ABSTRACT
Introduction
Although subjective cognitive decline (SCD) may be an early risk marker of Alzheimer’s Disease (AD), research on SCD among Hispanics/Latinos/as/x (henceforth Latinos/as) living in the U.S. is lacking. We investigated if the cross-sectional relationship of self-reported SCD with objective cognition varies as a function of ethnic background (Latinos/as versus Non-Hispanic Whites [NHWs]). Secondary analyses conducted solely within the Latino/a group investigated if informant reported SCD is associated with objective cognition and whether self-reported SCD is related to markers of brain health in a sub-sample of Latinos/as with available MRI data.
Methods
Eighty-three participants (≥60 years of age) without dementia (35 Latinos/as; 48 NHWs) completed the Mattis Dementia Rating Scale (MDRS) and the Subjective Cognitive Decline-Questionnaire (SCD-Q). Additionally, 22 Latino/a informants completed the informant-version of the SCD-Q. Hierarchical regression models investigated if ethnicity moderates the association of MDRS and SCD-Q scores after adjusting for demographics and depressive symptoms. Correlational analyses within the Latino/a group investigated self- and informant-reported associations of SCD-Q scores with objective cognition, and associations of self-reported SCD-Q scores with medial temporal lobe volume and thickness.
Results
Latinos/as had lower education and MDRS scores than NHWs. Higher SCD-Q scores were associated with lower MDRS scores only in Latinos/as. Within the Latino/a group, self, but not informant reported SCD was related to objective cognition. Medium to large effect sizes were found whereby higher self-reported SCD was associated with lower entorhinal cortex thickness and left hippocampal volume in Latinos/as.
Conclusions
The association of SCD and concurrent objectively measured global cognition varied by ethnic background and was only significant in Latinos/as. Self-reported SCD may be an indicator of cognitive and brain health in Latinos/as without dementia, prompting clinicians to monitor cognition. Future studies should explore if SCD predicts objective cognitive decline in diverse groups of Latinos/as living in the U.S.
The worldwide older adult population is expected to more than double to 98 million by the year 2060 (Colby & Ortman, Citation2015; Mather et al., Citation2015), with concomitant increases in the number of people living with Alzheimer’s disease (AD) (Barnes & Yaffe, Citation2011; Desa, Citation2010; Langa, Citation2018). Hispanics/Latinos/as/x, hereafter referred to as Latinos/as, are expected to comprise over 28% of the U.S. population (Colby & Ortman, Citation2015; U.S. Census Bureau, Citation2018) and are projected to remain the largest immigrant group, with 3.5 million living with AD by the year 2060 (Wu et al., Citation2016). As such, identifying early risk markers of AD can help with assessment and intervention efforts, and identify individuals who may need further diagnostic workup or follow-up. Unfortunately, the study of early risk markers of AD in Latinos/as has lagged in the U.S. (Babulal et al., Citation2019), despite numerous studies suggesting that they are at higher risk of developing mild cognitive impairment (MCI) and AD compared to non-Hispanic Whites (NHWs) (Black et al., Citation1999; Manly et al., Citation2008; Mayeda et al., Citation2016). Therefore, there is a need to fill this gap to improve diagnostic accuracy and early intervention (Rose, Citation2005) in this growing segment of the U.S. population.
Subjective cognitive decline (SCD), which refers to a person’s (or their informant’s) perception of decline in cognitive functioning (Rabin et al., Citation2017), has emerged as a potential early risk marker of AD (Jessen, Citation2014; Molinuevo et al., Citation2017; Verfaillie et al., Citation2019). Compared to those without self-reported SCD, individuals with normal cognition who report SCD have higher rates of cognitive impairment (Reisberg et al., Citation2010), more rapid rates of decline (Reisberg et al., Citation2010), higher risk of developing MCI (Gómez-Ramírez et al., Citation2020), and exhibit brain changes consistent with preclinical AD (Amariglio et al., Citation2012; Jessen, Citation2014), such as entorhinal cortex thinning (Holbrook et al., Citation2020; Jessen et al., Citation2006; D. Meiberth et al., Citation2015; Rabin et al., Citation2017) and reduced hippocampal volume (Holbrook et al., Citation2020; Perrotin et al., Citation2015; Rabin et al., Citation2017; Saykin et al., Citation2006; van der Flier & Scheltens, Citation2009). Approximately 11.2% of Latinos/as in the U.S. report SCD, a prevalence rate that is comparable to NHWs (10.8%) (Taylor et al., Citation2018).
There is limited and conflicting research on the relationship between SCD and cognition in Latinos/as. Our research group (Zlatar, Muniz, Espinoza, et al., Citation2018) previously found that symptoms of depression, rather than self-reports of SCD, were associated with objective cognition in a clinic-based sample of older Latinos/as living near the U.S.-Mexico border. Similar results were found in studies conducted in Spain (Alegret et al., Citation2015) and Brazil (Minett et al., Citation2008). However, these studies did not use validated SCD questionnaires (Zlatar, Muniz, Espinoza, et al., Citation2018), focused exclusively on SCD related to memory complaints (Alegret et al., Citation2015; Minett et al., Citation2008), or employed informal interviews to ascertain SCD (Minett et al., Citation2008). Conversely and consistent with previous findings in NHWs (Amariglio et al., Citation2012; Slot et al., Citation2019), studies conducted in Spain using a questionnaire developed specifically to measure SCD across various cognitive domains (i.e., the Subjective Cognitive Decline Questionnaire; SCD-Q) found associations between SCD and objective cognition (Sánchez-Benavides et al., Citation2018; Valech et al., Citation2015, Citation2018) as well as with risk markers of AD pathology (i.e., positive amyloid-beta status) (Sánchez‐Benavides et al., Citation2020). Sánchez-Benavides et al. (Citation2018) found that Spaniards who reported SCD performed significantly lower on episodic memory and attention/processing speed compared to those who did not report SCD, even after accounting for patient demographics, genetic factors, and mood. In the U.S., older Mexican American adults with SCD performed lower on tasks of global cognition, language, and attention than those without SCD (Hall et al., Citation2018).
Although individuals’ self-reported SCD is typically greater compared to that reported by their informants (Rabin et al., Citation2017; Valech et al., Citation2015), informant reports of cognitive decline better differentiate between cognitively normal and pre-AD groups in Spaniards (Valech et al., Citation2015, Citation2018). Moreover, informant reports are a stronger predictor of objective performance (Sánchez-Benavides et al., Citation2018), consistent with most research in NHWs (Rattanabannakit et al., Citation2016; Rueda et al., Citation2015; Slavin et al., Citation2010). This suggests that informant reports may be more useful than self-reports of SCD in providing information about early decline related to AD (Jessen et al., Citation2014; Valech et al., Citation2015). Nevertheless, findings from Spaniard populations cannot be generalized to Latinos/as living in the U.S., as cultural factors such as behavioral norms, values, language, traditions, and beliefs (Alarcón, Citation2009) may differ and impact SCD reporting (Rabin et al., Citation2017).
In summary, research is needed to better understand the relationship of culturally informed self- and informant- SCD reports with objective cognition in Latinos/as living in the U.S. This study used the Subjective Cognitive Decline Questionnaire (SCD-Q), originally developed in Spain (Rami et al., Citation2014), to measure SCD. We chose the SCD-Q since it was developed specifically to assess SCD in participants and their informants in Spanish, with an equivalent English version, and is in line with recommendations set forth by the SCD workgroup (Molinuevo et al., Citation2017). We evaluated if the relationship of self-reported SCD and global cognition varies as a function of ethnicity (Latinos/as compared to NHWs living in the U.S.) and whether self- or informant- SCD reports are more strongly associated with objective cognition within the Latino/a group. We also explored whether those who self-reported higher SCD (indicating greater cognitive complaints) had smaller medial temporal lobe volume/thickness in a subsample of Latinos/as with available brain MRI scans. Since we employed an SCD questionnaire validated in Spanish, we hypothesized that the association of self-reported SCD and global cognition would be moderated by ethnicity, and would be attenuated after adjusting for depression and demographic characteristics known to influence SCD and cognition (Alegret et al., Citation2015; Minett et al., Citation2008; Zlatar, Muniz, Espinoza, et al., Citation2018). Furthermore, consistent with previous research, we expected that informant-reports would be more strongly associated with objective cognition in Latinos/as compared to self-reports (Jessen et al., Citation2014; Valech et al., Citation2015). Lastly, we hypothesized that higher self-reported SCD would be related to lower brain volume and thickness in the medial temporal lobe in a subsample of Latinos/as with available brain MRI data (Peters, Citation2006; Rabin et al., Citation2017; Sánchez‐Benavides et al., Citation2020).
