ABSTRACT
Introduction: To estimate the direct and indirect costs of bladder cancer prior to and following cystectomy in a U.S. sample of patients.
Methods: This retrospective, observational analysis of de-identified patients with bladder cancer utilized the MarketScan Commercial Claims & Encounters and Health & Productivity Management databases. Adult patients with bladder cancer plus ≥ 1 claim for partial or radical cystectomy between 10/1/2015 – 12/31/20 (date of the cystectomy = index date) and who were continuously enrolled for 6 months pre- (baseline) and post-index (follow-up) were included in the sample. All-cause total healthcare costs and indirect costs associated with short-term and long-term disability (STD and LTD) employer claims were assessed during each of the 6-month baseline and follow-up periods.
Results: The study included N = 142 patients; mean age 56 ± 6 years, 76% (male), and 42% had a baseline Deyo-Charlson Comorbidity Index ≥ 2. Baseline mean total all-cause direct healthcare costs were $51,473 ± $48,560 (median: $36,202), and $99,524 ± 86,839 (median: $75,444) during follow-up. At baseline, 32% of patients had ≥ 1 STD claim, equating to a mean 134 ± 303 hours lost and $2,353 ± $6,445 in total payments per patient. Follow up STD claims increased 23.4% equating to a mean 218 ± 324 hours lost and $3,679 ± $7,795 per patient. Patient LTD claims increased from baseline to follow-up (1% to 3%), with post-cystectomy LTD claims resulting in 574 ± 490 hours lost, and $1,636 ± $1,429 in total payments. Over 85% of the population had a cystectomy related complication, the most common were genitourinary-related (47.9%) and infection/sepsis (33.1%).
Conclusions: Cystectomy was associated with complications and decreased work productivity post-surgery. Findings may aid to inform decisions regarding cystectomy vs. bladder preservation approaches, and underscores an ongoing need to further develop bladder preservation therapies within the bladder cancer treatment landscape.
Keywords:
Disclaimer
As a service to authors and researchers we are providing this version of an accepted manuscript (AM). Copyediting, typesetting, and review of the resulting proofs will be undertaken on this manuscript before final publication of the Version of Record (VoR). During production and pre-press, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal relate to these versions also.INTRODUCTION
Bladder cancer is the 4th most common cancer in men, the 12th most common cancer in women, and the 6th most common cancer in the United States (U.S.) overall.1 In the U.S. alone, there are more than 80,000 new cases of bladder cancer each year.2 A radical cystectomy with pelvic lymph node dissection involves excising the whole bladder and reproductive organs, and nearby lymph nodes.3 The intestines are used to perform urinary diversion either by a conduit or neobladder. A partial cystectomy involves removal of only part of the bladder and can preserve urine function. The goal of cystectomy is to eliminate the chance of bladder cancer from metastasizing to other parts of the body.
The appropriateness and timing of partial or radical cystectomy course of care depends on the type and stage of bladder cancer at the time of diagnosis.8,9 Most patients with bladder cancer (70%) present with non-muscle invasive bladder cancer (NMIBC) and the remaining present with MIBC or de novo metastatic disease.4-6 Patients with NMIBC are classified as low-, intermediate-, or high-risk. Those with intermediate or high risk, including those with high grade tumors and carcinoma in situ, are initially treated with intravesical therapies like bacillus Calmette–Guérin (BCG) or intravesical chemotherapy (e.g. gemcitabine and docetaxel) and radical cystectomy would be used as a salvage curative treatment for those with BCG-refractory disease.7 The standard of care treatment for MIBC for patients who are cisplatin-eligible, appropriate for cisplatin-based chemotherapy (without renal impairment, history of stroke/heart disease, and other exclusions), and strong enough for major surgery, is currently neoadjuvant cisplatin-based chemotherapy followed by radical cystectomy.1 However, patients with MIBC who are cisplatin-ineligible are also treated with curative-intent radical cystectomy (without neoadjuvant chemotherapy). Patients with NMIBC and MIBC with focal tumors may be able to undergo a partial cystectomy rather than radical cystectomy.
Overall, bladder cancer treatments are associated with a significant economic burden due to high resource utilization, inclusive of hospital, laboratory, and pharmacy costs,8 contributing to an estimated 6 billion dollars annually in the U.S.9-11 Radical cystectomies alone contribute to an estimated $29,000 per patient in direct medical costs.12 Because patients undergoing a radical cystectomy may take several weeks to months to recover,13 indirect costs accrue. Recovery time may lead to prolonged hospitalizations and reduced work productivity due to absences, short-term disability (STD), or long-term disability (LTD) leave.
