FIT and mtsDNA (7, 11), more CRC cases averted (27 vs. 16, 22) and higher LYG (214 vs. 157, 199). BBT yields fewer CRC deaths averted compared to colonoscopy (13, 15) but requires fewer colonoscopies (1,053 vs. 1,928).
Limitations
Due to limited data, the CAN-SCREEN model with longitudinal adherence leverages evidence-informed assumptions for the natural history and real-world longitudinal adherence to screening.
Conclusions
The CAN-SCREEN model demonstrates that amongst non-invasive CRC screening strategies, those with higher adherence yield more favorable health outcomes as measured by CRC deaths averted, CRC cases averted, and LYG.
Plain Language Summary
This study explored the impact of poor adherence to colorectal cancer (CRC) screening, where about one-third of people face barriers to screening. Common models don't consider real-world adherence, so we introduced the CAN-SCREEN model. It uses real-world data to determine how well a blood-based test (BBT) could work compared to existing tests.
We studied people starting CRC screening at age 45. The model looked at two adherence scenarios: assuming everyone follows guidelines, and using real-world data about how people follow screening guidelines over time. The BBT's performance was based on a recent study, and we compared it to existing methods using data from the literature.
Results per 1,000 simulated patients showed that the BBT outperforms two guideline-recommended stool-based tests, fecal immunochemical test (FIT) and the multitarget stool DNA (mtsDNA) test, with more CRC deaths averted (13) compared to FIT and mtsDNA (7, 11), more CRC cases averted (27 vs. 16, 22) and higher LYG (214 vs. 157, 199). BBT prevents less CRC deaths than colonoscopy (13 vs. 15), but it leads to fewer colonoscopies (1,053 compared to 1,928).
Despite some limitations due to limited data, our model relies on informed assumptions for the natural history of CRC and real-world adherence. In conclusion, our CAN-SCREEN model shows that CRC screening strategies combining good test performance with high adherence give better health outcomes. Adding a blood test, which could be easier for people to use, could save lives and reduce the number of colonoscopies needed.
Disclaimer
As a service to authors and researchers we are providing this version of an accepted manuscript (AM). Copyediting, typesetting, and review of the resulting proofs will be undertaken on this manuscript before final publication of the Version of Record (VoR). During production and pre-press, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal relate to these versions also.Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed cancer and the second leading cause of cancer-related mortality in the United States (US), among cancers affecting both men and women.Citation1 Although CRC incidence and mortality has decreased among older adults after the introduction of widespread screening in the US, recent trends indicate that it is increasing amongst adults younger than age 55.Citation1 Nevertheless, since CRC progresses relatively slowly and early treatment is successful in curing most CRCs, identifying and treating patients as part of a screening program has been shown to reduce CRC morbidity and mortality in clinical trials.Citation2 Therefore, it is evident that screening is an important tool for prevention of CRC related death and suffering, CRC detection, and CRC control.
Nonetheless, insufficient participation in colorectal cancer (CRC) screening programs significantly hinders the realization of the full potential of their individual and population health benefits. The barriers to completion of any of the multiple screening options available today in the US such as fears related to sedation and bowel preparation for colonoscopy or discomfort handling stool lead to approximately one third of people not being up to date.Citation3 Given this unmet need, newer testing modalities have been developed, including blood-based tests (BBTs), that have the potential to increase adherence due to their relative ease of collection. In particular, the Shield test developed by Guardant Health is a BBT that has been evaluated by a prospective registrational study in detecting signs of colorectal cancer compared to a screening colonoscopy in average-risk adults between the ages of 45 and 84 from across the US.Citation4 In the real-world clinical setting, laboratory orders of the first 10,000 screening age-eligible patients for the test has demonstrated an adherence rate, defined as the percentage of clinical tests ordered that resulted in the receipt of a blood sample, greater than 90%.Citation5
The current screening modalities that have been recommended by the US Preventive Services Task Force (USPSTF) include direct visualization tests, such as colonoscopy, and stool-based tests, such as fecal immunochemical test (FIT) and the multitarget stool DNA (mtsDNA) test.Citation6 This recommendation has been informed in part by simulation models developed by the National Cancer Institute (NCI)-sponsored Cancer Intervention and Surveillance Modeling Network (CISNET).Citation7, Citation8 The CISNET models for CRC are: Colorectal Cancer Simulated Population model for Incidence and Natural history (CRC-SPIN), MIcrosimulation SCreening Analysis (MISCAN), Simulation Model of Colorectal Cancer (SimCRC). While adherence has been incorporated into the CISNET models in multiple ways over time, there have been limitations to each. First the observed lack of adherence is not considered as part of the primary analysis of CISNET models used to inform USPSTF guidelines,Citation7, Citation8 limiting the ability to evaluate real-world population-level impacts of the existing screening options and newer modalities such as BBT. Second, a study by Peterse et al. which uses a version of the MISCAN model included a scenario analysis assuming imperfect longitudinal adherence.Citation9 However, the authors assumed that the imperfect adherence was the same for each test rather than differing between modalities. Third, as part of analyses conducted for the AGA, the CISNET models incorporate imperfect adherence by taking a weighted average of the results for the no intervention and intervention arms for a given modality which they termed the “participation rate.” This approach assumes that each simulated patient either adheres or not for their entire life based on the assumed rate.Citation10 Even a recent cost-effectiveness analysis from CISNET that assesses the performance of a BBT that meets the CMS minimum threshold of test performance did not take imperfect adherence into account.Citation11
Thus, this study introduces the Colorectal cANcer SCReening Economics and adherence (CAN-SCREEN) discrete-event simulation model to assess the effectiveness of the Shield blood-based test relative to existing CRC screening modalities. A key component of the DES model is incorporation of real-world adherence rates in order to generate health outcomes reflective of actual clinical utilization by individual patients over time.
