Abstract
Objectives: To evaluate the cost-effectiveness of budesonide/formoterol reliever and maintenance therapy compared with salmeterol/fluticasone plus salbutamol as reliever therapy for asthma patients ≥12 years from the societal perspective in China.
Methods: A Markov model was developed with three health states (non-exacerbation, exacerbation, and death) with a lifetime horizon. The exacerbation rates were obtained from a prospective cohort study conducted in Chinese asthma patients. Healthcare resources utilization data were estimated based on current clinical asthma management guidelines. Asthma-related mortality, cost input and utility values were derived from public database and literature. Model robustness was assessed with one-way sensitivity and probabilistic sensitivity analyses.
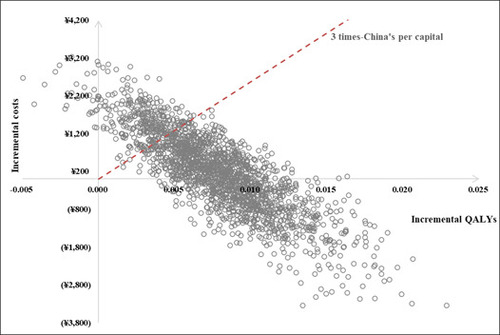
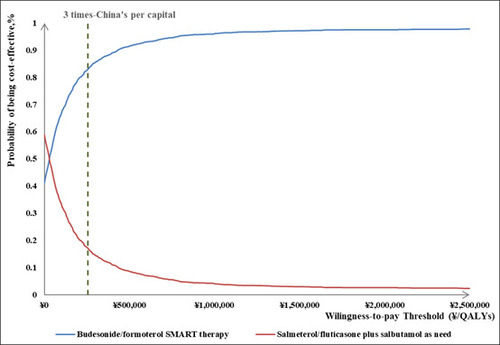
Results: Compared with salmeterol/fluticasone plus salbutamol, budesonide/formoterol reliever and maintenance therapy led to fewer exacerbation events (13.6 vs. 15.9) and 0.0077 quality-adjusted life years (QALY) gain at an additional cost of ¥196.38 over lifetime. The base case incremental cost-effectiveness ratio (ICER) was ¥25,409.98 per QALY gained. The variables that had most impact on the model output included drug costs and medication adherence. At a willingness-to-pay threshold of ¥257,094/QALY (3 times of gross domestic product per capita in China in 2022), the probability of budesonide/formoterol maintenance and reliever therapy being cost-effective versus salmeterol/fluticasone plus as-needed salbutamol was 83.00%.
Conclusion: From the societal perspective, budesonide/formoterol reliever and maintenance therapy is likely to be a cost-effective option compared with salmeterol/fluticasone plus as-needed salbutamol for Chinese asthma patients ≥12 years.
Disclaimer
As a service to authors and researchers we are providing this version of an accepted manuscript (AM). Copyediting, typesetting, and review of the resulting proofs will be undertaken on this manuscript before final publication of the Version of Record (VoR). During production and pre-press, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal relate to these versions also.Introduction
As a chronic heterogeneous disease, asthma affects the respiratory system, causing inflammation, shortness of breath, and chest tightness. Various factors could trigger asthma symptoms, including allergens, weather change, respiratory infections, and exercise. Effective management and treatment of asthma are crucial for controlling symptoms and preventing severe attacks[1]. In severe cases, asthma exacerbation can be life-threatening.
China faces a significant public health concern related to asthma. A recent countrywide survey estimated that the prevalence of asthma was 4.2% in individuals aged 20 years or older while the prevalence of airflow limitation asthma was 1.1%[2]. Findings from the Global Burden of Disease (GBD) study showed that the prevalence rate in China was 1.97% in 2019[3]. Asthma patients experience a considerable higher disease burden compared to individuals without asthma. The GBD study ranked asthma the 8th leading cause of disability-adjusted life years’s (DALY) burden in China[4]. Research conducted in China has shown that asthma is associated with higher economic burden. Total medical costs were hand in hand with asthma severity and higher medical resource utilization was related to poor asthma control[5]. Despite ongoing updates in asthma management strategy, overall asthma control remains less optimal. Findings from a recent study[6] showed that only slightly over half of asthma patients in China was considered in good control.
