ABSTRACT
Introduction: Rule induction tests such as the Wisconsin Card Sorting Test require executive control processes, but also the learning and memorization of simple stimulus–response rules. In this study, we examined the contribution of diminished learning and memorization of simple rules to complex rule induction test performance in patients with amnestic mild cognitive impairment (aMCI) or Alzheimer’s dementia (AD). Method: Twenty-six aMCI patients, 39 AD patients, and 32 control participants were included. A task was used in which the memory load and the complexity of the rules were independently manipulated. This task consisted of three conditions: a simple two-rule learning condition (Condition 1), a simple four-rule learning condition (inducing an increase in memory load, Condition 2), and a complex biconditional four-rule learning condition—inducing an increase in complexity and, hence, executive control load (Condition 3). Results: Performance of AD patients declined disproportionately when the number of simple rules that had to be memorized increased (from Condition 1 to 2). An additional increment in complexity (from Condition 2 to 3) did not, however, disproportionately affect performance of the patients. Performance of the aMCI patients did not differ from that of the control participants. In the patient group, correlation analysis showed that memory performance correlated with Condition 1 performance, whereas executive task performance correlated with Condition 2 performance. Conclusions: These results indicate that the reduced learning and memorization of underlying task rules explains a significant part of the diminished complex rule induction performance commonly reported in AD, although results from the correlation analysis suggest involvement of executive control functions as well. Taken together, these findings suggest that care is needed when interpreting rule induction task performance in terms of executive function deficits in these patients.
Memory impairments are characteristic for patients with Alzheimer’s disease. These impairments typically stand out in the early pre-dementia phase of Alzheimer’s disease, as is the case in the amnestic form of mild cognitive impairment (aMCI). In the later dementia stage of Alzheimer’s disease, impairments in other cognitive domains, such as executive functions (EF), also become apparent (e.g., Collie & Maruff, Citation2000; Koivunen et al., Citation2012). However, there is abundant evidence that subclinical decrements in EF may already be present in the aMCI stage (Chen et al., Citation2013). For example, in a recent study, Johns et al. (Citation2012) demonstrated that aMCI patients perform significantly worse than controls on executive function tests tapping functions such as working memory, inhibitory control, divided attention, planning ability, and verbal fluency. Whereas these changes in performance patterns do not necessarily suggest clinically impaired performance (i.e., performance 1.5 standard deviations (SD) or more below that of normative scores; see Winblad et al., Citation2004), performance levels generally are significantly worse than that of controls. Studies demonstrating impaired executive test performance in AD patients are numerous, reporting deficits in all executive functions (e.g., Belleville, Fouquet, Duchesne, Collins, & Hudon, Citation2014; Weintraub, Wicklund, & Salmon, Citation2012).
Evidence for the presence of EF decrements, however, is typically based on standardized neuropsychological tests, which are often not process-pure as they tap multiple cognitive functions. Tests such as the Wisconsin Card Sorting Test (WCST), for example, also require basic learning and memorization of the rules. Accordingly, in conditions such as aMCI and AD, it is possible that the characteristic learning and memorization deficits explain at least an important part of the diminished complex rule induction processes that are required for intact WCST performance. Some studies (indirectly) support this crucial role of learning and memorization processes. Papp, Snyder, Maruff, Bartkowiak, and Pietrzak (Citation2011) demonstrated that aMCI patients show diminished spatial learning as part of a complex visuospatial executive function task. Moreover, earlier studies suggested that memory deficits potentially play an important role in performing a rule induction task in patients with Korsakoff’s syndrome (e.g., Bardenhagen, Oscar-Berman, & Bowden, Citation2007) and in patients with posterior focal brain damage (e.g., Mimura, Citation1992). Comparable findings were reported recently in a study in younger and older volunteers, where both episodic and working memory processes were found to independently contribute to complex rule induction performance (Oosterman, Boeschoten, Eling, Kessels, & Maes, Citation2014). Other studies have demonstrated a unique correlational relationship between memory and EF performance in pathological (Baudic et al., Citation2006) and non-demented (Oosterman et al., Citation2010) aging. It was furthermore demonstrated that the medial temporal lobes, which are critically involved in memorization processes, are strong, independent neuroanatomical correlates of executive task performance, also in normal (Oosterman et al., Citation2008; Papp et al., Citation2014) and pathological (Oosterman, Oosterveld, Olde Rikkert, Claassen, & Kessels, Citation2012; Overdorp, Kessels, Claassen, & Oosterman, Citation2014) aging. These studies all suggest that memory may play a crucial rule in executive task performance. To our knowledge, however, studies are lacking that directly isolate this potentially confounding role of simple rule learning and memorization processes in complex rule induction test performance in aMCI and AD patients.