Methods
Participants
Participants were 83 community-dwelling research volunteers with a wide range of cognitive performances who were selected from existing parent studies at the University of California, San Diego’s (UCSD) Wellness Initiative for Senior Enrichment (WISE) Lab (NHWs) and the Shiley-Marcos Alzheimer’s Disease Research Center (ADRC) (Latinos/as). Individuals from both studies who had available Subjective Cognitive Decline Questionnaire (SCD-Q) and Mattis Dementia Rating Scale (MDRS) data were selected. Only individuals aged 60 and older, without major neurological (e.g., dementia, Parkinson’s disease) or psychiatric disorders (e.g., schizophrenia, bipolar disorder), major vascular events (e.g., stroke), diabetes, self-reported alcohol or drug abuse, actively treated cancers, recent or remote history of severe TBI, or history of chemotherapy in the past 6 months were included in the analytic sample. Additionally, participants from the WISE Lab parent study were excluded if they had poorly controlled medical problems (e.g., renal failure), a history of head injury with loss of consciousness in the past 6 months, and a fall within the past year resulting in hospitalization. The final analytic sample for this study consisted of 48 NHWs (Mage = 71.81 years, SDage = 4.27, rangeyears = 65–80) and 35 Latinos/as (Mage = 73.97 years, SDage = 7.06, rangeyears = 61–91). To generalize our findings to a more representative sample and replicate previous studies (e.g., Alegret et al., Citation2015; Minett et al., Citation2008; Valech et al., Citation2015; Zlatar, Muniz, Espinoza, et al., Citation2018), we included participants with a wide range of cognitive performances, including mostly cognitively normal individuals and some with MCI (NHW = 5 & Latino/a = 8). Those with frank dementia were excluded from analyses.
Both the WISE Lab and ADRC longitudinal parent studies performed comprehensive neuropsychological testing to determine cognitive status. The WISE lab parent study screened out dementia cases based on total scores below 32 on the modified version of the Telephone Interview for Cognitive Status (Knopman et al., Citation2010). Upon formal testing, all individuals enrolled in the WISE Lab study had T-scores greater than 40 on the MDRS, suggesting that no participants had dementia, while MCI was defined as performance greater than one standard deviation below normative expectations on at least two measures within a cognitive domain (Jak et al., Citation2009). The ADRC defined the clinical diagnoses of dementia and MCI based on extensive neuropsychological testing using the National Institute of Neurological and Communicative Disorders and Stroke (NINCDS) – Alzheimer’s Disease and Related Disorders Association (ADRDA) criteria or the National Institute on Aging – Alzheimer’s Association Criteria. These criteria require concern regarding cognitive change, impairment in one or more cognitive domains, preservation of independence in functional abilities and no dementia to diagnose MCI, and cognitive impairment in at least 2 cognitive domains which interfere with the ability to function for a diagnosis of dementia (Albert et al., Citation2011; McKhann et al., Citation2011). All data was reviewed by senior ADRC neurologists and the diagnosis was determined by a multidisciplinary team, which accounted for cultural considerations of clinical-neuropathological correlations (Soria et al., Citation2018).
Latino/a participants were mostly of Mexican descent (84.4% Mexican/Chicano, 3.1% Puerto Rican, 3.1% Central American, 9.4% Other) and were tested in their language of preference (37.1% in Spanish, 62.9% in English). Given that the Latino/a and NHW participants were recruited from different ongoing studies, only the Latino/a group had study partners (n = 22) who completed the informant version of the SCD-Q. Informant’s demographic data (e.g., age, education, gender, relationship to participant) was not included in this study because it was not collected contemporaneously to SCD-Q administration, which is not uncommon in the literature (e.g., refer to Edmonds et al., Citation2014; Rueda et al., Citation2015; Sánchez-Benavides et al., Citation2018 for articles that include informant reports without related demographics). Additionally, a small subset of participants in the Latino/a group (n = 12) underwent neuroimaging approximately 4 and a half years prior to collection of SCD reports and cognitive testing (Myears = 4.60, SDyears = 1.46, rangeyears = 1.00–6.34). The Institutional Review Board/Human Research Protections Program at UCSD approved this study, and all participants provided written informed consent.9
Measures
Participants completed a series of standard neuropsychological tests and questionnaires during their respective study visits in a quiet room. Trained study personnel administered all cognitive tests and were available to answer any questions. The language of test administration was determined by the participant’s stated preference and comfort level. For those whose preferred language was Spanish, trained bilingual and bicultural staff administered all cognitive tests and questionnaires in Spanish. Study partners completed the informant version of the SCD-Q when available.
Subjective cognitive decline (SCD)
Subjective cognitive decline was measured with the Subjective Cognitive Decline Questionnaire (SCD-Q) (Rami et al., Citation2014): The SCD-Q is a 24-item self- and informant-report measure of perceived cognitive decline over the past two years, covering domains of memory, language, and executive functioning (Rami et al., Citation2014; Valech et al., Citation2018). The forms are available in English and Spanish. Both the self-report “MyCog” and informant-report “TheirCog” forms were used in this study. NHWs completed the MyCog form, whereas Latino/a participants and their informants (when available) completed the MyCog and TheirCog forms, respectively. Items are endorsed for the perceived difficulty in areas such as learning new material, remembering past events, and recalling recent events compared to the last two years. Higher scores (range 0–24) are indicative of greater SCD. The SCD-Q was validated in Spain for use in populations with varying degrees of cognitive functioning, ranging from normal cognition to dementia. The SCD-Q demonstrates adequate reliability (Cronbach’s alpha of 0.90 and 0.93 for MyCog and TheirCog, respectively), internal validity, and sensitivity/specificity (MyCog 83%/87%; TheirCog 85%/80%) for discriminating between individuals with and without objective cognitive deficits (Rami et al., Citation2014). For this study, a professional translator modified the wording of the Spanish version items to maximize understanding by individuals of Mexican background. Supplementary Table 1 contains a side-by-side comparison of the items from the original questionnaire and items that were modified for this study.
We selected the SCD-Q because it follows recommendations from the SCD-Initiative Working Group (Molinuevo et al., Citation2017), that SCD measures used in research should a) be validated and developed with a similar target population (i.e., Spanish-speakers), b) incorporate informant in conjunction with self-reports, and c) determine SCD in relation to a shorter time frame to yield more reliable results (Jessen et al., Citation2014; Rabin et al., Citation2015). The SCD-Q differs from other SCD measures developed in the U.S., such as the Everyday Cognition Scale (ECog) (Farias et al., Citation2008), in that it is much briefer (i.e., 24 versus 39 items) and assesses a shorter time frame of perceived decline (2 versus 10 years). Research suggests that assessing a shorter time frame may yield more reliable results (Molinuevo et al., Citation2017) and have higher predictive value for future dementia (Jessen et al., Citation2014). Although the SCD-Q was not validated with U.S. residing Latinos/as, we believe it is the best tool currently available to assess SCD in Spanish.
Global cognition
Cognitive function was assessed with the Mattis Dementia Rating Scale (MDRS) (Mattis, Citation1988), an assessment of global cognitive function derived from measurement of attention, initiation/preservation, construction, conceptualization, and memory (Mattis, Citation1988). Higher scores (range 0–144) are indicative of better global cognitive function. For those who opted to be tested in Spanish, the Spanish version of the MDRS (Arnold et al., Citation1998) was administered.
Mood
Depression was assessed with the Geriatric Depression Scale (GDS) (Yesavage et al., Citation1982), a self-report questionnaire of depressive symptomatology experienced within the past week. Higher scores are indicative of worse depressive symptomatology. NHWs were administered the 30-item version (range 0–30) (Yesavage et al., Citation1982), whereas a majority of the Latinos/as were administered the 15-item version (range 0–15) (Lesher & Berryhill, Citation1994). For those who opted to be tested in Spanish, the Spanish form of the GDS 15-item (Martínez de La Iglesia et al., Citation2002) was administered. Since participants completed different versions of the GDS, we standardized GDS scores by deriving the 15-item total score for the NHW group (i.e., GDS-15). A cut–off score of 5 is indicative of depressive symptoms in elderly individuals (Bijl et al., Citation2006; Greenberg, Citation2012). Specifically, total scores of 5–8 suggest mild depression, 9–11 suggest moderate depression, and 12–15 suggest severe depression (Greenberg, Citation2012).
