ABSTRACT
Introduction: Historically, most studies about awake brain surgery have focused on language or motor functions. More recently, other cognitive functions have also been assessed. However, a clear overview of the neuropsychological tests or test paradigms that are used during such procedures is missing. The primary research question of this review is: What neuropsychological tests or paradigms are used during awake brain surgery? This review aims to give an extensive overview about the assessment of cognition during awake brain surgery.
Method: A systematic search was performed in PubMed and Embase. Studies about awake surgery that mentioned a specific test or test paradigm for assessing cognition were included in this review.
Results: The search yielded 4,052 articles. A manual selection for cognition in title and abstract resulted in 360 studies. Those were evaluated in full text; 212 articles described a cognitive task or paradigm. Further reference-list search yielded 20 more studies. In 207 of 232 studies, a test for assessment of language is reported. Tests for the visuospatial domain and motor and sensory functions are described in, respectively, only 23 and 20 studies. Tests for memory, calculation, emotions, or other cognitive functions are reported only in a minority of the included studies.
Conclusions: Tests for assessment of language functions during awake brain surgery are widely reported. Other cognitive functions are underexposed. There is a need for development of tests or paradigms for assessment of other cognitive functions so that the broad spectrum of cognition can be monitored during awake brain surgery.
Awake brain surgery has been performed frequently in the past several years, especially in patients suffering from epilepsy or a brain tumor in an eloquent area. Literature shows that awake brain surgery results in a more extensive resection (De Benedictis, Moritz-Gasser, & Duffau, Citation2010) and fewer neurological deficits (Hamer, Robles, Zwinderman, Duffau, & Berger, Citation2012).
Table 1. Studies included in the review, with cognitive domains monitored during surgery and test/paradigm used to assess the domain.
Table 2. Overview of described tests or paradigms used for measurement of different cognitive functions during awake brain surgery.
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of the systematic literature search.
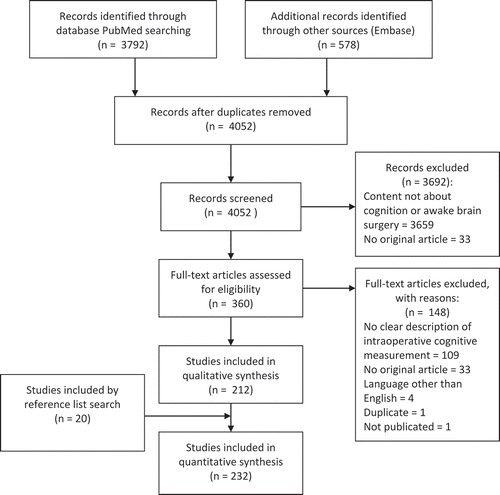
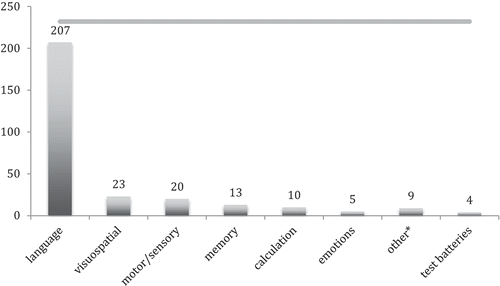
Figure 2. Number of studies reporting tests or paradigms per cognitive domain during awake brain surgery (total number of studies included in review = 232). *Other: executive functions, face recognition, musical skills, finger gnosis.
During awake brain surgery, cognitive functions are monitored. The use of neuropsychological tests during awake brain surgery is not comparable to a standard neuropsychological assessment. Such tests have to meet specific criteria. Administration has to be done during electrical stimulation, and consequently presentation time of stimuli has to be short. Furthermore, repeated measurements are usually necessary within one operation, so a test must have many stimuli, and possible learning effects should be minimalized.
Traditionally, awake surgery was usually done when a tumor was in a language area in the dominant hemisphere (Coello et al., Citation2013), or when motor functions needed to be monitored. There was much attention paid to preservation of language and motor functions (Grossman & Ram, Citation2013). Several reviews are available specifically addressing language mapping (e.g., De Witte & Mariën, Citation2013; Rofes & Miceli, Citation2014).
More recently, awake brain surgery has also been done in patients with lesions in different areas than language areas in the dominant hemisphere. This asks for other cognitive tasks or neuropsychological test paradigms. More and more often, tests for monitoring other cognitive functions are described: for example, visuospatial functions (Conner et al., Citation2016) or calculation (Kurimoto et al., Citation2006). However, an overview of different neuropsychological tests used during awake surgery is missing. A review of Duffau (Citation2010) gives an overview of mapping of nonlanguage functions during awake surgery. However, instead of tests, cognitive domains are described. More recently, Coello and colleagues (Citation2013) presented an overview of different tasks that can be used intraoperatively. In this study, specific parameters such as tumor location and functional networks were used to create a personalized protocol for a patient. However, both studies are not based on a systematic review of the literature, and the cognitive domains or tests are not described from a neuropsychological perspective.
The primary research question for this systematic research is therefore: What neuropsychological tests or paradigms are used during awake brain surgery in brain tumor or epilepsy patients? In contrast to the study of Coello (Citation2013), we include not only studies that describe brain tumor patients but also studies concerning epilepsy patients, so that we can review a wide range of tests used in awake surgeries. This overview will help clinicians in selecting tasks for monitoring cognition during awake brain surgery, taking into account the patient’s lesion and cognitive complaints. We hope to make clear which cognitive domains are best covered by different neuropsychological tests and which cognitive domains are not yet covered. Subsequently, this overview can be helpful in clarifying which domains are most in need of new test development so that cognition can be monitored in the most optimal way.
Method
A systematic literature search was conducted using PubMed and Embase up to February 2017.
We designed a framework for the search strategy, wherein we searched for diseases in combination with awake brain surgery (disease [e.g., tumor] AND procedure [e.g., neurosurgery]) AND (awake [e.g., local anesthesia]). For exact search strategies for PubMed and Embase, see Appendix.
A pilot search made clear that when adding a factor “cognition” to the search strategy, too many relevant articles were not found. Therefore, the author has chosen not to include this factor in our search, but screen for cognition manually in the first results.
Title and abstract screening and full-text reviews were based on the following inclusion criteria:
English language.
Original article (no review, letter to editor, etc.).
Intraoperative measurement of cognition during awake brain surgery.
Clear description of test or paradigm used during awake brain surgery.
Age of patients ≥18.
When there was any doubt about whether an article met the inclusion criteria (for example about intra- or extraoperatively measurement of cognition), the original author of the study was contacted, when possible.
After papers were selected from the literature search in PubMed and Embase, reference list searching was used to identify possible missing papers.
From each paper included in this review, a description of the cognitive domain that was monitored during awake brain surgery and a description of the test or neuropsychological test paradigm used was abstracted.
Results
The PubMed search yielded 3,792 results, and a second search in Embase resulted in 578 additional articles (see ). After removing duplicates, the author manually screened 4,052 articles for the factor cognition in title or abstract, and 360 papers were potentially relevant. Those 360 papers were assessed in full text for eligibility. This study included 212 papers. By reference list searching, another 20 papers were added.
As can be seen in , papers were excluded from this review because of multiple reasons (another language than English, type of article, content not about cognition or awake brain surgery, etc.). Some issues are worth mentioning regarding the process of exclusion. The majority of studies about awake surgery describe that the procedure starts with mapping sensory and motor areas, because these areas tend to have lower thresholds. This kind of mapping is not included in this review. Moreover, when somatosensory functions are assessed more extensively, this is frequently done by sensations described by patients themself, and no specific test is described (e.g., Duffau et al., Citation1999). Comparably, patients can be instructed to report any contractions, movements, or loss of control of voluntary movements (e.g., Kumar et al., Citation2014). Only studies that described a specific test or standardized procedure for measurement of somatosensory and motor functions were included. In addition, mapping of motor functions is also often done by motor-evoked potentials under general anesthesia. Those studies were also excluded from this review.
In addition, many studies report that mapping of a cognitive function (e.g., language) was done, but no description of the test or test paradigm was given (see, e.g., Almairac, Herbet, Moritz-Gasser, & Duffau, Citation2014). Those studies were also excluded. Studies that made use of extraoperative cortical mapping (e.g., by use of a grid) are likewise excluded. During extraoperative mapping there is usually much more time to administer cognitive tests. Therefore, this procedure is not comparable to intraoperative direct electrical stimulation. Studies that describe mapping under awake conditions but resection under general anesthesia could be included (e.g., Ojemann & Schoenfield-McNeill, Citation1999; Weber & Ojemann, Citation1995; Zamora, Corina, & Ojemann, Citation2016), but mapping and resection had to be done in the same operation.
The studies included in this review are presented in . For each study, the cognitive domains that were monitored during surgery are presented in the second column, followed by a description of the tests used to assess this cognitive domain in the third column. The description of the tests or test paradigms is as detailed as possible but is dependent on how much information is given in the original articles. If specific standardized neuropsychological tests are mentioned, those are presented in italic.
Naming famous faces is described as a test for language (naming test) but also as a test for face recognition. Therefore, the author assigned this test to both the language domain and the face-recognition domain.
In , the total number of studies that describe tests or paradigms per cognitive domain are presented. From the 232 studies included in this review, 207 studies describe one or more tests/paradigms for assessment of language functions. Only a minority of the included studies describe tests for other cognitive domains, whereby visuospatial and sensory or motor functions are best represented, followed by tests for memory and calculation. Tests for assessment of executive functions, face recognition, musical skills, and finger gnosis are reported only sporadically.
summaries the tests/paradigms used per cognitive function during awake brain surgery.
Discussion
In this review the author provides an overview of the neuropsychological tests or paradigms that are used during awake brain surgery for brain tumors or epilepsy. Results of this study show that language is by far the most tested cognitive domain in awake brain surgery. Almost 90% of the studies included in this review describe a test or test paradigm for the assessment of language functions. Monitoring of this function is done by very simple tasks such as counting, to more complex or specific tasks such as semantic associations or translating skills. In addition to more experimental paradigms, a variety of standardized neuropsychological tests, such as the Test de Dénomination Orale D’Images (DO80; Deloche & Hannequin, Citation1997), Boston Naming Test (Kaplan, Goodglass, & Weintraub, Citation2001), and Pyramid and Palmtree Test (Howard & Patterson, Citation1992), are used in monitoring language functions.
Tests for motor or sensory functions are less frequently mentioned. This does not automatically mean that those functions are only rarely monitored during awake brain surgery. Actually, many studies report that they monitor those functions, but this is mostly done by asking a patient to report any sensations or movements, and no specific task or paradigm is administered. Moreover, as mentioned earlier in the Method section, motor functions are also often monitored by motor-evoked potentials. So, unless the fact that motor and sensory functions are frequently monitored during awake brain surgery, only a few studies describe a specific test or paradigm for the measurement of these domains.