Methods
Decision Model
We developed the CAN-SCREEN discrete-event simulation model using Arena Version 16.20 by Rockwell Automation Technologies, Inc., integrated with Microsoft Excel to assess the performance of BBT compared to no screening (natural history), colonoscopy, FIT, and mtsDNA. To align with USPSTF guidelines, we simulated hypothetical cohorts of individuals without any known high-risk features for CRC such as family history of CRC (“average risk”) and were currently undergoing routine CRC screening as part of standard care who are between the age of 45 years and 74. Patients were simulated until death from CRC or other causes (lifetime horizon). For a given strategy we simulated 4,000 trials for each cohort of 10,000 individuals, and the average of all replicates was used to calculate outcomes. The number of trials was determined by assessing the stability in the estimation of outputs (Supplementary Figure 1). We compare the following primary outcomes for each strategy: life-years gained (LYG), number of colonoscopies, number of (non-colonoscopy) tests, CRC deaths averted, and CRC cases (overall and by stage). The outcomes for each screening modality were compared to the reference scenario of no screening.
Model structure and key assumptions
Natural history
The natural history component of the model consists of six health states present within the patient population, namely: no lesion, non-advanced adenoma (<10mm in size), advanced adenoma (AA; ≥10mm in size), pre-clinical CRC, clinical CRC, and death (). Detailed information on the model can be found in the Supplementary Material. The natural history component incorporates established parameters for adenoma initiation, adenoma growth, and the transition to pre-cancerous and CRC stages. Individuals in the no lesion, non-advanced adenoma, advanced adenoma, and pre-clinical CRC health states are assumed to have the same mortality rate as the general population, with age and sex both influencing occurrences (). Individuals with clinical CRC additionally experience mortality rates associated with cancer, with the stage of the tumor serving as a risk-modifying factor.
Figure 1. Natural history conceptual model.
Abbreviations: CRC, colorectal cancer; mm, millimeter
Conceptual model for the underlying natural history component of the simulation model (no screening arm). The natural history component consists of the following processes illustrated by dotted lines: 1. Adenoma initiation risk: Nonhomogeneous Poisson process based on age, sex, & tumor location (proximal/distal); 2. Adenoma growth: Continuous growth to 10mm (advanced adenoma [AA]) that varies by tumor location while AA growth rate calibrated to be consistent with 2021 CISNET dwell times;Citation8 3. Transition from AA to pre-clinical CRC: Calibrated logistic regression model dependent on age, sex, tumor location, and tumor size to be consistent with 2021 CISNET dwell times;Citation8 4. Pre-clinical stage progression: Calibrated rates to be consistent with 2021 CISNET sojourn times;Citation8 starting values for calibration were based on literature;Citation40, Citation41 5. Transition from pre-clinical CRC to clinical CRC: Calibrated constant transition rates to be consistent with CRC stage distribution (SEER – 1975-1979) and 2021 CISNET sojourn times while the starting values for calibration used transition probabilities from literature;Citation8 6. Clinical stage progression: Calibrated constant transition rate that increases by stage based on literature;Citation41 7. CRC Survival: Constant transition rate increasing by stage adjusted based on observed trends.Citation1, Citation30 Model also allows for competing risks of background and screening (colonoscopy) related mortality.
![Figure 1. Natural history conceptual model.Abbreviations: CRC, colorectal cancer; mm, millimeterConceptual model for the underlying natural history component of the simulation model (no screening arm). The natural history component consists of the following processes illustrated by dotted lines: 1. Adenoma initiation risk: Nonhomogeneous Poisson process based on age, sex, & tumor location (proximal/distal); 2. Adenoma growth: Continuous growth to 10mm (advanced adenoma [AA]) that varies by tumor location while AA growth rate calibrated to be consistent with 2021 CISNET dwell times;Citation8 3. Transition from AA to pre-clinical CRC: Calibrated logistic regression model dependent on age, sex, tumor location, and tumor size to be consistent with 2021 CISNET dwell times;Citation8 4. Pre-clinical stage progression: Calibrated rates to be consistent with 2021 CISNET sojourn times;Citation8 starting values for calibration were based on literature;Citation40, Citation41 5. Transition from pre-clinical CRC to clinical CRC: Calibrated constant transition rates to be consistent with CRC stage distribution (SEER – 1975-1979) and 2021 CISNET sojourn times while the starting values for calibration used transition probabilities from literature;Citation8 6. Clinical stage progression: Calibrated constant transition rate that increases by stage based on literature;Citation41 7. CRC Survival: Constant transition rate increasing by stage adjusted based on observed trends.Citation1, Citation30 Model also allows for competing risks of background and screening (colonoscopy) related mortality.](/cms/asset/ee9f70c1-a82d-443f-86b9-fa5d6c5b4101/ijme_a_2382036_f0001.jpg)
Table 1. Key natural history parameters.
Screening modalities
There are several guideline-recommended methods for CRC screening, including both visual examinations (e.g., colonoscopy) and high-sensitivity stool-based tests (e.g., FIT, mtsDNA) which vary in terms of their test performance characteristics (). We compared these existing modalities to BBT to determine their relative impact on the natural history (no screening) of CRC in a simulated average risk cohort. We focused on colonoscopy, FIT, and mtsDNA as comparators since these are the most commonly performed screening tests for CRC in the US and therefore excluded less common tests such as sigmoidoscopy, fecal occult blood test, CT colonography, or capsule endoscopy.Citation12 We initially considered screening interval testing with BBT every three years, similar to the recommended interval testing with mtsDNA. We assumed FIT was offered at an annual interval and colonoscopy had a 10-year interval.
