ABSTRACT
A.V. is a young herpes simplex encephalitis (HSE) survivor who suffered extensive bilateral damage to the medial temporal lobe (MTL) leading to a severe and pervasive form of anterograde amnesia. Structural Magnetic Resonance Imaging (MRI) revealed lesions that encompass the hippocampus and amygdala in both hemispheres and that extend more laterally in the right temporal lobe. At the same time, detailed neuropsychological testing showed that the disparity between A.V.’s preserved intellectual functioning (Full Scale IQ: 115) and severe memory deficit (Delayed Memory Index: 42) is one of the largest on record. Despite this deficit, A.V. has regained a higher level of functioning and autonomy compared to previously documented amnesic cases with major bilateral MTL lesions. As a millennial, one advantage which A.V. has over prior amnesic cases is fluency with digital technology – particularly the smartphone. The analysis of his phone and specific app usage showed a pattern that is consistent with the strategy to offload cognitive tasks that would normally be supported by the MTL. A.V.’s behavior is significant in terms of rehabilitation and may have broader implications at the societal level and for public health given the ubiquity of smartphone technology and its potential to become integrated with neural mnemonic functions.
Introduction
The classic case of the amnesic patient H.M. provided concrete evidence that damage to the hippocampus and neighboring medial temporal lobe (MTL) structures produces disturbances of declarative memory (Corkin, Citation2002). H.M. acquired a pervasive and irreversible form of anterograde amnesia at age 27, after undergoing a bilateral medial temporal lobectomy (Scoville & Milner, Citation1957). Performed in 1953, the surgery represented a novel, experimental approach to the treatment of epileptic convulsions. The procedure entailed the complete ablation of the hippocampus and neighboring MTL tissue in both hemispheres. As the postmortem examination of H.M.’s brain eventually demonstrated, the surgeon’s post-operative drawings do not match the actual lesion. Specifically, microscopic 3-D reconstruction of the brain revealed that the posterior half of the hippocampus (i.e., the periventricular portion) was preserved morphologically and histologically in both hemispheres (Annese et al., Citation2014). These postmortem anatomical findings, along with evidence that H.M. might have in fact retained some ability to acquire new knowledge (Corkin, Citation2002), do not lessen his importance for modern memory research (Squire, Citation2009). Rather, they underscore the need for additional validation based on new cases of bilateral MTL damage with selective loss of declarative memory.
In practice, cases of anterograde amnesia secondary to bilateral MTL lesions are extremely rare. Significant memory deficits can ensue following anoxia (Warren et al., Citation2012; Zola-Morgan et al., Citation1986) and traumatic brain injury (TBI) (Rosenbaum et al., Citation2005), but these etiologies produce an amnesia of variable severity, and real-life outcomes can vary in different patients (Duff et al., Citation2008). In the brain, anoxia-derived pathology produces localized damage that is typically confined within subfields of the hippocampus (Volpe & Hirst, Citation1983) leading to hippocampal atrophy (DiPaola et al., Citation2008), while TBI pathology often entails axonal damage and diffuse lesions in the deep white matter (Johnson et al., Citation2013). Other than in the instance of H.M.’s anomalous surgery (Dittrich, Citation2017), lesions that destroy large portions of the MTL in both hemispheres have only been observed in patients whose central nervous system was infected by the Herpes simplex virus (Hierons et al., Citation1978; Shaw & Alvord, Citation1997). Herpes simplex encephalitis (HSE) develops as a process of acute inflammation that culminates in the necrosis of gray and white matter. The nature and severity of cognitive deficits presented by HSE patients depend on the location of tissue necrosis (Kapur et al., Citation1994), with a severe memory impairment being one of the most common outcomes because of HSE’s predilection for attacking the MTL before advancing into adjacent structures within the limbic and paralimbic circuitry (Damasio & Van Hoesen, Citation1985). HSE lesion borders, as seen by MRI and in stained histological preparations, can be remarkably sharp and often follow neuroanatomical boundaries (Damasio & Van Hoesen, Citation1985; Feinstein et al., Citation2010; Hierons et al., Citation1978).
With the introduction in the early 1980’s of the antiviral drug acyclovir, the number of HSE cases developing significant CNS morbidity and mortality decreased substantially. Patients who are treated timely with acyclovir, tend to bear smaller, unilateral brain lesions resulting in milder cognitive deficits (Sköldenberg et al., Citation1984; Tranel et al., Citation2000; Tyler, Citation2004; Whitley et al., Citation1986). Consequently, there have been very few recent reports of HSE patients who developed severe and persistent anterograde amnesia following bilateral MTL damage. Among the most notable are the cases of patients B (Damasio et al., Citation2013; Damasio et al., Citation1985), E.P (Insausti et al., Citation2013; Stefanacci et al., Citation2000). Roger (Feinstein et al., Citation2010), and Clive Wearing (Wilson & Wearing, Citation1995; Wilson et al., Citation1995, Citation2008), all of whom, like H.M., made invaluable contributions to the field.
