ABSTRACT
Introduction: Motor planning is important for daily functioning. Deficits in motor planning can result in slow, inefficient, and clumsy motor behavior and are linked to disruptions in performance of activities of daily living in children with cerebral palsy (CP). However, the evidence in CP is primarily based on cross-sectional data. Method: Data are presented on the development of motor planning in children with CP using a longitudinal design with three measurement occasions, each separated by 1 year. Twenty-two children with CP (9 boys, 13 girls; age in years;months, M = 7;1, SD = 1;2) and 22 age-matched controls (10 boys, 12 girls, M = 7;1, SD = 1;3) participated. Children performed a bar transport task in which some conditions (“critical angles”) required participants to sacrifice initial posture comfort in order to achieve end-state comfort. Performance on critical trials was analyzed using linear growth curve modeling. Results: In general, children with CP showed poor end-state planning for critical angles. Importantly, unlike in controls, motor planning ability did not improve across the three measurement occasions in children with CP. Conclusion: These longitudinal results show that motor planning issues in CP do not resolve with development over childhood. Strategies to enhance motor planning are suggested for intervention.
Motor planning is a prerequisite of many daily life activities (e.g., Rosenbaum, Meulenbroek, & Vaughan, Citation2004). Deficits in motor planning can result in slow, inefficient, and sequential behaviors and were indicated to hinder proper performance of motor actions in children with cerebral palsy (CP) (Steenbergen, Jongbloed-Pereboom, Spruijt, & Gordon, Citation2013). These compromised motor planning abilities were repeatedly shown in children of different ages and adults with CP (Chen & Yang, Citation2007; Steenbergen et al., Citation2013). The consistency of this finding over different age groups suggests a disorder or a delay in the development of motor planning ability in children with CP compared to their typically developing peers (Craje, Aarts, Nijhuis-van der Sanden, & Steenbergen, Citation2010; Janssen & Steenbergen, Citation2011). However, the validity of such a conclusion from cross-sectional age-group data alone is debatable (Robinson, Schmidt, & Teti, Citation2005). Identifying age-related developmental change requires a longitudinal design (Robinson et al., Citation2005). The current study is the first to present longitudinal data on the development of motor planning over time in children with CP using a two-year follow-up design.
Motor planning can be described as the computational process of selecting a single pattern of behavior from many alternatives that allows the performer to achieve a task goal (e.g., Rosenbaum et al., Citation2004; Wolpert, Citation1997). An often used paradigm studying motor planning is the bar-transport task in which a bar has to be picked up and subsequently placed at another location in a specific orientation (e.g., Rosenbaum & Jorgensen, Citation1992). It has been frequently shown that in performing this task, the selection of the initial movement depends on how comfortable the posture will be at the end of the movement sequence (reviewed in Rosenbaum et al., Citation2004). Subjects will start a movement in an initially uncomfortable posture in order to end with a comfortable one. This phenomenon is known as the end-state comfort effect (e.g., Rosenbaum et al., Citation2004). Three important functional advantages to end a task with a comfortable posture have been indicated (Rosenbaum & Jorgensen, Citation1992): first, it maximizes the range of movements that can subsequently be performed; second, it enables more precision to be exerted (“precision hypothesis”, Short & Cauraugh, Citation1999); third, kinetically, moving from an uncomfortable posture to a comfortable posture requires less energy than moving toward an uncomfortable posture.