The goal of this study was therefore to test the hypothesis that in patients with aMCI and AD, diminished memorization of the rules explains an important part of the reduced performance on EF tasks tapping complex rule induction performance. For this, we used a task in which the number and the complexity of the rules were independently manipulated, in order to deduce the contribution of basic rule learning and memorization processes to executive task performance (Oosterman et al., Citation2014). Our hypothesis was that both patients with aMCI and those with AD would show impaired performance, particularly as the number of rules to be memorized increased, as this increases the memory load. Moreover, we expected that the performance decrements that resulted from this increase in basic rule learning and memorization processes (the increase in memory load) would account for a significant part of the performance in the complex executive control condition. We also expected that, when basic rule learning and memorization load was increased, the performance decline would be stronger in patients with AD than in aMCI patients. Finally, we expected to find a disproportionate performance decline in the dementia subgroup when the complexity was increased; this was expected because executive function deficits are likely to be more pronounced in patients with AD than in patients with aMCI.
Method
Participants
Patients with aMCI or AD were recruited via the memory clinic at the University Medical Center Utrecht between January 2010 and October 2011. The clinical diagnosis of aMCI or AD was established at a multidisciplinary meeting: aMCI (n = 26) was diagnosed according to the Winblad/Petersen criteria for single- or multi-domain amnestic MCI (Winblad et al., Citation2004); probable (n = 24) or possible (n = 15) AD was diagnosed according to the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association criteria (McKhann et al., Citation1984). The control group consisted of 32 older people matched for age and education, selected from a larger database on rule induction performance in older people, which consisted of volunteers recruited through advertisements and of acquaintances of the researchers (N = 58). Participants from this control group all had a Mini-Mental State Examination (Folstein, Folstein, & McHugh, Citation1975) score of 27 or higher (M = 28.5, SD = 0.9), in order to minimize the potential presence of undetected substantial cognitive impairment (Kukull et al., Citation1994). Data of 12 of these control participants have been used in a previous publication (Oosterman et al., Citation2014). Controls did not have a history of neurodegenerative disorders (including a dementia diagnosis), stroke, or severe depression. In addition, all completed a brief neuropsychological examination of tests measuring episodic memory and executive functioning, to ensure that no undetected cognitive impairment was present. Education level was classified using a 7-point ordinal rating scale (1 = less than primary education, 7 = university degree). This study was approved by the medical ethical committee of the University Medical Center Utrecht for the patients with aMCI or AD, and by the Institutional Review Board of the Radboud University in Nijmegen for the inclusion of controls. All participants gave informed consent.
All patients completed a neuropsychological assessment of tests measuring memory performance, executive function, and processing speed. Briefly, this examination comprised verbal episodic memory as measured with the Rey Auditory Verbal Learning Test (RAVLT; Van der Elst, Van Boxtel, Van Breukelen, & Jolles, Citation2005), visual associative memory as measured with the Visual Association Test (VAT; Lindeboom, Schmand, Tulner, Walstra, & Jonker, Citation2002), and executive functioning and processing speed as measured with animal fluency (Van der Elst, Van Boxtel, Van Breukelen, & Jolles, Citation2006a), the Trail Making Test (TMT; Reitan, Citation1958), the Stroop Color/Word Test (Van der Elst, Van Boxtel, Van Breukelen, & Jolles, Citation2006b), the Letter Digit Substitution Test (LDST; Van der Elst, Van Boxtel, Van Breukelen, & Jolles, Citation2006c), and the Digit Span Test (Wechsler, Citation1997). Performance levels of the aMCI and AD patients, together with the percentages of patients that revealed clinically impaired performance (using the established cut-off score of 1.5 SD below the normative mean; Winblad et al., Citation2004), are presented in . A further examination of the aMCI participants revealed that 10 participants showed isolated memory impairments, suggestive of single-domain aMCI, whereas 16 participants additionally showed evidence of executive function deficits and hence fulfilled clinical criteria for multi-domain aMCI (Winblad et al., Citation2004).
Table 1. Characteristics and neuropsychological task performance of the aMCI and AD participants.
Materials
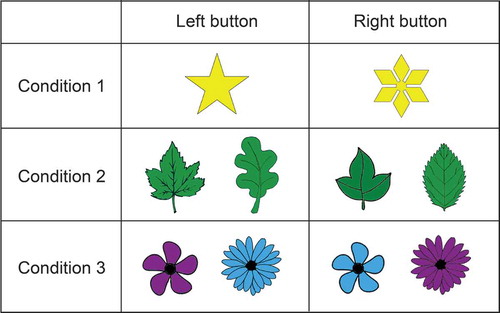
The employed rule induction task (see ) consists of three task conditions, in which participants have to induce the correct rule based on feedback. For Condition 1, a total of two different stimuli are employed (presented one at a time) that differ in one feature only (e.g., color: a white versus a purple balloon). Participants are instructed to respond using two keys on the keyboard; for each stimulus, only one key is correct (e.g., the left key for the white and the right key for the purple balloon; this is unknown to the participant). Hence, only two exemplars of the feature have to be memorized for accurate task performance. As only two exemplars are being used, the first condition is a simple 2-choice learning condition. In Condition 2, one of four different stimuli is presented; again these stimuli differ in one feature only, and, hence, four different exemplars have to be memorized (e.g., a red, an orange, a yellow, and a green pepper). In Condition 3 again four different stimuli have to be memorized. This time, however, each stimulus consists of a unique combination of two exemplars with two different features (color and shape); this combination is crucial for determining the correct response—e.g., purple (dark grey) flower/Shape 1 and blue (light grey) flower/Shape 2 are each mapped onto a left response, whereas purple (dark grey) flower/Shape 2 and blue (light grey) flower/Shape 1 are mapped onto a right response. This condition therefore requires biconditional learning, a process known to require executive control processes (e.g., see Haddon & Killcross, Citation2006). It was previously shown that this condition is predicted independently by episodic and working memory, together accounting for approximately 86% of the age-related variance of performance on this condition (Oosterman et al., Citation2014).