In this report, we present the results of the first neuroimaging and behavioral studies conducted with patient A.V., a newly identified HSE survivor who became amnesic at the age of 20. A.V.’s brain sustained bilateral, near-complete damage of the MTL, as confirmed by MRI. Formal neuropsychological testing confirmed that A.V. acquired a severe and selective form of anterograde amnesia while maintaining an otherwise high level of intellectual functioning. At the same time, observations of A.V.’s behavior in real-world settings revealed a remarkable adaptation that involves to a large extent the use of the smartphone. In conjunction with his mother’s consistent and insightful caregiving, A.V.’s use of his mobile device allows him to live independently, work, and cope in his daily life with what would otherwise be a devastating memory impairment, as discussed below.
Materials and methods
The study was approved by the Western Institutional Review Board (WIRB) and by the IRB at the University of California, San Diego (UCSD). Written informed consent was obtained from Patient A.V. prior to participation. All consenting procedures were performed in the presence of A.V.’s mother, who is also his primary caregiver. Throughout testing and during the scans, A.V. was regularly reminded that he could stop the session at any time.
Case overview
Patient A.V. is a fully right-handed (+100) Caucasian man who was between 28–30 years old at the time of this study. He grew up as an only child in a suburban American town. A.V. was raised by his mother in a single parent household with the support of his maternal grandparents and a large extended family, all of whom live nearby. A.V. showed normal development during childhood and adolescence. According to his mother, and as confirmed by medical records, A.V. had no significant health, developmental or neurological issues prior to the HSE infection.
A.V.’s family described him as imaginative, introspective, independent, and well-liked within his circle of friends. From an early age, A.V. showed a strong interest in technology and computers. He played video games and collected digital music in his free time. A.V. worked several part-time jobs in his high school years, mostly in the food service industry. His school grades were average or below average with a cumulative high school grade point average of 2.2 (his lowest grades were in Spanish and math). In contrast, he excelled in computer science, which he picked as an elective subject in high school. He competed for the school’s wrestling team throughout junior high and high school, and experienced his first serious romantic relationship during his final years of high school. After graduating, A.V. enrolled in a local community college, with the goal of transferring into a 4-year university program to obtain a degree in computer science so that he could pursue a career in information technology.
A.V. contracted HSE toward the end of his first semester in college, when he was 20 years old. The initial symptoms were fever, nausea, and lethargy. Eleven days later he was showing signs of incoherent thought and experienced visual hallucinations. At this stage, he could not recognize his mother. Two weeks after the onset of the symptoms, additional diagnostic tests confirmed HSE, and treatment with acyclovir was finally initiated. However, A.V.’s condition continued to deteriorate, and he became unresponsive. After he was hospitalized, the medical staff informed the family that A.V. might not survive. However, after three days of antiviral treatment A.V. slowly regained consciousness.
Several months after the encephalitis attack, A.V. did not recognize his close friends and family members. The initial years post-HSE were characterized by prolonged periods of poor attention and comprehension, and great difficulty in managing basic activities of daily living. Through nine subsequent months of physical, speech, and occupational therapy (he received outpatient therapy twice a week), A.V. slowly started making progress. However, while his overall cognition and level of functioning improved during rehabilitation therapy, he continued to show impairment in the formation of new declarative memories. A.V. showed some degree of knowledge that the cause of his faulty memory was “some kind of brain injury.” At the time of our study, A.V. recognized that his memory impairment was a burden that he would have to live with indefinitely, but he expressed satisfaction knowing that his participation in research could be beneficial to others. When we asked him if he was bothered by the long series of experiments in this study, he replied: “At least I know that if I can’t get better, I might be helping someone else in the future get better. That’s what makes all this testing worth doing.”
Neuroimaging
Patient A.V.’s brain scans were performed on a 3 Tesla (3T) General Electric MR750 using an 8-channel brain array coil and a 1.5T General Electric “HDX” Twinspeed EXCITE scanner using an 8-channel phased-array head coil. was generated from a 2D axial Spin Echo (SE) sequence on the 1.5T magnet (matrix size = 256×224; number of slices = 20; slice thickness = 5 mm). The MRI slices displayed in and S1 were sampled from a T1-weighted 3D FSPGR scan on the 3T magnet (matrix size = 320×320; number of slices = 168; section thickness = 1 mm; number of acquisitions = 2). The anatomical (FSPGR) T1-weighted MRI data, after registration into Talairach space (Talairach & Tournoux, Citation1988), was used to generate the cortical surface displayed in utilizing the FreeSurfer image analysis suite (http://surfer.nmr.mgh.harvard.edu). Some manual input into the automated cortical generation pipeline was necessary because A.V.’s scan departed from normal anatomy in two significant ways: firstly, large areas normally occupied by brain tissue (gray and white matter) were filled with CSF; secondly, automated identification of the AC was problematic because of severe tissue degeneration. provides a graphical reference to localize cross-sectional anatomy displayed in , and S1.