The incorporation of the expected end state of the motor system when selecting a starting posture implies that a prediction was made of the consequence of the action—i.e., that the action was internally represented or modeled (Wolpert, Citation1997). This forward modeling is necessary for fast and coordinated movements, as biological feedback loops are inherently slow (e.g., Bubic, von Cramon, & Schubotz, Citation2010). The internal representations of movements incorporate, for example, learned arm dynamics and trajectory information in calculating motor commands for reaching movements (Kawato, Citation1999; Wolpert & Kawato, Citation1998). Reach-to-grasp movements are thus not based just on the perceived spatial demands of the initial movement, but, rather, the intended goal of the entire action sequence is taken into account when starting a movement (Johnson‐Frey, McCarty, & Keen, Citation2004). A deficit in (the use of) motor representations may interfere with the ability to predict the outcome of a particular movement, thus compromising motor planning and execution (e.g., Caeyenberghs, Tsoupas, Wilson, & Smits-Engelsman, Citation2009; Gabbard, Caçola, & Bobbio, Citation2012). There is converging evidence that such an internal modeling deficit (Wilson & Butson, Citation2007) is related to the motor problems of children with developmental coordination disorder (DCD) (Adams, Lust, Wilson, & Steenbergen, Citation2014; Wilson, Ruddock, Smits-Engelsman, Polatajko, & Blank, Citation2013). Research has shown that an internal modeling deficit may also contribute to the impaired motor function of children with CP (e.g., Steenbergen, Crajé, Nilsen, & Gordon, Citation2009; Steenbergen & Gordon, Citation2006; Steenbergen et al., Citation2013). This was evidenced by a much reduced end-state comfort effect; in other words, the children did not incorporate the future state of the motor system, or the consequences of an action when first planning a movement. As internal models of movement contain information concerning the predicted end state of an intended movement, the finding that children with CP seem to start a movement without reference to an uncomfortable end posture may represent a deficit in processing internal movement representations (Adams, Steenbergen, Lust, & Smits-Engelsman, Citation2016; Ruddock et al., Citation2016; Wilson, Citation2014). They appear, instead, to use a less efficient iterative (step-by-step) planning strategy (Steenbergen et al., Citation2009; Steenbergen & Gordon, Citation2006).
Knowledge of the development of motor planning in children stems from cross-sectional age studies. In typically developing children, recent large-scale cross-sectional studies showed that this ability improves between the ages of 3 and 10 years (Jongbloed-Pereboom, Nijhuis-van der Sanden, Saraber-Schiphorst, Crajé, & Steenbergen, Citation2013; Scharoun & Bryden, Citation2014), confirming earlier studies (e.g., Weigelt & Schack, Citation2010). In children with CP only small-scale cross-sectional studies have been conducted, with a varying age span. Craje et al. (Citation2010) reported that children with CP aged 3–6 years did not differ with regard to the degree to which they showed motor planning. This finding was extended to the ages of 7–12 years in the study of Janssen and Steenbergen (Citation2011). Collectively, these studies indicated no age-related differences on motor planning ability in CP, unlike typically developing children. However, the results of Craje et al. (Citation2010) suggest that motor planning in CP may be amenable to training.
In the present study the development of motor planning in CP and typically developing peers was examined using a two-year follow-up design. This is the first longitudinal study of its type to follow up on cross-sectional studies that suggest a lack of development of motor planning in children with CP. The absence of developmental changes in CP would suggest a more persistent deficit in movement planning, whereas evidence for change with age would be more consistent with a delay.
Method
Participants
The longitudinal study consisted of three measurement waves, each separated by one year. A sample of 22 children with CP and 22 age-matched controls (also described in Lust, Wilson, & Steenbergen, Citation2016), aged between 5 and 9 years (age in years;months: M = 7;1, SD = 1;2) at the first measurement wave (Time 0) participated. Participant information is provided in . Children in the two groups were matched for age at Time 0. One child with CP entered the study during the second data wave and was only tested on two (instead of three) occasions, and one child with CP could not be tested at Measurement Wave 2 due to persevering non-compliance. The other children (n = 20) participated on all three measurement occasions.
Table 1. Participant characteristics per group.
The children with CP were recruited via the Dutch organization for parents of physically disabled children (“BOSK”) and a rehabilitation clinic, the Sint MaartensKliniek Nijmegen. Inclusion criteria were the ability to handle the bar used in the task and IQ > 70 (as indicated by the brief version of the Wechsler Nonverbal Scale of Ability (WNV, Wechsler & Naglieri, Citation2006) or attendance of a mainstream primary school. In three cases IQ estimates were obtained from file analysis. Median estimated IQ score in the CP group was 93 (min–max = 65–134). Asymmetry in hand function was measured using the Box and Block test (, gross dexterity; e.g., Jongbloed-Pereboom, Nijhuis-van der Sanden, & Steenbergen, Citation2013). The Gross Motor Function Classification System (GMFCS, Gorter, Van Tol, Van Schie, & Ketelaar, Citation2009) and the Manual Ability Classification System (MACS, Eliasson et al., Citation2006) were used to classify the movement ability of the participating children with CP ().