Neuroimaging data
Structural Magnetic Resonance Imaging (MRI) consisted of a high–resolution T1-weighted image acquired at UCSD (TE: 2.8 ms/3.8 ms; TR: 6.5 ms/8.5 ms; TI: 600 ms/500 ms; flip angle: 8°/10° matrix: 256 × 256; voxel size: 0.9375 mm × 0.9375 mm × 1.2000 mm; values separated by “/” are for 3.0 T data/1.5 T data). The FreeSurfer (version 6.0; http://surfer.nmr.mgh.harvard.edu/) pipeline (Fischl, Citation2012; Fischl et al., Citation2002, Citation2004) was used to derive automated brain volume and cortical thickness values for regions typically implicated in AD (i.e., hippocampal, parahippocampal, and entorhinal regions) to explore their associations with SCD-Q scores. MRI scans were collected on average 4.60 years (min years = 1.00, max years = 6.34) prior to the SCD-Q completion, hence our preliminary analyses explore if current SCD reports may be indicative of long-standing brain changes typically seen in AD.
Statistical analyses
All analyses were conducted using IBM SPSS Version 26.0 (IBM, Citation2019). Data were screened for normality by inspecting skewness and kurtosis limits (Field, Citation2009), and no significant outliers were detected for the MDRS, SCD-Q, and brain variables. Independent samples t-tests and chi-square tests were conducted to determine mean group differences in demographic characteristics, mood, SCD-Q, and cognitive scores among NHWs and Latinos/as.
Bivariate Pearson correlations were conducted to investigate demographic characteristics that may significantly influence MDRS scores (p < .05) to be entered as covariates in all models. (Maxwell et al., Citation2017; Tabachnick & Fidell, Citation2013). Since GDS scores are known to influence the association of SCD reporting and cognition (Buckley et al., Citation2016; Molinuevo et al., Citation2017; Zlatar et al., Citation2014; Zlatar, Muniz, Espinoza, et al., Citation2018), the GDS-15 was entered as an a-priori covariate in fully adjusted models regardless of statistical significance.
Effects of ethnicity in the association of self-reported SCD and global cognition
A hierarchical linear regression model was employed to investigate if the association of MDRS and SCD-Q scores is moderated by ethnicity. Regression blocks were defined as follows (all variables were centered): Block 1 = significant covariates (i.e., age, education); Block 2 = GDS-15; Block 3 = SCD-Q and ethnicity; Block 4 = SCD-Q x ethnicity. Follow-up linear regression models stratified by ethnic group were conducted to determine the strength and direction of associations between SCD and cognition for each group separately. All analyses investigating the association of SCD with cognition are reported as non-adjusted (no covariates), demographically adjusted (including significant covariates), and fully adjusted (including significant covariates and GDS-15 scores) models. Since the SCD-Q was developed in Spain and was not validated with NHWs living in the U.S., we also collected the Everyday Cognition Scale [ECog] (Farias et al., Citation2008), a widely used self-report measure of perceived decline in cognitively mediated daily activities. We used the ECog to validate our SCD-Q results within the NHW group. The ECog was not collected in the Latino/a sample.
Exploratory analyses
Self- versus informant-reported SCD in Latinos/as
Only Latinos/as with identified informants were included in this sub-sample. Twenty–two Latino/a participants and their informants completed the SCD-Q. Independent samples t-tests assessed for differences between self (i.e., MyCog) and informant (i.e., TheirCog) SCD-Q total scores. Subsequently, bivariate Pearson correlations explored the relationship between self and informant SCD-Q total scores with MDRS total scores.
Self-reported SCD and medial temporal lobe thickness and volume in Latinos/as
Only Latinos/as with available neuroimaging data were included in the sample (n = 12). Age was entered as an a-priori covariate due to its known effects on brain structure (Cole et al., Citation2019). Partial correlations evaluated whether self-reported SCD-Q total scores were associated with right and left hippocampal volume, right and left entorhinal thickness, and right and left parahippocampal thickness, adjusting for age. Since brain volume is influenced by total brain size (Kijonka et al., Citation2020; Voevodskaya et al., Citation2014), associations of self-reported SCD-Q with right and left hippocampal volume were additionally adjusted for intracranial volume. Due to the small sample included in this exploratory analysis, interpretation was based on effect size, which conveys information about the practical significance/importance of results (Lakens, Citation2013; Tabachnick & Fidell, Citation2013). We interpreted medium (r ≥ .30) and large (r ≥ .50) effect sizes as clinically meaningful. Refer to Supplementary Table 2 for descriptive statistics of medial temporal lobe regions in the Latino/a group.
Sensitivity analyses
Groups filtered based on lowest education level and MDRS scores
To ensure our analyses were not heavily influenced by ethnic group differences in cognitive performance and education level, we ran the fully adjusted models, stratified by ethnic group, excluding individuals with <12 years of education and those with MDRS scores < 132 from both groups (Green et al., Citation1995), which included 48 NHWs and 27 Latinos/as.
Running all models excluding participants with MCI
To ensure our analyses were not heavily influenced by cognitive status, all the analyses described above (i.e., effects of ethnicity in the association of self-reported SCD-Q and MDRS scores and exploratory analyses) were conducted including only participants diagnosed with normal cognition based on neuropsychological assessment and expert consensus as described in the methods section. This excluded 8 Latinos/as and 5 NHWs with an MCI diagnosis.
Results
Ethnic groups did not differ significantly on age, GDS-15 total scores, or SCD-Q total scores. NHWs had significantly more years of education and higher MDRS total scores and were more likely to be women compared to Latinos/as (). Bivariate Pearson correlations indicated that age (for Latinos/as only) and years of education (for NHWs and Latinos/as) were significantly correlated with MDRS total scores (Supplementary Table 3). As such, our demographically adjusted models corrected for age and education, while fully adjusted models additionally corrected for GDS-15 scores.
Table 1. Demographic characteristics by ethnic group (n = 83)
Effects of ethnicity in the association of self-reported SCD and global cognition (NHW n = 48; Latinos/as n = 35)
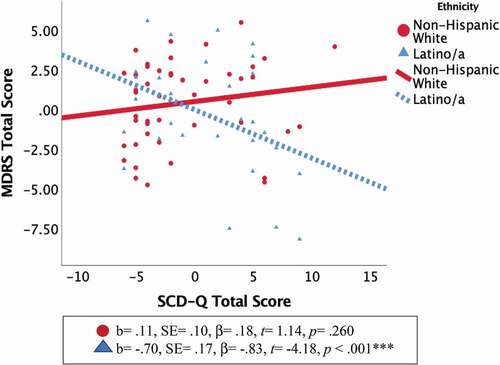
Fully adjusted hierarchical linear regression models (i.e., age, education, and GDS-15 scores) revealed a significant interaction between ethnic group and SCD-Q total scores on MDRS total scores. Follow-up regression analyses stratified by ethnicity showed that higher SCD-Q total scores were significantly correlated with lower MDRS total scores only in Latinos/as ( and ). The association of SCD-Q and MDRS total scores within Latinos/as remained significant after full covariate adjustment, increasing the magnitude of the association by ~30% (from non-adjusted to fully-adjusted model) rather than attenuating it. The results of the ECog corroborated those of the SCD-Q. Specifically, ECog total scores were not significantly correlated with MDRS scores in the NHW group after full covariate adjustment (b = −.008, SE = .028, β = −.042, t = −.269, p = .789).
Table 2. Effects of ethnicity in the association of SCD-Q with MDRS total scores and follow-up analyses stratified by ethnicity
Figure 1. Ethnic differences in the association of SCD-Q and MDRS total scores.
Exploratory analyses
Self- versus informant-reported SCD in Latinos/as (n = 22)
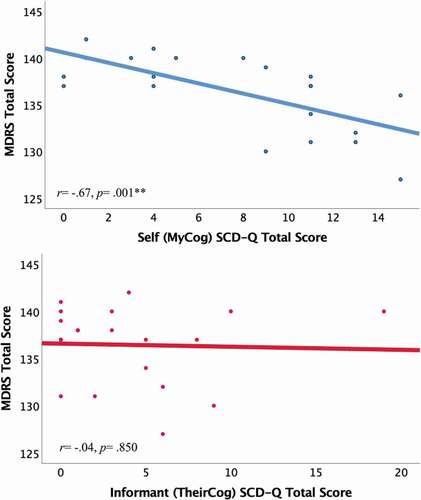
Independent samples t-test revealed no significant differences between self-reports (“MyCog”) and informant-reports (“TheirCog”) of SCD, although there was a trend for self-reported SCD scores (mean = 7.68, SD = 4.90) to be higher than informant-reports (mean = 4.68, SD = 5.30, p = .058, Cohen’s d = .58). Bivariate Pearson correlations revealed that higher SCD-Q total scores were significantly correlated with lower MDRS scores for self-reports (r = −.672, p = .001), but not for informant-reports (r = −.044, p = .850) ().