Radical cystectomies are associated with high rates of clinical complications following the procedure.14,15 Complications include poor cardiovascular, pulmonary, gastrointestinal, hematological, renal, metabolic, and neurological outcomes as well as infection.16 Some of the costliest complications were venous thromboembolism, infection, wound complications, and pulmonary complications.17 Further, the outcomes of cystectomy can negatively affect patients’ quality of life, including emotional, sexual and social functioning.13,18 Previous studies measured common postoperative complications of radical cystectomy and found that hospital costs of patients with postoperative complications following radical cystectomy are significantly higher than patients without postoperative complications, however, indirect costs were not included.16,17
The present study aimed to develop a better understanding of the full scope of surgical and post-surgical burden of cystectomy by quantifying the direct and indirect costs associated with a cystectomy procedure, as well as the frequency of post-operative complications, among a sample of patients with bladder cancer.
METHODS
Design and Data Source
This was a retrospective cohort study of the MarketScan Commercial Claims & Encounters Databases and the Health & Productivity Management (HPM) Databases between October 1, 2015, to December 31, 2020. The MarketScan Commercial Claims & Encounters Databases contain the inpatient, outpatient, and outpatient prescription-drug experience of several million employees and their dependents covered under a variety of fee-for-service and capitated health plans, including exclusive provider organizations (EPO), preferred provider organizations (PPOs), point of service (POS) plans, indemnity plans, and health maintenance organizations. The HPM databases contain workplace absence STD, LTD, and worker’s compensation data for a subset of employer clients that contribute data to the Commercial Database. These employers represent diverse industries, employing both hourly and salaried workers, and are geographically dispersed throughout the US. The data are linked to member healthcare claims.19
Sample Selection
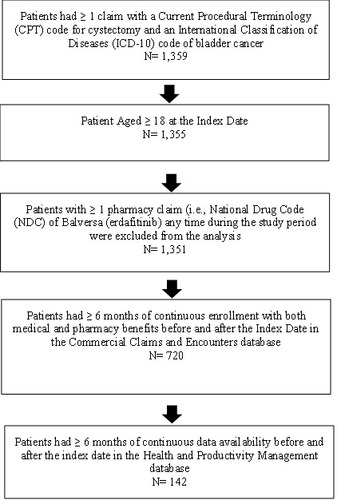
All patients were required to present ≥ 1 claim with an International Classification of Diseases, Tenth Revision (ICD-10) code for bladder cancer and a partial or radical cystectomy procedure on the same visit during the indexing which spanned April 1, 2016 to July 1, 2020 (which allowed for a minimum 6-month baseline and follow-up period). The earliest claim with a cystectomy procedure served as the study index date. Patients were required to be ≥ 18 years of age at index with ≥ 6 months of continuous enrollment with both medical and pharmacy benefits in the time preceding (baseline period) and following the index date (follow-up period). Utilization and costs incurred on the index date are included in the follow-up period. Additionally, patients were required to have ≥ 6 months of continuous data availability in the HPM database in the time preceding and following the index date. Patients were excluded if they presented with ≥ 1 pharmacy claim for erdafitinib any time during the study period, as this treatment is only indicated for metastatic or locally advanced urothelial cancer and not NMIBC or MIBC (Figure 1).
Study Measures
Patient Demographics and Clinical Characteristics
Demographic characteristics included patient age, gender, census divisions, insurance type, and employment status, all measured on the index date. Clinical characteristics including the Charlson Comorbidity Index (CCI) score and commonly reported comorbid conditions among patients with bladder cancer were measured via ICD-10 diagnoses during the 6-month baseline period.20 Bladder cancer-related chemotherapy treatments including cisplatin, doxorubicin, gemcitabine, methotrexate, and vinblastine, identified via procedure codes and/or National Drug Codes (NDC), were only measured during the 6-month baseline period.