Procedure
Participants were seated in front of a laptop and instructed to press one of two keys in response to a stimulus. On each trial one stimulus was presented; the stimulus remained on the screen until a response had been given by the participant. Participants received feedback about the accuracy of each response, to enable them to induce the rule. All conditions were administered in a fixed order: the test always started with Condition 1, followed by Conditions 2 and 3. Condition 1 consisted of 50 trials that were consecutively administered until the participant deduced the rule. For Conditions 2 and 3 a total of 100 trials were used. A successful induction of a rule was operationalized as eight consecutive correct responses, after which the condition terminated automatically; the number of trials needed to reach this criterion was recorded. If the criterion was not met after reaching a maximum number of trials (50 for Condition 1 and 100 for Conditions 2 and 3), this maximum number was recorded. Since the final condition contained both color and shape features, two versions of the same task were employed that had either color (Version 1) or shape (Version 2) as primary feature in Conditions 1 and 2, in order to rule out potential confounding effects of stimulus type. Condition 3 consisted of the same stimuli in both task versions. Participants completed one out of two versions; groups did not differ in the number of participants that completed the shape or the color version, χ2(2) = 1.80, p = .41.
Results
Due to non-normality of the data, non-parametric Kruskal-Wallis Tests (corrected for ties) were performed to test for group differences in the three conditions as well as the difference scores. These difference scores were computed to determine the extent to which the increase in basic rule learning and memorization load (from Condition 1 to 2) and in complexity (from Condition 2 to 3) contributed to diminished performance in the patient group. Significant results were followed by Mann-Whitney U tests (corrected for ties) to locate the significant differences between the groups. For Kruskall-Wallis tests, eta-squared was calculated as effect size using the following formula: η2 = χ2/(N – 1). Here, values of .01, .06 and .14 represent small, medium, and large effects (Cohen, Citation1988). For Mann-Whitney U tests, r was calculated as a measure of effect size. Values of .1, .3 and .5 represent small, medium, and large effect sizes, respectively (Cohen, Citation1988).
Next, analyses were repeated for those patients who still showed relatively intact rule learning and memorization processes as measured with the first two conditions of the rule induction task (see Results section). Kruskal-Wallis tests were repeated to test for potential differences in performance of these selected participant groups.
Finally, Spearman rank correlations were computed between the scores from the rule induction conditions and the neuropsychological test scores. As we were primary interested in the relationship of rule induction performance with memory and executive control, only neuropsychological tests measuring memory (RAVLT, VAT) and executive functions (Stroop Color/Word Card, TMT-B, Digit Span Backward, animal fluency) were included in this analysis. In case multiple neuropsychological test scores were significant correlated with a single rule induction condition, these scores were subjected to subsequent linear regression analysis with the stepwise selection method to identify those neuropsychological correlates uniquely predicting performance on the rule induction conditions. Here, if necessary, scores were normalized using Blom transformation. This analysis was restricted to the patient group, as the neuropsychological test scores were available only for aMCI and AD patients. We adopted a p-value of < .05 as criterion for statistical significance throughout, except when applying a Bonferroni correction for multiple comparisons.
Participant characteristics
Five patients with AD (mean MMSE = 21.8 ± 1.8, range =19–23) only completed the first condition, as the rule induction task was too difficult for them. The test results of these patients were therefore not included in the subsequent analyses. For one aMCI patient, the final condition was missing due to time constraints, and only the scores of Condition 1 and 2 were available. The analyses reported below are therefore based on a total of 26 aMCI and 34 AD patients (for Conditions 1 and 2) or 25 aMCI and 34 AD patients (for Condition 3). Characteristics of these participants are presented in . The groups did not differ with regard to age, education level, or sex distribution. A one-way ANOVA revealed a significant effect for the MMSE scores; Tukey’s post-hoc testing revealed that MMSE scores differed significantly between all groups (all p-values < .03).
Table 2. Characteristics and task performance of the control, aMCI, and AD participants.