Self-reported SCD and medial temporal lobe thickness and volume in Latinos/as (n = 12)
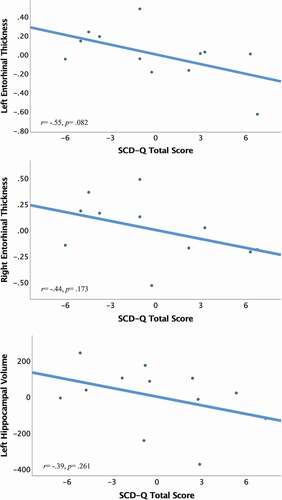
We found large and medium effect sizes when exploring the association of self-reported SCD-Q total scores with left (r = −.547, p = .082) and right entorhinal cortex thickness (r = −.442, p = .173), respectively. There was a medium effect size for the association of SCD-Q with left hippocampal volume (r = −.393, p = .261). Effect sizes in the parahippocampi and the right hippocampus were small (r’s ≤.30). Refer to for a visual depiction of the relationship between SCD-Q scores, left and right entorhinal cortex thickness, and left hippocampal volume in Latinos/as. Refer to Supplementary Table 4 for associations between informant reported SCD and medial temporal lobe thickness and volume in the Latino/a group.
Figure 3. Preliminary associations of self-reported SCD-Q, entorhinal cortex thickness, and left hippocampal volume in Latinos/as (n = 12).
Sensitivity analyses
Refer to for regression statistics and Supplementary Table 5 for demographic, mood, and cognitive characteristics across subgroups.
Table 3. Sensitivity analyses: association of SCD-Q scores with MDRS scores filtering groups based on lowest education level and MDRS scores <132
Groups filtered based on lowest education level and MDRS scores (NHW n = 48; Latinos/as n = 27)
After applying these filters (sample reduced from 83 to 75 participants, with 3 missing GDS-15 and 4 missing SCD-Q), ethnic groups did not differ significantly on age, GDS-15 total scores, SCD-Q total scores, MDRS total scores, or sex distribution. However, there was a trend for higher education in NHWs compared to Latinos/as (p = .051; Supplementary Table 5). The interaction between ethnicity and SCD-Q total scores approached significance in the fully adjusted model (b = −.312, SE = .158, β = −.288, t = −1.976, p = .052). An interrogation of the interaction analyses revealed that higher SCD-Q scores were significantly associated with lower MDRS scores in Latinos/as, but not in NHWs (), corroborating results from the full analytic sample. This indicates that the significant association of SCD-Q and MDRS scores in this small sample is not heavily influenced by group differences in education and global cognition.
Running all models excluding participants with MCI (NHW n = 43; Latinos/as n = 27)
In those with normal cognition only (sample reduced from 83 to 70 participants after removing MCI, with 1 participant missing SCD-Q and 1 missing GDS-15 data), the fully adjusted hierarchical linear regression model revealed a significant interaction between ethnic group and SCD-Q total scores on MDRS total scores (b = −.429, SE = .158, β = −.921, t = −2.710, p = .009). Consistent with findings of the full analytic sample, higher SCD-Q total scores were significantly associated with lower MDRS scores in Latinos/as (b = −.604, SE = .164, β = −.836, t = −3.68, p = .001) but not in NHWs (b = −.122, SE = .101, β = .20, t = 1.21, p = .235). In the Latino/a group only (sample reduced from 22 to 18 participants after removing MCI), independent samples t-tests revealed no significant differences between self-reports (“MyCog”) and informant-reports (“TheirCog”) of SCD (t = 1.77, p = .085, Cohen’s d = .59), although self-reported (mean = 6.94, SD = 5.00) SCD scores were still higher than informant-reports (mean = 4.11, SD = 4.57). Bivariate Pearson correlations revealed that higher SCD-Q total scores were significantly correlated with lower MDRS scores for self-reports (r = −.625, p = .006), but not for informant-reports (r = −.293, p = .238). Regarding the association of SCD-Q self-reports with medial temporal lobe volume and thickness (sample reduced from 12 to 10 participants after removing MCI), there were large effect sizes for left (r = −.578, p = .103) and right (r = −.564, p = .114) entorhinal cortex thickness, and a medium effect size for left hippocampal volume (r = −.397, p = .330). Overall, this indicates that the results obtained from the full analytic sample were not influenced by the few individuals diagnosed with MCI.
Discussion
This study evaluated if there are ethnic differences (Latino/a versus NHW) in the association of SCD reporting and global cognition. We found that ethnicity moderated the association of SCD and global cognition such that SCD reporting was associated with worse global cognition only within Latinos/as and not in NHWs. The pattern of results did not change when we excluded individuals with lower global cognitive performance (MDRS scores < 132), lower educational levels (<12 years), and MCI diagnosis. These preliminary findings suggest that among community samples of Latino/a older adults of mostly Mexican descent, higher SCD reporting may be indicative of lower global cognitive status (Jessen, Citation2014; Mendonça et al., Citation2016), consistent with recently published findings in a large and diverse community-based sample of U.S. residing Latinos/as (Zlatar et al., Citation2021) and with studies conducted in Spain (Sánchez-Benavides et al., Citation2018; Valech et al., Citation2015, Citation2018). This is conflicting with our prior work in a clinical sample of Latinos/as in the U.S., which found that SCD reflected depression symptoms rather than objective cognition (Zlatar, Muniz, Espinoza, et al., Citation2018). One potential explanation for the discrepant results is that, compared to our previous study (2018), our current participants, on average, reported lower levels of depressive symptoms (GDS-15 item raw score of 1.32 vs. 4.00 points) even though neither study excluded participants based on depression scores. Moreover, our current sample is community-based, rather than clinic-based, and therefore participants may not have been worried enough about their SCD to seek medical attention. Most importantly, the use of a validated SCD scale that was developed specifically to measure SCD in Spanish may have been better able to predict cognition than our prior 5-item scale (Zlatar, Muniz, Espinoza, et al., Citation2018).
Although we did not find an association of SCD with global cognition in NHWs, this is not surprising since the SCD-Q was developed in Spain and was not validated for use in NHWs living in the U.S., which may be considered a limitation of the current study. That said, we also collected the Everyday Cognition Scale (ECog) (Farias et al., Citation2008) on the NHW group and were able to replicate our findings (there was no association between ECog total scores and global cognition in the NHW group after adjusting for covariates), indicating that the lack of association between SCD and global cognition in the NHW sample is reliable. Moreover, previous cross-sectional studies by our research group and others using English-based measures of SCD with NHWs show similar findings suggesting that depression, rather than SCD, is related to cognition in some samples (Markova et al., Citation2017; Yates et al., Citation2015; Zlatar et al., Citation2014; Zlatar, Muniz, Galasko, et al., Citation2018). Given our small sample size, we cannot rule out the possibility that we did not have enough power to detect an association in NHWs. However, our Latino/a group was smaller than the NHW group and we detected strong effects. Unfortunately, there is no gold standard questionnaire to measure SCD, and utilizing a single, standardized questionnaire across samples and cultures may not be a practical or feasible solution (Molinuevo et al., Citation2017). It will be important for future studies to select SCD measures that have adequate psychometric properties, have been validated with different samples, measure several cognitive domains, and that follow the SCD-Initiative Working Group (Molinuevo et al., Citation2017) recommendations for how to operationalize SCD.
In contrast to our hypothesis, informant-based SCD reports were not significantly associated with participants’ global cognition within the Latino/a group, suggesting that self-reported SCD may be more sensitive to objective concurrent cognition than informant-reported SCD in Latino/a older adults without dementia. Due to the small sample size, these results are interpreted with caution and are presented to generate new hypotheses. Nonetheless, consistent with findings in NHWs, there was a trend for self-reports of SCD to be higher than informant-reports (Slavin et al., Citation2010), which is thought to reflect an overestimation of SCD by individuals who are aging normally (Edmonds et al., Citation2014). Given the exploratory nature of this analysis, larger studies should confirm these preliminary findings to determine the value of self- versus informant-SCD reports and explore their utility in predicting cognitive performance in Latinos/as.