Figure 1. Axial T2-weighted FLAIR scans of A.V.’s brain. a A.V.’s first MRI exam taken at the onset of HSE infection. b Recent scan taken during our study (8 years post-HSE). The early scan shows signal hyperintensities in the temporal lobes consistent with tissue inflammation. Even though the T2-weighted signal is abnormally bright, the shape of the temporal lobes can be delineated clearly, making it possible to evaluate the topography of the lesion with respect to the original anatomy. Areas of T2-weighted hyperintensity in the original clinical scan coincide with the borders of the lesion in the more recent scan. The right hemisphere is marked by the top blue line; the left hemisphere by the green line (see Fig. 2). Scale bars: 1 cm.
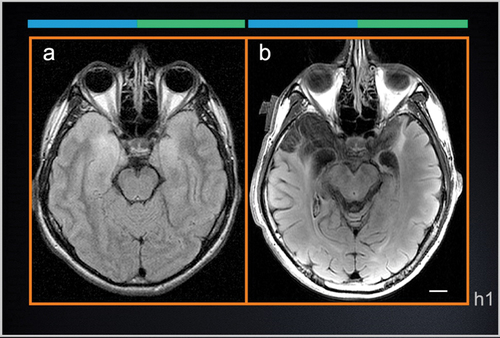
Figure 2. 3-dimensional (3-D) anatomical legend for cross-sectional images of A.V.’s brain. 3D reconstruction of A.V.’s right and left hemispheres of the brain were created to display the relative position of cross-sectional anatomical images of A.V.’s brain displayed in Figs. 1–3 and S1, S2 with respect to cortical anatomy and major surface landmarks. The white and gray lines indicate the plane of section for horizontal (h1-h5), sagittal (s1), and coronal slices (c1-c8). The right hemisphere is colored blue and the left hemisphere is green. Scale cube: 1 cm3.
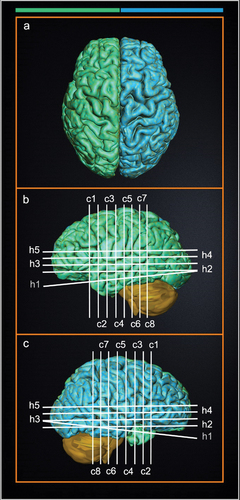
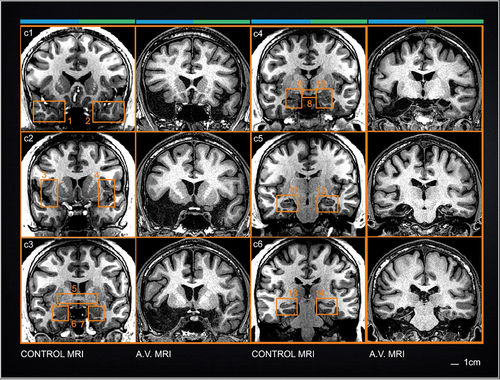
Figure 3. Cross-sectional coronal MRI images through the brain of patient A.V. and an age-matched healthy male. Coronal images provide a comprehensive view of the position of subcortical structures relative to the MTL. Numbered boxes outline major regions of interest in the control brain images to help classify missing or damaged structures (see text). At the top of each column, the blue and green line denote the right hemispheres and left hemispheres respectively (see Fig. 2). Scale bars: 1 cm.
Neuropsychological assessment
Patient A.V. completed several sessions of neuropsychological testing over a 3-year period (approximately 8 to 10 years post-HSE). On each occasion, the assessments took place in a quiet and well-lit room, and regular breaks were provided. In some cases, minor adjustments were made to the testing protocol to accommodate A.V.’s amnesia, including repetition of task instructions, when appropriate, to facilitate task comprehension. During the assessments, A.V. was separated from his smartphone and his mother so that he would not have access to any cues that might improve his performance. Throughout testing, he was cooperative and pleasant, and denied the presence of pain, fatigue, motor impairments, or any basic sensory deficits that might impact the results. During the tests, he appeared to put forth sufficient effort, which was further confirmed by his good performance on the Test of Memory Malingering () as well as his reliable digit span score (Table S1). All test results are considered valid unless otherwise noted. Additional results from this battery of neuropsychological tests are available in the Supplementary Materials.
Table 1. A.V.’s general cognition and intellectual functioning.
Table 2. A.V.’s anterograde memory.
Phone data recording
Phone usage statistics were derived from continuous monitoring with App Usage (a0soft Software, Hsinchu, Taiwan) for Android over a period of 100 days (March-June 2019). Data relative to phone and app usage time were exported as. csv files and examined in Excel (Microsoft, Redmond, WA). Resulting graphs were exported to Photoshop (Adobe, San Jose, CA)
Results
In the sections that follow, we examine A.V.’s brain pathology as seen in MRI images and ascertain his cognitive state based on a detailed battery of standardized neuropsychological tests. We also provide a structured account of A.V.’s most salient naturalistic behaviors and describe the utilization of his personal smartphone based on the analysis of his phone- and app-usage.