Direct Costs
All-cause and bladder cancer-related costs were measured during the baseline and follow-up periods. Costs associated with the index are included in the follow-up period. Cost categories included physician office/clinic, emergency room visits, hospitalizations, and 30-day readmissions, other outpatient, pharmacy, total medical costs, and total healthcare costs. Bladder cancer-related costs included any inpatient admission with a primary diagnosis of bladder cancer, any outpatient claim with a bladder cancer diagnosis anywhere on the claim, and any prescription claim for a treatment indicated for bladder cancer. Healthcare costs were calculated from adjudicated claims and include both insurer and health plan payments, as well as patient cost-sharing in the form of copayment, deductible, and coinsurance. Costs were adjusted for inflation to 2021 dollars using the medical care component of the Consumer Price Index obtained from the U.S. Bureau of Labor Statistics.21
Indirect Costs
Indirect costs were assessed during the baseline and the follow-up periods and included work loss outcomes attributable to absence, STD, and LTD. Measures of indirect costs for absence, STD, and LTC included the number of patients with ≥ 1 claim, the total number of work hours lost, and the estimated productivity loss attributable to work hours lost. Costs attributable to STD and LTD were based on the total paid amounts appearing on STD and LTD claims. Costs attributable to time lost due to absence was estimated by multiplying the number of days absent by a percentage of an estimated daily wage based on the 2021 age-, sex-, and geographic region-adjusted mean wage rate reported by the US Bureau of Labor Statistics,24 which is the standard approach to monetizing the absence data.23,25,26
Post-Cystectomy Complications
The proportion of patients with a post-cystectomy complications were assessed through ICD-10 diagnosis and/or procedures codes occurring on any medical claim during the follow up period and further reported by those occurring between 0-30 days post-cystectomy and 31-180 days post-cystectomy. The strategy for identifying and codifying post-cystectomy complication follows Peyton et al. methodology.14 Complications were categorized as cardiac, gastrointestinal, genitourinary, hemorrhage/bleeding, infection and sepsis, miscellaneous and surgical, neurologic, renal-related (includes renal failure, metabolic disturbances, dehydration, failure to thrive, and malnutrition), respiratory, thrombosis/embolic, and wound and hernia.14
To examine the presence of any bias among the subset of patients appearing in the HPM database, cystectomy complication rates of the final study sample were compared to rates of a larger sample of patients with bladder cancer receiving a cystectomy appearing in the MarketScan commercial database who did not appear in the HPM database.
Statistical Analyses
All analyses were descriptive in nature; means and standard deviations (SD) were presented for continuous variables, while frequencies and proportions were presented for categorical variables.
RESULTS
Sample Characteristics
A total of 142 patients with bladder cancer met all sample selection criteria (Figure 1). The majority of patients were male gender (76%), with a mean ± SD age of 56 ± 6 years (Table 1).
Patients presented a mean ± SD CCI of 2 ± 1, with the majority of patients (61%) presented with a CCI score ≥ 2. The most common baseline comorbidities include hypertension (48%), tobacco use (36%), cardiovascular disease (21%), anxiety (16%), and gastroesophageal reflux disease (GERD; 14%). Common previous bladder cancer related treatments include cisplatin (42%), gemcitabine (23%), methotrexate (23%), doxorubicin (22%), and vinblastine (22%; Table 1).
Direct Costs
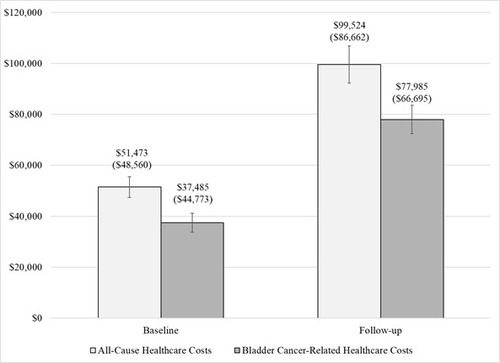
During the baseline period, the total mean ± SD direct healthcare costs were $51,473 ± $48,560 (median: $36,202; IQR: $54,012), which increased two-fold to $99,524 ± $86,839 (median: $75,444; IQR: $63,309) during the post-index period (Figure 2). Baseline total healthcare costs were driven by costs associated with physician office visits (mean ± SD $41,843 ± $45,068; median: $25,748; IQR: $44,404 Table 2).
Bladder-cancer related healthcare costs contributed to 78% of the total mean follow-up healthcare costs ($77,985 ± $66,685; median: $67,028; IQR: $59,536).
A supplemental analysis of patients with at least 1 claim of neoadjuvant treatment with cisplatin during the baseline period (N = 59 patients) confirmed patients receiving such treatment were driving the baseline physician costs. Patients receiving neoadjuvant treatment had total mean ± SD all-cause healthcare costs and bladder cancer-related costs of $74,211± $38,845 (median: $70,356; IQR: $47,143) and $58,169 ± $35,687 (median: $56,450; IQR: $45,171), respectively, per patient at baseline (Supplementary Table 1).
During follow-up, inpatient costs, including costs associated with the cystectomy, contributed to 77% of total healthcare costs (mean ± SD: $76,727 ± $76,724; median: $58,201; $59,530). Further, 30% of total follow-up healthcare costs were associated with cystectomy complications, $29,864 ± $39,554 (median: $16,083; IQR: $32,910).