Current treatment strategies recommended by the Global Initiative for Asthma (GINA) Strategy Report aim to reduce symptoms, prevent mortality and exacerbations, and improve lung function[1]. Compelling evidence[7-9] has shown that inhaled corticosteroids (ICS) and bronchodilators (such as long-acting β2-agonist, LABA and short-acting β2-agonist, SABA) are the cornerstone of asthma management. As a result, the guidelines recommend single inhaler maintenance plus reliever therapy (SMART), which consists of ICS and formoterol (one kind of LABA)[1]. SMART allows for better individualized asthma treatment as it could be used for relief alone or as maintenance therapy in combination with relief therapy as needed, effectively addressing inflammatory fluctuations. In recent years, novel therapies, such as targeted biologic therapy, including anti-IgE and anti-IL-5, are available for patients with uncontrolled asthma to improve clinical outcomes. In patients experiencing the disease exacerbation, oral corticosteroids or systemic corticosteroids may be prescribed. Non-pharmacological interventions, such as patient education, smoking cessation, and self-management strategies including exercise, are also crucial components of asthma management.
Budesonide/formoterol and salmeterol/fluticasone are the two most commonly prescribed drugs in clinical practice for asthma in China. Budesonide and fluticasone are corticosteroids that help reduce inflammation in the airways, while formoterol and salmeterol are LABAs that expand the airways and provide bronchodilation. Of these, budesonide/formoterol combination, known as one kind of maintenance and reliever therapy (SMART), is recommended as the first treatment for moderate to severe asthma by GINA[1] and is the only available medication for SMART therapy in China so far. As for patients using salmeterol/fluticasone, it is still necessary to take SABA as reliever medication, for which salbutamol is most commonly used in China. While the effectiveness and clinical value of both treatments are well documented, there is limited research investigating their economic value, specifically in Chinese population. Prior cost-effectiveness analyses were primarily focused on young children[10, 11]. Therefore, we conducted this health economic evaluation comparing budesonide/formoterol with fluticasone/salmeterol for adolescent and adult asthmatics in China to support clinical drug usage decision making.
Methods
Overview of the model
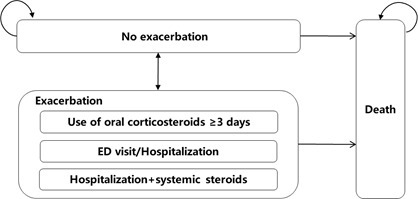
A Markov model was developed with the Microsoft Excel to estimate cost and quality-adjusted life-years (QALYs) of budesonide/formoterol reliever and maintenance therapy compared with salmeterol/fluticasone plus salbutamol as reliever therapy in asthma patients ≥12 years. Patients entered the model from no exacerbation state and could transit among three mutually exclusive health states (no exacerbation, exacerbation, and death) at each cycle (Figure 1). The asthma exacerbation state was further divided into three substates according to the severity and healthcare resource use: use of oral corticosteroids ≥3 days, emergency department (ED) visit or hospitalization, and hospitalization with systemic steroids. Considering that asthma is an incurable chronic disease, a lifetime horizon was selected to take into account the impact of disease on various aspects across patients’ whole life, e.g. quality of life, productivity, etc. The cycle length was set as 2 weeks to generally cover the recovery period after an exacerbation, which was been validated by a panel of clinical experts. Half-cycle correction and an annual discounting rate of 5% was applied to both costs and QALYs.
The model was developed from the Chinese societal perspective, with both direct healthcare costs and indirect costs considered. Costs were expressed in the 2023 Chinese Yuan (¥ and 1US$=7.0467¥).
Exacerbation rates data inputs
The exacerbation rates were derived from a prospective real-world observational study[12] that of 164 adolescent patients aged 12-17 years with persistent asthma who were assigned to receive budesonide/formoterol combination therapy (N = 82) or salmeterol/fluticasone plus terbutaline as need (N = 82) for a period of six months. The proportion of males was 63.4% and 59.75%, respectively, for these two patient groups. All patients in both treatment and control group were observed with the baseline forced expiratory volume in 1 second (FEV1) ≥ 50% and the mean FEV1 < 80% predicted, meanwhile had taken an as-needed reliever SABA for >4 of the last week, but with no more than 8 inhalations per day, which corresponds to the definition of moderate asthma in National Asthma Education and Prevention Program (NAEPP) Asthma Guidelines[13]. The number of patients reported with severe asthma exacerbations, which was defined as asthma deteriorations that lead to hospitalization/ED therapy and/or usage of oral corticosteroid therapy for a minimum of three days in this study was used to calculate the bi-weekly exacerbation rates (Table 1). In view of the difference in the population age between the source study and our model setting, it is assumed that the exacerbation risk of asthma patients beyond 18 years is similar to those aged 12-17 years, which has been validated by Chinese clinical experts. Assumption was also made that the efficacy of terbutaline and salmeterol as reliever therapy was the same.