Table 2. Test performance characteristics.
All screening tests have the potential to yield false negative, true negative, false positive, or true positive results. In cases where a positive result is obtained in a stool or blood test (regardless of its accuracy), the simulated patient can undergo a colonoscopy along with polypectomy which is highly accurate for CRC and advanced polyps (subject to their adherence to diagnostic colonoscopy).Citation10 This procedure either provides potentially curative treatment by removing neoplasia (adenomas or CRC) or incurs additional resource utilization and exposure to potential complications in the case of false positive results without affecting the natural history of CRC. Patients who undergo a colonoscopy which identified neoplasia will then be subject to a colonoscopy surveillance schedule that is more frequent than the screening interval for people without adenomas per USPSTF guidelines (patients without neoplastic findings revert back to their original modality and its respective screening interval). We assumed a recommended surveillance interval is 1 year for patients diagnosed with CRC, 3 years for patients with advanced adenoma, and 5 years for patients with non-advanced adenoma.Citation13 Patients had the opportunity to continue with the surveillance schedule through age 85. During the surveillance period we assumed that patients would have 100% adherence to the receiving a colonoscopy.
Adherence
Recent studies and real-world data suggest that adherence to CRC screening tests varies over time, and thus it is may be more accurate to model adherence on a longitudinal basis.Citation14–17 As such, we used an approach similar to D’Andrea et al. (2020) to incorporate longitudinal adherence into the screening component of our model.Citation18
According to the National Health Interview Survey (NHIS), a maximum of 67.2% of individuals aged 50 to 75 in 2018 were up to date with colorectal cancer (CRC) screening,Citation3 primarily by having had a colonoscopy within the past 10 years. This information was used to fine-tune the longitudinal adherence component of the model, ensuring it reflects the overall adherence to colonoscopy over a 10-year period. Subsequently, the calibrated longitudinal adherence component of the model was utilized to simulate adherence patterns for alternative screening strategies. To elaborate, if individuals eligible for screening decline a colonoscopy in the first year, they are offered a colonoscopy in subsequent years to encourage their adherence. The goal is to ensure that up to 67.2% of these individuals become up to date with CRC screening via colonoscopy within a 10-year duration (Supplementary Table 1).
To implement the longitudinal adherence approach, two assumptions were made. Firstly, individuals who adhere with screening in the first year will maintain the same one-time adherence rate in subsequent scheduled screenings. For instance, if their adherence rate for the initial colonoscopy is 38.4%, it will remain 38.4% for the next round of colonoscopy. Secondly, screening options are annually provided to non-adherent individuals. However, the one-time adherence rates for these individuals gradually decline at a constant rate (represented by ) for each year they remain non-adherent. For example, if a colonoscopy is delayed by
years (where
ranges from 1 to 10), the adherence rate would be calculated as
.
The introduction of a decline rate was necessary to ensure that the simulated number of individuals up to date with colonoscopy screening aligns with the observed rate (67.2%), rather than approaching 100%. In this case, it was estimated that a decline rate of 39.5% ( = 0.395) would appropriately calibrate the model for colonoscopy. This decline rate was calculated by iteratively increasing the rate
from 0 until the cumulative percentage of adherence individuals after 10 years for colonoscopy matched the calibration target of 67.2%. Consequently, the same decline rate was applied to the corresponding one-time adherence rates of all other screening strategies when individuals were overdue for their screenings (Supplementary Table 2). Results for longitudinal adherence of each test for 10-year period is shown in Supplementary Table 3. So that the cumulative adherence doesn’t increase above the calibration target we set the adherence rate for 0% for year 11 onward for all tests for those who have continued to not adhere for 10 consecutive years.
We present results for the full adherence scenario (including full adherence to both screening tests and diagnostic colonoscopy), for comparison with existing models, and under the primary assumption of longitudinal adherence (which also incorporates imperfect diagnostic colonoscopy adherence).
Model calibration
Calibration was conducted to estimate unknown parameters of underlying disease progression, aiming to align with observed health metrics. This calibration process was executed using the natural history component of the model without any interventions to ensure that the disease progression, gauged by adenoma dwell time, sojourn time, or stage distributions, aligns with recent trends. Adenoma dwell and sojourn time refer to the duration from the non-advanced to the early pre-clinical phase of cancer and from pre-clinical until it becomes detectable as CRC, respectively. This timeframe is intricately tied to the shift from an initial pre-cancerous condition to specific CRC stages identifiable in clinical settings. To refine accuracy, we implemented a two-step calibration process, targeting adjustments to both these temporal aspects and CRC staging. The primary aim was to harmonize our model's metrics with established CISNET models,Citation7, Citation8 while also utilizing SEER data to define the cancer stage distribution at baseline (further details can be found in the Supplementary Material).Citation19
Parameter input distributions
The model was constructed with a probabilistic framework to comprehensively account for uncertainties surrounding input parameters within the analysis. For each input with inherent uncertainty, a specific probability distribution was designated. During model execution, values for these probabilistic inputs were chosen randomly, drawing from their respective probability distributions. To ensure thoroughness, the model underwent 4,000 replications, capturing a breadth of potential outcomes. The average results across these 4,000 simulations were then documented.