Tests for visuospatial functions are also reported in just a few studies, followed by tests for memory, calculation, and emotions. Tests for the measurement of other cognitive functions such as face recognition, executive functions, musical skills, and finger gnosis are rare.
Only four studies included in this review describe a complete test battery that can be used during awake brain surgery. Two of them assess multiple cognitive domains (De Witte et al. Citation2015; Skrap, Marin, Ius, Fabbro, & Tomasino, Citation2016); the others focus on language (De Witte et al., Citation2015) and motor/sensory functions (Becker et al., Citation2016).
It is remarkable that in many studies only one cognitive domain is tested, sometimes with just one test (for example, only object naming). The minority of the studies included in this review describe monitoring of multiple cognitive functions and usage of different cognitive tests in an awake brain surgery.
It is also remarkable that language takes such an important part of the monitoring of cognition during awake brain surgery, while other cognitive functions are clearly underexposed. Of course, impairment in language functions can cause numerous participation problems in daily life, and preservation of this function is very important. On the other hand, deficits in, for example, executive functions may also have a great impact on daily life (Chan, Shum, Toulopoulou, & Chen, Citation2008), and preserved executive functions are important for self-regulation (Hofmann, Schmeichel, & Baddeley, Citation2012). A recent systematic review about neurocognitive functioning prior to antitumor treatment shows that the majority of patients have impairment in any cognitive domain. Especially executive functioning is frequently impaired (Van Kessel, Baumfalk, Van Zandvoort, Robe, & Snijders, Citation2017). While many patients have executive impairments prior to surgery, it is paradoxical that the monitoring of executive functions is only reported in such a small number of studies included in this review (Plaza, Du Boullay, Perrault, Chaby, & Capelle, Citation2014; Wager et al., Citation2013). Possibly, the fact that executive functions are less concrete than, for example, language and motor functions makes it more difficult to imagine the consequenses of deficits in this cognitive domain. Permanent deficits in language and motor functions are clearly not acceptable for patients, surgeons, and society. However, deficits in executive functions may have an impact on daily functioning and communication with others that is at least as big as the problems deriving from language disorders. Although assessment of executive functions seems difficult, some aspects of this cognitive domain such as inhibition can be perfectly tested intraoperatively by administration of, for example, an (adjusted) Stroop Color Word Test (Wager et al., Citation2013).
The majority of the studies included in this review describe experimental tasks or paradigms, and just a minority of the studies make use of well-known standardized tests (example DO80, line bisection task) or modification of those tests. This is not surprising, given that tests that are used during awake brain surgery must meet specific criteria. Because of the short simulation time, items of the test should have a short presentation time. Furthermore, because of the sometimes-long duration of the surgery, many comparable stimuli with a comparable level of difficulty are needed.On the other hand, learning effects should be limited as much as possible. It is also important that the responses on the test items are simple and clear and do not have a high chance level. Tests that require a yes/no answer are therefore unsuitable. Rofes and Miceli (Citation2014) give a detailed description of the design of intraoperative tasks specific for language mapping. Besides language-specific recommendations, they also state that tasks should be short (both in presentation of stimuli and in response from patients) and that the tasks should be sensitive enough to detect subtle changes. While specificity is important during regular neuropsychological assessment, sensitivity is most important in monitoring cognition during the intraoperative phase, so that changes in cognitive functioning can be detected. It is important to realize that intraoperative cognitive functioning is always compared to preoperative functioning, and therefore an extensive preoperative neuropsychological assessment is indispensable. It is clear that most standardized neuropsychological tests cannot be simply used during awake brain surgery. Keeping the abovementioned criteria in our minds, there is a need for development of new tests or paradigms (or modifications of existing tests) for the cognitive domains that are underexposed up to this point.
A limitation of this study may be the exclusion of studies that do not give a description of the tests or paradigms used for monitoring cognition during awake brain surgery. Although it was the specific goal of this study to include only studies that gave a description of how different cognitive functions are measured during awake brain surgery, this may distort the overall picture of measurement of cognition in such procedures. Many studies do mention that cognition is monitored, but because of a lack of further explanation of the way this was done, those studies were excluded from this review. For example, this review makes clear that only a few formalized tests for monitoring motor and sensory functions are described in different studies. On the other hand, this does not mean that these functions are also monitored only in a small proportion of the surgeries. Furthermore, it is plausible that in clinical practice, more specific tests are administered during awake brain surgery, but that those groups have not yet published about their procedures.
A strength of this study is the extensive systematic search of the literature. This is the first systematic review about monitoring cognition during awake brain surgery at test or neuropsychological paradigm level. Furthermore, the full width of cognition instead of a specific cognitive function, such as language, is studied.
Monitoring cognition during awake brain surgery is done more and more, and can usually not be captured in a strict protocol because of different locations of lesions and, as a result, different (possible) cognitive deficits. Individual patient care is needed, and most standardized neuropsychological tests cannot be used. This review can help clinicians in selecting tests that can be used during such a procedure so that optimal cognitive monitoring can be achieved. Sharing knowledge about this specific topic is important, and there is a need for scientific publications about new methods that are used in clinical care.
In summary, monitoring of language functions during awake brain surgery has been studied extensively, and many different tests or paradigms for assessing language functions have been described. Although there is no doubt about the importance of preserving language functions, it is remarkable that tests for other cognitive domains receive much less attention. The results of this review make clear that development of new tests for several cognitive domains is needed, so that cognition can be comprehensively monitored in awake brain surgeries.
Acknowledgments
The author would like to thank Paulien Wiersma for her support in constructing a search strategy.
Disclosure statement
No potential conflict of interest was reported by the author.
References
- Abel, T. J., Hebb, A. O., & Silbergeld, D. L. (2009). Cortical stimulation mapping in a patient with foreign accent syndrome: Case report. Clinical Neurology and Neurosurgery, 111(1), 97–101.
- Alimohamadi, M., Shirani, M., Moharari, R. S., Pour-Rashidi, A., Ketabchi, M., Khajavi, M., … Amirjamshidi, A. (2016). Application of awake craniotomy and intraoperative brain mapping for surgical resection of insular gliomas of the dominant hemisphere. World Neurosurgery, 92, 151–158. doi:10.1016/j.wneu.2016.04.079
- Almairac, F., Herbet, G., Moritz-Gasser, S., De Champfleur, N. M., & Duffau, H. (2015). The left inferior fronto-occipital fasciculus subserves language semantics: A multilevel lesion study. Brain Structure and Function, 220(4), 1983–1995.
- Almairac, F., Herbet, G., Moritz-Gasser, S., & Duffau, H. (2014). Parietal network underlying movement control: Disturbances during subcortical electrostimulation. Neurosurgical Review, 37(3), 513–517.
- Bai, H. M., Wang, W. M., Li, T. D., He, H., Shi, C., Guo, X. F., … Wang, S. S. (2011). Three core techniques in surgery of neuroepithelial tumors in eloquent areas: Awake anaesthesia, intraoperative direct electrical stimulation and ultrasonography. Chinese Medical Journal, 124(19), 3035–3041.
- Bandur, D. L., Parrent, A. G., & Stevens, D. A. (2007). A tailored approach to language mapping in epilepsy surgery: Some preliminary findings. Journal of Medical Speech-Language Pathology, 15(2), 107–118.
- Bartha, L., Knosp, E., Pfisterer, W., & Benke, T. (2000). Intra- and perioperative monitoring of language functions in patients with tumours in the left perisylvian area. Aphasiology, 14(8), 779–793.
- Bartolomeo, P., De Schotten, M. T., & Duffau, H. (2007). Mapping of visuospatial functions during brain surgery: A new tool to prevent unilateral spatial neglect. Neurosurgery, 61(6), E1340.
- Bartoš, R., Jech, R., Vymazal, J., Petrovický, P., Vachata, P., Hejčl, A., … Sameš, M. (2009). Validity of primary motor area localization with fMRI versus electric cortical stimulation: A comparative study. Acta Neurochirurgica, 151(9), 1071–1080. doi:10.1007/s00701-009-0368-4
- Becker, J., Jehna, M., Steinmann, E., Mehdorn, H. M., Synowitz, M., & Hartwigsen, G. (2016). The sensory-motor profile awake—A new tool for pre-, intra-, and postoperative assessment of sensory-motor function. Clinical Neurology and Neurosurgery, 147, 39–45.
- Bello, L., Acerbi, F., Giussani, C., Baratta, P., Taccone, P., & Songa, V. (2006). Intraoperative language localizationin multilingual patients with gliomas. Neurosurgery, 59(1), 115–125.
- Bello, L., Gallucci, M., Fava, M., Carrabba, G., Giussani, C., Acerbi, F., … Gaini, S. M. (2007). Intraoperative subcortical languagetract mapping guides surgical removalof gliomas involving speech areas. Neurosurgery, 60(1), 67–82.
- Bello, L., Gambini, A., Castellano, A., Carrabba, G., Acerbi, F., Fava, E., … Falini, A. (2008). Motor and language DTI Fiber Tracking combined with intraoperative subcortical mapping for surgical removal of gliomas. Neuroimage, 39(1), 369–382. doi:10.1016/j.neuroimage.2007.08.031
- Benzagmout, M., Gatignol, P., & Duffau, H. (2007). Resection of World Health Organization Grade II gliomas involving Broca’s area: Methodological and functional considerations. Neurosurgery, 61(4), 741–753.
- Bertani, G., Fava, E., Casaceli, G., Carrabba, G., Casarotti, A., Papagno, C., … Bello, L. (2009). Intraoperative mapping and monitoring of brain functions for the resection of low-grade gliomas: Technical considerations. Neurosurgical Focus, 27(4), E4.
- Bilotta, F., Stazi, E., Titi, L., Lalli, D., Delfini, R., Santoro, A., & Rosa, G. (2014). Diagnostic work up for language testing in patients undergoing awake craniotomy for brain lesions in language areas. British Journal of Neurosurgery, 28(3), 363–367.
- Bizzi, A., Blasi, V., Falini, A., Ferroli, P., Cadioli, M., Danesi, U., … Broggi, G. (2008). Presurgical functional MR imaging of language and motor functions: Validation with intraoperative electrocortical mapping. Radiology, 248(2), 579–589. doi:10.1148/radiol.2482071214
- Borius, P.-Y., Giussani, C., Draper, L., & Roux, F.-E. (2012). Sentence translation in proficient bilinguals: A direct electrostimulation brain mapping. Cortex, 48(5), 614–622.