Overall, the study reported a 93% adherence of maintenance therapy[12]. Prior research[14] showed that, relative to perfect adherence to maintenance therapy, adherence of 89% for ICS was associated with a 25% increase in asthma exacerbation. Since our literature review found no study examining the relationship between adherence to ICS/LABA and asthma exacerbation, we assumed the similar increased risk of asthma exacerbation in patients who did not adhere to ICS therapy. An exponential function was fitted to model the relationship between decrease in treatment adherence and the hazard ratio of asthma exacerbations (See Supplemental Material Figure S1). The medication adherence to budesonide/formoterol reliever and maintenance therapy and salmeterol/fluticasone were obtained from in-depth interviews of a panel of clinical experts. The key parameters regarding exacerbation rates and adherence rates are summarized in Table 1.
Mortality data inputs
For mortality, the age-specific mortality rate for Chinese population[15] by different age groups was used to estimate the transition probabilities from no exacerbation state to death. We assumed no increased risk of death in patients with good asthma control, which was also confirmed by our panel of clinical experts. For patients who experience the disease exacerbation, the exacerbation-related mortality by 3 age groups (12-16 years, 17-44 years and ≥ 45 years) from the study conducted by Watson et al[16] was used to differentiate the mortality of hospitalized asthma patients from that of those non-hospitalized. The model also used the hospitalization-related mortality with calibration, i.e., taking the maximum between the age-specific natural mortality and the exacerbation-related mortality.
Cost and health resource utilization data inputs
From the societal perspective, both direct medical costs and indirect costs were included in the model calculation. Key cost parameters are listed in Table 2.
The direct medical costs consist of drug acquisition, clinical monitoring follow-ups, costs associated with exacerbation treatment and adverse event (AE) management. Drug acquisition costs were calculated based on drug unit price, dosage, and the administration frequency. Given that drug procurement at provincial and municipal levels across China are typically fulfilled through bidding or competitive procurement processes and the bid prices vary across regions, the most recent average winning bid prices in all available provinces in China, obtained from a public drug price database[17], was used in the model. The mean inhalation of maintenance budesonide/formoterol and salmeterol/fluticasone was twice daily. The number of as-needed inhalations for salbutamol per day was obtained from the study conducted by Vogelmeier et al[18], a subgroup analysis on Asian populations of the COSMOS study, in which 206 asthma patients aged ≥ 16 years received salmeterol/fluticasone propionate 50 µg/250 µg bid plus salbutamol as need.
The bi-weekly monitoring cost for the regular following-up visits included the registration and examination fees. Patients with exacerbations were assumed to receive additional tests besides as-needed medication. The chosen three rescuing methods involved various health resources with different application frequency. The disease management costs per exacerbation were calculated as the weighted average of all the related health resource costs (See Supplemental Material Table S1). Costs for outpatient and inpatient services related to the exacerbation treatment were obtained from the official medical service fee schedules and were supplemented with panel interviews of clinical experts where data were not available.
The costs associated with productivity loss were estimated as the product of missed paid workdays and average daily wage. The daily wage was available from the National Bureau of Statistics of China[19]. For patients aged 12-20 years, the model included the productivity loss of their parents rather than their own. For asthmatics aged 20 years and above, only those employed incurred indirect costs for missed workdays. The employment rates across different age groups were available from the population census report in 2020[15]. These assumption on patients’ productivity was based on the Singaporean asthma economic burden study[20]. The average asthma-related work loss in patients with no exacerbations and their caregivers were also sourced from this Singapore study, and were calculated as the weighted average of absenteeism and presenteeism days. For patients with asthma exacerbation, the missed workdays were counted as one day for those non-hospitalized to obtain outpatient prescription and 6.4 days for those hospitalized to receive inpatient care[21]. Only those under the retirement age incurred indirect costs for missed workdays.