In the Latino/a culture, respect for older persons and their experiences is highly valued (Beyene et al., Citation2002). Furthermore, family responsibilities and perceived societal roles may influence the perception of cognitive issues within this population (Cuevas & Zuñiga, Citation2020). More specifically, Latino/a culture highlights the importance of family loyalty and support. These values and experiences, in addition to potential discrimination within the healthcare system (Cuevas & Zuñiga, Citation2020), may have resulted in informants underreporting SCD to protect their family members. Moreover, the informant’s education levels, acculturation, health literacy about cognitive decline, personal exposure to dementia, and comorbidity with other physical or mental conditions could have influenced SCD reports (Lee et al., Citation2020; Morrell et al., Citation2019). For instance, some Latino/a individuals may believe that cognitive decline is expected with aging given lower health literacy about dementia (Laditka et al., Citation2011). It will be important to incorporate sociocultural measures (e.g., acculturation, language proficiency, bilingualism, access to healthcare, knowledge about and exposure to Alzheimer’s disease, perceived social support) in future research of SCD and cognition in Latinos/as to better understand their influence on SCD reporting.
Lastly, on a small sub-sample (n = 12) of Latinos/as with available brain MRI data, we found that higher self-reported SCD-Q scores were linked to lower thickness in the left and right entorhinal cortex and lower left hippocampal volume an average of 4.6 years prior to SCD-Q data collection. Although not statistically significant, these medium to large effect sizes suggest that the self-report version of the SCD-Q may be sensitive to neural signatures of AD (Holbrook et al., Citation2020; Meiberth et al., Citation2015; Meiberth et al., Citation2020) and possibly identify those who are at risk (Jessen, Citation2014; Sánchez‐Benavides et al., Citation2020). This is consistent with previous literature implicating reduced entorhinal cortex thickness (Holbrook et al., Citation2020) and hippocampal volume loss (Holbrook et al., Citation2020; van der Flier & Scheltens, Citation2009) as biomarkers for early detection of AD. Additionally, progressive decrease in entorhinal and hippocampal volumes has been associated with the trajectory from normal cognition to MCI and dementia in Latinos/as living in the U.S. (Burke et al., Citation2018). Given our small sample size, these preliminary findings are interpreted with caution but can help propel new studies investigating the utility of SCD to not only predict cognition but biomarkers of AD to improve early identification and clinical outcomes in Latinos/as.
An important limitation of this study is the small sample size, particularly for informant reports and brain MRI data. Furthermore, MRI data were collected on average 4.6 years prior to the SCD-Q completion rather than contemporaneously. Thus, the exploratory analysis of associations between SCD-Q scores and medial temporal lobe thickness and volume within Latinos/as reflects long-standing brain changes typically seen in AD that may be associated with later SCD reporting. Larger studies with the simultaneous collection of neuroimaging and neuropsychological data are needed to confirm our findings. A second limitation is that a majority of the Latino/a participants were of Mexican descent. As such, results may not generalize to other Latino/a subgroups living in the U.S. A third limitation is that this study was cross-sectional in nature. Thus, future research on the ability of SCD to predict cognitive decline and progression to MCI and AD with diverse Latino/a samples is also warranted. Furthermore, we modified some of the SCD-Q items to ease readability by individuals of Mexican background. Future research should validate the SCD-Q with larger populations of Latinos/as living in the U.S. Given the need to investigate culturally relevant variables that may modify the association of SCD and cognition in Latinos/as to address disparities, acculturation factors (e.g., where education was obtained, quality of education, time spent in the U.S., fluency in English, socioeconomic status, health literacy) should also be considered in future studies to better characterize the association of SCD and cognition.
In conclusion, our study fills an important gap in the literature by indicating that the SCD-Q may reflect current global cognition in a community sample of Latinos/as without dementia. This is especially important for the Latino/a population who are more likely to experience socioeconomic stressors (Gallagher-Thompson et al., Citation2006; Morales et al., Citation2002) and barriers to healthcare (Azar et al., Citation2017), making it difficult to receive proper diagnosis and treatment. The SCD-Q could be used as a tool to identify individuals who may be experiencing cognitive difficulties for referral to comprehensive neuropsychological testing and continued monitoring.
Supplemental Material
Download PDF (39.8 KB)Supplemental Material
Download PDF (9.5 KB)Supplemental Material
Download PDF (11.1 KB)Supplemental Material
Download PDF (11.8 KB)Supplemental Material
Download PDF (22.5 KB)Acknowledgments
Contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. We also acknowledge the University of California, San Diego’s Strategic Enhancement of Excellence through Diversity (SEED) Fellowship to Marina Zaher Nakhla. We would like to thank the Alzheimer’s Disease Research Center for supporting data collection efforts. Furthermore, we would like to thank all research participants for volunteering their time and effort to propel scientific knowledge. Finally, we thank the research assistants at the University of California, San Diego’s WISE Lab (Caitlin Knight, Allison Williams, Maya Eshel) for their help. Study data were collected and managed using REDCap electronic data capture tools hosted at the University of California, San Diego CTRI [UL1TR001442].
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Alarcón, R. D. (2009). Culture, cultural factors and psychiatric diagnosis: Review and projections. World Psychiatry, 8(3), 131. https://doi.org/https://doi.org/10.1002/j.2051-5545.2009.tb00233.x
- Albert, M. S., DeKosky, S. T., Dickson, D., Dubois, B., Feldman, H. H., Fox, N. C., Gamst, A., Holtzman, D. M., Jagust, W. J., & Petersen, R. C. (2011). The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging‐Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia, 7(3), 270–279. https://doi.org/https://doi.org/10.1016/j.jalz.2011.03.008
- Alegret, M., Rodríguez, O., Espinosa, A., Ortega, G., Sanabria, A., Valero, S., Hernández, I., Rosende-Roca, M., Vargas, L., & Abdelnour, C. (2015). Concordance between subjective and objective memory impairment in volunteer subjects. Journal of Alzheimer’s Disease, 48(4), 1109–1117. https://doi.org/https://doi.org/10.3233/JAD-150594
- Amariglio, R. E., Becker, J. A., Carmasin, J., Wadsworth, L. P., Lorius, N., Sullivan, C., Maye, J. E., Gidicsin, C., Pepin, L. C., Sperling, R. A., Johnson, K. A., & Rentz, D. M. (2012). Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia, 50(12), 2880–2886. https://doi.org/https://doi.org/10.1016/j.neuropsychologia.2012.08.011
- Arnold, B. R., Cuellar, I., & Guzman, N. (1998). Statistical and clinical evaluation of the Mattis Dementia Rating Scale–Spanish Adaptation: An initial investigation. The Journals of Gerontology: Series B, 53B(6), P364–P369. https://doi.org/https://doi.org/10.1093/geronb/53B.6.P364
- Azar, M., Zhu, C., DeFeis, B., Gu, Y., Ornstein, K., Lawless, S., & Cosentino, S. (2017). Increased reporting accuracy of Alzheimer’s disease symptoms in Caribbean Hispanic informants. Alzheimer Disease and Associated Disorders, 31(4), 328. https://doi.org/https://doi.org/10.1097/WAD.0000000000000199
- Babulal, G. M., Quiroz, Y. T., Albensi, B. C., Arenaza-Urquijo, E., Astell, A. J., Babiloni, C., Bahar-Fuchs, A., Bell, J., Bowman, G. L., & Brickman, A. M. (2019). Perspectives on ethnic and racial disparities in Alzheimer’s disease and related dementias: Update and areas of immediate need. Alzheimer’s & Dementia, 15(2), 292–312. https://doi.org/https://doi.org/10.1016/j.jalz.2018.09.009
- Barnes, D. E., & Yaffe, K. (2011). The projected effect of risk factor reduction on Alzheimer’s disease prevalence. The Lancet Neurology, 10(9), 819–828. https://doi.org/https://doi.org/10.1016/S1474-4422(11)70072-2