- Brandling-Bennett, E. M., Bookheimer, S. Y., Horsfall, J. L., Moftakhar, P., Sedrak, M., Barkulis, C. T., … Bergsneider, M. (2012). A paradigm for awake intraoperative memory mapping during forniceal stimulation. Neurocase, 18(1), 26–38. doi:10.1080/13554794.2010.547509
- Breshears, J., Sharma, M., Anderson, N. R., Rashid, S., & Leuthardt, E. C. (2010). Electrocorticographic frequency alteration mapping of speech cortex during an awake craniotomy: Case report. Stereotactic and Functional Neurosurgery, 88(1), 11–15. doi:10.1159/000260074
- Bunyaratavej, K., Sangtongjaraskul, S., Lerdsirisopon, S., & Tuchinda, L. (2016). Continuous physical examination during subcortical resection in awake craniotomy patients: Its usefulness and surgical outcome. Clinical Neurology and Neurosurgery, 147, 34–38.
- Burbridge, M., & Raazi, M. (2013). Awake craniotomy in a developmentally delayed blind man with cognitive deficits. Canadian Journal of Anesthesia/Journal Canadien D’anesthésie, 60(4), 399–403.
- Chacko, A. G., Thomas, S. G., Babu, K. S., Daniel, R. T., Chacko, G., Prabhu, K., … Korula, G. (2013). Awake craniotomy and electrophysiological mapping for eloquent area tumours. Clinical Neurology and Neurosurgery, 115(3), 329–334.
- Chan, R. C., Shum, D., Toulopoulou, T., & Chen, E. Y. (2008). Assessment of executive functions: Review of instruments and identification of critical issues. Archives of Clinical Neuropsychology, 23(2), 201–216. doi:10.1016/j.acn.2007.08.010
- Chang, E. F., Breshears, J. D., Raygor, K. P., Lau, D., Molinaro, A. M., & Berger, M. S. (2017). Stereotactic probability and variability of speech arrest and anomia sites during stimulation mapping of the language dominant hemisphere. Journal of Neurosurgery, 126(1), 114–121.
- Chan-Seng, E., Moritz-Gasser, S., & Duffau, H. (2014). Awake mapping for low-grade gliomas involving the left sagittal stratum: Anatomofunctional and surgical considerations: Clinical article. Journal of Neurosurgery, 120(5), 1069–1077.
- Coello, A. F., Duvaux, S., De Benedictis, A., Matsuda, R., & Duffau, H. (2013). Involvement of the right inferior longitudinal fascicle in visual hemiagnosia: A brain stimulation mapping study: Case report. Journal of Neurosurgery, 118(1), 202–205.
- Coello, A. F., Moritz-Gasser, S., Martino, J., Martinoni, M., Matsuda, R., & Duffau, H. (2013). Selection of intraoperative tasks for awake mapping based on relationships between tumor location and functional networks: A review. Journal of Neurosurgery, 119(6), 1380–1394.
- Conner, A. K., Glenn, C., Burks, J. D., McCoy, T., Bonney, P. A., Chema, A. A., … Sughrue, M. (2016). The use of the target cancellation task to identify eloquent visuospatial regions in awake craniotomies: Technical note. Cureus, 8(11), e883.
- Corrivetti, F., Herbet, G., Moritz-Gasser, S., & Duffau, H. (2017). Prosopagnosia Induced by a left anterior temporal lobectomy following a right temporo-occipital resection in a multicentric diffuse low-grade glioma. World Neurosurgery, 97, 756–e1.
- Costello, T. G. (2014). Awake craniotomy and multilingualism: Language testing during anaesthesia for awake craniotomy in a bilingual patient. Journal of Clinical Neuroscience, 21(8), 1469–1470.
- Danks, R. A., Aglio, L. S., Gugino, L. D., & Black, P. M. (2000). Craniotomy under local anesthesia and monitored conscious sedation for the resection of tumors involving eloquent cortex. Journal of Neuro-Oncology, 49(2), 131–139.
- De Benedictis, A., Moritz-Gasser, S., & Duffau, H. (2010). Awake mapping optimizes the extent of resection for low‐grade gliomas in eloquent areas. Neurosurgery, 66(6), 1074–1084. doi:10.1227/01.NEU.0000369514.74284.78
- De Witte, E., & Mariën, P. (2013). The neurolinguistic approach to awake surgery reviewed. Clinical Neurology and Neurosurgery, 115(2), 127–145. doi:10.1016/j.clineuro.2012.09.015
- De Witte, E., Satoer, D., Colle, H., Robert, E., Visch-Brink, E., & Mariën, P. (2015). Subcortical language and non-language mapping in awake brain surgery: The use of multimodal tests. Acta Neurochirurgica, 157(4), 577–588.
- De Witte, E., Satoer, D., Robert, E., Colle, H., Verheyen, S., Visch-Brink, E., & Mariën, P. (2015). The Dutch Linguistic Intraoperative Protocol: A valid linguistic approach to awake brain surgery. Brain and Language, 140, 35–48.
- Della Puppa, A., De Pellegrin, S., d’Avella, E., Gioffrè, G., Munari, M., Saladini, M., … Semenza, C. (2013). Right parietal cortex and calculation processing: Intraoperative functional mapping of multiplication and addition in patients affected by a brain tumor: Clinical article. Journal of Neurosurgery, 119(5), 1107–1111.
- Della Puppa, A., De Pellegrin, S., Lazzarini, A., Gioffrè, G., Rustemi, O., Cagnin, A., … Semenza, C. (2015). Subcortical mapping of calculation processing in the right parietal lobe. Journal of Neurosurgery, 122(5), 1038–1041.
- Deloche, G., & Hannequin, D. (1997). Test de dénomination orale d’images: DO 80. Éd. du Centre de psychologie appliquée.
- Duffau, H., Capelle, L., Sichez, J. P., Faillot, T., Abdennour, L., Law Koune, J.-D., … Fohanno, D. (1999). Intra-operative direct electrical stimulations of the central nervous system: The Salpêtrière experience with 60 patients. Acta Neurochirurgica, 141(11), 1157–1167.
- Duffau, H., Denvil, D., Lopes, M., Gasparini, F., Cohen, L., Capelle, L., & Van Effenterre, R. (2001). Intraoperative mapping of the cortical areas involved in multiplication and subtraction: An electrostimulation study in a patient with a left parietal glioma. Journal of Neurology, Neurosurgery & Psychiatry, 73(6), 733–738. doi:10.1136/jnnp.73.6.733
- Duffau, H., Bauchet, L., Lehéricy, S., & Capelle, L. (2001). Functional compensation of the left dominant insula for language. Neuroreport, 12(10), 2159–2163.
- Duffau, H., Lopes, M., Denvil, D., & Capelle, L. (2001). Delayed onset of the supplementary motor area syndrome after surgical resection of the mesial frontal lobe: A time course study using intraoperative mapping in an awake patient. Stereotactic and Functional Neurosurgery, 76(2), 74–82.
- Duffau, H., Capelle, L., Sichez, N., Denvil, D., Lopes, M., Sichez, J.-P., … Fohanno, D. (2002). Intraoperative mapping of the subcortical language pathways using direct stimulations. Brain, 125(1), 199–214.
- Duffau, H., Denvil, D., & Capelle, L. (2002). Long term reshaping of language, sensory, and motor maps after glioma resection: A new parameter to integrate in the surgical strategy. Journal of Neurology, Neurosurgery & Psychiatry, 72(4), 511–516.
- Duffau, H., Gatignol, P., Denvil, D., Lopes, M., & Capelle, L. (2003). The articulatory loop: Study of the subcortical connectivity by electrostimulation. Neuroreport, 14(15), 2005–2008.
- Duffau, H., Capelle, L., Denvil, D., Sichez, N., Gatignol, P., Taillandier, L., … Van Effenterre, R. (2003). Usefulness of intraoperative electrical subcortical mapping during surgery for low-grade gliomas located within eloquent brain regions: Functional results in a consecutive series of 103 patients. Journal of Neurosurgery, 98(4), 764–778. doi:10.3171/jns.2003.98.4.0764
- Duffau, H., Velut, S., Mitchell, M.-C., Gatignol, P., & Capelle, L. (2004). Intra-operative mapping of the subcortical visual pathways using direct electrical stimulations. Acta Neurochirurgica, 146(3), 265–270. doi:10.1007/s00701-003-0199-7
- Duffau, H., Gatignol, P., Mandonnet, E., Peruzzi, P., Tzourio-Mazoyer, N., & Capelle, L. (2005). New insights into the anatomo-functional connectivity of the semantic system: A study using cortico-subcortical electrostimulations. Brain, 128(4), 797–810.
- Duffau, H., Lopes, M., Arthuis, F., Bitar, A., Sichez, J. P., Van Effenterre, R., & Capelle, L. (2005). Contribution of intraoperative electrical stimulations in surgery of low grade gliomas: A comparative study between two series without (1985–96) and with (1996–2003) functional mapping in the same institution. Journal of Neurology, Neurosurgery & Psychiatry, 76(6), 845–851.
- Duffau, H., Gatignol, P., Mandonnet, E., Capelle, L., & Taillandier, L. (2008). Intraoperative subcortical stimulation mapping of language pathways in a consecutive series of 115 patients with Grade II glioma in the left dominant hemisphere. Journal of Neurosurgery, 109(3), 461–471.
- Duffau, H., Gatignol, P., Moritz-Gasser, S., & Mandonnet, E. (2009). Is the left uncinate fasciculus essential for language? Journal of Neurology, 256(3), 382–389.
- Duffau, H. (2010). Awake surgery for nonlanguage mapping. Neurosurgery, 66(3), 523–529.
- Duffau, H. (2012). Awake surgery for incidental WHO grade II gliomas involving eloquent areas. Acta Neurochirurgica, 154(4), 575–584.
- Fujii, M., Maesawa, S., Motomura, K., Futamura, M., Hayashi, Y., Koba, I., & Wakabayashi, T. (2015). Intraoperative subcortical mapping of a language-associated deep frontal tract connecting the superior frontal gyrus to Broca’s area in the dominant hemisphere of patients with glioma. Journal of Neurosurgery, 122(6), 1390–1396.
- Gao, H., Bai, H.-M., Han, L.-X., Li, T.-D., Wang, G.-L., & Wang, W.-M. (2015). Brain cancer surgery in the language areas of Mandarin-Cantonese bilinguals. Journal of Cancer Research and Therapeutics, 11(2), 415. doi:10.4103/0973-1482.151932
- Gao, H., Bai, H., Han, L., Li, T., Wang, W., Liu, Y., … Wang, W. (2016). Language-associated cortical regions in non-proficient Chinese–English bilinguals with glioma. Journal of Neurolinguistics, 39, 49–56.