The effect of rule induction task version
As two task versions were employed (a “shape” and a “color” version), simple univariate tests were performed to see whether task version influenced potential group differences on the rule induction conditions. ANOVAs with task version (shape/color) and group (controls/aMCI/AD) as between-subjects variables, age as covariate (as age differences were present between participants completing the color and those completing the shape version), and task performance as dependent variables showed a main effect of task version on Condition 2 performance, F(1, 85) = 6.17, p = .015, ηp2 = .07. A further examination indicated that more trials were, on average, needed to complete the color (M = 49.3, SE = 4.5) than the shape (M = 33.7, SE = 4.3) condition. However, this effect was not modulated by group, nor were any other interaction effects between group and task version significant (all p-values > .180). It is therefore unlikely that potential group effects were dependent on the type of stimulus (shape or color) employed.
Rule induction task performance: main results
Two control participants failed to solve Condition 1 within the maximum number of trials, two failed to solve Condition 2, and seven failed to solve Condition 3. The corresponding numbers were higher in the aMCI and AD groups: four AD patients were unable to solve the first condition, three aMCI and nine AD patients the second, and seven aMCI and 16 AD patients the third condition. In the primary analysis, these participants were nonetheless included.
Kruskal-Wallis tests showed a significant group difference for Condition 2 and for Condition 3 (see ), suggesting medium effect sizes. Further Mann-Whitney U tests (Bonferroni corrected: p < .017) showed that the AD patients performed worse on both Condition 2 (Z = −3.21, p < .01, r = −.40) and Condition 3 (Z = −2.76, p < .01, r = −.34) compared to the controls, indicating medium effect sizes. AD patients, furthermore, performed worse than aMCI patients on Condition 2, although this effect did not survive Bonferroni correction (Z = −2.31, p = .021. r = −.30). No differences were found between the controls and aMCI patients.
Further analyses of the difference scores (see ) revealed significant group differences when the number of simple rules to be memorized was increased (from Condition 1 to 2), but not when the complexity of the rule was increased (from Condition 2 to 3). Mann-Whitney U tests showed one significant effect for the Condition 2–1 difference score, namely that an increase in rule learning and memorization load induced worse performance in patients with AD compared to controls (Z = −3.01, p = .003, r = −.37), again showing a moderate effect size.
Restricting the analyses to those participants who had, at least, successfully completed the first condition (30 control participants, 26 aMCI, and 30 AD patients), showing intact ability to understand the task instructions and the capability to induce the rule based on feedback, did not alter the results: group differences were present only for Condition 2 (p = .003), Condition 3 (p = .023), and the Condition 2–1 difference score (p = .005). Similarly, an analysis of the Condition 3–2 difference score, while only including participants (30 control participants, and 23 aMCI and 25 AD patients) who had successfully completed Condition 2 (since those with the maximum number of trials on Condition 2 cannot show a performance decline on Condition 3), did not change the results: the difference score still did not differ between the groups (p = .38). A final, very strict, analysis restricted to those participants who had successfully completed Condition 3 (25 control participants, 18 aMCI and 18 AD patients), showed largely the same results, with significant group differences only on Condition 2 (p = .007) and on the Condition 2–1 difference score (p = .021). No significant group differences were found for Condition 3 (p = .17).
Additional performance-based subgroup comparisons
Next, we computed standardized z-scores based on the average performance level of the control group. Using a cut-off score of 1.5 SD below the normative mean showed that one aMCI patient performed in the impaired range on Condition 1, and six in the impaired range on Condition 2. Of all AD patients, two performed in the impaired range on Condition 1, seven in the impaired range on Condition 2, and four performed in the impaired range on both conditions. None of the patients showed an impaired performance on Condition 3; all scores fell within 1.5 SD of the control mean.
To examine whether impaired performance on Condition 1 and/or 2 may obscure the patients’ executive function deficits in Condition 3, a final analysis was performed including only those patients with relatively intact performance on Conditions 1 and 2. Kruskal-Wallis tests revealed that the performance of these selected patient groups (aMCI = 19 patients, AD = 21 patients) and of the control group differed only for Condition 2, χ2(2) = 6.95, p = .031, η2 = .10; no group differences were found for Condition 1, χ2(2) = 2.55, p = .28, η2 = .04, Condition 3, χ2(2) = 5.01, p = .08, η2 = .07, or the Condition 2–1, χ2(2) = 4.15, p = .13, η2 = .06, and the Condition 3–2, χ2(2) = 3.62, p = .16, η2 = .05, difference scores.
Correlation analysis
Spearman rank correlations, calculated between the rule induction scores and the neuropsychological outcomes, showed only a few significant results (see ). Better performance on the RAVLT-immediate recall was associated with fewer trials needed to complete Condition 1, whereas better performance on executive tests (animal fluency and Stroop Color/Word card completion time) was associated with a reduction in trials required to complete Condition 2. A single positive correlation between the Digit Span Backward score and Condition 3 performance was observed. Subsequent linear regression analysis for Condition 2, including animal fluency and Stroop Color/Word completion time as predictors, showed that animal fluency performance predicted the number of trials needed to complete Condition 2 (β = −0.49, p < .01), F(1, 53) = 16.7, p < .001.
Table 3. Correlations between the rule induction task conditions and the neuropsychological test scores in the aMCI and AD participants.