Beta distributions, being bound between zero and one, and were used to propagate uncertainty in each screening test’s adherence, incompleteness rate, sensitivity and specificity parameters. The and
parameters of the associated beta distributions were derived from the estimated mean and standard error (SE) via the method of moments; these were calculated as follows:
Model validation
We validated the model by comparing the results with those from existing published models including those used to support USPSTF recommendations.Citation7, Citation8, Citation18 For the validation analyses we used a cohort of 10,000 simulated individuals (with 4,000 replications) that began screening at 45. We compared the following outcomes: CRC cases, CRC deaths, adenoma dwell time (i.e., non-advanced adenoma to pre-clinical cancer dwell time), and overall dwell time (i.e., non-advanced adenoma to clinical cancer dwell time). Also we compare our results for CRC deaths averted with a recent analysis from CISNET performed for the American Gastroenterological Association (AGA).Citation10
Sensitivity analyses
We performed one-way sensitivity analyses for key features of the screening strategies as identified in the literature: AA sensitivityCitation20, Citation21 and the one-time adherenceCitation18, Citation22 component of the longitudinal adherence model. The outcomes of LYG, number of CRC cases averted, CRC deaths averted, and number of colonoscopies performed for non-invasive stool-based tests and BBT were assessed by varying alternative base case values of these parameters by a relative +/-20% for the primary longitudinal adherence scenario (i.e., for Shield AA sensitivity of 13% we assess 10.4% to 15.6%). The alternative base case values were 80% one-time adherence for Shield and 60% for FIT and mtsDNA. The numbers were chosen for two reasons: (1) given the high one-time adherence for Shield is 90% we would be unable to assess a relative change of +20% and (2) The recent CISNET modeling performed for the AGA found that a blood based test would need to have at least 80% one-time adherence to be superior to FIT at 60% adherence.Citation10 These analyses allow us to gauge the relative importance of changes in AA sensitivity as compared to the one-time adherence component of the longitudinal adherence model.
We also conducted a sensitivity analysis to evaluate the effect of lowering the follow-up diagnostic colonoscopy adherence to 56.1% for the non-invasive tests (FIT, mtsDNA, and BBT) based on an alternative literature source.Citation23 As analysed in the recent analysis from CISNET performed for the AGA, we evaluated the CRC deaths averted for a version of the BBT assuming full adherence that meets the CMS test performance criteria for coverage of a BBT (sensitivity = 74%, non-AA sensitivity = 10%, AA sensitivity = 10%, specificity = 90%).
Although we assumed three-year interval testing for BBT in the CAN-SCREEN model, the frequency of programmatic screening with BBT has yet to be established and requires a long-term clinical study. We undertook additional sensitivity analyses to evaluate outcomes when BBT was performed at 1-, 2-, 4-, and 5-year intervals to compare to 3-year interval testing. These results can be used to evaluate an efficiency ratio based on a trade-off between resource utilization such as number of colonoscopies that need to be performed and outcomes such as CRC deaths averted.
Results
Results from model validation
displays how the results of our calibrated model compare to other simulation models from CISNET. Despite variations in modeling assumptions, our model still produces estimates for adenoma and overall dwell times that fall within the range of these existing models; however, the estimated CRC cases and deaths in the no screening arm are lower for CAN-SCREEN.
Table 3. Validation results for the CAN-SCREEN model under the no screening scenario compared to other simulation models.
Full adherence results
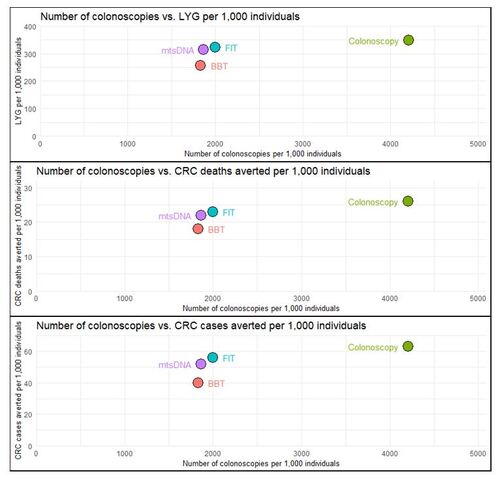
Results for the alternative screening strategies compared to no screening using the full adherence model are presented in . In this scenario, the colonoscopy strategy achieved 349 LYG per 1,000, averted the most deaths due to CRC per 1,000 (26), and averted the most CRC cases per 1,000 (63). However, it did so by having the highest resource intensiveness as measured by the number of colonoscopies per 1,000 simulated patients (4,208). The stool-based tests, FIT and mtsDNA, achieved fewer LYG per 1,000 (322 and 313, respectively), averted fewer CRC deaths per 1,000 (23 and 22), and averted fewer CRC cases per 1,000 (56 and 52). However, these outcomes were achieved with roughly half as many colonoscopies being performed per 1,000 screened of 1,997 and 1,863 for FIT and mtsDNA, respectively. Lastly, the BBT achieved the least amount of LYG per 1,000 (256), CRC deaths averted (18), and CRC cases averted (40), albeit with the least number of colonoscopies required per 1,000 (1,831). The number of non-colonoscopy tests performed per 1,000 was highest for FIT due to its annual screening interval (10,873), followed by mtsDNA (4,315) and BBT (4,292). Stage-specific CRC case results for full and longitudinal adherence are provided in the Supplementary Material.
Figure 2. Full Adherence Outcomes per 1,000 Simulated Individuals.
Abbreviations: BBT, blood-based test; CRC, colorectal cancer; FIT, fecal immunochemical test; LYG, life-years gained; mtsDNA, multitarget stool DNA
This figure shows the life-years gained, CRC deaths averted, and CRC cases averted all compared to a common measure of the number of colonoscopies per 1,000 simulated individuals. All screening strategies are considered in reference to “no screening” under the assumption of full adherence.