- Gatignol, P., Capelle, L., Le Bihan, R., & Duffau, H. (2004). Double dissociation between picture naming and comprehension: An electrostimulation study. Neuroreport, 15(1), 191–195.
- Gharabaghi, A., Berger, M. F., Tatagiba, M., & Karnath, H.-O. (2006). The role of the right superior temporal gyrus in visual search—Insights from intraoperative electrical stimulation. Neuropsychologia, 44(12), 2578–2581. doi:10.1016/j.neuropsychologia.2006.04.006
- Gil Robles, S., Gelisse, P., Vergani, F., Moritz-Gasser, S., Rigau, V., Coubes, P., … Duffau, H. (2008). Discrepancies between preoperative stereoencephalography language stimulation mapping and intraoperative awake mapping during resection of focal cortical dysplasia in eloquent areas. Stereotactic and Functional Neurosurgery, 86(6), 382–390.
- Gil Robles, S. G., Gatignol, P., Capelle, L., Mitchell, M. C., & Duffau, H. (2005). The role of dominant striatum in language: A study using intraoperative electrical stimulations. Journal of Neurology, Neurosurgery & Psychiatry, 76(7), 940–946.
- Giussani, C., Pirillo, D., & Roux, F.-E. (2010). Mirror of the soul: A cortical stimulation study on recognition of facial emotions: Clinical article. Journal of Neurosurgery, 112(3), 520–527.
- Giussani, C., Roux, F.-E., Bello, L., Lauwers-Cances, V., Papagno, C., Gaini, S. M., … Démonet, J.-F. (2009). Who is who: Areas of the brain associated with recognizing and naming famous faces: Clinical article. Journal of Neurosurgery, 110(2), 289–299.
- Gonen, T., Sela, G., Yanakee, R., Ram, Z., & Grossman, R. (2017). Surgery-independent language function decline in patients undergoing awake craniotomy. World Neurosurgery, 99, 674–679.
- Gras-Combe, G., Moritz-Gasser, S., Herbet, G., & Duffau, H. (2012). Intraoperative subcortical electrical mapping of optic radiations in awake surgery for glioma involving visual pathways: Clinical article. Journal of Neurosurgery, 117(3), 466–473.
- Grossman, R., & Ram, Z. (2013). awake Craniotomy in Glioma surgery. European Association of NeuroOncology Magazine, 4(1), 27–33.
- Gupta, D. K., Chandra, P. S., Ojha, B. K., Sharma, B. S., Mahapatra, A. K., & Mehta, V. S. (2007). Awake craniotomy versus surgery under general anesthesia for resection of intrinsic lesions of eloquent cortex—A prospective randomised study. Clinical Neurology and Neurosurgery, 109(4), 335–343.
- Haglund, M. M., Berger, M. S., Shamseldin, M., Lettich, E., & Ojemann, G. A. (1994). Cortical localization of temporal lobe language sites in patients with gliomas. Neurosurgery, 34(4), 567–576.
- Haglund, M. M., Ojemann, G. A., Schwartz, T. W., & Lettich, E. (1994). Neuronal activity in human lateral temporal cortex during serial retrieval from short-term memory. The Journal of Neuroscience, 14(3), 1507–1515.
- Hamberger, M. J., Goodman, R. R., Perrine, K., & Tamny, T. (2001). Anatomic dissociation of auditory and visual naming in the lateral temporal cortex. Neurology, 56(1), 56–61.
- Hamberger, M. J., McClelland, S., McKhann, G. M., Williams, A. C., & Goodman, R. R. (2007). Distribution of auditory and visual naming sites in nonlesional temporal lobe epilepsy patients and patients with space‐occupying temporal lobe lesions. Epilepsia, 48(3), 531–538.
- Hamberger, M. J., Seidel, W. T., Goodman, R. R., & McKhann, G. M. (2010). Does cortical mapping protect naming if surgery includes hippocampal resection? Annals of Neurology, 67(3), 345–352.
- Hamberger, M. J., Seidel, W. T., Goodman, R. R., Perrine, K., & McKhann, G. M. (2003). Temporal lobe stimulation reveals anatomic distinction between auditory naming processes. Neurology, 60(9), 1478–1483.
- Hamberger, M. J., Seidel, W. T., Mckhann, G. M., Perrine, K., & Goodman, R. R. (2005). Brain stimulation reveals critical auditory naming cortex. Brain, 128(11), 2742–2749.
- Hamer, P. C. D. W., Robles, S. G., Zwinderman, A. H., Duffau, H., & Berger, M. S. (2012). Impact of intraoperative stimulation brain mapping on glioma surgery outcome: A meta-analysis. Journal of Clinical Oncology, 30(20), 2559–2565. doi:10.1200/JCO.2011.38.4818
- Henry, R. G., Berman, J. I., Nagarajan, S. S., Mukherjee, P., & Berger, M. S. (2004). Subcortical pathways serving cortical language sites: Initial experience with diffusion tensor imaging fiber tracking combined with intraoperative language mapping. Neuroimage, 21(2), 616–622.
- Herbert, G., Lafargue, G., Almairac, F., Moritz-Gasser, S., Bonnetblanc, F., & Duffau, H. (2015). Disrupting the right pars opercularis with electrical stimulation frees the song: Case report. Journal of Neurosurgery, 123(6), 1401–1404. doi:10.3171/2014.12.JNS141829
- Herbert, G., Lafargue, G., Moritz-Gasser, S., Bonnetblanc, F., & Duffau, H. (2015). Interfering with the neural activity of mirror-related frontal areas impairs mentalistic inferences. Brain Structure and Function, 220(4), 2159–2169. doi:10.1007/s00429-014-0777-x
- Herholz, K., Reulen, H.-J., Von Stockhausen, H.-M., Thiel, A., Llmberger, J., Kessler, J., … Heiss, W.-D. (1997). Preoperative activation and intraoperative stimulation of language-related areas in patients with glioma. Neurosurgery, 41(6), 1253–1262.
- Hofmann, W., Schmeichel, B. J., & Baddeley, A. D. (2012). Executive functions and self-regulation. Trends in Cognitive Sciences, 16(3), 174–180.
- Holmes, M. D., Ojemann, G. A., & Lettich, E. (1996). Neuronal activity in human right lateral temporal cortex related to visuospatial memory and perception. Brain Research, 711(1–2), 44–49.
- Howard, D., & Patterson, K. E. (1992). The Pyramids and Palm Trees Test: A test of semantic access from words and pictures. Thames Valley Test Company.
- Huang, M., Baskin, D. S., & Fung, S. (2016). Glioblastoma presenting with pure alexia and palinopsia involving the left inferior occipital gyrus and visual word form area evaluated with functional magnetic resonance imaging and diffusion tensor imaging tractography. World Neurosurgery, 89, 725–e5.
- Ille, S., Sollmann, N., Hauck, T., Maurer, S., Tanigawa, N., Obermueller, T., … Krieg, S. M. (2015a). Impairment of preoperative language mapping by lesion location: A functional magnetic resonance imaging, navigated transcranial magnetic stimulation, and direct cortical stimulation study. Journal of Neurosurgery, 123(2), 314–324.
- Ille, S., Sollmann, N., Hauck, T., Maurer, S., Tanigawa, N., Obermueller, T., … Krieg, S. M. (2015b). Combined noninvasive language mapping by navigated transcranial magnetic stimulation and functional MRI and its comparison with direct cortical stimulation. Journal of Neurosurgery, 123(1), 212–225.
- Ilmberger, J., Eisner, W., Schmid, U., & Reulen, H.-J. (2001). Performance in picture naming and word comprehension: Evidence for common neuronal substrates from intraoperative language mapping. Brain and Language, 76(2), 111–118.
- Ilmberger, J., Ruge, M., Kreth, F.-W., Briegel, J., Reulen, H.-J., & Tonn, J.-C. (2008). Intraoperative mapping of language functions: A longitudinal neurolinguistic analysis. Journal of Neurosurgery, 109(4), 583–592.
- Ishikawa, T., Muragaki, Y., Maruyama, T., Kayoko, A. B. E., & Kawamata, T. (2017). Roles of the Wada Test and functional magnetic resonance imaging in identifying the language-dominant hemisphere among patients with gliomas located near speech areas. Neurologia Medico-Chirurgica, 57(1), 28–34. doi:10.2176/nmc.oa.2016-0042
- Jiménez de la Peña, M. J., Robles, S. G., Rodríguez, M. R., Ocaña, C. R., & Martínez de Vega, V. M. (2013). Cortical and subcortical mapping of language areas: Correlation of functional MRI and tractography in a 3T scanner with intraoperative cortical and subcortical stimulation in patients with brain tumors located in eloquent areas. Radiología (English Edition), 55(6), 505–513. doi:10.1016/j.rxeng.2012.01.001
- Joswig, H., Bratelj, D., Brunner, T., Jacomet, A., Hildebrandt, G., & Surbeck, W. (2016). Awake craniotomy: First-year experiences and patient perception. World Neurosurgery, 90, 588–596.
- Kamada, K., Todo, T., Masutani, Y., Aoki, S., Ino, K., Morita, R. T., … Saito, N. (2007). Visualization of the frontotemporal language fibers by tractography combined with functional magnetic resonance imaging and magnetoencephalography. Journal of Neurosurgery, 106(1), 90–98.
- Kaplan, E., Goodglass, H., & Weintraub, S. (2001). Boston naming test. Pro-ed.
- Kawashima, A., Krieg, S. M., Faust, K., Schneider, H., Vajkoczy, P., & Picht, T. (2013). Plastic reshaping of cortical language areas evaluated by navigated transcranial magnetic stimulation in a surgical case of glioblastoma multiforme. Clinical Neurology and Neurosurgery, 115(10), 2226–2229. doi:10.1016/j.clineuro.2013.07.012
- Khan, O. H., Herbet, G., Moritz-Gasser, S., & Duffau, H. (2014). The role of left inferior fronto-occipital fascicle in verbal perseveration: A brain electrostimulation mapping study. Brain Topography, 27(3), 403–411.
- Kim, J.-H., Amankulor, N. M., Peck, K. K., Brennan, N., Gutin, P. H., & Holodny, A. I. (2014). Resection of glioma in an fMRI-defined “split” Broca’s area. Neurocase, 20(5), 481–486.
- Kin, H., Ishikawa, E., Takano, S., Ayuzawa, S., Matsushita, A., Muragaki, Y., … Matsumura, A. (2013). Language areas involving the inferior temporal cortex on intraoperative mapping in a bilingual patient with glioblastoma. Neurologia Medico-Chirurgica, 53(4), 256–258.
- Kinoshita, M., De Champfleur, N. M., Deverdun, J., Moritz-Gasser, S., Herbet, G., & Duffau, H. (2015). Role of fronto-striatal tract and frontal aslant tract in movement and speech: An axonal mapping study. Brain Structure and Function, 220(6), 3399–3412.