Utilities data inputs
The literature review found no available utility values for Chinese asthma patients, so we extracted utility value based on a meta-analysis[22] of utilities in asthmatics and assigned the utility to patients with “no exacerbation” state in the model. This meta-analysis included 40 studies and used a random-effect model to estimate the utility measured with different instruments. Since almost half of the involved studies were performed on European country, the uncertainty from the utility parameter was been explored in the probabilistic sensitivity analysis.
Disutility values for exacerbations with different management (use of oral corticosteroids ≥ 3 days, ED visit or hospitalization, and hospitalization with systemic steroids) were soured from a previous study[23]. As the disutility of an exacerbation in need of ED visit or hospitalization, while no systemic steroids, was not reported in this study, the value was assumed to be -0.15, which is the mid-point between those requiring systemic steroids and those requiring hospitalization with systemic steroids therapy. The disutility was only considered for the duration of exacerbation. Both the baseline utilities and the utility loss data are showed in Table 3.
Model assumptions
The model was simulated with the following key assumptions:
• Patients experiencing asthma exacerbations were assumed to transit back to “no exacerbation” or “death” state at the end of a simulation cycle[11, 24-26].
• A tri-monthly follow-up visit was assumed for patients in no exacerbation state as routine clinical follow-up as recommended by the latest Chinese asthma management clinical guidelines[27].
• None of patients with exacerbation in need of hospitalization would be admitted to intensive care unit (ICU).
• The simulated population were assumed to be in stable condition with age, i.e. the exacerbation rate used in the model would keep constant across the time horizon. This handling method was common in other economic models for asthma patients with a Markov model design and a lifetime horizon[25, 26].
• Salbutamol was chosen for the cost calculation since it was validated by clinical experts as the most frequently used as-needed SABA in China.
• Treatment adherence was assumed to affect both efficacy and drug costs in the model.
• The treatment adherence of as-needed therapy was assumed to be 100%.
Model outputs
The per capita costs and effectiveness over lifetime were calculated for each treatment regimen. The effectiveness outcomes include the number of exacerbation events, life years, and quality-adjusted life years (QALYs). The incremental cost-effectiveness ratio was also estimated to inform the cost-effectiveness of the interventions.
Uncertainty analysis
Both one-way sensitivity analysis (OWSA) and probabilistic sensitivity analysis (PSA) were performed to assess the robustness of the model. In the OWSA, one parameter input varied at a time to identify variables that had the greatest impact on the estimated ICER. Parameters were varied between the 95% CI, which were obtained from literature or estimated based on pre-specified distributions of the variables. The PSA was performed with Monte Carlo simulation of 2,000 iterations. Key parameters were sampled to vary concurrently according to the pre-specified distributions. The cost-effectiveness acceptability curve (CEAC) was presented to estimate the probability of budesonide/formoterol reliever and maintenance therapy being cost-effective under different thresholds compared with salmeterol/fluticasone plus salbutamol as reliever therapy. The main variables included in the sensitivity analyses and the 95% CI used, as well as the distributions are showed in Table 2.
China's volume-based procurement (VBP) program is a government-led policy for medicines and medical consumables with a long history of clinical use and high usage volumes. Through centralized procurement and negotiation, individual provinces can purchase medicines and medical consumables on a large scale at lower prices, thereby reducing medical costs and improving the accessibility of these medicines and consumables. Given that budesonide/formoterol and salmeterol/fluticasone have already been included in the VBP program in several provinces in China, which means that lowest possible prices are set, an exploratory scenario analysis was conducted to investigate the impacts of VBP on the model outcomes. The model was rerun with the unit prices for budesonide/formoterol and salmeterol/fluticasone replaced with the average of available VBP prices.
Result
Base case analysis
Compared with salmeterol/fluticasone plus salbutamol, budesonide/formoterol reliever and maintenance therapy resulted in fewer exacerbation events (13.56 vs 15.89) and 0.0077 QALY gains at an additional ¥196.38 total cost over lifetime (Table 4). The incremental cost-effectiveness ratio (ICER) of budesonide/formoterol reliever and maintenance therapy was ¥ 25,409.98 per QALY gained.