Indirect Costs
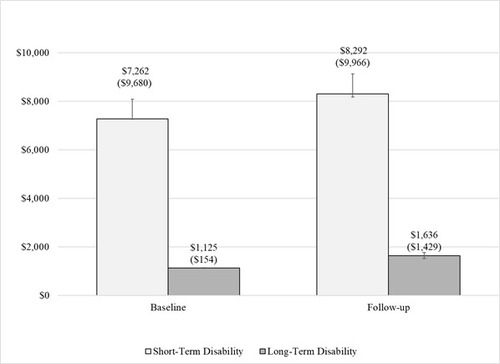
Compared to the baseline period, the proportion of patients claiming work loss during the follow-up period increased for STD claims (32% to 44%), LTD claims (1% to 3%) and workplace absence (5% to 6%). Among the full sample, mean STD costs increased 56% from the baseline through the follow up period from $2,353 ± $6,445 (median: $0) to $3,679 ± $7,795 (median: $0). Among patients with ≥1 STD claim, baseline and follow-up mean ± SD STD costs were $7,263 ± $9,680 and $8,292 ± $9,966, respectively (Figure 3). Among patients with ≥1 LTD claim, baseline and follow-up mean ± SD LTD costs were $1,125 ± $154 and $1,636 ± $1,429, respectively.
Post Cystectomy Complications
During the 6-month follow-up period, 85% of patients, experienced a post-cystectomy complication. Overall, the most common complications were genitourinary (48%), followed by renal-related (40%), gastrointestinal-related (34%), and infection/sepsis (33%; Table 3). During the first 30 days following a cystectomy, 73% of patients experienced a post-cystectomy complication, while 59% of patients experienced a post-cystectomy complication during days 31-180. The most common complications occurring during the first 30 days were renal-related (36%), genitourinary (30%) and hemorrhage/bleeding (23%; Table 3), while, genitourinary (29%), gastrointestinal (23%) and infection/sepsis (22%) were the most common complications during the 31-180-day post-cystectomy period. Further analyses comparing complication rates to the non-HPM sample revealed that those patients who do not appear in the HPM database had similar rates and types of cystectomy complications as those in the current sample (Supplementary Table 2).
DISCUSSION
This retrospective, observational database study was conducted to describe patient characteristics, and direct and indirect costs in patients with bladder cancer who recently underwent a cystectomy procedure. Results from the present study demonstrate the notable economic burden associated with a cystectomy procedure for the treatment of bladder cancer. Cystectomy introduced considerable additional costs in the follow-up period. Further, workplace absences along with short- and long-term disability claims following a cystectomy contributed notable indirect costs, which are likely attributable to the known post-surgical burden following the procedure. A majority of patients included in the present study presented a complication following the cystectomy, and with only a 6-month follow-up period, rates of complication may be an underestimation of year-over-year complication rates and costs.
Recently, data supporting bladder preservation has increased the attention for these alternate therapies.27 Intravesical gemcitabine and docetaxel,28 systemic pembrolizumab,29 nadofaregene firadenovec-vncg,30 and N-80331 have all shown activity as potential agents to treat BCG-refractory NMIBC, with pembrolizumab and nadofaragene firadenovec having FDA approval for this indication. Retrospective studies of similar oncological outcomes also offer support of alternate therapies to cystectomy.32,33 However, trimodality therapy (TMT) with chemoradiation, is also not without its own cost considerations. Prior cost-effectiveness studies comparing TMT to radical cystectomy have suggested that TMT may be associated with higher short-term and long-term costs,34,35 which may limit the cost-effectiveness of this therapy. One limitation of prior studies is that they have not accounted for the indirect costs associated with therapies, which could potentially be higher with cystectomy given the high rates of complication and longer recovery times associated with this intervention. Overall, our study adds to the literature by quantifying the costs associated radical local therapies for bladder cancer and further extends this literature by estimating indirect cost associated with cystectomy.
Previous studies have examined the indirect costs associated with workplace absence among patients with other bladder and pelvic conditions, including overactive bladder and endometriosis,36 37 25 38 though there is limited evidence evaluating the impact of a cystectomy on these outcomes. However, new research by Ahlschlager et al. finds 17.5% and 18.8% absenteeism, defined as the percent of work time lost due to health, of a combined sample of NMIBC and MIBC patients after bladder preserving and radical cystectomy, respectively; these findings are lower than the percent work time lost attributing to indirect costs in this study.39 Similar to the present study, between 49.4% - 56.0% of patients have been shown to file a STD claim following a cancer diagnosis,14, 16 and this proportion increases following a surgical intervention.