- Kinoshita, M., Nakajima, R., Shinohara, H., Miyashita, K., Tanaka, S., Okita, H., … Hayashi, Y. (2016). Chronic spatial working memory deficit associated with the superior longitudinal fasciculus: A study using voxel-based lesion-symptom mapping and intraoperative direct stimulation in right prefrontal glioma surgery. Journal of Neurosurgery, 125(4), 1024–1032.
- Krieg, S. M., Schnurbus, L., Shiban, E., Droese, D., Obermueller, T., Buchmann, N., … Ringel, F. (2013). Surgery of highly eloquent gliomas primarily assessed as non-resectable: Risks and benefits in a cohort study. BMC Cancer, 13(1), 51.
- Krieg, S. M., Sollmann, N., Hauck, T., Ille, S., Meyer, B., & Ringel, F. (2014). Repeated mapping of cortical language sites by preoperative navigated transcranial magnetic stimulation compared to repeated intraoperative DCS mapping in awake craniotomy. BMC Neuroscience, 15(1), 20.
- Kuchcinski, G., Mellerio, C., Pallud, J., Dezamis, E., Turc, G., Rigaux-Viode, O., … Oppenheim, C. (2015). Three-tesla functional MR language mapping: Comparison with direct cortical stimulation in gliomas. Neurology, 84(6), 560–568.
- Kumabe, T., Nakasato, N., Suzuki, K., Sato, K., Sonoda, Y., Kawagishi, J., & Yoshimoto, T. (1998). Two-staged resection of a left frontal astrocytoma involving the operculum and insula using intraoperative neurophysiological monitoring—Case report—. Neurologia Medico-Chirurgica, 38(8), 503–507.
- Kumar, A., Chandra, P. S., Sharma, B. S., Garg, A., Rath, G. K., Bithal, P. K., & Tripathi, M. (2014). The role of neuronavigation-guided functional MRI and diffusion tensor tractography along with cortical stimulation in patients with eloquent cortex lesions. British Journal of Neurosurgery, 28(2), 226–233.
- Kurimoto, M., Asahi, T., Shibata, T., Takahashi, C., Nagai, S., Hayashi, N., … Endo, S. (2006). Safe removal of glioblastoma near the angular gyrus by awake surgery preserving calculation ability-case report. Neurologia Medico-Chirurgica, 46(1), 46–50.
- Kurimoto, M., Takaiwa, A., Nagai, S., Hayashi, N., & Endo, S. (2010). Anomia for people’s names after left anterior temporal lobe resection. Neurologia Medico-Chirurgica, 50(1), 36–40.
- Leblanc, R., Robitaille, Y., Andermann, F., & Ptito, A. (1995). Retained language in dysgenic cortex: Case report. Neurosurgery, 37(5), 992–997.
- Leclercq, D., Duffau, H., Delmaire, C., Capelle, L., Gatignol, P., Ducros, M., … Lehéricy, S. (2010). Comparison of diffusion tensor imaging tractography of language tracts and intraoperative subcortical stimulations: Clinical article. Journal of Neurosurgery, 112(3), 503–511. doi:10.3171/2009.8.JNS09558
- Li, T., Bai, H., Wang, G., Wang, W., Lin, J., Gao, H., … Xie, X. (2015). Glioma localization and excision using direct electrical stimulation for language mapping during awake surgery. Experimental and Therapeutic Medicine, 9(5), 1962–1966.
- Lima, G. L. O., Dezamis, E., Corns, R., Rigaux-Viode, O., Moritz-Gasser, S., Roux, A., … Pallud, J. (2017). Surgical resection of incidental diffuse gliomas involving eloquent brain areas. Rationale, functional, epileptological and oncological outcomes. Neurochirurgie, 63(3), 250–258.
- Low, D., Ng, I., & Ng, W. (2007). Awake craniotomy under local anaesthesia and monitored conscious sedation for resection of brain tumours in eloquent cortex-outcomes in 20 patients. Annals-Academy of Medicine Singapore, 36(5), 326.
- Lu, J., Wu, J., Yao, C., Zhuang, D., Qiu, T., Hu, X., … Zhou, L. (2013). Awake language mapping and 3-Tesla intraoperative MRI-guided volumetric resection for gliomas in language areas. Journal of Clinical Neuroscience, 20(9), 1280–1287.
- Lubrano, V., Draper, L., & Roux, F.-E. (2010). What makes surgical tumor resection feasible in Broca’s area? Insights into intraoperative brain mapping. Neurosurgery, 66(5), 868–875.
- Lubrano, V., Filleron, T., Démonet, J.-F., & Roux, F.-E. (2014). Anatomical correlates for category‐specific naming of objects and actions: A brain stimulation mapping study. Human Brain Mapping, 35(2), 429–443.
- Lubrano, V., Prod’homme, K., Démonet, J.-F., & Köpke, B. (2012). Language monitoring in multilingual patients undergoing awake craniotomy: A case study of a German–English–French trilingual patient with a WHO grade II glioma. Journal of Neurolinguistics, 25(6), 567–578.
- Lubrano, V., Roux, F.-E., & Démonet, J.-F. (2004). Writing-specific sites in frontal areas: A cortical stimulation study. Journal of Neurosurgery, 101(5), 787–798.
- Lucas, T. H., II, Drane, D. L., Dodrill, C. B., & Ojemann, G. A. (2008). Language reorganization in aphasics: An electrical stimulation mapping investigation. Neurosurgery, 63(3), 487–497.
- Lurito, J. T., Lowe, M. J., Sartorius, C., & Mathews, V. P. (2000). Comparison of fMRI and intraoperative direct cortical stimulation in localization of receptive language areas. Journal of Computer Assisted Tomography, 24(1), 99–105.
- Mack, P. F., Perrine, K., Kobylarz, E., Schwartz, T. H., & Lien, C. A. (2004). Dexmedetomidine and neurocognitive testing in awake craniotomy. Journal of Neurosurgical Anesthesiology, 16(1), 20–25.
- Magrassi, L., Bongetta, D., Bianchini, S., Berardesca, M., & Arienta, C. (2010). Central and peripheral components of writing critically depend on a defined area of the dominant superior parietal gyrus. Brain Research, 1346, 145–154.
- Maldonado, I. L., Moritz-Gasser, S., De Champfleur, N. M., Bertram, L., Moulinié, G., & Duffau, H. (2011). Surgery for gliomas involving the left inferior parietal lobule: New insights into the functional anatomy provided by stimulation mapping in awake patients: Clinical article. Journal of Neurosurgery, 115(4), 770–779.
- Maldonado, I. L., Moritz-Gasser, S., & Duffau, H. (2011). Does the left superior longitudinal fascicle subserve language semantics? A brain electrostimulation study. Brain Structure and Function, 216(3), 263–274.
- Mandonnet, E., Nouet, A., Gatignol, P., Capelle, L., & Duffau, H. (2007). Does the left inferior longitudinal fasciculus play a role in language? A brain stimulation study. Brain, 130(3), 623–629. doi:10.1093/brain/awl361
- Matsuda, R., Coello, A. F., De Benedictis, A., Martinoni, M., & Duffau, H. (2012). Awake mapping for resection of cavernous angioma and surrounding gliosis in the left dominant hemisphere: Surgical technique and functional results: Clinical article. Journal of Neurosurgery, 117(6), 1076–1081.
- Matsuda, R., Moritz-Gasser, S., Duvaux, S., Coello, A. F., Martinoni, M., & Duffau, H. (2014). The persistent crucial role of the left hemisphere for language in left-handers with a left low grade glioma: A stimulation mapping study. Acta Neurochirurgica, 156(4), 661–670.
- Mazerand, E., Le Renard, M., Hue, S., Lemée, J.-M., Klinger, E., & Menei, P. (2017). Intraoperative subcortical electrical mapping of the optic tract in awake surgery using a virtual reality headset. World Neurosurgery, 97, 424–430. doi:10.1016/j.wneu.2016.10.031
- Mehta, V., Danks, R. A., & Black, P. M. (2000, August). Cortical mapping under local anesthesia for tumor resection. Seminars in Neurosurgery, 11(03), 0287–0300.
- Metellus, P., Boussen, S., Guye, M., & Trebuchon, A. (2017). Successful insular glioma removal in a deaf signer patient during an awake craniotomy procedure. World Neurosurgery, 98, 883.e1–883.e5.
- Meyer, F. B., Bates, L. M., Goerss, S. J., Friedman, J. A., Windschitl, W. L., Duffy, J. R., … O’Neill, B. P. (2001, July). Awake craniotomy for aggressive resection of primary gliomas located in eloquent brain. Mayo Clinic Proceedings, 76(7), 677–687. Elsevier.
- Mikuni, N., Okada, T., Enatsu, R., Miki, Y., Hanakawa, T., Urayama, S.-I., … Hashimoto, N. (2007). Clinical impact of integrated functional neuronavigation and subcortical electrical stimulation to preserve motor function during resection of brain tumors. Journal of Neurosurgery, 106(4), 593–598.
- Milea, D., Lobel, E., Lehéricy, S., Duffau, H., Rivaud-Péchoux, S., Berthoz, A., & Pierrot-Deseilligny, C. (2002). Intraoperative frontal eye field stimulation elicits ocular deviation and saccade suppression. Neuroreport, 13(10), 1359–1364. doi:10.1097/00001756-200207190-00029
- Miozzo, M., Williams, A. C., McKhann, G. M., & Hamberger, M. J. (2017). Topographical gradients of semantics and phonology revealed by temporal lobe stimulation. Human Brain Mapping, 38(2), 688–703.
- Moritz-Gasser, S., & Duffau, H. (2009). Evidence of a large-scale network underlying language switching: A brain stimulation study: Case report. Journal of Neurosurgery, 111(4), 729–732.
- Moritz-Gasser, S., Herbet, G., & Duffau, H. (2013). Mapping the connectivity underlying multimodal (verbal and non-verbal) semantic processing: A brain electrostimulation study. Neuropsychologia, 51(10), 1814–1822.
- Morrison, M. A., Tam, F., Garavaglia, M. M., Golestanirad, L., Hare, G. M. T., Cusimano, M. D., … Graham, S. J. (2016). A novel tablet computer platform for advanced language mapping during awake craniotomy procedures. Journal of Neurosurgery, 124(4), 938–944. doi:10.3171/2015.4.JNS15312
- Motomura, K., Fujii, M., Maesawa, S., Kuramitsu, S., Natsume, A., & Wakabayashi, T. (2014). Association of dorsal inferior frontooccipital fasciculus fibers in the deep parietal lobe with both reading and writing processes: A brain mapping study: Case report. Journal of Neurosurgery, 121(1), 142–148.