Performance of the children with CP was compared to a gender- and age-matched control group of typically developing children from mainstream Dutch primary schools. Median estimated IQ score in the control group was 100 (min–max = 80–133), as measured by the brief version of the WNV (Wechsler & Naglieri, Citation2006). These children had no reported motor problems. The absence of motor disabilities was confirmed by results on the Box and Block test that were within the normal range (, Jongbloed-Pereboom et al., Citation2013).
All children were invited by an information letter to their parents, who signed and returned an informed consent to allow their child’s participation and publication of the data. The study was approved by the local ethics committee (ECG2012-1304–027) and the medical research ethics committee (MREC; NL 40,355.091.12).
Apparatus
The bar transport task (e.g., Noten, Wilson, Ruddock, & Steenbergen, Citation2014) consisted of a vertically placed rectangular panel (30 × 30 cm) on which a bar (width: 25 cm; diameter: 2.5 cm) was presented at different rotation angles. The bar itself was colored red at one end and yellow at the other and could rotate freely around its center axis. A cylinder-shaped holder (height: 5 cm; diameter of fitting hole: 3 cm) was placed in front of the panel. The child was required to pick up the bar and place it into the holder with either the red or yellow end, as indicated by the instructions.
Procedure
Each child was seated in front of the apparatus at arm’s length from the bar. The holder was placed centrally between the child and the panel. Children were instructed to use their less affected hand (CP) or their preferred hand (controls) to grasp the bar at the center using a power grip and subsequently place it in the holder. They were free to choose an overhand (pronated) or underhand (supinated) grip to grasp the bar. Once the bar was placed in the holder, the experimenter registered whether the thumb side of the participant’s hand was pointing upward (comfortable end state) or downward (uncomfortable). One practice trial (red end of the bar at 90°, with the instruction to place the bar in the holder with the red end) preceded the 32 experimental trials. Eight orientations of the bar were used; 0°, 60°, 90°, 120°, 180°, 240°, 270°, or 300° from upright. Each angle occurred four times: twice with the instruction to place the red end of the bar into the holder and twice with the instruction to place the yellow end into the holder. The order of trials was randomized but was held constant across subjects.
Control trials were defined as those in which the bar could be picked up from the panel with an overhand (pronated) grip in order to end in a comfortable thumb-up posture after placing the bar in the holder. Critical trials (or “change trials”, e.g., Weigelt & Schack, Citation2010) were defined as trials in which the bar had to be picked up from the panel with an uncomfortable underhand (supinated) grip in order to end in a comfortable thumb-up posture. These trials are critical for the assessment of motor planning as they require the participant to sacrifice initial comfort.
Data analyses
The proportion of comfortable end postures at each measurement wave was calculated for control trials and critical trials in each group.
To compare groups on the proportion of trials that ended in a comfortable posture at each measurement wave, we used Mann-Whitney U Tests.
To compare change trajectories in motor planning over time for each group, the proportion of comfortable end postures on critical trials was analyzed using the linear mixed model procedure in SPSS–Version 23 (SPSS Inc). A multilevel linear growth model was estimated using measurement occasion (i.e., time = 0, 1, 2) as a Level-1 (within-person) predictor and group (control/CP) as a Level-2 (between-person) predictor. The intercept (proportion comfortable end postures in critical trial at Time 0), group, time, and Group × Time interaction were modeled as fixed effects. Also individual differences in the intercept and slope of time were modeled by these being entered as random effects.
Results
We hypothesized that at first measurement, the children with CP would show a smaller proportion of comfortable end postures than controls specifically for critical trials. Furthermore, we predicted that the control group would show a steeper increase in the proportion of comfortably ended critical trials over time than the children with CP.
Mann-Whitney U tests showed no group difference on critical trials at Time 0 (p = .228, ). However, at Time 1 and Time 2 the mean proportion of critical trials that ended in a comfortable posture was higher in the control group than in the group of children with CP (p < .001, ). This suggests different developmental trajectories over a period of 2 years for children with CP compared to controls. As expected, on control trials there was no significant difference between children with and without CP at either measurement occasion (p > . 320, see ).
Table 2. Mann-Whitney U test results.