- Beyene, Y., Becker, G., & Mayen, N. (2002). Perception of aging and sense of well-being among Latino elderly. Journal of Cross-Cultural Gerontology, 17(2), 155–172. https://doi.org/https://doi.org/10.1023/A:1015886816483
- Bijl, D., Van Marwijk, H. W. J., Adér, H. J., Beekman, A. T. F., & De Haan, M. (2006). Test-characteristics of the GDS-15 in screening for major depression in elderly patients in general practice. Clinical Gerontologist, 29(1), 1–9. https://doi.org/https://doi.org/10.1300/J018v29n01_01
- Black, S. A., Espino, D. V., Mahurin, R., Lichtenstein, M. J., Hazuda, H. P., Fabrizio, D., Ray, L. A., & Markides, K. S. (1999). The influence of noncognitive factors on the Mini-Mental State Examination in older Mexican-Americans: Findings from the Hispanic EPESE. Journal of Clinical Epidemiology, 52(11), 1095–1102. https://doi.org/https://doi.org/10.1016/S0895-4356(99)00100-6
- Buckley, R. F., Villemagne, V. L., Masters, C. L., Ellis, K. A., Rowe, C. C., Johnson, K., Sperling, R., & Amariglio, R. (2016). A conceptualization of the utility of subjective cognitive decline in clinical trials of preclinical Alzheimer’s disease. Journal of Molecular Neuroscience, 60(3), 354–361. https://doi.org/https://doi.org/10.1007/s12031-016-0810-z
- Burke, S. L., Rodriguez, M. J., Barker, W., Greig-Custo, M. T., Rosselli, M., Loewenstein, D. A., & Duara, R. (2018). Relationship between cognitive performance and measures of neurodegeneration among hispanic and white non-hispanic individuals with normal cognition, mild cognitive impairment, and dementia. Journal of the International Neuropsychological Society, 24(2), 176–187. https://doi.org/https://doi.org/10.1017/S1355617717000820
- Colby, S. L., & Ortman, J. M. (2015). Projections of the size and composition of the US population: 2014 to 2060: Population estimates and projections. U.S. Census Bureau
- Cole, J. H., Marioni, R. E., Harris, S. E., & Deary, I. J. (2019). Brain age and other bodily ‘ages’: Implications for neuropsychiatry. Molecular Psychiatry, 24(2), 266–281. https://doi.org/https://doi.org/10.1038/s41380-018-0098-1
- Cuevas, H., & Zuñiga, J. (2020). Latinx with type 2 diabetes: Perceptions of cognitive health. Journal of Immigrant and Minority Health, 23(2), 1–7. https://doi.org/https://doi.org/10.1007/s10903-020-00995-7
- Desa, U. N. (2010). United Nations Department of Economic and Social Affairs/Population Division (2009b): World population prospects: The 2008 revision. https://www.un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/files/documents/2020/Jan/un_2008_world_population_prospects-2008_revision_volume-ii.pdf
- Edmonds, E. C., Delano-Wood, L., Galasko, D. R., Salmon, D. P., & Bondi, M. W. (2014). Subjective cognitive complaints contribute to misdiagnosis of mild cognitive impairment. Journal of the International Neuropsychological Society, 20(8), 836. https://doi.org/https://doi.org/10.1017/S135561771400068X
- Farias, S. T., Mungas, D., Reed, B. R., Cahn-Weiner, D., Jagust, W., Baynes, K., & DeCarli, C. (2008). The measurement of everyday cognition (ECog): Scale development and psychometric properties. Neuropsychology, 22(4), 531. https://doi.org/https://doi.org/10.1037/0894-4105.22.4.531
- Field, A. (2009). Discovering statistics using SPSS (3rd ed.). SAGE Publications Inc.
- Fischl, B. (2012). FreeSurfer. Neuroimage, 62(2), 774–781. https://doi.org/https://doi.org/10.1016/j.neuroimage.2012.01.021
- Fischl, B., Salat, D. H., Busa, E., Albert, M., Dieterich, M., Haselgrove, C., Van Der Kouwe, A., Killiany, R., Kennedy, D., & Klaveness, S. (2002). Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron, 33(3), 341–355. https://doi.org/https://doi.org/10.1016/S0896-6273(02)00569-X
- Fischl, B., Van Der Kouwe, A., Destrieux, C., Halgren, E., Ségonne, F., Salat, D. H., Busa, E., Seidman, L. J., Goldstein, J., & Kennedy, D. (2004). Automatically parcellating the human cerebral cortex. Cerebral Cortex, 14(1), 11–22. https://doi.org/https://doi.org/10.1093/cercor/bhg087
- Gallagher-Thompson, D., Shurgot, G. R., Rider, K., Gray, H. L., McKibbin, C. L., Kraemer, H. C., Sephton, S. E., & Thompson, L. W. (2006). Ethnicity, stress, and cortisol function in Hispanic and non-Hispanic white women: A preliminary study of family dementia caregivers and noncaregivers. The American Journal of Geriatric Psychiatry, 14(4), 334–342. https://doi.org/https://doi.org/10.1097/01.JGP.0000206485.73618.87
- Gómez-Ramírez, J., Ávila-Villanueva, M., & Fernández-Blázquez, M. Á. (2020). Selecting the most important self-assessed features for predicting conversion to mild cognitive impairment with random forest and permutation-based methods. Scientific Reports, 10(1), 1–15. https://doi.org/https://doi.org/10.1038/s41598-020-77296-4
- Green, R. C., Woodard, J. L., & Green, J. (1995). Validity of the Mattis Dementia Rating Scale for detection of cognitive impairment in the elderly. Journal of Neuropsychiatry and Clinical Neurosciences, 7(3), 357–360. https://doi.org/https://doi.org/10.1176/jnp.7.3.357
- Greenberg, S. A. (2012). The geriatric depression scale (GDS). Best Practices in Nursing Care to Older Adults, 4(1), 1–2.
- Hall, J. R., Wiechmann, A., Johnson, L. A., Edwards, M., & O’Bryant, S. E. (2018). Characteristics of cognitively normal Mexican-Americans with cognitive complaints. Journal of Alzheimer’s Disease, 61(4), 1485–1492. https://doi.org/https://doi.org/10.3233/JAD-170836
- Holbrook, A. J., Tustison, N. J., Marquez, F., Roberts, J., Yassa, M. A., Gillen, D. L., & Alzheimer’s Disease Neuroimaging Initiative §. (2020). Anterolateral entorhinal cortex thickness as a new biomarker for early detection of Alzheimer’s disease. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring, 12(1), 1–11. doi: https://doi.org/10.1002/dad2.12068
- IBM Corp. Released (2019). IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp.
- Jak, A. J., Bondi, M. W., Delano-Wood, L., Wierenga, C., Corey-Bloom, J., Salmon, D. P., & Delis, D. C. (2009). Quantification of five neuropsychological approaches to defining mild cognitive impairment. The American Journal of Geriatric Psychiatry, 17(5), 368–375. doi: https://doi.org/10.1097/JGP.0b013e31819431d5
- Jessen, F. (2014). Subjective and objective cognitive decline at the pre-dementia stage of Alzheimer’s disease. European Archives of Psychiatry and Clinical Neuroscience, 264(1), 3–7. https://doi.org/https://doi.org/10.1007/s00406-014-0539-z
- Jessen, F., Amariglio, R. E., Van Boxtel, M., Breteler, M., Ceccaldi, M., Chételat, G., Dubois, B., Dufouil, C., Ellis, K. A., & van der Flier, W. M. (2014). A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimer’s & Dementia, 10(6), 844–852. https://doi.org/https://doi.org/10.1016/j.jalz.2014.01.001
- Jessen, F., Feyen, L., Freymann, K., Tepest, R., Maier, W., Heun, R., Schild, -H.-H., & Scheef, L. (2006). Volume reduction of the entorhinal cortex in subjective memory impairment. Neurobiology of Aging, 27(12), 1751–1756. https://doi.org/https://doi.org/10.1016/j.neurobiolaging.2005.10.010
- Kijonka, M., Borys, D., Psiuk-Maksymowicz, K., Gorczewski, K., Wojcieszek, P., Kossowski, B., Marchewka, A., Swierniak, A., Sokol, M., & Bobek-Billewicz, B. (2020). Whole brain and cranial size adjustments in volumetric brain analyses of sex-and age-related trends. Frontiers in Neuroscience, 14, 278. https://doi.org/https://doi.org/10.3389/fnins.2020.00278
- Knopman, D. S., Roberts, R. O., Geda, Y. E., Pankratz, V. S., Christianson, T. J. H., Petersen, R. C., & Rocca, W. A. (2010). Validation of the telephone interview for cognitive status-modified in subjects with normal cognition, mild cognitive impairment, or dementia. Neuroepidemiology, 34(1), 34–42. https://doi.org/https://doi.org/10.1159/000255464
- Laditka, J. N., Laditka, S. B., Liu, R., Price, A. E., Wu, B., Friedman, D. B., Corwin, S. J., Sharkey, J. R., Tseng, W., & Hunter, R. (2011). Older adults’ concerns about cognitive health: Commonalities and differences among six United States ethnic groups. Ageing & Society, 31(7), 1202–1228. https://doi.org/https://doi.org/10.1017/S0144686X10001273
- Lakens, D. (2013). Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Frontiers in Psychology, 4(26), 863. https://doi.org/https://doi.org/10.3389/fpsyg.2013.00863
- Langa, K. M. (2018). Cognitive aging, dementia, and the future of an aging population. In Future directions for the demography of aging: Proceedings of a workshop.