The cost and clinical impact of the cystectomy procedure was also evident in analysis, with high costs of inpatient hospitalization and over 85% of patients experiencing a complication in the 6-month period following the procedure. It should be noted that measures of dispersion (standard deviation, IQR) were high for cost measures, indicating substantial variability in costs among this sample, but also in line with economic analyses leveraging healthcare data.40-43 Inpatient hospitalization costs associated with the cystectomy procedure and related complications were the main drivers of the increase in direct healthcare costs in the post-cystectomy period. A previous study by Peyton et al. reported that approximately 75% of patients receiving a cystectomy experienced a complication during the first 30 days following a cystectomy,14 which aligns with the rate observed in the current study for the first 30-days following the procedure (72.5%). Interestingly, Peyton et al. reported infection/sepsis as the most common complication post-cystectomy (34.7%), whereas the current study identified renal-related complications as the most common complication (35.9%).17 However, Peyton et al.’s sample was entirely patients who received a radical cystectomy whereas the present study includes both partial and radical cystectomy. The differences in complication rates between the studies may also be attributable to the level of detail in the underlying data source, with Peyton et al. leveraging a cystectomy registry database, whereas the present study utilized administrative claims data. Lower rates of select complications observed in the current study, such as infections (21.8%), could be due to improvements to surgical techniques, as a recent study evaluated annual trends in cystectomy complications from 2006-2018 and reported a decrease in urinary tract infections.15 Further, as the MarketScan commercial claims are largely generated from employer claims, the present sample of patients with bladder cancer are likely younger (mean age of 56) than samples appearing in other investigations.
One potential alternate and possibly more cost-effective and better tolerated approach under investigation for MIBC is the use of risk-enabled therapy.44 This approach has utilized systemic anti-cancer therapy with chemotherapy and/or immunotherapy with subsequent disease surveillance among those with clinical complete response. Here omission of any radical therapy in the appropriately selected patients may be a clinically effective approach and would remove the most costly intervention (radical therapy – either cystectomy or TMT) in MIBC management.
Limitations and Strengths
There are several limitations to be noted. First, this study was limited to only those individuals <65 years of age with continuous commercial health coverage, so results may not be generalizable to the overall population of patients with bladder cancer, who present an average age of diagnosis of 73,45 and particularly to patients with bladder cancer receiving Medicare benefits or without insurance at all. A large proportion (71%) of the sample was employed full time so results may not be generalizable to a retired, underemployed, or unemployed population. Also, patients in the HPM database represent a small subset of the overall sample available in the MarketScan databases, which resulted in a total of just 142 patients included in the sample, though this is not atypical for analyses using the MarketScan indirect cost data.26 Regardless, all study results may not translate to the overall population found in the MarketScan database. However, a supplemental analysis of a subset of patients with bladder cancer receiving a cystectomy procedure who did not appear in the HPM database was conducted, and those patients had similar rates and types of cystectomy complications. Additionally, for some patients in the HPM database, costs directly associated with absences were unavailable, therefore an approach in which imputation of costs was adopted similar to other published analyses conducted using these data,23 though these estimates will not be a perfect reflection of the actual costs associated with a workplace absence. Specific reasons for the time missed from work may also not be clearly delineated in the database, and bladder cancer may not necessarily account for all observed productivity losses. It is also difficult to discern NMIBC and MIBC patient subtypes in claims data, and the treatment pathways prior to cystectomy are likely different across these bladder cancer subtypes. Also, the current sample included patients who received either a partial or a full cystectomy procedure and does not distinguish between the two groups or report results separately for the two groups, though recent analyses demonstrate comparable costs between the two procedures.8 Further, this study does not differentiate costs between radical cystectomy with conduit and radical cystectomy with neobladder, and future research of associated costs, complications, and patient burden should consider this distinction.
Finally, STD and LTD were lower than anticipated for the sample. For a subset of patients, a portion of the data collection period coincided with the COVID-19 pandemic, during which time there was a restriction in access to care in the U.S. This may have also affected indirect costs, as patients were more likely to telework during this period, potentially decreasing the likelihood of filing for disability.
A strength of our study is that while previous studies have relied on transurethral resection index dates,46 this study’s cystectomy index date allows for a more precise measure of cystectomy-related complication.