One-way sensitivity analysis
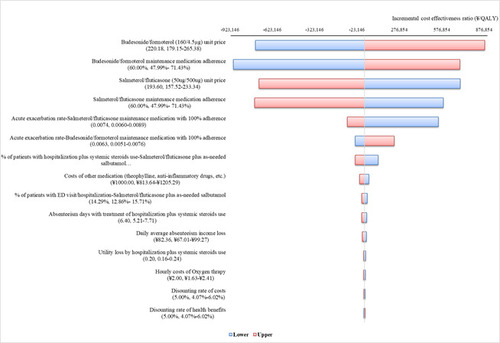
Findings from the OWSA (Figure 2) indicated that patient adherence to medication and drug costs had highest impact on the model output.
Probabilistic sensitivity analysis
The PSA findings indicated that when the cost-effective threshold was set at three times China's per capita gross domestic product (GDP), budesonide/formoterol reliever and maintenance therapy had an 83.00% probability of being considered cost-effectiveness and a 41.05% probability of dominance. The results of both sensitivity analyses are illustrated in Figures 2, 3 and 4.
Scenario analysis
When the VBP pricing was applied in the model (¥183.4 per unit for budesonide/formoterol and ¥159.8 per unit for salmeterol/fluticasone), the model projected a net gain of 0.0077 QALYs at the saving ¥90.53 when budesonide/formoterol maintenance and reliever therapy was used instead of salmeterol/fluticasone plus salbutamol as reliever therapy, i.e., budesonide/formoterol SMART therapy emerged as the dominant strategy over salmeterol/fluticasone plus salbutamol.
Discussion
Prior health economics studies on asthma management in China primarily had been primarily focusing on pediatric population[11, 24]. This is likely due to asthma being more prevalent in children and effective treatment during childhood can lead to long-term benefits[28, 29]. Nevertheless, asthma in adult population presents a significant healthcare burden and its clinical management remains less optimal[5]. Since budesonide/formoterol and salmeterol/fluticasone have been most widely used in clinical practice for many years, we conducted this economic evaluation to support patients' clinical medication choices.
Our study is the first economic evaluation focusing on moderate asthmatic patients aged 12 and above. Our model projects that asthma management with budesonide/formoterol in lieu of salmeterol/fluticasone could reduce acute exacerbations by 14.68% and gain an additional 0.0077 QALYs over lifetime at an additional cost of ¥196.38. With the threshold of three times China's per capita GDP, budesonide/formoterol should be considered cost-effective and the sensitivity analysis supports robustness of the model. In general, our findings are consistent with previous economic evaluations in middle-income countries, where budesonide‐formoterol SMART therapy was cost-effective. Buendía et al.[30] conducted a probabilistic Markov model analysis, comparing low-dose inhaled budesonide-formoterol as both maintenance and reliever therapy (SMART) to fixed combination of low dose ICS-LABA with as-needed SABA in adolescent and adults with moderate persistent asthma in Colombia. This study reported that patients with SMART therapy resulted in a higher probability of surviving free of exacerbation (0.84 for SMART and 0.79 for fixed combination) and a potential gain of 1.27 QALYs. A $4 reduction in total discounted cost per person-year compared to a fixed combination of ICS and LABA was also reported, indicating the cost-effectiveness of the budesonide-formoterol SMART regimen. Similarly, our study found that low-dose inhaled budesonide-formoterol was a cost-effective option for moderate persistent asthma management. This alignment with Buendía et al.'s results strengthens the evidence supporting the economic benefits of combination therapies in asthma management. The consistency across different healthcare settings and economic conditions suggests that these findings may be generalizable to other middle-income countries. In our study, the transition probability parameters and cost parameters used in the model calculations are based on the Chinese population and treatment scenarios in China, therefore, our findings provide relevant evidence to support medication choices for Chinese asthma patients.