Finally, we examined these neuropsychological correlates in relation to the rule induction difference scores. This analysis showed that performance on the animal fluency (ρ = −0.31, p = .02) and Stroop Color/Word (ρ = 0.31, p = .02) test was still associated with the Condition 2–1 difference score, whereas the association between the Digit Span Backward test and the Condition 3–2 difference score was lost (ρ = 0.22, p = .09). Again, animal fluency (β = −0.42, p = .001), but not the Stroop Color/Word test, predicted the Condition 2–1 difference score in subsequent regression analysis, F(1, 53) = 11.3, p < .001.
Discussion
The goal of the present study was to assess the effect of an increase in simple rule learning and memorization load on the one hand, and an increase in rule complexity on the other hand, on rule induction performance in patients with aMCI or AD. Overall, the results indicate that performance of AD patients declined most pronouncedly as the number of simple rules that had to be memorized increased (i.e., from Condition 1 to Condition 2). Although their performance declined further with an increase in complexity in Condition 3, this decline was comparable to the decline observed in the other groups. Taken together, these results indicate that diminished learning and memorization of simple rules accounts for an important part of the performance decline on complex rule induction tasks using multi-featured stimuli in patients with AD.
Several studies have reported on performance deficiencies of aMCI and AD patients on rule induction tasks. Most of these studies employed the WCST, showing that aMCI and early AD patients completed fewer categories and made more errors than did controls (Chen et al., Citation2009). Another study, however, noticed that large interindividual differences exist among patients with mild AD. At a group level, patients performed worse than did control participants, but only few patients actually displayed clinically relevant impaired WCST performance (Stokholm, Vogel, Gade, & Waldemar, Citation2006). This is in line with the current findings, showing similar performance decrements in AD and in the controls when complexity was increased. Furthermore, when we restricted the analyses to those patients who revealed relatively intact performance on the first two memory conditions, group differences on the most complex condition (Condition 3) were also no longer significant. These findings support the idea that AD profoundly affects simple rule learning and memorization processes, and that these processes consequently limit their performance on more complex rule induction tests.
The currently observed mild decrements on a complex rule induction condition (Condition 3) are in agreement with observations of previous studies showing mild WCST deficiencies in the very early stages of AD (Perry, Watson, & Hodges, Citation2000). Some studies indicate, furthermore, that the traditional “frontal” performance markers on the WCST, such as the number of perseverative responses, may be relatively intact in AD (Stokholm et al., Citation2006). In the present study, limited evidence for the assumption of impaired rule induction performance in the pre-dementia stage (aMCI) was also found. As a group, these patients did not perform worse than did the controls, in spite of the fact that cognitive impairment was obviously present in this group (as indicated by their reduced MMSE scores and by the fact that the patients displayed clinically impaired neuropsychological test performance). Also, the most marked difference (approaching significance) between the aMCI and AD subgroups was found on the primary “memory” Condition (Condition 2), but not on Condition 3, which incorporated the increase in executive control processes by increasing complexity of the rules. These findings are in line with the observation that memory performance, but not executive functioning, may be a strong predictor of future conversion from MCI to dementia (DeCarli et al., Citation2004; Peters, Villeneuve, & Belleville, Citation2014). As such, in the more advanced AD stage learning and memorization processes are likely to play important roles in complex rule acquisition performance. Nonetheless, as was noted in a study showing increased costs in patients with Alzheimer’s disease when switching between two rules (Belleville, Bherer, Lepage, Chertkow, & Gauthier, Citation2008), part of the diminished performance of patients with AD on standardized tasks such as the WCST does likely reflect genuine diminished executive control (see also Lange et al., Citation2016). Following this line of reasoning, it should be stressed that, in contrast to the experimental rule induction task employed in the current study, in the WCST category stimuli and the cards assorted by the patient are constantly visible during the test session. This is likely to reduce memory load, suggesting that the involvement of memory processes may be higher in the current experimental rule induction task than is normally the case in the WCST.