To directly compare the trajectories of the mean proportion of critical trials that ended in a comfortable posture, we used a multilevel linear growth curve model. Parameter estimates for this model are presented in . The fixed effects showed no significant effect of group (p = .207). This indicated that at the first measurement occasion there was no difference between groups. On average, both groups showed an initial level of ending approximately 30% of critical trials in a comfortable posture. Over the 2 years of data collection, the control group showed a significant .13 unit increase in the proportion of comfortably ended critical trials. The children with CP deviated significantly from this slope, such that they had a significantly more negative slope. A simple slopes analysis was conducted to probe this significant interaction between group and change over time. This showed that the children with CP did not show a change in mean proportion of comfortable end postures in critical trials over time, B = –.04, t(−1.08), p = .292, 95% CI [−1.10, .03]. The model predicted change over time per group (with 95% CIs) is displayed in .
Table 3. Parameter estimates for linear growth model of proportion of critical trials ended in a comfortable posture as a function of group.
Figure 1. Model predicted change over time per group in mean comfortable end postures for critical trials. Gray lines represent 95% confidence intervals.
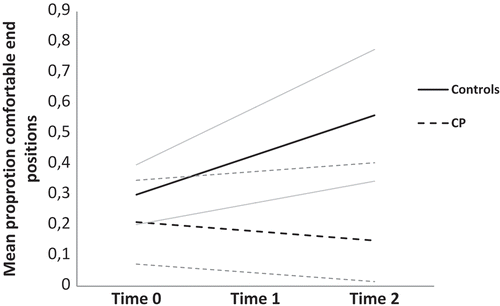
The significant interaction between group and change over time remained when controlling for mean age and mean proportion of comfortable end postures over time (averaged over the three measurement occasions) by these being added as between-person covariates, B(group by time) = –.15, t(17.90) = −2.89, p = .010, 95% CI [–.26, –.04].
The between-subjects random effects (reflecting within-group variability with regard to individual intercepts and slopes) are shown in the lower panel of . At the beginning of the study there was considerable variation within each group with regard to the mean proportion of comfortably ended trials (estimated intercept variance is .05, p = .010). The variability of time slopes within each group (.02) was not significant. Also, there was no linear relationship between intercept differences and slope differences (p = .257). The within-subjects random effects showed that the deviation of observed data points from the individual-specific fitted lines was negligible (p = .289) and that there was no tendency of adjacent residuals to be correlated (p = .496).
Discussion
Motor planning is a prerequisite for optimal performance in many daily life activities (e.g., Rosenbaum et al., Citation2004). During these activities, goal postures are modeled internally in a predictive or anticipatory manner, yielding effects such as the end-state comfort (e.g., Rosenbaum et al., Citation2004). Cross-sectional studies have shown that children with CP show a reduced tendency in comparison with their peers to sacrifice initial comfort in order to end at a comfortable end posture (Craje et al., Citation2010; Janssen & Steenbergen, Citation2011). However, longitudinal data are needed to discern whether these trends are more likely to reflect a developmental lag or a more disrupted pattern of development (Robinson et al., Citation2005). The longitudinal study presented here showed that over a two-year period, the motor planning abilities of a group of children with CP (N = 22, aged 5–9 years at Time 0) showed no evidence of improvement. Motor planning was tested using a bar transport task (Noten et al., Citation2014) on three measurement occasions separated by 1 year. Our growth curve modeling results were in broad agreement with our expectations. On control trials, children with CP performed similarly to controls, showing that they could complete the basic task demands. In contrast, however, children with CP, unlike controls, planned less for end-state comfort on critical trials and showed no development in this ability with age. These results suggested a disrupted pattern of development in CP.
Our findings corroborate earlier cross-correlational findings that fail to show age differences in CP on measures of motor planning (Craje et al., Citation2010; Janssen & Steenbergen, Citation2011). That is, the difference in performance between children with CP and controls increases rather than decreases over time. Our two-year longitudinal data thus showed an absence of spontaneous improvement in motor planning over time. This could be explained by neurophysiological abnormalities in affected brain areas (e.g., Kurz, Becker, Heinrichs-Graham, & Wilson, Citation2014). However, preliminary evidence has also indicated that motor planning in children with CP may be amendable to improvement by training, suggesting sufficient plasticity in the system for training to be effective (Cabral-Sequeira, Coelho, & Teixeira, Citation2016; Craje et al., Citation2010). The absence in the present study of developmental change over time without specific training might thus partly be explained by insufficient environmental stimulation. Important in this respect is that current intervention programs predominantly use physical training, whereas converging evidence in adults with acquired brain damage showed two promising new techniques to stimulate damaged networks in the brain that can be used to remediate motor planning: Motor Imagery (MI; internal rehearsal of a future motor action without overt motor output) and Action Observation (AO; observation of the action performed by someone else). MI and AO are important means by which learning of complex motor tasks can be established, and they share common neurophysiological networks with motor planning (e.g., Vogt, Rienzo, Collet, Collins, & Guillot, Citation2013). Training of motor skills via MI and AO intervention was successful in adults with stroke (Page, Levine, & Leonard, Citation2007), and accumulating evidence indicates that it is also useful to train motor planning in children with congenital motor disorders such as CP and DCD (e.g., Sgandurra et al., Citation2013; Wilson et al., Citation2016). The extent to which the development of this ability can be improved and accelerated with guided therapeutic intervention based on MI and AO in the present group of children warrants further study.