- Mukae, N., Mizoguchi, M., Mori, M., Hashiguchi, K., Kawaguchi, M., Hata, N., … Hashizume, M. (2017). The usefulness of arcuate fasciculus tractography integrated navigation for glioma surgery near the language area; Clinical Investigation. Interdisciplinary Neurosurgery, 7, 22–28.
- Nakajima, R., Okita, H., Kinoshita, M., Miyashita, K., Nakada, M., Yahata, T., … Hayashi, Y. (2014). Direct evidence for the causal role of the left supplementary motor area in working memory: A preliminary study. Clinical Neurology and Neurosurgery, 126, 201–204.
- Nguyen, H. S., Sundaram, S. V., Mosier, K. M., & Cohen-Gadol, A. A. (2011). A method to map the visual cortex during an awake craniotomy: Technical note. Journal of Neurosurgery, 114(4), 922–926.
- Nowacki, A., Seidel, K., Schucht, P., Schindler, K., Abela, E., Heinemann, D., … Pollo, C. (2015). Induction of fear by intraoperative stimulation during awake craniotomy: Case presentation and systematic review of the literature. World Neurosurgery, 84(2), 470–474.
- Ogawa, H., Kamada, K., Kapeller, C., Prueckl, R., Takeuchi, F., Hiroshima, S., … Guger, C. (2017). Clinical impact and implication of real-time oscillation analysis for language mapping. World Neurosurgery, 97, 123–131.
- Ojemann, G. A. (1978). Organization of short-term verbal memory in language areas of human cortex: Evidence from electrical stimulation. Brain and Language, 5(3), 331–340.
- Ojemann, G. A., & Whitaker, H. A. (1978). Language localization and variability. Brain and Language, 6(2), 239–260.
- Ojemann, G. A. (1979). Individual variability in cortical localization of language. Journal of Neurosurgery, 50(2), 164–169.
- Ojemann, G., & Mateer, C. (1979). Human language cortex: Localization of memory, syntax, and sequential motor-phoneme identification systems. Science, 205(4413), 1401–1403.
- Ojemann, G. A., & Dodrill, C. B. (1985). Verbal memory deficits after left temporal lobectomy for epilepsy: Mechanism and intraoperative prediction. Journal of Neurosurgery, 62(1), 101–107. doi:10.3171/jns.1985.62.1.0101
- Ojemann, G., Ojemann, J., Lettich, E. R. E. E. G. T., & Berger, M. (1989). Cortical language localization in left, dominant hemisphere: An electrical stimulation mapping investigation in 117 patients. Journal of Neurosurgery, 71(3), 316–326.
- Ojemann, J. O., & Silbergeld, D. L. (1995). Cortical stimulation mapping of phantom limb rolandic cortex: Case report. Journal of Neurosurgery, 82(4), 641–644.
- Ojemann, G. A., & Schoenfield-McNeill, J. (1999). Activity of neurons in human temporal cortex during identification and memory for names and words. The Journal of Neuroscience, 19(13), 5674–5682.
- Ojemann, J. G., Ojemann, G. A., & Lettich, E. (2002). Cortical stimulation mapping of language cortex by using a verb generation task: Effects of learning and comparison to mapping based on object naming. Journal of Neurosurgery, 97(1), 33–38.
- Otani, N., Bjeljac, M., Muroi, C., Weniger, D., Khan, K. H. A. N., Wieser, H.-G., … Yonekawa, Y. (2005). Awake surgery for glioma resection in eloquent areas. Neurologia Medico-Chirurgica, 45(10), 501–511.
- Pallud, J., & Dezamis, E. (2017). Functional and oncological outcomes following awake surgical resection using intraoperative cortico-subcortical functional mapping for supratentorial gliomas located in eloquent areas. Neurochirurgie, 63(3), 208–218.
- Papagno, C., Gallucci, M., Casarotti, A., Castellano, A., Falini, A., Fava, E., … Caramazza, A. (2011). Connectivity constraints on cortical reorganization of neural circuits involved in object naming. Neuroimage, 55(3), 1306–1313.
- Papagno, C., Miracapillo, C., Casarotti, A., Romero Lauro, L. J., Castellano, A., Falini, A., … Bello, L. (2010). What is the role of the uncinate fasciculus? Surgical removal and proper name retrieval. Brain, 134(2), 405–414.
- Papagno, C., Pisoni, A., Mattavelli, G., Casarotti, A., Comi, A., Fumagalli, F., … Bello, L. (2016). Specific disgust processing in the left insula: New evidence from direct electrical stimulation. Neuropsychologia, 84, 29–35.
- Peraud, A., Ilmberger, J., & Reulen, H.-J. (2004). Surgical resection of gliomas WHO grade II and III located in the opercular region. Acta Neurochirurgica, 146(1), 9–18.
- Peraud, A., Meschede, M., Eisner, W., Ilmberger, J., & Reulen, H. J. (2002). Surgical resection of grade II astrocytomas in the superior frontal gyrus. Neurosurgery, 50(5), 966–977.
- Pereira, L. C. M., Oliveira, K. M., L ‘Abbate, G. L., Sugai, R., Ferreira, J. A., & Da Motta, L. A. (2009). Outcome of fully awake craniotomy for lesions near the eloquent cortex: Analysis of a prospective surgical series of 79 supratentorial primary brain tumors with long follow-up. Acta Neurochirurgica, 151(10), 1215–1230. doi:10.1007/s00701-009-0363-9
- Perrine, K., Devinsky, O., Uysal, S., Luciano, D. J., & Dogali, M. (1994). Left temporal neocortex mediation of verbal memory: Evidence from functional mapping with cortical stimulation. Neurology, 44(10), 1845.
- Petrovich- Brennan, N. M., Whalen, S., De Morales Branco, D., O’Shea, J. P., Norton, I. H., & Golby, A. J. (2007). Object naming is a more sensitive measure of speech localization than number counting: Converging evidence from direct cortical stimulation and fMRI. Neuroimage, 37, S100–S108.
- Petrovich, N. M., Holodny, A. I., Brennan, C. W., & Gutin, P. H. (2004). Isolated translocation of Wernicke’s area to the right hemisphere in a 62-year-man with a temporo-parietal glioma. American Journal of Neuroradiology, 25(1), 130–133.
- Picht, T., Kombos, T., Gramm, H. J., Brock, M., & Suess, O. (2006). Multimodal protocol for awake craniotomy in language cortex tumour surgery. Acta Neurochirurgica, 148(2), 127–138.
- Picht, T., Krieg, S. M., Sollmann, N., Rösler, J., Niraula, B., Neuvonen, T., … Ringel, F. (2013). A comparison of language mapping by preoperative navigated transcranial magnetic stimulation and direct cortical stimulation during awake surgery. Neurosurgery, 72(5), 808–819. doi:10.1227/NEU.0b013e3182889e01
- Pinsker, M. O., Nabavi, A., & Mehdorn, H. M. (2007). Neuronavigation and resection of lesions located in eloquent brain areas under local anesthesia and neuropsychological-neurophysiological monitoring. min-Minimally Invasive Neurosurgery, 50(5), 281–284.
- Plaza, M., Du Boullay, V., Perrault, A., Chaby, L., & Capelle, L. (2014). A case of bilateral frontal tumors without “frontal syndrome”. Neurocase, 20(6), 671–683.
- Plaza, M., Gatignol, P., Cohen, H., Berger, B., & Duffau, H. (2007). A discrete area within the left dorsolateral prefrontal cortex involved in visual–Verbal incongruence judgment. Cerebral Cortex, 18(6), 1253–1259. doi:10.1093/cercor/bhm169
- Plaza, M., Gatignol, P., Leroy, M., & Duffau, H. (2009). Speaking without Broca’s area after tumor resection. Neurocase, 15(4), 294–310.
- Pu, S., Li, Y.-N., Wu, C.-X., Wang, Y.-Z., Zhou, X.-L., & Jiang, T. (2011). Cortical areas involved in numerical processing: An intraoperative electrostimulation study. Stereotactic and Functional Neurosurgery, 89(1), 42–47.
- Quiñones-Hinojosa, A., Ojemann, S. G., Sanai, N., Dillon, W. P., & Berger, M. S. (2003). Preoperative correlation of intraoperative cortical mapping with magnetic resonance imaging landmarks to predict localization of the Broca area. Journal of Neurosurgery, 99(2), 311–318.
- Rapport, R. L., Tan, C. T., & Whitaker, H. A. (1983). Language function and dysfunction among Chinese- and English-speaking polyglots: Cortical stimulation, Wada Testing, and clinical studies. Brain and Language, 18(2), 342–366.
- Rech, F., Herbet, G., Moritz-Gasser, S., & Duffau, H. (2016). Somatotopic organization of the white matter tracts underpinning motor control in humans: An electrical stimulation study. Brain Structure and Function, 221(7), 3743–3753.
- Rech, F., Herbet, G., Moritz‐Gasser, S., & Duffau, H. (2014). Disruption of bimanual movement by unilateral subcortical electrostimulation. Human Brain Mapping, 35(7), 3439–3445.
- Riva, M., Casarotti, A., Comi, A., Pessina, F., & Bello, L. (2016). Brain and music: An intraoperative stimulation mapping study of a professional opera singer. World Neurosurgery, 93, 486.e13–486.e18.
- Riva, M., Fava, E., Gallucci, M., Comi, A., Casarotti, A., Alfiero, T., … Bello, L. (2016). Monopolar high-frequency language mapping: Can it help in the surgical management of gliomas? A comparative clinical study. Journal of Neurosurgery, 124(5), 1479–1489. doi:10.3171/2015.4.JNS14333
- Rofes, A., & Miceli, G. (2014). Language mapping with verbs and sentences in awake surgery: A review. Neuropsychology Review, 24(2), 185–199.
- Rofes, A., Spena, G., Miozzo, A., Fontanella, M. M., & Miceli, G. (2015). Intraoperative language tasks in awake surgery: A three-task approach for prefrontal tumors. Journal of Neurosurgical Sciences, 59, 337–349.
- Roland, J., Brunner, P., Johnston, J., Schalk, G., & Leuthardt, E. C. (2010). Passive real-time identification of speech and motor cortex during an awake craniotomy. Epilepsy & Behavior, 18(1–2), 123–128.
- Roux, F.-E., Boetto, S., Sacko, O., Chollet, F., & Trémoulet, M. (2003). Writing, calculating, and finger recognition in the region of the angular gyrus: A cortical stimulation study of Gerstmann syndrome. Journal of Neurosurgery, 99(4), 716–727.
- Roux, F.-E., Borsa, S., & Démonet, J.-F. (2009). “The Mute Who Can Sing”: A cortical stimulation study on singing: Clinical article. Journal of Neurosurgery, 110(2), 282–288.