CONCLUSIONS
Our study provides real-world evidence of the substantial burden radical cystectomy imposes on patients with bladder cancer and to society, which is associated with increased direct healthcare costs, along with decreased work productivity following the procedure and its complications. By including indirect costs associated with cystectomy, this analysis begins to provide a broader characterization of the economic burden of bladder cancer in the U.S. These analyses highlight the notable post-surgical clinical burden associated with this procedure. Future research should examine the longer-term impacts of radical cystectomy from both a direct and indirect costs perspective, as some complications and disability may extend beyond six months, in addition to examining the indirect costs associated with other treatment modalities. Regardless, results demonstrate the need for additional bladder-sparing treatment options within the bladder cancer treatment landscape, which may aid to improve patient quality of life and reduce direct and indirect healthcare costs.
Transparency
Declaration of funding
This study was funded by Janssen Pharmaceuticals.
Declaration of financial/other relationships
AI, HB, LE are employees of Janssen. JT and BA are employees of Inovalon, which received consulting fees from Janssen.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Authors' contributions
JT, AI, LE, BA participated in conception and design of study; JT, AI and BA oversaw the statistical analysis; all authors reviewed and participated in interpretation of findings. All authors contributed to the development of the manuscript. All authors read and approved the final manuscript.
Table 1. Baseline Demographic and Clinical Characteristics
Table 2. Specific Healthcare Costs at Baseline and Follow-Up
Table 3. Post-Cystectomy Complications During the Follow-up Period
Supplemental Material
Download MS Word (57 KB)Supplemental Material
Download MS Word (67.6 KB)REFERENCES
- National Cancer Institute. Bladder Cancer Treatment (PDQ®)–Health Professional Version. Updated 01/18/2023. Accessed 06/13/2023, https://www.cancer.gov/types/bladder/hp/bladder-treatment-pdq
- National Cancer Institute. Cancer Stat Facts: Bladder Cancer. Accessed 06/13/2023, https://seer.cancer.gov/statfacts/html/urinb.html
- Perera M, McGrath S, Sengupta S, Crozier J, Bolton D, Lawrentschuk N. Pelvic lymph node dissection during radical cystectomy for muscle-invasive bladder cancer. Nat Rev Urol. Nov 2018;15(11):686-692. doi: 10.1038/s41585-018-0066-1.
- Fernandez-Gomez J, Madero R, Solsona E, et al. Predicting nonmuscle invasive bladder cancer recurrence and progression in patients treated with bacillus Calmette-Guerin: the CUETO scoring model. J Urol. Nov 2009;182(5):2195-203. doi: 10.1016/j.juro.2009.07.016.
- Sylvester RJ, van der Meijden AP, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. Mar 2006;49(3):466-5; discussion 475-7. doi: 10.1016/j.eururo.2005.12.031.
- Chen S, Zhu S, Cui X, et al. Identifying non-muscle-invasive and muscle-invasive bladder cancer based on blood serum surface-enhanced Raman spectroscopy. Biomed Opt Express. Jul 1 2019;10(7):3533-3544. doi: 10.1364/BOE.10.003533.
- Powles T, Bellmunt J, Comperat E, et al. Bladder cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. Mar 2022;33(3):244-258. doi: 10.1016/j.annonc.2021.11.012.
- Bagheri I, Shan Y, Klaassen Z, et al. Comparing Costs of Radical Versus Partial Cystectomy for Patients Diagnosed With Localized Muscle-Invasive Bladder Cancer: Understanding the Value of Surgical Care. Urology. Jan 2021;147:127-134. doi: 10.1016/j.urology.2020.08.058.
- Williams SB, Howard LE, Foster ML, et al. Estimated Costs and Long-term Outcomes of Patients With High-Risk Non-Muscle-Invasive Bladder Cancer Treated With Bacillus Calmette-Guerin in the Veterans Affairs Health System. JAMA Netw Open. Mar 1 2021;4(3):e213800. doi: 10.1001/jamanetworkopen.2021.3800.
- Nguyen PL, Gu X, Lipsitz SR, et al. Cost implications of the rapid adoption of newer technologies for treating prostate cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 04/20/2011 2011;29(12) doi: 10.1200/JCO.2010.31.1217.
- Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. Jan 19 2011;103(2):117-28. doi: 10.1093/jnci/djq495.
- Hoeh B, Flammia RS, Hohenhorst L, et al. Regional differences in total hospital costs for radical cystectomy in the United States. Surg Oncol. Jun 2023;48:101924. doi: 10.1016/j.suronc.2023.101924.