Brain anatomy and pathology
Axial MRI slices from the first diagnostic scans that were collected in the early stages of A.V.’s HSE infection (), show areas of MR signal hyper-intensity that foreshadow the drastic tissue damage that would follow and that was clearly demarcated in the scans performed in the context of our study (). The MTL was the epicenter of the inflammatory process and subsequent necrosis, but additional damage was also evident in the basal forebrain, subgenual and subcallosal anterior cingulate (Figure 3, Sections c1-c2), and the insular cortex (see Fig. S1 in Supplementary Materials). The MTL lesion is bilateral and spans its entire length involving primarily the hippocampal formation, the parahippocampal gyrus, entorhinal and perirhinal cortex, medial temporopolar cortex, as well as the central, basal and lateral nuclei of the amygdala.
Anteriorly, the lesion is grossly uneven across hemispheres. The right temporal lobe, where the temporal pole and lateral temporal gyri are missing, is clearly more damaged () than the left temporal lobe. Medially and more posteriorly, the lesion is symmetrical, except that a thin band of tissue attributable to the subiculum and hippocampal tail can be discerned in the left hemisphere (Fig. 3, levels c5 and c6) whereas these structures cannot be seen in the right hemisphere.
White matter association tracts within the temporal lobes were clearly impacted by the infection, including the uncinate fasciculus, which connects limbic structures to the frontal lobes, and the inferior longitudinal fasciculus, which connects the temporal and occipital lobes. The lateral ventricles were not enlarged, and T2-weighted FLAIR scans did not reveal any areas of signal hyperintensity in the deep white matter. The gyral pattern of the frontal, parietal, and occipital lobes appear morphologically normal. Cerebellar size and anatomy also look normal for someone of A.V.’s age. In summary, there is no evidence of atrophy or gross morphological changes in areas not directly impacted by HSE-related pathology.
The borders of the MTL lesion are best appreciated in the coronal (frontal) plane. Pathologic anatomy as seen in the 3T T1-weighted MRI images is described at multiple levels in the following sections. Cross sectional anatomy is displayed based on Talairach stereotaxic coordinates in the Y (frontal) axis (Talairach & Tournoux, Citation1988). A.V.’s MRI images are compared to a qualitatively similar dataset from an age-matched, neurologically healthy male subject.
Level c1 (approx. Talairach Y coordinate: −15 mm). The planum polare (Brodmann area 38) and the entire lateral temporal cortex (boxes 1 and 2) of the right hemisphere are missing. The superior and middle temporal gyri are preserved in the left hemisphere with only partial damage of the planum polare.
Level c2 (approx. Talairach Y coordinate: −7 mm). Brodmann area 25 (the subgenual and subcallosal anterior cingulate) are damaged bilaterally. Based on visible anatomical landmarks, we deduce that the olfactory area and the piriform cortex are at least partially compromised. The area of the anterior entorhinal cortex shows drastic necrosis. These slices also show the severe gray matter damage in the insula (boxes 3 and 4; see Figure S1 in Supplemental materials)
Level c3 (approx. Talairach Y coordinate: 0 mm). The anterior commissure (AC), a transversal band of fibers that connects temporal lobe structures including the amygdalae, is severely atrophic (, box 5). Entorhinal and perirhinal cortices (Brodmann’s areas 35 and 36) as well as the basal and lateral nuclei of the amygdala are destroyed bilaterally (, boxes 6–7). Only a small island of tissue belonging to the superior temporal gyrus remains in the right temporal lobe, while on the left, all temporal isocortex seems to have been largely spared by the infection.
Level c4 (approx. Talairach Y coordinate: +8 mm). The mammillary bodies, a clear anatomical landmark at this level, appear intact (, box 8). Conversely, HSE pathology affected the body of the fornix, the amygdala, the entorhinal cortex, and portions of the anterior hippocampus (, boxes 9–10). A.V.’s MTL damage at this level includes the entorhinal cortex, the anterior hippocampus (CA1 and subiculum), as well as the lateral nucleus and the basolateral complex of the amygdala. The lesion also includes most of the inferior temporal lobe (through the rhinal sulcus) and the transition area between the inferior temporal gyrus and fusiform gyrus. The fornix, which is the major output tract of the hippocampus, shows significant atrophy compared to the control subject.
Level c5 (approx. Talairach Y coordinate: +16 mm). The lesion extends into the fusiform gyrus of the right hemisphere where the hippocampal formation, subiculum, and entorhinal cortex are unequivocally damaged. In the left hemisphere, the collateral sulcus is identifiable, and the parahippocampal gyrus is discernable, although the latter is atrophic and shows some “clipping,” a sign of partial necrosis. The entorhinal cortex is coextensive with the medial portion of the parahippocampal gyrus and therefore portions of it might remain, but the MRI images do not contain sufficient detail to localize its distinctive cellular anatomy. The appearance of the subiculum, the cornu ammonis (CA1-CA4), and dentate gyrus depart from normal anatomy bilaterally (, boxes 11 and 12) but more clearly in the right hemisphere.