Our results on motor planning were limited to the bar transport task. Although this is an established paradigm to measure motor planning, a recent study in children with DCD suggested that the bar transport task may not require enough precision to provoke the end-state comfort effect (Adams, Ferguson, Lust, Steenbergen, & Smits-Engelsman, Citation2016). However, studies that manipulated the precision of the movement following the reaching and grasping of the bar showed that these manipulations did not affect the initial grasp of children with either CP or DCD (Chen & Yang, Citation2007; Wilmut, Byrne, & Barnett, Citation2013). Indeed, the proportions of comfortable end postures in the present study do not deviate much from those reported in Craje et al. (Citation2010) and Jongbloed-Pereboom et al. (Citation2013), where a wooden sword had to be placed into a wooden box with a tighter fit than the fit of the bar in the cylinder. It is therefore unlikely that the lack of development in motor planning in children with CP can be explained by the amount of precision needed for placing the bar. Critical trials in the bar task are less demanding in terms of the postural comfort that is sacrificed to reach end-state comfort than in the sword task (Adams, Ferguson et al., Citation2016), and yet both tasks show deficits in CP. This suggests that motor planning issues in CP are evident across different levels of movement complexity.
Future studies would benefit from a longer follow-up period to address developmental change over an extended age period, providing an even stronger test of the delay-versus-deviance hypothesis in motor planning in CP. Also, the underlying causes for impaired motor planning remain to be defined. Notwithstanding this, as it is known that motor planning abilities are an important prerequisite for many daily life activities (Steenbergen & Gordon, Citation2006), our finding of a lack of spontaneous development in children with CP emphasized the need for early intervention, for example via motor imagery training or action observation training (e.g., Buccino et al., Citation2012; Cabral-Sequeira et al., Citation2016; Steenbergen et al., Citation2013). As sustained deficits in motor planning will have long-term effects on daily functioning of children with CP, interventions that target this constraint may enhance motor skill development.
In sum, this is the first study that examines the development of motor planning in children with CP using a longitudinal design. We have shown not only that motor planning ability is compromised in children with CP, but also that this ability does not develop naturally within the course of 2 years, as it does in controls. Development across the life span and the extent to which motor planning in these children is amendable to training are topics for future inquiry.
Acknowledgement
We thank the children and their parents for their participation. We also thank Pauline Aarts (Sint Maartens Kliniek), Johannes Verheijden (BOSK), and several schools for special education in the Netherlands for their cooperation. Peter Koval is greatly appreciated for his help with conducting the growth curve modeling analysis. We thank the expert reviewers and Duncan Canine for their helpful comments on the paper.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Adams, I. L. J., Ferguson, G. D., Lust, J. M., Steenbergen, B., & Smits-Engelsman, B. C. M. (2016). Action planning and position sense in children with developmental coordination disorder. Human Movement Science, 46, 196–208.
- Adams, I. L. J., Lust, J. M., Wilson, P. H., & Steenbergen, B. (2014). Compromised motor control in children with DCD : A deficit in the internal model? – A systematic review. Neuroscience and Biobehavioral Reviews, 47, 225–244.
- Adams, I. L. J., Steenbergen, B., Lust, J. M., & Smits-Engelsman, B. C. M. (2016). Motor imagery training for children with developmental coordination disorder – study protocol for a randomized controlled trial. BMC Neurology, 16(1). doi:10.1186/s12883-016-0530-6
- Bubic, A., von Cramon, D. Y., & Schubotz, R. I. (2010). Prediction, cognition and the brain. Frontiers in Human Neuroscience, 4, 25.