- Cleveland Clinic. Cystectomy. Updated 11/17/2022. Accessed 06/13/2023, https://my.clevelandclinic.org/health/treatments/21049-cystectomy
- Peyton CC, Reich RR, Tang D, et al. Identifying and Codifying Complications after Radical Cystectomy: Comparison of Administrative Diagnostic and Procedure Codes, and Clinical Chart Review. J Urol. Nov 2019;202(5):913-919. doi: 10.1097/JU.0000000000000398.
- Chua KJ, Patel HV, Srivastava A, et al. Annual trends of cystectomy complications: A contemporary analysis of the NSQIP database. Urol Oncol. May 26 2023; doi: 10.1016/j.urolonc.2023.03.014.
- Weinberg L, Aitken SAA, Kaldas P, et al. Postoperative complications and hospital costs following open radical cystectomy: A retrospective study. PLoS One. 2023;18(2):e0282324. doi: 10.1371/journal.pone.0282324.
- Mossanen M, Krasnow RE, Lipsitz SR, et al. Associations of specific postoperative complications with costs after radical cystectomy. BJU international. 2018;121(3):428-436.
- Mak KS, Smith AB, Eidelman A, et al. Quality of Life in Long-term Survivors of Muscle-Invasive Bladder Cancer. Int J Radiat Oncol Biol Phys. Dec 1 2016;96(5):1028-1036. doi: 10.1016/j.ijrobp.2016.08.023.
- Merative. Merative MarketScan Research Databases. Accessed June 17, 2024, https://www.merative.com/documents/brief/marketscan-explainer-general
- Barone B, Finati M, Cinelli F, et al. Bladder Cancer and Risk Factors: Data from a Multi-Institutional Long-Term Analysis on Cardiovascular Disease and Cancer Incidence. J Pers Med. Mar 13 2023;13(3) doi: 10.3390/jpm13030512.
- U.S. Bureau of Labor Statistics. Measuring Price Change in the CPI: Medical care. Updated 02/10/2023. Accessed 06/13/2023, https://www.bls.gov/cpi/factsheets/medical-care.htm
- Goetzel RZ, Long SR, Ozminkowski RJ, Hawkins K, Wang S, Lynch W. Health, absence, disability, and presenteeism cost estimates of certain physical and mental health conditions affecting U.S. employers. J Occup Environ Med. Apr 2004;46(4):398-412. doi: 10.1097/01.jom.0000121151.40413.bd.
- Settipane RA, Kreindler JL, Chung Y, Tkacz J. Evaluating direct costs and productivity losses of patients with asthma receiving GINA 4/5 therapy in the United States. Ann Allergy Asthma Immunol. Dec 2019;123(6):564-572 e3. doi: 10.1016/j.anai.2019.08.462.
- Statistics UBoL. Consumer Price Index: Medical Care. Medical care in U.S. city average, all urban consumers, not seasonally adjusted. . https://www.bls.gov/cpi/factsheets/medical-care.htm
- Soliman AM, Taylor HS, Bonafede M, Nelson JK, Castelli-Haley J. Incremental direct and indirect cost burden attributed to endometriosis surgeries in the United States. Fertil Steril. May 2017;107(5):1181-1190 e2. doi: 10.1016/j.fertnstert.2017.03.020.
- Jerry M, Arcona S, McMorrow D, Schwartz H, Princic N, Sasane R. Work Loss and Direct and Indirect Costs Associated with Parkinson's Disease. Clinicoecon Outcomes Res. 2023;15:309-319. doi: 10.2147/CEOR.S398509.
- Miron B, Hawley JE, Geynisman DM, et al. Clinical Trial Considerations for Bladder Preservation in Muscle-Invasive Bladder Cancer. Advances in Oncology. 2022;2(1):213-225.
- Steinberg RL, Thomas LJ, Brooks N, et al. Multi-Institution Evaluation of Sequential Gemcitabine and Docetaxel as Rescue Therapy for Nonmuscle Invasive Bladder Cancer. J Urol. May 2020;203(5):902-909. doi: 10.1097/ju.0000000000000688.
- Balar AV, Kamat AM, Kulkarni GS, et al. Pembrolizumab monotherapy for the treatment of high-risk non-muscle-invasive bladder cancer unresponsive to BCG (KEYNOTE-057): an open-label, single-arm, multicentre, phase 2 study. Lancet Oncol. Jul 2021;22(7):919-930. doi: 10.1016/s1470-2045(21)00147-9.
- Boorjian SA, Alemozaffar M, Konety BR, et al. Intravesical nadofaragene firadenovec gene therapy for BCG-unresponsive non-muscle-invasive bladder cancer: a single-arm, open-label, repeat-dose clinical trial. Lancet Oncol. Jan 2021;22(1):107-117. doi: 10.1016/s1470-2045(20)30540-4.