- Lee, G. J., Do, C., & Suhr, J. A. (2020). Effects of personal dementia exposure on subjective memory concerns and dementia worry. Aging, Neuropsychology, and Cognition, 28(6), 1–16. https://doi.org/https://doi.org/10.1080/13825585.2020
- Lesher, E. L., & Berryhill, J. S. (1994). Validation of the Geriatric Depression Scale-Short Form among inpatients. Journal of Clinical Psychology, 50(2), 256–260. John Wiley & Sons. https://doi.org/https://doi.org/10.1002/1097-4679(199403)50:2<256::AID-JCLP2270500218>3.0.CO;2-E
- Manly, J. J., Tang, M., Schupf, N., Stern, Y., Vonsattel, J. G., & Mayeux, R. (2008). Frequency and course of mild cognitive impairment in a multiethnic community. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society, 63(4), 494–506. https://doi.org/https://doi.org/10.1002/ana.21326
- Markova, H., Andel, R., Stepankova, H., Kopecek, M., Nikolai, T., Hort, J., Thomas-Antérion, C., & Vyhnalek, M. (2017). Subjective cognitive complaints in cognitively healthy older adults and their relationship to cognitive performance and depressive symptoms. Journal of Alzheimer’s Disease, 59(3), 871–881. https://doi.org/https://doi.org/10.3233/JAD-160970
- Martínez de La Iglesia, J., Onís Vilches, M., Dueñas Herrero, R., Albert Colomer, C., Aguado Taberné, C., & Luque Luque, R. (2002). Versión española del cuestionario de Yesavage abreviado (GDS) para el despistaje de depresión en mayores de 65 años: Adaptación y validación. Medifam, 12(10), 26–40. https://doi.org/https://doi.org/10.4321/S1131-57682002001000003
- Mather, M., Jacobsen, L. A., & Pollard, K. M. (2015). Population bulletin: Aging in the United States. Population Reference Bureau, 70(2), 1–19. https://www.prb.org/wp-content/uploads/2016/01/aging-us-population-bulletin-1.pdf
- Mattis, S. (1988). Dementia rating scale (DRS). Psychological Assessment Resources.
- Maxwell, S. E., Delaney, H. D., & Kelley, K. (2017). Designing experiments and analyzing data: A model comparison perspective. Routledge.
- Mayeda, E. R., Glymour, M. M., Quesenberry, C. P., & Whitmer, R. A. (2016). Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimer’s & Dementia, 12(3), 216–224. https://doi.org/https://doi.org/10.1016/j.jalz.2015.12.007
- McKhann, G. M., Knopman, D. S., Chertkow, H., Hyman, B. T., Jack, C. R., Jr., Kawas, C. H., Klunk, W. E., Koroshetz, W. J., Manly, J. J., & Mayeux, R. (2011). The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia, 7(3), 263–269. https://doi.org/https://doi.org/10.1016/j.jalz.2011.03.005
- Meiberth, D., Scheef, L., Wolfsgruber, S., Boecker, H., Block, W., Träber, F., Erk, S., Heneka, M. T., Jacobi, H., & Spottke, A. (2015). Cortical thinning in individuals with subjective memory impairment. Journal of Alzheimer’s Disease, 45(1), 139–146. https://doi.org/https://doi.org/10.3233/JAD-142322
- Meiberth, D. U., Hu, X., Schild, A., Spottke, A., Brosseron, F., Buerger, K., Fließbach, K., Heneka, M. T., Kilimann, I., & Laske, C. (2020). Decreased cortical thickness in individuals with subjective cognitive decline with and without CSF‐AD‐pathology: Data from the DELCODE Study: Neuroimaging/Optimal neuroimaging measures for early detection. Alzheimer’s & Dementia, 16(S5), e044741. https://doi.org/https://doi.org/10.1002/alz.044741
- Mendonça, M. D., Alves, L., & Bugalho, P. (2016). From subjective cognitive complaints to dementia: Who is at risk?: A systematic review. American Journal of Alzheimer’s Disease & Other Dementias®, 31(2), 105–114. https://doi.org/https://doi.org/10.1177/1533317515592331
- Minett, T. S. C., Da Silva, R. V., Ortiz, K. Z., & Bertolucci, P. H. F. (2008). Subjective memory complaints in an elderly sample: A cross‐sectional study. International Journal of Geriatric Psychiatry: A Journal of the Psychiatry of Late Life and Allied Sciences, 23(1), 49–54. https://doi.org/https://doi.org/10.1002/gps.1836
- Molinuevo, J. L., Rabin, L. A., Amariglio, R., Buckley, R., Dubois, B., Ellis, K. A., Ewers, M., Hampel, H., Klöppel, S., & Rami, L. (2017). Implementation of subjective cognitive decline criteria in research studies. Alzheimer’s & Dementia, 13(3), 296–311. https://doi.org/https://doi.org/10.1016/j.jalz.2016.09.012
- Morales, L. S., Lara, M., Kington, R. S., Valdez, R. O., & Escarce, J. J. (2002). Socioeconomic, cultural, and behavioral factors affecting Hispanic health outcomes. Journal of Health Care for the Poor and Underserved, 13(4), 477. https://doi.org/https://doi.org/10.1177/104920802237532
- Morrell, L., Camic, P. M., & Genis, M. (2019). Factors associated with informant-reported cognitive decline in older adults: A systemised literature review. Dementia, 18(7–8), 2760–2784. https://doi.org/https://doi.org/10.1177/1471301218759836
- Perrotin, A., De Flores, R., Lamberton, F., Poisnel, G., La Joie, R., De La Sayette, V., Mezenge, F., Tomadesso, C., Landeau, B., & Desgranges, B. (2015). Hippocampal subfield volumetry and 3D surface mapping in subjective cognitive decline. Journal of Alzheimer’s Disease, 48(s1), S141–S150. https://doi.org/https://doi.org/10.3233/JAD-150087
- Peters, R. (2006). Ageing and the brain. Postgraduate Medical Journal, 82(964), 84–88. https://doi.org/https://doi.org/10.1136/pgmj.2005.036665
- Rabin, L. A., Smart, C. M., & Amariglio, R. E. (2017). Subjective cognitive decline in preclinical Alzheimer’s disease. Annual Review of Clinical Psychology, 13(1), 369–396. https://doi.org/https://doi.org/10.1146/annurev-clinpsy-032816-045136
- Rabin, L. A., Smart, C. M., Crane, P. K., Amariglio, R. E., Berman, L. M., Boada, M., Buckley, R. F., Chételat, G., Dubois, B., & Ellis, K. A. (2015). Subjective cognitive decline in older adults: An overview of self-report measures used across 19 international research studies. Journal of Alzheimer’s Disease, 48(s1), S63–S86. https://doi.org/https://doi.org/10.3233/JAD-150154
- Rami, L., Mollica, M. A., García-Sanchez, C., Saldaña, J., Sanchez, B., Sala, I., Valls-Pedret, C., Castellví, M., Olives, J., & Molinuevo, J. L. (2014). The subjective cognitive decline questionnaire (SCD-Q): A validation study. Journal of Alzheimer’s Disease, 41(2), 453–466. https://doi.org/https://doi.org/10.3233/JAD-132027
- Rattanabannakit, C., Risacher, S. L., Gao, S., Lane, K. A., Brown, S. A., McDonald, B. C., Unverzagt, F. W., Apostolova, L. G., Saykin, A. J., & Farlow, M. R. (2016). The cognitive change index as a measure of self and informant perception of cognitive decline: Relation to neuropsychological tests. Journal of Alzheimer’s Disease, 51(4), 1145–1155. https://doi.org/https://doi.org/10.3233/JAD-150729
- Reisberg, B., Shulman, M. B., Torossian, C., Leng, L., & Zhu, W. (2010). Outcome over seven years of healthy adults with and without subjective cognitive impairment. Alzheimer’s & Dementia, 6(1), 11–24. https://doi.org/https://doi.org/10.1016/j.jalz.2009.10.002
- Rose, K. M. (2005). Mild cognitive impairment in Hispanic Americans: An overview of the state of the science. Archives of Psychiatric Nursing, 19(5), 205–209. https://doi.org/https://doi.org/10.1016/j.apnu.2005.07.002