Level c6 (approx. Talairach Y coordinate: +23 mm). The posterior portion of the left parahippocampal gyrus shows normal morphology (refer to box 14), whereas its counterpart on the right hemisphere shows clear signs of necrosis. The necrosis extends posteriorly beyond level c6 in the right hemisphere, impacting the tail of the hippocampus as well as the lingual and fusiform gyri in the occipital cortex (levels c7 and c8 in Fig. S1, Supplementary materials).
Neuropsychology
Neuropsychological assessments were administered when patient A.V. was between the age of 28 and 30 (i.e., 8 to 10 years after his brain injury). We measured his general cognition and intellectual functioning (), anterograde memory (), retrograde memory, procedural memory (), working memory, attention, and executive functioning (Table S1), speech and language (Table S2), visuospatial and motor functioning (Table S3), and olfactory function. Selected tests were repeated approximately once every year for three years in order to ascertain the consistency of the results (Table S4).
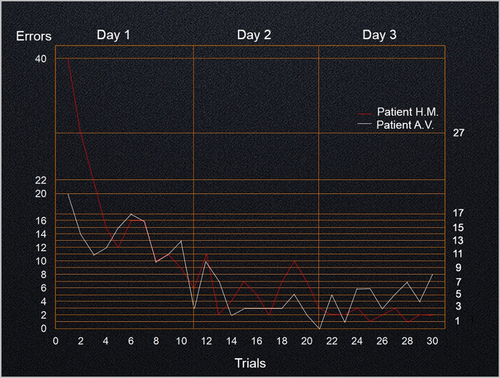
Figure 4. Number of errors using the mirror tracing task. Patients A.V. and H.M. completed 30 trials, 10 consecutive trials each day, over 3 consecutive days using their right (dominant) hand. H.M.’s average completion times were calculated based on Milner(Citation1962) (see figure 10 of Milner, Citation1962; note that data from H.M’s first trial was not included in this figure).
General cognition and intellectual functioning
A.V.’s intelligence is above average, with a Full Scale Intelligence Quotient of 115 on the Wechsler Adult Intelligence Scale (WAIS-IV; ). We measured a significant discrepancy between his superior perceptual reasoning (standard score: 138) and average verbal comprehension (standard score: 105), indicating that his strength lies within the domain of perceptual reasoning (99th percentile), including visuospatial processing, visual-motor coordination, abstract problem solving, and fluid intelligence. His reading skills, as assessed with the Wide Range Achievement Test (WRAT-4; ), were consistent with his 12.5 years of education. On the Mini Mental State Exam (MMSE-2), he performed within the normal range but was specifically impaired on memory-related questions, including orientation in time and space.
Anterograde memory
While the majority of A.V.’s cognitive functions were well preserved, there was significant deterioration of his performance on standardized measures of anterograde memory (). A.V. showed a severe deficit in learning verbal as well as nonverbal information across multiple memory tests that included word lists, short stories, pictures, and shapes. He was severely impaired at recalling information, both immediately and after delays of 20 to 30 minutes. Following these delays, A.V. was unable to recall any of the information that had been presented to him (resulting in a raw score of 0 across multiple tests). His recognition scores were similarly in the impaired range. In fact, his performance did not improve if he was asked to pick words that had been read to him earlier from a list that also included new words. On a “yes or no” recognition test he showed a bias for yes responses (with 7 hits and 17 false positives) and a high intrusion rate (27 intrusions, which place him in the severely impaired range), highlighting his attempt to name words that were not in the list. A.V. showed no evidence that repetition helped with the learning of new declarative information. On the California Verbal Learning Test, he recalled 6 out of 16 words on the 1st trial and only 5 out of 16 words on the 5th trial. However, for the words that he did recall, he showed a significant recency effect (i.e., he was better at remembering the words that were presented last and closer to the recall test). Finally, A.V.’s anterograde memory deficit was stable across three separate exams that were administered over three consecutive years (Table S4).
A.V.’s scores on standardized memory tests were compared to analogous scores from H.M. and four notable HSE amnesic patients with significant bilateral MTL damage. Comparative values relative to each patient’s Full Scale IQ, memory index, working memory, immediate- and delayed- verbal and visual memory scores are listed in . The differential between the Full Scale IQ on the Wechsler Adult Intelligence Scale (FSIQ/WAIS-IV) and the Delayed Memory Index on the Wechsler Memory Scale (DMI/WMS-IV) is an indicator of the severity and specificity of cognitive impairment in the memory domain. When calculated as such (FSIQ 115 – DMI 42 = 73), A.V.’s memory impairment ranked as the most severe among the amnesic patients in , including H.M.