In the consideration of study perspectives in health economic evaluation, the healthcare system perspective only includes all medical costs within the healthcare system, while the societal perspective considers all direct medical costs, direct non-medical costs, and indirect costs, which is considered to better reflect the overall economic burden of the disease. Economic evaluations from the societal perspective is also recommended by the current China Guidelines for Pharmacoeconomic Evaluations (2020) as a necessity for public decision-making[31]. Given that asthma is a chronic disease, our study was designed from the perspective of society to account for multifaceted patient burden. It is also worth noting that our model results showed that budesonide/formoterol remain cost-effective when only direct medical costs were included. In addition, both budesonide/formoterol and salmeterol/fluticasone are now increasingly accessible via the VBP, which is a common procurement model in China's healthcare system. Under this model, the government or insurance institutions negotiate prices by purchasing a specific quantity of drugs from designated healthcare service providers (such as hospitals or clinics). Procurement entities buy drugs from suppliers based on pre-agreed quantities and prices to control healthcare expenditures and ensure reasonable cost-effectiveness. The primary objective of VBP is to reduce drug procurement costs, improve the efficiency of the healthcare system, and promote the rational use of medications. As both budesonide/formoterol and salmeterol/fluticasone are part of China's government-led procurement in a number of provinces and major metropolitan cities, our scenario analysis incorporates the prices under VBP. The results showed that, if we apply the drug price as negotiated via process of volume-based procurement, budesonide/formoterol reliever and maintenance therapy would emerge as an advantageous strategy, providing evidence to support medication selection for most patients.
Our study has several limitations. Firstly, the assumptions used in the calculation of exacerbation rate introduced parameter uncertainty. Although the assumption on the consistence of the exacerbation risk of 12-17 years patients and adult patients has been validated by clinical experts, and previous study of Asian asthma patients aged 16 and above has shown that the average annual exacerbation rate under budesonide/formoterol combination therapy is similar to that reported in the source study, the data gap on head-to-head comparison still exists. It is suggested that further studies could be conducted on a larger sample scale to provide more support and insights on economic studies on asthma medications in the future. Meanwhile, the model assumes that the impact of salbutamol and terbutaline, on exacerbation rates in patients receiving salmeterol/fluticasone remains identical. Prior meta-analyses[32] has scrutinized the comparative clinical efficacy and safety profiles of these medications in bronchial asthma, revealing no statistically significant differences in enhancing pulmonary function, specifically FEV1 and peak expiratory flow (PEF), between terbutaline and salbutamol. However, pulmonary function only represents one facet of the multifactorial landscape influencing asthma exacerbation rates. The limited number of included literatures (15 studies) and the dated published date also constrain the generalizability of the study conclusion. Secondly, the severity of asthma was assumed to remain constant throughout lifetime, which did not take into the consideration of variation of clinical course of asthma. Nevertheless, this assumption was routinely used in modeling-based studies and also concurred by the panel of clinical experts. Thirdly, the utility data in our study were sourced from research conducted in non-Chinese population, which may introduce bias or errors. Fourthly, the medication adherence data was estimated based on feedback from a panel of clinical experts, which also added parameter uncertainties. Fifth, due to data limitations, our model only considered emergency room visits and hospitalizations during acute exacerbations, without accounting for cases where acute exacerbations may resolve on their own. Additionally, the model did not consider the impact of environmental, social, and emotional stress on patients, nor did it account for the presence of co-morbidities. Lastly, the readers should be cautioned that our scenario analysis was based solely on the average VBP prices currently available, which is not guaranteed price for these two drugs in the future, hence limiting the generalizability of our scenario analysis.
Conclusion
From the societal perspective, budesonide/formoterol reliever and maintenance therapy is likely to be a cost-effective option compared with salmeterol/fluticasone plus as-needed salbutamol for Chinese asthma patients ≥12 years.
Transparency
Declaration of funding
No funding was provided for this study.
Declaration of financial/other relationships
The authors declare that they have no competing interests.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Authors’ contributions
JX and MZ contributed to the concept and design of the study. KZ contributed to model construction, data analysis and data interpretation. KZ, XX and CZ contributed to data acquisition.KZ, XX and CZ contributed to the drafting of this manuscript. All authors read and approved the final manuscript.
Acknowledgements
We thank Xiaohan Hu for his helpful comments on this study and the writing of this manuscript.
Table 1 Key efficacy parameter inputs (exacerbation rates and distributions of patients received different exacerbation management strategies)
Table 2 Key cost parameter inputs (direct and indirect costs)
Table 3 Key utility parameter inputs
Table 4 Results from Base Case Cost-effectiveness Analysis
Figure 2 OWSA Tornado Diagram. Negative values of cost per QALY gained from the tornado plot should be interpreted with caution as a negative cost per QALY gained may be caused by negative incremental costs (a good outcome) or negative incremental QALYs (a bad outcome).
Supplemental Material
Download MS Word (44 KB)References
- Asthma GIf. Global Strategy for Asthma Management and Prevention,2021. 2021.