- Roux, F.-E., Boukhatem, L., Draper, L., Sacko, O., & Démonet, J.-F. (2009). Cortical calculation localization using electrostimulation: Clinical article. Journal of Neurosurgery, 110(6), 1291–1299. doi:10.3171/2008.8.JNS17649
- Roux, F.-E., Boulanouar, K., Lotterie, J.-A., Mejdoubi, M., LeSage, J. P., & Berry, I. (2003). Language functional magnetic resonance imaging in preoperative assessment of language areas: Correlation with direct cortical stimulation. Neurosurgery, 52(6), 1335–1347.
- Roux, F.-E., Dufor, O., Giussani, C., Wamain, Y., Draper, L., Longcamp, M., & Démonet, J.-F. (2009). The graphemic/motor frontal area Exner’s area revisited. Annals of Neurology, 66(4), 537–545. doi:10.1002/ana.21804
- Roux, F.-E., Dufor, O., Lauwers-Cances, V., Boukhatem, L., Brauge, D., Draper, L., … Démonet, J.-F. (2011). Electrostimulation mapping of spatial neglect. Neurosurgery, 69(6), 1218–1231.
- Roux, F.-E., Durand, J.-B., Djidjeli, I., Moyse, E., & Giussani, C. (2016). Variability of intraoperative electrostimulation parameters in conscious individuals: Language cortex. Journal of Neurosurgery, 126(5), 1641–1652.
- Roux, F.-E., Durand, J.-B., Réhault, E., Planton, S., Draper, L., & Démonet, J.-F. (2014). The neural basis for writing from dictation in the temporoparietal cortex. Cortex, 50, 64–75.
- Roux, F. E., Lubrano, V., Lauwers-Cances, V., Trémoulet, M., Mascott, C. R., & Démonet, J. F. (2004). Intra-operative mapping of cortical areas involved in reading in mono-and bilingual patients. Brain, 127(8), 1796–1810. doi:10.1093/brain/awh204
- Roux, F.-E., Lubrano, V., Lotterie, J.-A., Giussani, C., Pierroux, C., & Démonet, J.-F. (2007). When “abegg” is read and (“A, B, E, G, G”) is not: A cortical stimulation study of musical score reading. Journal of Neurosurgery, 106(6), 1017–1027. doi:10.3171/jns.2007.106.6.1017
- Roux, F.-E., Minkin, K., Durand, J.-B., Sacko, O., Réhault, E., Tanova, R., & Démonet, J.-F. (2015). Electrostimulation mapping of comprehension of auditory and visual words. Cortex, 71, 398–408.
- Roux, F.-E., & Trémoulet, M. (2002). Organization of language areas in bilingual patients: A cortical stimulation study. Journal of Neurosurgery, 97(4), 857–864.
- Rutten, G.-J. (2015). Speech hastening during electrical stimulation of left premotor cortex. Brain and Language, 141, 77–79.
- Sacko, O., Lauwers-Cances, V., Brauge, D., Sesay, M., Brenner, A., & Roux, F.-E. (2011). Awake craniotomy vs surgery under general anesthesia for resection of supratentorial lesions. Neurosurgery, 68(5), 1192–1199.
- Saito, T., Muragaki, Y., Maruyama, T., Tamura, M., Nitta, M., Tsuzuki, S., … Kawamata, T. (2016). Difficulty in identification of the frontal language area in patients with dominant frontal gliomas that involve the pars triangularis. Journal of Neurosurgery, 125(4), 803–811.
- Saito, T., Muragaki, Y., Miura, I., Tamura, M., Maruyama, T., Nitta, M., … Okada, Y. (2014). Functional plasticity of language confirmed with intraoperative electrical stimulations and updated neuronavigation: Case report of low-grade glioma of the left inferior frontal gyrus. Neurologia Medico-Chirurgica, 54(7), 587–592. doi:10.2176/nmc.cr.2013-0248
- Saito, T., Tamura, M., Muragaki, Y., Maruyama, T., Kubota, Y., Fukuchi, S., … Iseki, H. (2014). Intraoperative cortico-cortical evoked potentials for the evaluation of language function during brain tumor resection: Initial experience with 13 cases: Clinical article. Journal of Neurosurgery, 121(4), 827–838.
- Sakurada, K., Sato, S., Sonoda, Y., Kokubo, Y., Saito, S., & Kayama, T. (2007). Surgical resection of tumors located in subcortex of language area. Acta Neurochirurgica, 149(2), 123–130. doi:10.1007/s00701-006-0857-7
- Sallard, E., Duffau, H., & Bonnetblanc, F. (2012). Ultra-fast recovery from right neglect after ‘awake surgery’for slow-growing tumor invading the left parietal area. Neurocase, 18(1), 80–90.
- Sanai, N., Mirzadeh, Z., & Berger, M. S. (2008). Functional outcome after language mapping for glioma resection. New England Journal of Medicine, 358(1), 18–27.
- Santini, B., Talacchi, A., Squintani, G., Casagrande, F., Capasso, R., & Miceli, G. (2012). Cognitive outcome after awake surgery for tumors in language areas. Journal of Neuro-Oncology, 108(2), 319–326.
- Sarubbo, S., Latini, F., Panajia, A., Candela, C., Quatrale, R., Milani, P., … Cavallo, M. A. (2011). Awake surgery in low-grade gliomas harboring eloquent areas: 3-year mean follow-up. Neurological Sciences, 32(5), 801–810.
- Sarubbo, S., Latini, F., Sette, E., Milani, P., Granieri, E., Fainardi, E., & Cavallo, M. A. (2012). Is the resection of gliomas in Wernicke’s area reliable? Acta Neurochirurgica, 154(9), 1653–1662. doi:10.1007/s00701-012-1416-z
- Sato, S., Oka, H., Utsuki, S., Shimizu, S., Suzuki, S., Fujii, K., … Obonai, A. (2006). Utilization of personal digital assistants (PDA) for intraoperative naming tasks in awake surgery. min-Minimally Invasive Neurosurgery, 49(1), 58–59.
- Scarone, P., Gatignol, P., Guillaume, S., Denvil, D., Capelle, L., & Duffau, H. (2009). Agraphia after awake surgery for brain tumor: New insights into the anatomo-functional network of writing. Surgical Neurology, 72(3), 223–241. doi:10.1016/j.surneu.2008.10.074
- Schapiro, R., Ferson, D., Prabhu, S. S., Tummula, S., Wefel, J., & Rao, G. (2012). A technique for mapping cortical areas associated with speech arrest. Stereotactic and Functional Neurosurgery, 90(2), 118–123. doi:10.1159/000335500
- Schucht, P., Moritz‐Gasser, S., Herbet, G., Raabe, A., & Duffau, H. (2013). Subcortical electrostimulation to identify network subserving motor control. Human Brain Mapping, 34(11), 3023–3030.
- Schwartz, T. H., Ojemann, G. A., Haglund, M. M., & Lettich, E. (1996). Cerebral lateralization of neuronal activity during naming, reading and line-matching. Cognitive Brain Research, 4(4), 263–273.
- Seeck, M., Pegna, A. J., Ortigue, S., Spinelli, L., Dessibourg, C. A., Delavelle, J., … Villemure, J. G. (2006). Speech arrest with stimulation may not reliably predict language deficit after epilepsy surgery. Neurology, 66(4), 592–594. doi:10.1212/01.wnl.0000199254.67398.a7
- Shinoura, N., Yamada, R., Tabei, Y., Otani, R., Itoi, C., Saito, S., & Midorikawa, A. (2011). Left or right temporal lesion might induce aggression or escape during awake surgery, respectively: Role of the amygdala. Acta Neuropsychiatrica, 23(3), 119–124.
- Sierpowska, J., Gabarrós, A., Fernandez-Coello, A., Camins, À., Castañer, S., Juncadella, M., … Rodríguez-Fornells, A. (2015). Morphological derivation overflow as a result of disruption of the left frontal aslant white matter tract. Brain and Language, 142, 54–64. doi:10.1016/j.bandl.2015.01.005
- Sierpowska, J., Gabarrós, A., Fernandez-Coello, A., Camins, À., Castañer, S., Juncadella, M., … Rodríguez-Fornells, A. (2017). Words are not enough: Nonword repetition as an indicator of arcuate fasciculus integrity during brain tumor resection. Journal of Neurosurgery, 126(2), 435–445. doi:10.3171/2016.2.JNS151592
- Sierpowska, J., Gabarrós, A., Ripollés, P., Juncadella, M., Castañer, S., Camins, Á., … Rodríguez-Fornells, A. (2013). Intraoperative electrical stimulation of language switching in two bilingual patients. Neuropsychologia, 51(13), 2882–2892.
- Signorelli, F., Ruggeri, F., Iofrida, G., Isnard, J., Chirchiglia, D., Lavano, A., … Guyotat, J. (2007). Indications and limits of intraoperative cortico-subcortical mapping in brain tumor surgery: An analysis of 101 consecutive cases. Journal of Neurosurgical Sciences, 51(3), 113.
- Skrap, M., Marin, D., Ius, T., Fabbro, F., & Tomasino, B. (2016). Brain mapping: A novel intraoperative neuropsychological approach. Journal of Neurosurgery, 125(4), 877–887.
- Southwell, D. G., Hervey-Jumper, S. L., Perry, D. W., & Berger, M. S. (2016). Intraoperative mapping during repeat awake craniotomy reveals the functional plasticity of adult cortex. Journal of Neurosurgery, 124(5), 1460–1469. doi:10.3171/2015.5.JNS142833
- Spena, G., Costi, E., Panciani, P. P., Roca, E., Migliorati, K., & Fontanella, M. M. (2015). Acute functional reactivation of the language network during awake intraoperative brain mapping. Neurocase, 21(3), 403–407. doi:10.1080/13554794.2014.910306
- Spena, G., Garbossa, D., Panciani, P. P., Griva, F., & Fontanella, M. M. (2013). Purely subcortical tumors in eloquent areas: Awake surgery and cortical and subcortical electrical stimulation (CSES) ensure safe and effective surgery. Clinical Neurology and Neurosurgery, 115(9), 1595–1601. doi:10.1016/j.clineuro.2013.02.006
- Spena, G., Gatignol, P., Capelle, L., & Duffau, H. (2006). Superior longitudinal fasciculus subserves vestibular network in humans. Neuroreport, 17(13), 1403–1406.
- Spena, G., Nava, A., Cassini, F., Pepoli, A., Bruno, M., D’Agata, F., … Versari, P. (2010). Preoperative and intraoperative brain mapping for the resection of eloquent-area tumors. A prospective analysis of methodology, correlation, and usefulness based on clinical outcomes. Acta Neurochirurgica, 152(11), 1835–1846.