- Buccino, G., Arisi, D., Gough, P., Aprile, D., Ferri, C., Serotti, L., … Fazzi, E. (2012). Improving upper limb motor functions through action observation treatment: A pilot study in children with cerebral palsy. Developmental Medicine and Child Neurology, 54(9), 822–828.
- Cabral-Sequeira, A. S., Coelho, D. B., & Teixeira, L. A. (2016). Motor imagery training promotes motor learning in adolescents with cerebral palsy: Comparison between left and right hemiparesis. Experimental Brain Research, 234(6), 1515–1524.
- Caeyenberghs, K., Tsoupas, J., Wilson, P. H., & Smits-Engelsman, B. C. M. (2009). Motor imagery development in primary school children. Developmental Neuropsychology, 34(1), 103–121.
- Chen, Y., & Yang, T.-F. (2007). Effect of task goals on the reaching patterns of children with cerebral palsy. Journal of Motor Behavior, 39(4), 317–324.
- Craje, C., Aarts, P., Nijhuis-van der Sanden, M., & Steenbergen, B. (2010). Action planning in typically and atypically developing children (unilateral cerebral palsy). Research in Developmental Disabilities, 31(5), 1039–1046.
- Eliasson, A.-C., Krumlinde-Sundholm, L., Rösblad, B., Beckung, E., Arner, M., Ohrvall, A.-M., & Rosenbaum, P. (2006). The Manual Ability Classification System (MACS) for children with cerebral palsy: Scale development and evidence of validity and reliability. Developmental Medicine and Child Neurology, 48(7), 549–554.
- Gabbard, C., Caçola, P., & Bobbio, T. (2012). The ability to mentally represent action is associated with low motor ability in children: A preliminary investigation. Child: Care, Health and Development, 38(3), 390–393.
- Gorter, J. W., Van Tol, E., Van Schie, P., & Ketelaar, M. (2009). GMFCS – E & R gross motor function classification system expanded and revised. Retrieved from https://canchild.ca/system/tenon/assets/attachments/000/000/067/original/GMFCS-ER_Translation-Dutch.pdf
- Janssen, L., & Steenbergen, B. (2011). Typical and atypical (cerebral palsy) development of unimanual and bimanual grasp planning. Research in Developmental Disabilities, 32(3), 963–971.
- Johnson‐Frey, S., McCarty, M., & Keen, R. (2004). Reaching beyond spatial perception: Effects of intended future actions on visually guided prehension. Visual Cognition, 11(2–3), 371–399.
- Jongbloed-Pereboom, M., Nijhuis-van der Sanden, M., Saraber-Schiphorst, N., Crajé, C., & Steenbergen, B. (2013). Anticipatory action planning increases from 3 to 10 years of age in typically developing children. Journal of Experimental Child Psychology, 114(2), 295–305.
- Jongbloed-Pereboom, M., Nijhuis-van der Sanden, M. W. G., & Steenbergen, B. (2013). Norm scores of the box and block test for children ages 3–10 years. The American Journal of Occupational Therapy, 67(3), 312–318.
- Kawato, M. (1999). Internal models for motor control and trajectory planning. Current Opinion in Neurobiology, 9, 718–727.
- Kurz, M. J., Becker, K. M., Heinrichs-Graham, E., & Wilson, T. W. (2014). Neurophysiological abnormalities in the sensorimotor cortices during the motor planning and movement execution stages of children with cerebral palsy. Developmental Medicine and Child Neurology, 56(11), 1072–1077.
- Lust, J. M., Wilson, P. H., & Steenbergen, B. (2016). Motor imagery difficulties in children with cerebral palsy: A specific or general deficit? Research in Developmental Disabilities, 57, 102–111.
- Noten, M., Wilson, P., Ruddock, S., & Steenbergen, B. (2014). Mild impairments of motor imagery skills in children with DCD. Research in Developmental Disabilities, 35(5), 1152–1159.
- Page, S. J., Levine, P., & Leonard, A. (2007). Mental practice in chronic stroke: Results of a randomized, placebo-controlled trial. Stroke, 38, 1293–1297.
- Robinson, K., Schmidt, T., & Teti, D. M. (2005). Issues in the use of longitudinal and cross-sectional designs. In D. M. Teti, Ed. Handbook of research methods in developmental science (pp. 1–20). Oxford: Blackwell Publishing Ltd.