One aspect that should be discussed concerns the precise cognitive functions that underlie the deficits in rule induction performance in AD patients. Although we can only hint at the cognitive processes involved in our experimental rule induction task, some suggestions can be made. First of all, our groups did not differ in Condition 1 performance, indicating that both control and patient groups were able to use feedback in order to induce simple rules. Differences between groups were, however, apparent once the number of simple rules to be induced was increased (Condition 2). A previous study demonstrated that performance on this condition in healthy younger and older participants was related to visual episodic memory performance (Oosterman et al., Citation2014). Since AD is often characterized by a severe and early decline in episodic memory, one possibility is that these deficits underlie the deficient performance of our patients on Condition 2. Nonetheless, there is also evidence that Alzheimer patients may already use higher-order cognitive control processes to compensate for deficits in performing relatively easy tasks (Gould et al., Citation2006). With regard to the current study, this might suggest that the severe memory deficits in these patients trigger the involvement of executive control processes in relatively easy task conditions, such as Condition 2. Consequently, performance deficits on Condition 2 could, next to memory deficits, reflect insufficient compensation by presumably malfunctioning higher-order executive control processes. From this perspective, Condition 3 might not have been sensitive enough to detect an increase in executive control load in AD, as Condition 2 performance already loaded significantly on executive control processes in this patient group. If this is the case, then executive function deficits are still a crucial ingredient underlying impaired rule induction performance in AD, but only at a different level than is commonly assumed for complex task such as the WCST. Stated otherwise, Alzheimer patients may fail this task because their memory deficits, combined with the inefficient executive compensatory mechanisms, limit the learning and memorization of simple rules, not because they have a failure in shifting between rules. Our correlation analysis provides some support for these assumptions, showing that performance on a memory test was the only correlate of Condition 1 performance, whereas executive function (but not memory) tests were associated with the number of trials needed to perform Condition 2. Also in line with this reasoning is the finding that a decline in neuropsychological test performance was not associated with reduced Condition 3 performance, suggesting that executive function performance no longer has a differentiable association with complex rule induction performance in our patients (even though better Digit Span Backward performance was unexpectedly associated with more trials needed to complete Condition 3, this association did not survive correction for Condition 2 performance, through the Condition 3–2 difference score). However, these analyses were restricted to the aMCI and AD groups and tell us nothing therefore regarding the rule induction decline that occurs from normal aging to aMCI and AD. Future studies are needed that specifically try to isolate the most important mechanisms underlying the performance deficits in AD, for example by examining individual (process-pure) cognitive functions in relation to rule induction performance in these patients.
A point of caution is that a large number of patients, but also several control participants, were unable to complete all conditions, particularly Condition 3. The resulting relatively large SD in the control group potentially explains why all AD and aMCI patients performed within normal limits (defined as less than 1.5 SD below the average performance of the controls) on Condition 3. This might be due to the fact that we adopted a maximum number of 100 trials for this condition and thereby introduced a floor effect (i.e., performance could not get worse). One could argue that this limit in the number of trials could explain the absence of any group differences on the final condition, and that the use of more trials might have revealed a significant effect where an increase in rule complexity disproportionately affected performance of AD (and perhaps even of aMCI) patients. However, the task at hand was already challenging for some AD patients. As a result, it is doubtful whether the use of more than 100 trials would have resulted in a different pattern of results. Nonetheless, when we restricted the analyses to those patients who had successfully completed Condition 3, results still indicated that the two groups differed significantly when the memory load was increased, but not when the executive control load was increased. It should be noted that on the third condition the patients with AD actually performed significantly worse than did the controls, but that this difference disappeared after simple rule learning and memorization processes were accounted for. This finding of significant group differences for the uncorrected Condition 3 scores also contradicts the idea of a floor effect.
Finally, as all conditions were administered in a fixed order, task familiarity may have played a role, potentially explaining why no disproportional performance decline was found on the final condition. The extent to which this has affected the current findings is unclear, considering the fact that familiarity of the final condition is questionable since this condition introduced a new form of learning—that is, biconditional learning. Also, it is not clear why familiarity effects should be larger in the AD group, if this factor is to explain the fact that an increase in complexity did not disproportionately affect performance of these patients.
To summarize, diminished learning and memorization of simple rules may be important constructs underlying reduced complex rule induction performance in AD. The AD patients showed the most marked decline in performance when memory load was increased, not when the engagement of executive control processes was increased by increasing the complexity of the rules. Hence, caution is needed when interpreting executive function performance of patients with AD—and even those with aMCI—on tasks that require rule induction abilities. Further studies are needed using a larger number of trials on the complex rule induction condition (and preferably also on other conditions) to confirm our finding that an increase in rule complexity truly does not disproportionately affect performance of AD (or even aMCI) patients compared to control participants.
Acknowledgments
Members of the Utrecht Vascular Cognitive Impairment Study Group involved in the present study (in alphabetical order by department): University Medical Center Utrecht, the Netherlands, Department of Neurology: E. van den Berg, G.J. Biessels, S.M. Heringa, L.J. Kappelle, I. Verhage, I. Wielaard; Department of Radiology/Image Sciences Institute: J. de Bresser; Department of Geriatrics: H.L. Koek, J.E. de Wit; Hospital Diakonessenhuis Zeist, the Netherlands: M. Hamaker, R. Faaij, M. Pleizier, E. Vriens.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Bardenhagen, F. J., Oscar-Berman, M., & Bowden, S. C. (2007). Rule knowledge aids performance on spatial and object alternation tasks by alcoholic patients with and without Korsakoff’s amnesia. Neuropsychiatric Disease and Treatment, 3, 907–918. doi:10.2147/NDT.S1425
- Baudic, S., Barba, G. D., Thibaudet, M. C., Smagghe, A., Remy, P., & Traykov, L. (2006). Executive function deficits in early Alzheimer’s disease and their relations with episodic memory. Archives of Clinical Neuropsychology, 21, 15–21. doi:10.1016/j.acn.2005.07.002
- Belleville, S., Bherer, L., Lepage, E., Chertkow, H., & Gauthier, S. (2008). Task switching capacities in persons with Alzheimer’s disease and mild cognitive impairment. Neuropsychologia, 46, 2225–2233. doi:10.1016/j.neuropsychologia.2008.02.012
- Belleville, S., Fouquet, C., Duchesne, S., Collins, D. L., & Hudon, C. (2014). Detecting early preclinical Alzheimer’s disease via cognition, neuropsychiatry, and neuroimaging: Qualitative review and recommendations for testing. Journal of Alzheimer’s Disease, 42, S375–S382. doi:10.3233/JAD-141470
- Chen, T.-F., Chen, Y.-F., Cheng, T.-W., Hua, M.-S., Liu, H.-M., & Chiu, M.-J. (2009). Executive dysfunction and periventricular diffusion tensor changes in amnesic mild cognitive impairment and early Alzheimer’s disease. Human Brain Mapping, 30, 3826–3836. doi:10.1002/hbm.20810
- Chen, N.-C., Chang, C.-C., Lin, K.-N., Huang, C.-W., Chang, W.-N., Chang, Y.-T., … Wang, P.-N. (2013). Patterns of executive dysfunction in amnestic mild cognitive impairment. International Psychogeriatrics, 25, 1181–1189. doi:10.1017/S1041610213000392
- Cohen, J. (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Hillsdale, NJ: Erlbaum.