- Chamie K, Chang SS, Gonzalgo M, et al. Final clinical results of pivotal trial of IL-15RαFc superagonist N-803 with BCG in BCG-unresponsive CIS and papillary nonmuscle-invasive bladder cancer (NMIBC). American Society of Clinical Oncology; 2022.
- Zlotta AR, Ballas LK, Niemierko A, et al. Radical cystectomy versus trimodality therapy for muscle-invasive bladder cancer: a multi-institutional propensity score matched and weighted analysis. Lancet Oncol. Jun 2023;24(6):669-681. doi: 10.1016/s1470-2045(23)00170-5.
- Swinton M, Mariam NBG, Tan JL, et al. Bladder-Sparing Treatment With Radical Dose Radiotherapy Is an Effective Alternative to Radical Cystectomy in Patients With Clinically Node-Positive Nonmetastatic Bladder Cancer. J Clin Oncol. Sep 20 2023;41(27):4406-4415. doi: 10.1200/jco.23.00725.
- Seisen T, Sun M, Lipsitz SR, et al. Comparative Effectiveness of Trimodal Therapy Versus Radical Cystectomy for Localized Muscle-invasive Urothelial Carcinoma of the Bladder. Eur Urol. Oct 2017;72(4):483-487. doi: 10.1016/j.eururo.2017.03.038.
- Golla V, Shan Y, Farran EJ, et al. Long term cost comparisons of radical cystectomy versus trimodal therapy for muscle-invasive bladder cancer. Urol Oncol. Jun 2022;40(6):273.e1-273.e9. doi: 10.1016/j.urolonc.2022.01.007.
- Durden E, Walker D, Gray S, Fowler R, Juneau P, Gooch K. The Direct and Indirect Costs Associated With Overactive Bladder Within a Commercially-Insured Population in the United States. J Occup Environ Med. Sep 2018;60(9):847-852. doi: 10.1097/JOM.0000000000001367.
- Cong Z, Tran O, Nelson J, Silver M, Chung K. Productivity Loss and Indirect Costs for Patients Newly Diagnosed with Early- versus Late-Stage Cancer in the USA: A Large-Scale Observational Research Study. Appl Health Econ Health Policy. Nov 2022;20(6):845-856. doi: 10.1007/s40258-022-00753-w.
- Parasuraman S, Thiel E, Park J, Teschemaker A. Productivity loss outcomes and costs among patients with cholangiocarcinoma in the United States: an economic evaluation. J Med Econ. Jan-Dec 2023;26(1):454-462. doi: 10.1080/13696998.2023.2187604.
- Ahlschlager L, McCabe S, Deal AM, et al. The effect of treatment on work productivity in patients with bladder cancer. Urol Oncol. Jun 2023;41(6):293.e15-293.e21. doi: 10.1016/j.urolonc.2023.01.020.
- Dormont B, Milcent C. The sources of hospital cost variability. Health Economics. 2004;13(10):927-939. doi: 10.1002/hec.935.
- Jacobs K, Roman E, Lambert J, et al. Variability drivers of treatment costs in hospitals: A systematic review. Health Policy. 2022;12(2):75-86. doi: 10.1016/j.healthpol.2021.12.004.
- Mihaylova B, Briggs A, O'Hagan A, Thompson S. Review of statistical methods for analysing healthcare resources and costs. Health Economics. 2011;20(8):897-916. doi: 10.1002/hec.1653.
- Malehi A, Pourmotahari F, Angali K. Statistical models for the analysis of skewed healthcare cost data: a simulation study. Health Economics Review. 2015;27(5) doi: 10.1186/s13561-015-0045-7.
- Geynisman DM, Broughton E, Hao Y, Zhang Y, Le T, Huo S. Real-world treatment patterns and clinical outcomes among patients with advanced urothelial carcinoma in the United States. Urol Oncol. May 2022;40(5):195.e1-195.e11. doi: 10.1016/j.urolonc.2021.11.014.
- American Cancer Society. Key Statistics for Bladder Cancer. Accessed June 17th, 2024, https://www.cancer.org/cancer/types/bladder-cancer/about/key-statistics.html
- Malangone-Monaco E, Wilson K, Diakun D, Tayama D, Satram S, Ogale S. Cost of cystectomy-related complications in patients with bladder cancer in the United States. Curr Med Res Opin. Jul 2020;36(7):1177-1185. doi: 10.1080/03007995.2020.1758927.