- Rosenbaum, D. A., & Jorgensen, M. (1992). Planning macroscopic aspects of manual control. Human Movement Science, 11, 61–69.
- Rosenbaum, D. A., Meulenbroek, R. G. J., & Vaughan, J. (2004). What is the point of motor planning? International Journal of Sport and Exercise Psychology, 2(4), 439–469.
- Ruddock, S., Caeyenberghs, K., Piek, J., Sugden, D., Hyde, C., Morris, S., … Wilson, P. (2016). Coupling of online control and inhibitory systems in children with atypical motor development: A growth curve modelling study. Brain and Cognition, 109, 84–95.
- Scharoun, S. M., & Bryden, P. J. (2014). The development of end- and beginning-state comfort in a cup manipulation task. Developmental Psychobiology, 56(3), 407–420.
- Sgandurra, G., Ferrari, A., Cossu, G., Guzzetta, A., Fogassi, L., & Cioni, G. (2013). Randomized trial of observation and execution of upper extremity actions versus action alone in children with unilateral cerebral palsy. Neurorehabilitation and Neural Repair, 27(9), 808–815.
- Short, M. W., & Cauraugh, J. H. (1999). Precision hypothesis and the end-state comfort effect. Acta Psychologica, 100(3), 243–252.
- Steenbergen, B., Crajé, C., Nilsen, D. M., & Gordon, A. M. (2009). Motor imagery training in hemiplegic cerebral palsy: A potentially useful therapeutic tool for rehabilitation. Developmental Medicine and Child Neurology, 51(9), 690–696.
- Steenbergen, B., & Gordon, A. (2006). Review Activity limitation in hemiplegic cerebral palsy : Evidence for disorders in motor planning. Developmental Medicine & Child Neurology, 48, 780–783.
- Steenbergen, B., Jongbloed-Pereboom, M., Spruijt, S., & Gordon, A. M. (2013). Impaired motor planning and motor imagery in children with unilateral spastic cerebral palsy: Challenges for the future of pediatric rehabilitation. Developmental Medicine and Child Neurology, 55(s4 4), 43–46.
- Vogt, S., Rienzo, F. D., Collet, C., Collins, A., & Guillot, A. (2013). Multiple roles of motor imagery during action observation. Frontiers in Human Neuroscience, 7, 807.
- Wechsler, D., & Naglieri, J. A. (2006). Wechsler Non Verbal scale of ability (WNV). Amsterdam: Pearson Inc., Dutch edition Pearson Assessment and Information B.V.
- Weigelt, M., & Schack, T. (2010). The development of end-state comfort planning in preschool children. Experimental Psychology, 57(6), 476–482.
- Wilmut, K., Byrne, M., & Barnett, A. L. (2013). Reaching to throw compared to reaching to place: A comparison across individuals with and without developmental coordination disorder. Research in Developmental Disabilities, 34(1), 174–182.
- Wilson, P. H. (2014). Neurocognitive processing deficits in children with developmental coordination disorder. In J. Cairney (Ed.), Developmental coordination disorder in children: Consequences and concurrent problems (pp. 138–166). Toronto: University of Toronto Press.
- Wilson, P. H., Adams, I. L. J., Caeyenberghs, K., Thomas, P., Smits-Engelsman, B., & Steenbergen, B. (2016). Motor imagery training enhances motor skill in children with DCD: A replication study. Research in Developmental Disabilities, 57, 54–62.
- Wilson, P. H., & Butson, M. L. (2007). Deficits underlying DCD. In R. H. Geuze (Ed.), Developmental coordination Disorder: A review of current approaches (pp. 111–137). Marseille: Solal Press.
- Wilson, P. H., Ruddock, S., Smits-Engelsman, B., Polatajko, H., & Blank, R. (2013). Understanding performance deficits in developmental coordination disorder: A meta-analysis of recent research. Developmental Medicine and Child Neurology, 55(3), 217–228.
- Wolpert, D. M. (1997). Computational approaches to motor control. Trends in Cognitive Sciences, 1(6), 209–216.
- Wolpert, D. M., & Kawato, M. (1998). Multiple paired forward and inverse models for motor control. Neural Networks, 11(7–8), 1317–1329.