Longitudinal adherence results
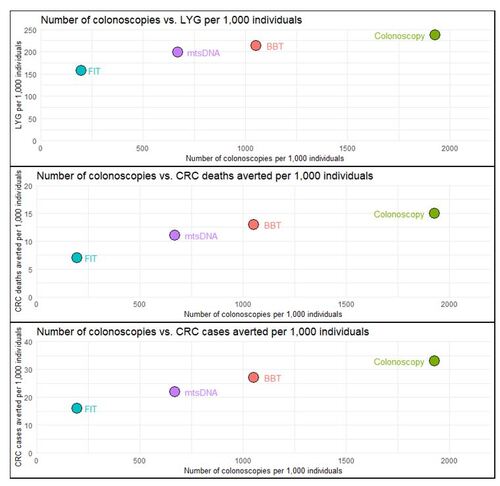
The results for the model assuming longitudinal adherence compared to no screening are provided in . Under this real-world scenario, colonoscopy achieved the most LYG per 1,000 (237) compared to BBT (214), or the stool-based tests (mtsDNA: 199 and FIT: 157). Similarly, colonoscopy achieved the highest number of CRC deaths averted per 1,000 followed by BBT (colonoscopy: 15, BBT: 13) and lastly by the stool-based tests (mtsDNA: 11 and FIT: 7). The number of CRC cases averted per 1,000 was highest for colonoscopy (33) followed by BBT (27), while again the stool-based tests were lower (mtsDNA: 22 and FIT: 16). While colonoscopy achieved the best clinical outcomes, BBT led to nearly half the number of colonoscopies required per 1,000 (colonoscopy: 1,928 vs. BBT: 1,053). The stool-based tests had lower colonoscopy resource utilization (mtsDNA: 670 and FIT: 197). Due to its higher longitudinal adherence (arising from high initial one-time adherence), BBT had the highest number of non-colonoscopy tests performed per 1,000 (5,140), followed by mtsDNA (3,269), and FIT (2,216).
Figure 3. Longitudinal Adherence Outcomes per 1,000 Simulated Individuals.
Abbreviations: BBT, blood-based test; CRC, colorectal cancer; FIT, fecal immunochemical test; LYG, life-years gained; mtsDNA, multitarget stool DNA
This figure shows the life-years gained, CRC deaths averted, and CRC cases averted all compared to a common measure of the number of colonoscopies per 1,000 simulated individuals. All screening strategies are considered in reference to no screening under the assumption of longitudinal adherence.
Scenario analysis results
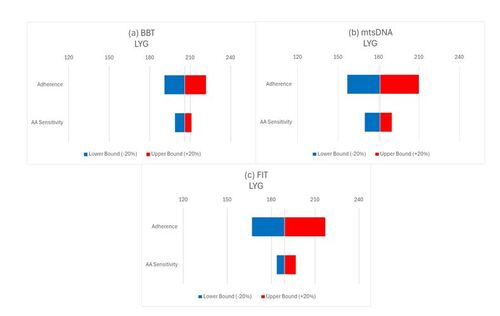
Results of the sensitivity analyses which vary AA sensitivity and one-time adherence component of the longitudinal adherence model by a relative +/- 20% of the longitudinal adherence scenario for non-invasive screening modalities in terms of LYG are shown in . The results demonstrate that the same magnitude change in adherence has a much larger impact in terms of LYG compared to AA sensitivity for FIT and mtsDNA while the opposite was the case for BBT. The results are similar in terms of number of CRC cases averted and CRC deaths averted (see Supplementary Material).
Figure 4. Sensitivity analysis of changes in adherence to BBT, mtsDNA, and FIT compared to each test’s AA sensitivity in terms of LYG.
Abbreviations: AA, advanced adenoma; BBT, blood-based test; CRC, colorectal cancer; FIT, fecal immunochemical test; LYG, life-years gained; mtsDNA, multitarget stool DNA
These figures demonstrate the relative importance of a +/-20% change of the one-time initial adherence parameter in the longitudinal adherence component of the CAN-SCREEN model compared to the same relative change for AA sensitivity in terms of LYG for each screening modality.
Reducing the adherence to diagnostic colonoscopy assumption from 76.2% to 56.1% did not change the relative order of performance between the non-invasive tests (1. BBT, 2. mtsDNA, 3. FIT) in terms of CRC deaths averted, cases averted, and LYG (see Supplementary Material). The CAN-SCREEN model under full adherence produces results in line with the CISNET results for a CMS reimbursable blood test as considered by the AGACitation10, providing further validation of the CAN-SCREEN model as shown in . In addition, we evaluated the efficiency ratio (ER) for each testing interval as the incremental number of lifetime colonoscopies needed to avert each additional CRC death. As shown in the Supplementary Material, 3-year testing interval for Shield provides a reasonable balance of the benefits and harms for CRC screening.
Table 4. Validation results for CRC deaths averted for the CAN-SCREEN model compared to CISNET models
Discussion
When factoring in real-world adherence it is projected that a BBT, with the clinically demonstrated test performance characteristics of the Shield test, will be superior to existing guideline-recommended non-invasive options in terms of LYG, CRC cases averted, and CRC deaths averted. These clinical benefits are also achieved at a much lower level of resource intensiveness compared to colonoscopy, as measured by the number of colonoscopies performed. Nonetheless, the improved longitudinal adherence performance that a BBT will likely have will tend to result in more non-colonoscopy tests being performed compared to other options. Furthermore, the sensitivity analyses demonstrate that (longitudinal) adherence is a more significant contributor to CRC clinical outcomes than AA sensitivity.