- Lin J, Wang W, Chen P, Zhou X, Wan H, Yin K, et al. Prevalence and risk factors of asthma in mainland China: The CARE study. Respiratory Medicine. 2018;137:48-54. doi: 10.1016/j.rmed.2018.02.010.
- Huang K, Yang T, Xu J, Yang L, Zhao J, Zhang X, et al. Prevalence, risk factors, and management of asthma in China: a national cross-sectional study. The Lancet. 2019;394(10196):407-18. doi: 10.1016/s0140-6736(19)31147-x.
- Liu M, Gan H, Lin Y, Lin R, Xue M, Zhang T, et al. Prevalence and Disability-Adjusted Life Year Rates of Asthma in China: Findings from the GBD Study 2019 of the G20. International Journal of Environmental Research and Public Health. 2022;19(22). doi: 10.3390/ijerph192214663.
- Yang X, Zhang T, Yang X, Jiang J, He Y, Wang P. Medical resource utilization and the associated costs of asthma in China: a 1-year retrospective study. BMC Pulmonary Medicine. 2023;23(1). doi: 10.1186/s12890-023-02685-0.
- Lommatzsch M, Buhl R, Korn S. The Treatment of Mild and Moderate Asthma in Adults. Deutsches Ärzteblatt international. 2020. doi: 10.3238/arztebl.2020.0434.
- Papi A, Brightling C, Pedersen SE, Reddel HK. Asthma. The Lancet. 2018;391(10122):783-800. doi: 10.1016/s0140-6736(17)33311-1.
- Castillo JR, Peters SP, Busse WW. Asthma Exacerbations: Pathogenesis, Prevention, and Treatment. The Journal of Allergy and Clinical Immunology: In Practice. 2017;5(4):918-27. doi: 10.1016/j.jaip.2017.05.001.
- Chapman KR, An L, Bosnic-Anticevich S, Campomanes CM, Espinosa J, Jain P, et al. Asthma patients' and physicians’ perspectives on the burden and management of asthma. Respiratory Medicine. 2021;186. doi: 10.1016/j.rmed.2021.106524.
- QIN Qiong SW-h, LEI Wei, GONG Yin-hua, MIAO Li-yan, BAO Jian-an, GU Bao-chen, CHEN Rong. Economic and device adherence evaluation of different doses of budesonide and formoterol inhalant on patients with asthma. Chinese Journal of Hospital Pharmacy [Internet]. 2016; 36(24):[2202-5 pp.]. Available from: doi: 10.13286/j.cnki.chinhosppharmacyj.2016.24.17.
- Wang X, Fang H, Shen K, Liu T, Xie J, Liu Y, et al. Cost-effectiveness analysis of double low-dose budesonide and low-dose budesonide plus montelukast among pediatric patients with persistent asthma receiving Step 3 treatment in China. Journal of Medical Economics. 2020;23(12):1630-9. doi: 10.1080/13696998.2020.1830410.
- Jiang PZ, Lanyu; Yao, Zezhong. Budesonide/formoterol versus salmeterol/fluticasone for asthma in children: an effectiveness and safety analysis. Journal of Comparative Effectiveness Research. 2021;10(17):1283-9. doi: 10.2217/cer-2021-0142.
- Cloutier MM, Baptist AP, Blake KV, Brooks EG, Bryant-Stephens T, DiMango E, et al. 2020 Focused Updates to the Asthma Management Guidelines: A Report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group. Journal of Allergy and Clinical Immunology. 2020;146(6):1217-70. doi: 10.1016/j.jaci.2020.10.003.
- Williams LK, Peterson EL, Wells K, Ahmedani BK, Kumar R, Burchard EG, et al. Quantifying the proportion of severe asthma exacerbations attributable to inhaled corticosteroid nonadherence. Journal of Allergy and Clinical Immunology. 2011;128(6):1185-91.e2. doi: 10.1016/j.jaci.2011.09.011.
- CHINA POPULATION CENSUS YEARBOOK 2020 (BOOK1): China Statistics Press; 2020.
- Watson L, Turk F, James P, Holgate ST. Factors associated with mortality after an asthma admission: A national United Kingdom database analysis. Respiratory Medicine. 2007;101(8):1659-64. doi: 10.1016/j.rmed.2007.03.006.
- Information of Drug Winning Bid: DRUGDATAEXP. Available from: https://data.yaozh.com/yaopinzhongbiao.