- Rueda, A. D., Lau, K. M., Saito, N., Harvey, D., Risacher, S. L., Aisen, P. S., Petersen, R. C., Saykin, A. J., Farias, S. T., & Initiative, A. D. N. (2015). Self-rated and informant-rated everyday function in comparison to objective markers of Alzheimer’s disease. Alzheimer’s & Dementia, 11(9), 1080–1089. https://doi.org/https://doi.org/10.1016/j.jalz.2014.09.002
- Sánchez-Benavides, G., Grau-Rivera, O., Suárez-Calvet, M., Minguillon, C., Cacciaglia, R., Gramunt, N., Falcon, C., Camí, J., Operto, G., Skouras, S., Fauria, K., Brugulat-Serrat, A., Salvadó, G., Polo, A., Tenas, L., Marne, P., Gotsens, X., Menchón, T., Soteras, A., & Study, A. (2018). Brain and cognitive correlates of subjective cognitive decline-plus features in a population-based cohort. Alzheimer’s Research & Therapy, 10(1), 123. https://doi.org/https://doi.org/10.1186/s13195-018-0449-9
- Sánchez‐Benavides, G., Salvadó, G., Arenaza‐Urquijo, E. M., Grau‐Rivera, O., Suárez‐Calvet, M., Milà‐Alomà, M., González‐de‐Echávarri, J. M., Minguillon, C., Crous‐Bou, M., & Niñerola‐Baizán, A. (2020). Quantitative informant‐and self‐reports of subjective cognitive decline predict amyloid beta PET outcomes in cognitively unimpaired individuals independently of age and APOE ε4. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring, 12(1), 1–10. https://doi.org/https://doi.org/10.1002/dad2.12127
- Saykin, A. J., Wishart, H. A., Rabin, L. A., Santulli, R. B., Flashman, L. A., West, J. D., McHugh, T. L., & Mamourian, A. C. (2006). Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology, 67(5), 834–842. https://doi.org/https://doi.org/10.1212/01.wnl.0000234032.77541.a2
- Slavin, M. J., Brodaty, H., Kochan, N. A., Crawford, J. D., Trollor, J. N., Draper, B., & Sachdev, P. S. (2010). Prevalence and predictors of “subjective cognitive complaints” in the Sydney Memory and Ageing Study. The American Journal of Geriatric Psychiatry, 18(8), 701–710. https://doi.org/https://doi.org/10.1097/JGP.0b013e3181df49fbGet
- Slot, R. E. R., Sikkes, S. A. M., Berkhof, J., Brodaty, H., Buckley, R., Cavedo, E., Dardiotis, E., Guillo‐Benarous, F., Hampel, H., & Kochan, N. A. (2019). Subjective cognitive decline and rates of incident Alzheimer’s disease and non–Alzheimer’s disease dementia. Alzheimer’s & Dementia, 15(3), 465–476. https://doi.org/https://doi.org/10.1016/j.jalz.2018.10.003
- Soria, J. A., Huisa, B. N., Edland, S. D., Litvan, I., Peavy, G. M., Salmon, D. P., Hansen, L. A., Galasko, D. R., Brewer, J. B., & González, H. M. (2018). Clinical-neuropathological correlations of Alzheimer’s disease and related dementias in Latino volunteers. Journal of Alzheimer’s Disease, 66(4), 1539–1548. https://doi.org/https://doi.org/10.3233/JAD-180789
- Tabachnick, B. G., & Fidell, L. S. (2013). Using multivariate statistics ( Issue 6). Pearson Education.
- Taylor, C. A., Bouldin, E. D., & McGuire, L. C. (2018). Subjective cognitive decline among adults aged≥ 45 years—United States, 2015–2016. Morbidity and Mortality Weekly Report, 67(27), 753. https://doi.org/https://doi.org/10.1016/j.jalz.2009.10.002
- U.S. Census Bureau. (2018). Hispanic population to reach 111 million by 2060. Census Infographics and Visualizations.
- Valech, N., Mollica, M. A., Olives, J., Tort, A., Fortea, J., Lleo, A., Belén, -S.-S., Molinuevo, J. L., & Rami, L. (2015). Informants’ perception of subjective cognitive decline helps to discriminate preclinical Alzheimer’s disease from normal aging. Journal of Alzheimer’s Disease, 48(s1), S87–S98. https://doi.org/https://doi.org/10.3233/JAD-150117
- Valech, N., Tort-Merino, A., Coll-Padrós, N., Olives, J., Leon, M., Rami, L., & Molinuevo, J. L. (2018). Executive and language subjective cognitive decline complaints discriminate preclinical Alzheimer’s disease from normal aging. Journal of Alzheimer’s Disease, 61(2), 689–703. https://doi.org/https://doi.org/10.3233/JAD-170627
- van der Flier, W. M., & Scheltens, P. (2009). Hippocampal volume loss and Alzheimer disease progression. Nature Reviews Neurology, 5(7), 361–362. https://doi.org/https://doi.org/10.1038/nrneurol.2009.94
- Verfaillie, S. C. J., Timmers, T., Slot, R. E. R., van der Weijden, C. W. J., Wesselman, L. M. P., Prins, N. D., Sikkes, S. A. M., Yaqub, M., Dols, A., Lammertsma, A. A., Scheltens, P., Ossenkoppele, R., Van Berckel, B. N. M., & van der Flier, W. M. (2019). Amyloid-β load Is related to worries, but not to severity of cognitive complaints in individuals with subjective cognitive decline: The SCIENCe project. Frontiers in Aging Neuroscience, 11, 7. https://doi.org/https://doi.org/10.3389/fnagi.2019.00007
- Voevodskaya, O., Simmons, A., Nordenskjöld, R., Kullberg, J., Ahlström, H., Lind, L., Wahlund, L.-O., Larsson, E.-M., Westman, E., & Initiative, A. D. N. (2014). The effects of intracranial volume adjustment approaches on multiple regional MRI volumes in healthy aging and Alzheimer’s disease. Frontiers in Aging Neuroscience, 6, 264. https://doi.org/https://doi.org/10.3389/fnagi.2014.00264
- Wu, S., Vega, W. A., Resendez, J., & Jin, H. (2016). Latinos & Alzheimer’s disease: New numbers behind the crisis. USC Edward R. Roybal Institute on Aging and the Latinos against Alzheimer’s Network, 1–31. https://www.usagainstalzheimers.org/sites/default/files/Latinos-and-AD_USC_UsA2-Impact-Report.pdf
- Yates, J. A., Clare, L., Woods, R. T., & Matthews, F. E. (2015). Subjective memory complaints are involved in the relationship between mood and mild cognitive impairment. Journal of Alzheimer’s Disease, 48(s1), S115–S123. https://doi.org/https://doi.org/10.3233/JAD-150371
- Yesavage, J. A., Brink, T. L., Rose, T. L., Lum, O., Huang, V., Adey, M., & Leirer, V. O. (1982). Development and validation of a geriatric depression screening scale: A preliminary report. Journal of Psychiatric Research, 17(1), 37–49. https://doi.org/https://doi.org/10.1016/0022-3956(82)90033-4
- Zlatar, Z. Z., Moore, R. C., Palmer, B. W., Thompson, W. K., & Jeste, D. V. (2014). Cognitive complaints correlate with depression rather than concurrent objective cognitive impairment in the successful aging evaluation baseline sample. Journal of Geriatric Psychiatry and Neurology, 27(3), 181–187. https://doi.org/https://doi.org/10.1177/0891988714524628
- Zlatar, Z. Z., Muniz, M., Galasko, D., & Salmon, D. P. (2018). Subjective cognitive decline correlates with depression symptoms and not with concurrent objective cognition in a clinic-based sample of older adults. The Journals of Gerontology: Series B, 73(7), 1198–1202. https://doi.org/https://doi.org/10.1093/geronb/gbw207
- Zlatar, Z. Z., Muniz, M. C., Espinoza, S. G., Gratianne, R., Gollan, T. H., Galasko, D., & Salmon, D. P. (2018). Subjective cognitive decline, objective cognition, and depression in older Hispanics screened for memory impairment. Journal of Alzheimer’s Disease, 63(3), 949–956. https://doi.org/https://doi.org/10.1017/S104161021200213X
- Zlatar, Z. Z., Tarraf, W., González, K. A., Vásquez, P. M., Marquine, M. J., Lipton, R. B., Gallo, L. C., Khambaty, T., Zeng, D., & Youngblood, M. E. (2021). Subjective cognitive decline and objective cognition among diverse US Hispanics/Latinos: Results from the Study of Latinos‐Investigation of Neurocognitive Aging (SOL‐INCA). Alzheimer’s & Dementia, 1–10. https://doi.org/https://doi.org/10.1002/alz.12381