To our knowledge this is the first simulation model for CRC screening that incorporates longitudinal adherence to assess the outcomes of a BBT screening strategy with test performance characteristics based on the Shield screening assay. Although, other simulation modeling studies have evaluated CRC screening strategies using the longitudinal adherence approach for older screening modalities such as the methylated SEPT9 DNA testCitation18 or mtsDNA.Citation18 These studies intuitively demonstrate that under this assumption, less invasive and easier to perform modalities with higher adherence can achieve an “effective sensitivity” approaching the reference standard test of colonoscopy. A study by Peterse et al. that leveraged a version of the MISCAN model (part of the CISNET consortium) used to validate our results included a scenario analysis with a similar longitudinal adherence approach that was consistent with NHIS data.Citation9 However, in the Peterse et al. study the imperfect adherence was assumed to be the same for each test and thus didn’t assess the potential differential level of adherence between modalities. Therefore, the authors found that the imperfect longitudinal adherence assumption merely reduced the effectiveness of all screening modalities without significantly affecting the incremental cost-effectiveness ratios.
Typically, simulation modeling studies that incorporate imperfect adherence to the CRC screening tests do so by assuming imperfect adherence to each offer of a screening test at its specified interval (so called “one-time adherence”). For example, a recent study by Kisiel at al. estimated the relative cost-effectiveness of a BBT for CRC compared to mtsDNA according to alternative one-time adherence rates.Citation24 The data used to inform this initial adherence are similar to what was used in the initial adherence of our longitudinal adherence scenario, which capture the proportion of patients that adhere to a given screening test offer in a specified follow-up window, (e.g., one year). However, these models assume that patients will not be offered a given test or otherwise have the possibility of becoming adherent to the screening test until the next recommended interval. Furthermore, this assumption doesn’t incorporate evidence that patient participation in repeat CRC screening rounds tends to diminish over time.Citation25
Despite the clear impact of adherence on the realization of improved clinical outcomes from CRC screening, some studies suggest that AA sensitivity could be an even greater factor.Citation20, Citation21 This question of the relative importance of adherence or AA sensitivity on projected clinical outcomes is key since the BBT evaluated in this study has lower AA sensitivity than the other modalities evaluated while it is projected to have higher longitudinal adherence. However, the study by Carroll et al.Citation21, which suggested the relative importance of AA sensitivity over adherence, did not include a scenario of imperfect longitudinal adherence, rather only imperfect adherence at each screening interval (“one-time adherence”). Therefore, it is difficult to compare the results from these studies that use a more simplistic approach to modeling imperfect adherence to what has been demonstrated by the CAN-SCREEN model.
Although we have demonstrated the importance of incorporating imperfect longitudinal adherence into simulation models of CRC screening strategies, it is still important to include scenarios with full adherence in order to compare results between studies and to support aspirational guidelines such as those recommended by the USPSTF. While the CISNET modeling report used to support the USPSTF’s most recent 2021 guidelines, only assesses full adherence in the base case, they do demonstrate the impact of various types of imperfect adherence as part of scenario analyses.Citation8 Additionally, when they perform analyses that assess the relative efficiency of alternative screening strategies via the efficiency ratio or the incremental number of colonoscopies performed divided by the LYG (similar to an incremental cost effectiveness ratio [ICER] calculated in cost-effectiveness analysis), they do so separately for each modality. This is in recognition of the fact that there are differences in the characteristics of the tests (i.e., invasiveness, ease of use) beyond simply test performance that their base case analyses of full adherence do not reflect. Therefore, comparisons between classes of modalities using this metric are not performed. Nonetheless, in terms of validation, we have demonstrated that the CAN-SCREEN model produces similar dwell times, albeit with fewer CRC cases and deaths for the scenario of no screening compared to the CISNET8 models for the non-BBT tests and note that the results agree under the full adherence scenario as well. We also showed that in recent analyses performed by CISNET for the AGA, the CAN-SCREEN results are in line with CISNET under the unrealistic assumption of full adherence. While the analyses conducted for the AGA incorporate imperfect adherence by simply taking a weighted average of the results for the no intervention and intervention arms for a given modality (“participation rate”), they did not consider the scenario of real-world longitudinal adherence.
Despite the strengths of the CAN-SCREEN model there are some key limitations. First, like the other models discussed, the CAN-SCREEEN model ignores the serrated polyp pathway in the adenoma-carcinoma sequence due to lack of published data and understanding of its natural history, even though it is estimated to potentially account for up to 30% of colorectal cancer cases.Citation26 Second, we assumed that the test performance characteristics (i.e., sensitivity and specificity) remained constant over time (i.e., assumed that no neoplasia were systematically missed) for each interval due to a lack of longitudinal data. This assumption may overestimate the sensitivity and false positivity of all considered tests at subsequent rounds. This potential bias would also have a greater affect for tests with shorter intervals such as annual FIT. Third, although we assumed a single value for the one-time adherence performance for each test, there is substantial heterogeneity in the literature depending on the specific population and setting studied and real-world longitudinal adherence is not well-known.Citation14–17 Fourth, due to the computational-intensiveness of the DES model, we did not perform a probabilistic sensitivity analysis. However, we did perform one-way sensitivity analyses on key parameters to demonstrate their relative importance. Lastly, in the face of growing incidence of CRC amongst younger adults, the current lifetime risk of developing CRC in the absence of screening in the US is unknown.Citation1 Thus while our model produces dwell time results consistent with those from CISNET, they may not reflect the complete extent of this worrying phenomenon.
Conclusions
The CAN-SCREEN model demonstrates that CRC screening strategies combining clinically meaningful performance with high adherence yield more favorable CRC-related health outcomes. Existing models that assume 100% adherence are limited in their ability to accurately predict population level health outcomes. A validated blood-based CRC test is a screening modality that maximizes adherence and is likely to be more preferred by patients. Incorporation of this test as an additional strategy will avert deaths in the unscreened population.