- Vogelmeier C NI, Ekelund J. Budesonide/formoterol maintenance and reliever therapy in Asian patients (aged ≥16 years) with asthma: a sub-analysis of the COSMOS study Clinical Drug Investigation. 2012;32(7):439-49. doi: 10.2165/11598840-000000000-00000.
- Nationwide Per Capita Income: National Bureau of Statistics of China. Available from: https://data.stats.gov.cn/english/easyquery.htm?cn=C01.
- Finkelstein EA, Lau E, Doble B, Ong B, Koh MS. Economic burden of asthma in Singapore. BMJ Open Respiratory Research. 2021;8(1). doi: 10.1136/bmjresp-2020-000654.
- Lin H-C, Kao S, Wen H-C, Wu C-S, Chung C-L. Length of Stay and Costs for Asthma Patients by Hospital Characteristics—A Five-Year Population-Based Analysis. Journal of Asthma. 2009;42(7):537-42. doi: 10.1080/02770900500214783.
- Oh B-C, Lee J-E, Nam JH, Hong J-Y, Kwon S-H, Lee E-K. Health-related quality of life in adult patients with asthma according to asthma control and severity: A systematic review and meta-analysis. Frontiers in Pharmacology. 2022;13. doi: 10.3389/fphar.2022.908837.
- Lloyd A, Price D, Brown R. The impact of asthma exacerbations on health-related quality of life in moderate to severe asthma patients in the UK. Primary Care Respiratory Journal. 2007;16(1):22-7. doi: 10.3132/pcrj.2007.00002.
- Wang XF, Honghao; Shen, Kunling; Liu, Tianyi; Xie, Jipan; Liu, Yuantao; Zhong, Jia; Wu, Eric; Zhou, Wei; Wu, Bin. The cost-effectiveness of low-dose budesonide as a Step 2 treatment for pediatric asthma in China. Journal of Comparative Effectiveness Research. 2020;9(16):1141-51. doi: 10.2217/cer-2020-0102.
- FitzGerald JM, Arnetorp S, Smare C, Gibson D, Coulton K, Hounsell K, et al. The cost-effectiveness of as-needed budesonide/formoterol versus low-dose inhaled corticosteroid maintenance therapy in patients with mild asthma in the UK. Respiratory Medicine. 2020;171. doi: 10.1016/j.rmed.2020.106079.
- Buendía JA, Guerrero Patiño D, Talamoni HL. Cost‐utility of as‐needed combination low‐dose budesonide‐formoterol in adolescents mild asthma. Pediatric Pulmonology. 2021;56(12):3699-705. doi: 10.1002/ppul.25645.
- Society AgoCT. Guidelines for bronchial asthma prevent and management(2020 edition). Chinese Journal of Tuberculosis and Respiratory Diseases. 2020;32(12). doi: 10.3760/cma.j.cn112147-20200618-00721.
- The Subspecialty Group of Respiratory Diseases TSoP, Chinese Medical Association; The Editorial Board, Chinese Journal of Pediatrics. Guideline for the diagnosis and optimal management of asthma in children(2016). Chinese Journal of Pediatrics. 2016;54(3). doi: 10.3760/cma.j.issn.0578-1310.2016.03.003.
- Zhang YTY. A study of standardized management of childhood asthma on parents' knowledge, beliefs and behaviors Asthma control and medication adherence. Chinese Remedies & Clinics [Internet]. 2021; 21(8):[1327-9 pp.].
- Buendía JA, Patiño DG. SMART therapy in adolescent and adults patients with moderate persistent asthma: a cost-utility analysis. Journal of Asthma. 2021;59(12):2367-74. doi: 10.1080/02770903.2021.2019266.
- Liu GH, Shanlian; Wu, Jiuhong; Wu, Jing; Dong, Zhaohui; Li, Hongchao. China Guidelines for Pharmacoeconomic Evaluations 2020 (Chinese-English Version). Beijing: China Market Press; 2020.
- CHEN Jia-Huan XX, CAI Ping, LU Xiao-Lan, SUN Yue. Meta-analysis of Clinical Efficacy and Safety of Terbutaline and Salbutamol in the Treatment of Bronchial Asthma. China Journal of Pharmaceutical Economics. 2020;15(8):50-5. doi: 10.12010/j.issn.1673-5846.2020.08.011.