- Collie, A., & Maruff, P. (2000). The neuropsychology of preclinical Alzheimer’s disease and mild cognitive impairment. Neuroscience and Biobehavioral Reviews, 24, 365–374. doi:10.1016/S0149-7634(00)00012-9
- DeCarli, C., Mungas, D., Harvey, D., Reed, B., Weiner, M., Chui, H., & Jagust, W. (2004). Memory impairment, but not cerebrovascular disease, predicts progression of MCI to dementia. Neurology, 63, 220–227. doi:10.1212/01.WNL.0000130531.90205.EF
- Folstein, M. F., Folstein, S. E., & McHugh, P. R. (1975). “Mini-Mental State”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12, 189–198. doi:10.1016/0022-3956(75)90026-6
- Gould, R. L., Arroyo, B., Brown, R. G., Owen, A. M., Bullmore, E. T., & Howard, R. J. (2006). Brain mechanisms of successful compensation during learning in Alzheimer disease. Neurology, 67, 1011–1017. doi:10.1212/01.wnl.0000237534.31734.1b
- Haddon, J. E., & Killcross, S. (2006). Prefrontal cortex lesions disrupt the contextual control of response conflict. Journal of Neuroscience, 26, 2933–2940. doi:10.1523/JNEUROSCI.3243-05.2006
- Johns, E. K., Phillips, N. A., Belleville, S., Goupil, D., Babins, L., Kelner, N., … Chertkow, H. (2012). The profile of executive functioning in amnestic mild cognitive impairment: Disproportionate deficits in inhibitory control. Journal of the International Neuropsychological Society, 18, 1‒15. doi:10.1017/S1355617712000069
- Koivunen, J., Karrasch, M., Scheinin, N. M., Aalto, S., Vahlberg, T., Någren, K., … Rinne, J. O. (2012). Cognitive decline and amyloid accumulation in patients with mild cognitive impairment. Dementia and Geriatric Cognitive Disorders, 34, 31–37. doi:10.1159/000341580
- Kukull, W. A., Larson, E. B., Teri, L., Bowen, J., McCormick, W., & Pfanschmidt, M. L. (1994). The Mini-Mental State Examination score and the clinical diagnosis of dementia. Journal of Clinical Epidemiology, 47, 1061–1067. doi:10.1016/0895-4356(94)90122-8
- Lange, F., Kröger, B., Steinke, A., Seer, C., Dengler, R., & Kopp, B. (2016). Decomposing card-sorting performance: Effects of working memory load and age-related changes. Neuropsychology, 30, 579–590. doi:10.1037/neu0000271
- Lindeboom, J., Schmand, B., Tulner, L., Walstra, G., & Jonker, C. (2002). Visual association test to detect early dementia of the Alzheimer type. Journal of Neurology, Neurosurgery, and Psychiatry, 73, 126–133. doi:10.1136/jnnp.73.2.126
- McKhann, G., Drachman, D., Folstein, M., Katzman, R., Price, D., & Stadlan, E. M. (1984). Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology, 34, 939–944. doi:10.1212/WNL.34.7.939
- Mimura, M. (1992). Deficits of problem-solving ability in patients with focal brain damage: Neuropsychological investigation of prediction and hypothesis behavior. The Keio Journal of Medicine, 41, 87–98. doi:10.2302/kjm.41.87
- Oosterman, J. M., Boeschoten, M. S., Eling, P. A., Kessels, R. P. C., & Maes, J. H. (2014). Simple and complex rule induction performance in young and older adults: Contribution of episodic memory and working memory. Journal of the International Neuropsychological Society, 20, 333–341. doi:10.1017/S1355617713001446
- Oosterman, J. M., Oosterveld, S., Olde Rikkert, M. G., Claassen, J. A., & Kessels, R. P. (2012). Medial temporal lobe atrophy relates to executive dysfunction in Alzheimer’s disease. International Psychogeriatrics, 24, 1474–1482. doi:10.1017/S1041610212000506
- Oosterman, J. M., Vogels, R. L. C., van Harten, B., Gouw, A. A., Poggesi, A., Scheltens, P., … Scherder, E. J. (2010). Assessing mental flexibility: Neuroanatomical and neuropsychological correlates of the Trail Making Test in elderly people. Clinical Neuropsychologist, 24, 203–219. doi:10.1080/13854040903482848
- Oosterman, J. M., Vogels, R. L. C., van Harten, B., Gouw, A. A., Scheltens, P., Weinstein, H. C., & Scherder, E. J. A. (2008). The role of white matter hyperintensities and medial temporal lobe atrophy in age-related executive dysfunctioning. Brain and Cognition, 68, 128–133. doi:10.1371/journal.pone.0021688