Table 3. Standardized Full Scale IQ and memory test scores for A.V. and other amnesic patients with bilateral MTL lesions.
Each patient in demonstrated preserved working memory (Baddeley, Citation2012) as well as the ability to retain at least some verbal and visual information during immediate recall but not following the 30-minute delay.
Retrograde memory
A.V.’s retrograde memory of the period prior to his HSE infection appeared to be relatively well preserved. A.V. was presented with pictures of 30 people who became famous before his injury (pre-HSE), 30 people who became famous during the years following his injury (post-HSE), and 30 people who were not famous. In this modified famous-faces task, A.V. named 11 of the 60 famous faces correctly, 10 from the pre-HSE period and only 1 from the post-HSE period. He did not recognize any of the non-famous faces. Curiously, when A.V. was shown photos of the researchers that he had been working with in the days preceding the famous faces task, A.V. expressed some degree of familiarity, but he provided only vague explanations for recognizing these people.
A.V.’s performance on the “Information” and “Vocabulary” subtests of the WAIS-IV, was in the average range, suggesting that his remote semantic memories and general knowledge base were largely preserved. We also administered the Iowa Autobiographical Memory Questionnaire (IAMQ), an interview-based questionnaire with norms, developed to measure retrieval of specific personal knowledge from the past (Tranel & Jones, Citation2006). Sections 1 and 5 of the IAMQ were administered in order to assess his recollection of autobiographical memories from two separate time periods: from birth to 18 years of age (pre-HSE), and from the year of his hospitalization to the year prior to the interview (post-HSE). Only answers that were validated by A.V.’s mother were marked as correct answers. A.V. performed within normal limits (<1 standard deviation from the normative mean) when he had to recall autobiographical memories from his early childhood up to the year prior to his brain injury. He correctly recalled the names and locations of his schools and workplaces, as well as the names of his teachers, friends, and family members. He also demonstrated preserved memories for specific events like the passing of one of his school teachers. His retrograde memory included events that happened closer to his HSE infection, like his high school graduation party, prom night, and his relationship with his high school girlfriend. In contrast, autobiographical memories from the post-HSE period were largely missing and his performance was highly defective (>8 standard deviations below the normative mean).
Procedural memory
A mirror-tracing test conducted with patient H.M. suggested that some forms of learning do not depend on the hippocampus. Successive trials demonstrated that H.M. was able to learn new motor skills despite having no conscious recollection of the previous sessions or prior training (Milner, Citation1962, Citation1965). This landmark experiment provided evidence for the existence of multiple memory systems in the human brain. To test A.V.’s procedural memory, we presented him with the same mirror-tracing test and compared the results to H.M.’s results. Accordingly, A.V. was asked to trace the outline of a five-pointed star aided only by the reflection of his hand in a mirror. The same test was repeated ten times on three consecutive days to assess his capacity to improve based on repetition and training ().
A.V. showed a marked reduction of errors and completion time over the 3-day test period, evidencing preserved procedural learning. A.V. made half as many errors as H.M did in the first set of trials on Day 1. Improvement in A.V.’s performance appeared to plateau by the end of Day 2. Overall, A.V. completed the mirror tracing task faster than H.M., which explains why his learning curve is more gradual in . Average completion times (in seconds) for A.V. and H.M., respectively, were the following: Day 1: 42 vs 157; Day 2: 25 vs 96; Day 3: 23 vs 67. Like H.M., A.V. did not explicitly remember having completed previous trials.
Observations of A.V.’s naturalistic behaviors
In addition to formal neuropsychological testing in the laboratory setting, two authors (JA and JSF) visited A.V. in his hometown for one week and recorded facts about his daily life. The visit afforded the opportunity to witness first-hand how A.V. functions in real-world settings. We also conducted extensive interviews with his mother and other family members to substantiate our observations and contextualize the results.
A.V. lives alone in a single-family home that is located a few miles from the house he grew up in. His mother lives in a separate house a block away. During the early stages of his recovery, A.V. needed constant supervision, even with very basic tasks (e.g., choosing appropriate clothing for the weather, keeping up with his personal hygiene, and preparing meals); now he is largely able to perform these tasks on his own. His mother continues to provide oversight and assistance, often remotely, using text messaging.
A.V.’s mother presides over important business and safety issues, but she also occasionally resolves simple, practical problems that can nevertheless impact A.V.’s quality of life; for instance, she makes sure his refrigerator and pantry are stocked with healthy foods, something that A.V. may not do consistently. Having trained as a health professional, A.V.’s mother is exceptionally perceptive and resourceful as a caregiver. Her main objective, as she aptly describes it, is to reinforce behaviors and to teach A.V. skills that can ultimately bolster his independence, a strategy that has proven very effective so far.