- Suess, O., Picht, T., Kuehn, B., Mularski, S., Brock, M., & Kombos, T. (2007). Neuronavigation without rigid pin fixation of the head in left frontotemporal tumor surgery with intraoperative speech mapping. Operative Neurosurgery, 60(4), 330–338.
- Talacchi, A., Squintani, G. M., Emanuele, B., Tramontano, V., Santini, B., & Savazzi, S. (2013). Intraoperative cortical mapping of visuospatial functions in parietal low-grade tumors: Changing perspectives of neurophysiological mapping. Neurosurgical Focus, 34(2), E4.
- Tamura, Y., Ogawa, H., Kapeller, C., Prueckl, R., Takeuchi, F., Anei, R., … Kamada, K. (2016). Passive language mapping combining real-time oscillation analysis with cortico-cortical evoked potentials for awake craniotomy. Journal of Neurosurgery, 125(6), 1580–1588.
- Tate, M. C., Herbet, G., Moritz-Gasser, S., Tate, J. E., & Duffau, H. (2014). Probabilistic map of critical functional regions of the human cerebral cortex: Broca’s area revisited. Brain, 137(10), 2773–2782.
- Taylor, M. D., & Bernstein, M. (1999). Awake craniotomy with brain mapping as the routine surgical approach to treating patients with supratentorial intraaxial tumors: A prospective trial of 200 cases. Journal of Neurosurgery, 90(1), 35–41.
- Teixidor, P., Gatignol, P., Leroy, M., Masuet-Aumatell, C., Capelle, L., & Duffau, H. (2007). Assessment of verbal working memory before and after surgery for low-grade glioma. Journal of Neuro-Oncology, 81(3), 305–313.
- Thiebaut de Schotten, M., Urbanski, M., Duffau, H., Volle, E., Lévy, R., Dubois, B., & Bartolomeo, P. (2005). Direct evidence for a parietal-frontal pathway subserving spatial awareness in humans. Science, 309(5744), 2226–2228.
- Tomasino, B., Marin, D., Maieron, M., D’Agostini, S., Medeossi, I., Fabbro, F., … Luzzatti, C. (2015). A multimodal mapping study of conduction aphasia with impaired repetition and spared reading aloud. Neuropsychologia, 70, 214–226. doi:10.1016/j.neuropsychologia.2015.02.023
- Vallar, G., Bello, L., Bricolo, E., Castellano, A., Casarotti, A., Falini, A., … Papagno, C. (2014). Cerebral correlates of visuospatial neglect: A direct cerebral stimulation study. Human Brain Mapping, 35(4), 1334–1350.
- Van Buren, J. M., Fedio, P., & Frederick, C. G. (1978). Mechanism and localization of speech in the parietotemporal cortex. Neurosurgery, 2(3), 233–239.
- Van Geemen, K., Herbet, G., Moritz‐Gasser, S., & Duffau, H. (2014). Limited plastic potential of the left ventral premotor cortex in speech articulation: Evidence from intraoperative awake mapping in glioma patients. Human Brain Mapping, 35(4), 1587–1596.
- Van Kessel, E., Baumfalk, A. E., Van Zandvoort, M. J., Robe, P. A., & Snijders, T. J. (2017). Tumor-related neurocognitive dysfunction in patients with diffuse glioma: A systematic review of neurocognitive functioning prior to anti-tumor treatment. Journal of Neuro-Oncology, 134(1), 9–18. doi:10.1007/s11060-017-2503-z
- Vassal, F., Schneider, F., Sontheimer, A., Lemaire, -J.-J., & Nuti, C. (2013). Intraoperative visualisation of language fascicles by diffusion tensor imaging-based tractography in glioma surgery. Acta Neurochirurgica, 155(3), 437–448.
- Vassal, M., Le Bars, E., Sylvie Moritz-Gasser, S. T., Menjot, N., & Duffau, H. (2010). Crossed aphasia elicited by intraoperative cortical and subcortical stimulation in awake patients: Clinical article. Journal of Neurosurgery, 113(6), 1251–1258. doi:10.3171/2010.6.JNS10719
- Vidorreta, J. G., Garcia, R., Moritz‐Gasser, S., & Duffau, H. (2011). Double dissociation between syntactic gender and picture naming processing: A brain stimulation mapping study. Human Brain Mapping, 32(3), 331–340.
- Viegas, C., Moritz-Gasser, S., Rigau, V., & Duffau, H. (2011). Occipital WHO grade II gliomas: Oncological, surgical and functional considerations. Acta Neurochirurgica, 153(10), 1907–1917.
- Wager, M., Du Boisgueheneuc, F., Pluchon, C., Bouyer, C., Stal, V., Bataille, B., … Gil, R. (2013). Intraoperative monitoring of an aspect of executive functions: Administration of the Stroop test in 9 adult patients during awake surgery for resection of frontal glioma. Neurosurgery, 72, ons169–ons181.
- Walker, J. A., Quiñones-Hinojosa, A., & Berger, M. S. (2004). Intraoperative speech mapping in 17 bilingual patients undergoing resection of a mass lesion. Neurosurgery, 54(1), 113–118.
- Wang, X., Wang, -Y.-Y., Jiang, T., Wang, Y.-Z., & Wu, C.-X. (2013). Direct evidence of the left caudate’s role in bilingual control: An intra-operative electrical stimulation study. Neurocase, 19(5), 462–469. doi:10.1080/13554794.2012.701635
- Weber, P. B., & Ojemann, G. A. (1995). Neuronal recordings in human lateral temporal lobe during verbal paired associate learning. Neuroreport, 6(4), 685–689. doi:10.1097/00001756-199503000-00025
- Wu, C. X., Pu, S., Lin, Y., Wang, Y. Z., Jiang, T., Xie, J., … Wang, X. Y. (2008). Fractionated resection on low grade gliomas involving Broca’s area and insights to brain plasticity. Chinese Medical Journal, 121(20), 2026–2030.
- Wu, J., Lu, J., Zhang, H., Zhang, J., Yao, C., Zhuang, D., … Zhou, L. (2015). Direct evidence from intraoperative electrocortical stimulation indicates shared and distinct speech production center between Chinese and English languages. Human Brain Mapping, 36(12), 4972–4985. doi:10.1002/hbm.22991
- Xie, T., Zhang, D., Wu, Z., Chen, L., & Zhu, X. (2015). Classifying multiple types of hand motions using electrocorticography during intraoperative awake craniotomy and seizure monitoring processes—Case studies. Frontiers in Neuroscience, 9, 353.
- Yamao, Y., Matsumoto, R., Kunieda, T., Arakawa, Y., Kobayashi, K., Usami, K., … Miyamoto, S. (2014). Intraoperative dorsal language network mapping by using single‐pulse electrical stimulation. Human Brain Mapping, 35(9), 4345–4361.
- Yordanova, Y. N., Moritz-Gasser, S., & Duffau, H. (2011). Awake surgery for WHO Grade II gliomas within “noneloquent” areas in the left dominant hemisphere: Toward a “supratotal” resection: Clinical article. Journal of Neurosurgery, 115(2), 232–239.
- Yousry, T. A., Schmid, U. D., Jassoy, A. G., Schmidt, D., Eisner, W. E., Reulen, H. J., … Lissner, J. (1995). Topography of the cortical motor hand area: Prospective study with functional MR imaging and direct motor mapping at surgery. Radiology, 195(1), 23–29.
- Zamora, L., Corina, D., & Ojemann, G. (2016). Human temporal cortical single neuron activity during working memory maintenance. Neuropsychologia, 86, 1–12.
- Zemmoura, I., Herbet, G., Moritz‐Gasser, S., & Duffau, H. (2015). New insights into the neural network mediating reading processes provided by cortico‐subcortical electrical mapping. Human Brain Mapping, 36(6), 2215–2230.
- Zhang, X., Zhang, G., Yu, T., Ni, D., Cai, L., Qiao, L., … Li, Y. (2013). Surgical treatment for epilepsy involving language cortices: A combined process of electrical cortical stimulation mapping and intra-operative continuous language assessment. Seizure, 22(9), 780–786. doi:10.1016/j.seizure.2013.06.006
- Zhang, Z., Jiang, T., Xie, J., Liu, F. S., Li, S. W., Qiao, H., & Wang, Z. C. (2008). Surgical strategies for glioma involving language areas. Chinese Medical Journal, 121(18), 1800–1805.
Appendix
Search strategy used for Pubmed and Embase
PubMed
(“Brain Neoplasms”[MeSH:noexp] OR brain neoplasm*[Title/Abstract] OR brain tumor*[Title/Abstract] OR brain tumour*[Title/Abstract] OR “Glioma”[Mesh] OR glioma*[Title/Abstract] OR glioblastoma*[Title/Abstract] OR “Epilepsy”[MAJR] OR epilepsy[Title/Abstract] OR epilepsies[Title/Abstract]) AND (“Neurosurgical Procedures”[Mesh] OR “Cytoreduction Surgical Procedures”[Mesh] OR Cytoreduction Surgical Procedure*[Title/Abstract] OR reduction[Title/Abstract] OR debulking[Title/Abstract] OR remove[Title/Abstract] OR removal[Title/Abstract] OR surgery[Title/Abstract] OR craniotomy[Title/Abstract]) AND (“Anesthesia, Local”[Mesh] OR local anesthesia[Title/Abstract] OR local anaesthesia[Title/Abstract] OR “Transcranial Direct Current Stimulation”[Mesh] OR stimulat*[Title/Abstract] OR awake[Title/Abstract] OR penfield[Title/Abstract] OR “Monitoring, Intraoperative”[Mesh] OR intraoperative monitoring[Title/Abstract])
Embase
“brain tumor”/de OR brain AND neoplasm*:ti,ab OR brain AND tumour*:ti,ab OR “glioma”/exp OR glioma*:ti,ab OR glioblastoma*:ti,ab OR “epilepsy”/mj OR “epilepsy”:ti,ab OR epilepsies:ti,ab AND “cytoreductive surgery”/exp OR “neurosurgery”/exp OR cytoreductive AND surgery:ti,ab OR reduction:ti,ab OR debulking:ti,ab OR remove:ti,ab OR removal:ti,ab OR surgery:ti,ab OR craniotomy:ti,ab AND “local anesthesia”/exp OR “local anaesthesia”:ti,ab OR “transcranial direct current stimulation”/exp OR stimulat*:ti,ab OR awake:ti,ab OR penfield:ti,ab OR “neuromonitoring”/exp OR intraoperative AND monitoring:ti,ab AND [embase]/lim NOT [medline]/lim AND (“article”/it OR “article in press”/it OR “review”/it) AND [english]/lim