Transparency
Declaration of funding
Declaration of financial/other interests
SF, EYD, NZ, GS, VR, AAT, CE, and AD are employees of, and have stock ownership in, Guardant Health, Inc. BS and GT are employees of Cytel, Inc. Cytel, Inc. received consulting fees from Guardant Health, Inc. for the purposes of conducting this research. WMG is a paid consultant for Guardant Health, Inc., Karius, Inc., and Diacarta, Inc. and receives research support from LucidDx Technologies.
Reviewer disclosures
Peer reviewers on this manuscript have received an honorarium from JME for their review work but have no other relevant financial relationships to disclose.
Author Contributions
SF, EYD, NZ, VR, AD, BS, and GT were involved in the conception and design. BS developed the microsimulation model. All authors were involved in the interpretation of the data. SF drafted the manuscript. All authors revised it critically for intellectual content. Lastly, all authors read and gave approval of the final manuscript and agree to be accountable for all aspects of the work.
Acknowledgements
Abbey Poirier from Cytel, Inc. supported this research through project management and assisted in the model development.
Previous presentations
Part of the material in this manuscript for an earlier version of the CAN-SCREEN model was presented at the AcademyHealth Annual Research Meeting, held in Seattle, WA, June 24-27, 2023 and the American College of Gastroenterology Annual Scientific Meeting, held in Vancouver, BC, October 20-25, 2023.
Supplemental Material
Download MS Word (1.3 MB)Additional information
Funding
References
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. Ca Cancer J Clin. 2023;73(1):17-48.
- Bretthauer M, Løberg M, Wieszczy P, et al. Effect of Colonoscopy Screening on Risks of Colorectal Cancer and Related Death. N Engl J Med. 2022;387(17):1547-1556. doi: 10.1056/NEJMoa2208375.
- National Center for Health Statistics. Health, United States, 2019. Hyattsville, MD. 2021. doi: 10.15620/cdc:100685. Published online 2019.
- Chung DC, Gray DM, Singh H, et al. A cell-free DNA blood-based test for colorectal cancer screening. N Engl J Med. 2024;390(11):973-983.
- Raymond V, Foster G, Hong Y, et al. S295 Implementation of Blood-Based Colorectal Cancer Screening: Real-World Clinical Experience. Off J Am Coll Gastroenterol ACG. 2023;118(10S):S218.
- Davidson KW, Barry MJ, Mangione CM, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. Jama. 2021;325(19):1965-1977.
- Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of Benefits, Burden, and Harms of Colorectal Cancer Screening Strategies: Modeling Study for the US Preventive Services Task Force. JAMA. 2016;315(23):2595-2609. doi: 10.1001/jama.2016.6828.
- Knudsen AB, Rutter CM, Peterse EFP, et al. Colorectal Cancer Screening: An Updated Modeling Study for the US Preventive Services Task Force. JAMA. 2021;325(19):1998-2011. doi: 10.1001/jama.2021.5746.
- Peterse EFP, Meester RGS, de Jonge L, et al. Comparing the Cost-Effectiveness of Innovative Colorectal Cancer Screening Tests. JNCI J Natl Cancer Inst. 2020;113(2):154-161. doi: 10.1093/jnci/djaa103.
- van den Puttelaar R, de Lima PN, Knudsen AB, et al. Effectiveness and Cost-Effectiveness of Colorectal Cancer Screening With a Blood Test That Meets the Centers for Medicare & Medicaid Services Coverage Decision. Gastroenterology. Published online 2024.
- Nascimento De Lima P, Van Den Puttelaar R, Knudsen AB, et al. Characteristics of a cost-effective blood test for colorectal cancer screening. JNCI J Natl Cancer Inst. Published online June 6, 2024:djae124. doi: 10.1093/jnci/djae124.
- Fisher DA, Princic N, Miller-Wilson LA, Wilson K, Fendrick AM, Limburg P. Utilization of a Colorectal Cancer Screening Test Among Individuals With Average Risk. JAMA Netw Open. 2021;4(9):e2122269. doi: 10.1001/jamanetworkopen.2021.22269.
- Gupta S, Lieberman D, Anderson JC, et al. Recommendations for Follow-Up After Colonoscopy and Polypectomy: A Consensus Update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2020;158(4):1131-1153.e5. doi: 10.1053/j.gastro.2019.10.026.
- Gellad ZF, Stechuchak KM, Fisher DA, et al. Longitudinal Adherence to Fecal Occult Blood Testing Impacts Colorectal Cancer Screening Quality. Off J Am Coll Gastroenterol ACG. 2011;106(6):1125. doi: 10.1038/ajg.2011.11.
- Cyhaniuk A, Coombes ME. Longitudinal adherence to colorectal cancer screening guidelines. Am J Manag Care. 2016;22(2):105-111.
- van der Vlugt M, Grobbee EJ, Bossuyt PM, et al. Adherence to colorectal cancer screening: four rounds of faecal immunochemical test-based screening. Br J Cancer. 2017;116(1):44-49. doi: 10.1038/bjc.2016.399.
- Arevalo M, Sutton SK, Abdulla R, et al. Longitudinal adherence to annual colorectal cancer screening among Black persons living in the United States enrolled in a community-based randomized trial. Cancer. n/a(n/a). doi: 10.1002/cncr.35169.
- D’Andrea E, Ahnen DJ, Sussman DA, Najafzadeh M. Quantifying the impact of adherence to screening strategies on colorectal cancer incidence and mortality. Cancer Med. 2020;9(2):824-836. doi: 10.1002/cam4.2735.