Upon witnessing A.V.’s daily routine the observer gains an impression of normalcy that is atypical for an HSE survivor with such a severe memory deficit. displays A.V.’s weekly schedule. It is representative of his routine habits and commitments over the past year. He works 6 days a week (averaging 30 hours) at two different jobs, one with a landscaping and cleaning crew, the other in a catering business. His day off is Friday, when he rests and catches up with household tasks and errands like doing the laundry and gardening. He regularly sleeps six hours at night and wakes up around the same time every morning without having to set an alarm. Throughout the day, he takes one or more rest breaks, as needed. According to his mother, these breaks are very important for his functioning and well-being. He plays a ball game three days a week, mixing with a regular group of people at the local community center. The ball game is his main form of physical exercise and one of the occasions in which he engages in social interactions. He eats one or two meals a day, but not at any regular time, and snacks throughout the day. A.V. visits his grandparents regularly and attends church together with his extended family on Sunday followed by brunch at the local diner, a family tradition.
A.V. drives alone to work and to visit his relatives. He has been driving for over a decade with no reported accidents or traffic citations. During this time, A.V.’s mother can cite only two instances in which he needed her help with directions: one time he wanted to be sure he knew where he could turn to get to his destination since the police rerouted all traffic due to an accident; the other is when he made a left turn instead of a right and realized he had made an error. Indeed, our own observations confirmed that he has no difficulties driving and finding his way in the local environment. We toured the neighborhood in the car and observed how he recognized many landmarks in the area, including his former schools, the location of his workplace (both past and present), and the homes where his relatives live. A.V. also drove us for ten miles on the freeway and on secondary roads to return to his home after a day trip. On that occasion, we could confirm that he is able to navigate relatively long distances without the aid of GPS, maps, or his mother’s assistance. These observations were validated by the analysis of A.V.’s use of smartphone apps, as explained below.
Table 4. Typical week in A.V.’s life.
A.V.’s smartphone use
At the beginning of his recovery, A.V. wrote reminders on Post-It© notes. It took two years for him to resume the use of his personal computer (PC) which he used to access his music collection, photos, and the internet. He also used the PC to keep track of his tasks and to store information related to past and upcoming events. Following the introduction of smartphones, in the years after his injury, A.V. began using these portable devices and has since owned four different Android smartphones (HTC Hero, Galaxy S3, S4, and S7).
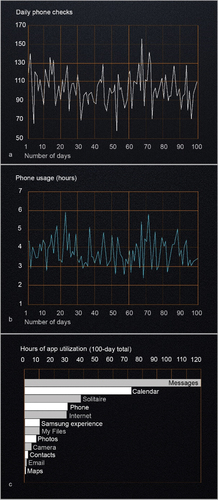
In order to determine how A.V. utilizes his smartphone, we collected statistics relative to his phone and app usage over a 100-day period. The results of the analyses are displayed in . Based on smartphone use data, A.V. activated (checked) his phone 103 times per day on average (), spending an average of 3 hours and 3 minutes on apps and 18 minutes on phone calls each day. In total, his average daily mobile phone use was 3 hours and 21 minutes, with most days ranging between 2 to 4 hours of use (). The five apps most heavily utilized were messages, calendar, solitaire, phone, and internet browser, which together comprised 87% of his total app usage (). He accessed his text messages ~ 84 times/day and spent more time on messages than any other application (averaging 1 hour and 11 minutes per day). Most text messages were to and from his mother, and he also received daily banking notifications, family “group” texts, and promotions from retailers. Throughout the day, A.V. used his calendar apps to receive notifications and alerts about upcoming events and commitments. A.V. uses notifications and reminders also at work to help with the timely completion of his assignments. It appears he also uses the calendar as a running diary to send notifications to his “future self” but we have not yet probed into the nature of these personal notes. Over the 100-day period, he accessed his calendar more than any other app (averaging 84 times/day). The game solitaire was the third most used app. He plays intermittently throughout the day (~5 times/day). Among the apps that he did not use, the most notable are e-mail and navigation maps, the latter possibly reflecting confidence in his navigation abilities, as we were able to ascertain by observing his driving behavior.
Figure 5. A.V.’s phone and app usage over a 100-day period. The number of times A.V. checked his phone and the daily length of time he used his phone over a 100-day period in figures 5a and 5b, respectively. Figure 5c shows the use of individual apps (in hours) during the same 100-day period. Only apps that were used for one hour or longer were included. A.V. used his phone an average of 3.18 hours/day (average U.S. user data ranges from 2.4 to 3.4 hours/day(Annie, Citation2019; Comscore, Citation2018; Kemp, Citation2020)) and he spent 69% of app usage time on the top 3 apps (average U.S. user percentage is 77%). A.V. played games on his phone for an average of 21 minutes/day (average U.S. user data is 23 minutes/day).