- Overdorp, E. J., Kessels, R. P., Claassen, J. A., & Oosterman, J. M. (2014). Cognitive impairments associated with medial temporal atrophy and white matter hyperintensities: An MRI study in memory clinic patients. Frontiers in Aging Neuroscience, 6, 98. doi:10.3389/fnagi.2014.00098
- Papp, K. V., Kaplan, R. F., Springate, B., Moscufo, N., Wakefield, D. B., Guttmann, C. R., & Wolfson, L. (2014). Processing speed in normal aging: Effects of white matter hyperintensities and hippocampal volume loss. Neuropsychology, Development, and Cognition. Section B, Aging, Neuropsychology and Cognition, 21, 197–213. doi:10.1080/13825585.2013.795513
- Papp, K. V., Snyder, P. J., Maruff, P., Bartkowiak, J., & Pietrzak, R. H. (2011). Detecting subtle changes in visuospatial executive function and learning in the amnestic variant of mild cognitive impairment. PloS ONE, 6, e21688. doi:10.1371/journal.pone.0021688
- Perry, R. J., Watson, P., & Hodges, J. R. (2000). The nature and staging of attention dysfunction in early (minimal and mild) Alzheimer’s disease: Relationship to episodic and semantic memory impairment. Neuropsychologia, 38, 252–271. doi:10.1016/S0028-3932(99)00079-2
- Peters, F., Villeneuve, S., & Belleville, S. (2014). Predicting progression to dementia in elderly subjects with mild cognitive impairment using both cognitive and neuroimaging predictors. Journal of Alzheimer’s Disease, 38, 307–318. doi:10.3233/JAD-130842
- Reitan, R. M. (1958). Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual and Motor Skills, 8, 271–276. doi:10.2466/pms.1958.8.3.271
- Stokholm, J., Vogel, A., Gade, A., & Waldemar, G. (2006). Heterogeneity in executive impairment in patients with very mild Alzheimer’s disease. Dementia and Geriatric Cognitive Disorders, 22, 54–59. doi:10.1159/000093262
- Van der Elst, W., Van Boxtel, M. P., Van Breukelen, G. J., & Jolles, J. (2005). Rey’s verbal learning test: Normative data for 1855 healthy participants aged 24-81 years and the influence of age, sex, education, and mode of presentation. Journal of the International Neuropsychological Society, 11, 290–302. doi:10.1017/S1355617705050344
- Van der Elst, W., Van Boxtel, M. P., Van Breukelen, G. J., & Jolles, J. (2006a). Normative data for the Animal, Profession and Letter M Naming verbal fluency tests for Dutch speaking participants and the effects of age, education, and sex. Journal of the International Neuropsychological Society, 12, 80–89. doi:10.1017/S1355617706060115
- Van der Elst, W., Van Boxtel, M. P., Van Breukelen, G. J., & Jolles, J. (2006b). The Stroop color-word test: Influence of age, sex, and education; and normative data for a large sample across the adult age range. Assessment, 13, 62–79. doi:10.1177/1073191105283427
- Van der Elst, W., Van Boxtel, M. P., Van Breukelen, G. J., & Jolles, J. (2006c). The letter digit substitution test: Normative data for 1,858 healthy participants aged 24-81 from the Maastricht Aging Study (MAAS): Influence of age, education, and sex. Journal of Clinical and Experimental Neuropsychology, 28, 998–1009. doi:10.1080/13803390591004428
- Wechsler, D. (1997). WAIS-III administration and scoring manual. San Antonio, TX: The Psychological Corporation.
- Weintraub, S., Wicklund, A. H., & Salmon, D. P. (2012). The neuropsychological profile of Alzheimer disease. Cold Spring Harbor Perspectives in Medicine, 2, a006171. doi:10.1101/cshperspect.a006171
- Winblad, B., Palmer, K., Kivipelto, M., Jelic, V., Fratiglioni, L., Wahlund, L. O., … Petersen, R. C. (2004). Mild cognitive impairment - Beyond controversies, towards a consensus: Report of the International Working Group on Mild Cognitive Impairment. Journal of Internal Medicine, 256, 240–246. doi:10.1111/j.1365-2796.2004.01380.x