- Surveillance E. SEER* Stat Database: Incidence—SEER 9 Regs Public‐Use. National Cancer Institute Bethesda, MD; 2004.
- Putcha G, Carroll LN, Fransen S, Chandra T, Piscitello A. Abstract 2240: Interception versus prevention in cancer screening: Results from the CRC-MAPS model. Cancer Res. 2022;82(12_Supplement):2240. doi: 10.1158/1538-7445.AM2022-2240.
- Carroll LN, Piscitello A, Chandra T, Putcha G. Adenoma detection improves clinical outcomes across adherence scenarios for a CRC screening blood test meeting CMS performance targets: Results from the CRC-MAPS model. In: Gastroenterology. Vol 162. WB SAUNDERS CO-ELSEVIER INC 1600 JOHN F KENNEDY BOULEVARD, STE 1800 …; 2022:S952-S952.
- Fisher DA, Karlitz JJ, Jeyakumar S, et al. Real-world cost-effectiveness of stool-based colorectal cancer screening in a Medicare population. J Med Econ. 2021;24(1):654-664. doi: 10.1080/13696998.2021.1922240.
- Mohl JT, Ciemins EL, Miller-Wilson LA, Gillen A, Luo R, Colangelo F. Rates of follow-up colonoscopy after a positive stool-based screening test result for colorectal cancer among health care organizations in the US, 2017-2020. JAMA Netw Open. 2023;6(1):e2251384-e2251384.
- Kisiel JB, Fendrick AM, Ebner DW, et al. Estimated impact and value of blood-based colorectal cancer screening at varied adherence compared with stool-based screening. J Med Econ. 2024;27(1):746-753. doi: 10.1080/13696998.2024.2349467.
- Liang PS, Wheat CL, Abhat A, et al. Adherence to Competing Strategies for Colorectal Cancer Screening Over 3 Years. Am J Gastroenterol. 2016;111(1):105-114. doi: 10.1038/ajg.2015.367.
- East JE, Vieth M, Rex DK. Serrated lesions in colorectal cancer screening: detection, resection, pathology and surveillance. Gut. 2015;64(6):991-1000.
- Bureau UC. Age and Sex Composition in the United States: 2021. Census.gov. Accessed January 23, 2024. https://www.census.gov/data/tables/2021/demo/age-and-sex/2021-age-sex-composition.html
- Atkin WS, Edwards R, Kralj-Hans I, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. The Lancet. 2010;375(9726):1624-1633. doi: 10.1016/S0140-6736(10)60551-X.
- United States Life Tables, 2020. National Center for Health Statistics (U.S.); 2022. doi: 10.15620/cdc:118055.
- O’Connell JB, Maggard MA, Ko CY. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst. 2004;96(19):1420-1425.
- Gatto NM, Frucht H, Sundararajan V, Jacobson JS, Grann VR, Neugut AI. Risk of perforation after colonoscopy and sigmoidoscopy: a population-based study. J Natl Cancer Inst. 2003;95(3):230-236.
- Wang L, Mannalithara A, Singh G, Ladabaum U. Low rates of gastrointestinal and non-gastrointestinal complications for screening or surveillance colonoscopies in a population-based study. Gastroenterology. 2018;154(3):540-555.
- Singal AG, Gupta S, Skinner CS, et al. Effect of Colonoscopy Outreach vs Fecal Immunochemical Test Outreach on Colorectal Cancer Screening Completion: A Randomized Clinical Trial. JAMA. 2017;318(9):806-815. doi: 10.1001/jama.2017.11389.
- Akram A, Juang D, Bustamante R, et al. Replacing the Guaiac Fecal Occult Blood Test With the Fecal Immunochemical Test Increases Proportion of Individuals Screened in a Large Healthcare Setting. Clin Gastroenterol Hepatol. 2017;15(8):1265-1270.e1. doi: 10.1016/j.cgh.2017.01.025.
- Miller-Wilson LA, Rutten LJF, Van Thomme J, Ozbay AB, Limburg PJ. Cross-sectional adherence with the multi-target stool DNA test for colorectal cancer screening in a large, nationally insured cohort. Int J Colorectal Dis. 2021;36(11):2471-2480. doi: 10.1007/s00384-021-03956-0.
- Corley DA, Jensen CD, Quinn VP, et al. Association Between Time to Colonoscopy After a Positive Fecal Test Result and Risk of Colorectal Cancer and Cancer Stage at Diagnosis. JAMA. 2017;317(16):1631-1641. doi: 10.1001/jama.2017.3634.
- Jensen CD, Corley DA, Quinn VP, et al. Fecal Immunochemical Test Program Performance Over 4 Rounds of Annual Screening. Ann Intern Med. 2016;164(7):456-463. doi: 10.7326/M15-0983.
- Kingsley J, Karanth S, Revere FL, Agrawal D. Cost Effectiveness of Screening Colonoscopy Depends on Adequate Bowel Preparation Rates – A Modeling Study. PLoS ONE. 2016;11(12):e0167452. doi: 10.1371/journal.pone.0167452.
- Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Multitarget Stool DNA Testing for Colorectal-Cancer Screening. N Engl J Med. 2014;370(14):1287-1297. doi: 10.1056/NEJMoa1311194.
- Costi R, Leonardi F, Zanoni D, Violi V, Roncoroni L. Palliative care and end-stage colorectal cancer management: The surgeon meets the oncologist. World J Gastroenterol. 2014;20(24):7602-7621. doi: 10.3748/wjg.v20.i24.7602.
- Excellence (NICE) NI for C. Colorectal cancer prevention: colonoscopic surveillance in adults with ulcerative colitis, Crohn’s disease or adenomas. Published online 2011.