Discussion
Brain imaging and neuropsychological testing confirmed that A.V. sustained near-complete bilateral lesions of the MTL leading to severe anterograde amnesia. Observations of A.V.’s naturalistic behavior revealed a high level of real-world functioning that was unexpected based on the history of prior cases with extensive bilateral MTL lesions and comparably dense anterograde amnesia. The prospect for MTL-lesioned patients like A.V. and the others in , is that of a gravely impoverished experience of life and very limited autonomy. A review of published case descriptions, corroborated by additional information obtained from researchers and close family members, would indicate that none of the other amnesic patients with comparable bilateral MTL lesions were able to live alone, like A.V. does without constant supervision. These other amnesic patients were severely limited in their daily activities; they did not work and could not maintain any regular occupations after their brain injury (Damasio et al., Citation1985; Feinstein et al., Citation2010; Insausti et al., Citation2013; Schapiro et al., Citation2014; Wilson et al., Citation2008). We were also able to ascertain that none of the HSE patients featured in used digital tools to help manage their daily life or to receive aid from their caregivers (Wilson & Wearing, Citation1995).
Thus, it is possible to draw a clear demarcation line between A.V. and the other patients in terms of real-world functioning and degree of autonomy. Yet, shows that A.V.’s anterograde memory deficit is more severe. Furthermore, access to qualified caregiving, family support, and self-motivation does not seem to differentiate A.V. from the other cases in and cannot satisfactorily account for the distinction in performance. The distinguishing element that emerged from the comparative review of the cases in is A.V.’s fluency with digital technology that can be ascribed to his young age, predisposition, and preserved cognitive toolkit. Before the brain injury, A.V. was a healthy, active teenager who displayed a keen interest in video gaming and an aptitude toward computer programming.
Although the technology has evolved significantly since the times before the onset of A.V.’s amnesia, his computer literacy spared him the need to learn new terminology and complex skills which are needed to utilize personal computers (Glisky et al., Citation1986). Moreover, thanks to his preserved procedural memory, and excellent visuospatial skills and reasoning, he can easily navigate modern smartphone operation. Although previous studies suggests that electronic memory aids are less effective in individuals with severe memory impairment (Stapleton et al., Citation2007), it has been possible to train amnesic patients on the use of commercially-available smartphones based on preserved implicit memory (Svoboda & Richards, Citation2009). In A.V.’s case, no formal training was necessary. To date, A.V. has switched over to four different smartphones (all Android systems), making it necessary for him to familiarize himself with new hardware and software. Preserved procedural memory () may explain A.V.’s ability to use new phones and to keep up with operating system updates and new apps. His high level of executive functioning (Table S1, Supplemental Materials) and superior visuospatial skills (Table S3, Supplemental Materials) may have further contributed to his acquisition of smartphone skills on new devices. Smartphone operating systems are designed to be intuitive if spatial relationships between graphic elements can be learned. A.V. clearly mastered these new skills and developed some degree of semantic knowledge in the process. This would indicate that as long as procedural memory supports the use of the device, declarative memory functions can be offloaded to the appropriate apps. In this context, it is worth noting that semantic learning is possible even in the presence of severe anterograde amnesia (Gordon Hayman et al., Citation1993; Rosenbaum et al., Citation2005).
The two apps that A.V. uses the most and that he accesses with the same frequency (~84 times per day) were “calendar” and “messages.” As expected, calendar entries were frequent, and they were paired with notifications. Notifications alert him in advance of a pending task, but also motivate him to start off on his activities and to keep up with his schedule. Text messaging is equally effective in this regard. This explains the high usage of the app and the fact that most texts are between Additionally, A.V. and his mother. Indeed, texting is their main channel of communication and the way she provides her oversight, remotely, on A.V.’s commitments, activities, and whereabouts, as well as pure companionship. A.V. and his mother regularly review his schedule together in case there is a need to resolve any potential conflict or errors in the calendar entries. As such, A.V.’s mother contributes to A.V.’s memory with her own knowledge and stored information and together they engage in recurrent processes that update his memory system (Wegner, Citation1986; Wegner et al., Citation1991).
A.V.’s app usage statistics revealed that he does not use navigation maps in any significant way. This was unexpected because A.V.’s MTL lesion encompasses neural structures known to play a critical role in navigation and the construction of spatial maps (Moser et al., Citation2008). Nevertheless, his lack of map utilization corroborates our observations of A.V.’s ability to drive alone without GPS-based navigation in real-world settings, which raises the question of how this function is supported in the absence of the MTL? Patients with only partial damage to the hippocampus can retain some degree of spatial memory (Bohbot & Corkin, Citation2007; Maguire et al., Citation2006), but A.V.’s MRI shows near-complete destruction of the hippocampus and parahippocampal cortex. With this lesion, it is unclear whether A.V.’s brain can still build new cognitive maps of the environment, or whether his navigation relies on a chain of responses to sequential stimuli (i.e., familiar landmarks) that guide him to his destination (O’keefe & Nadel, Citation1978; West et al., Citation2023). This strategy is consistent with neuropsychological evidence of A.V.’s superior visuospatial skills but tracing these skills to specific factors in A.V.’s pre-morbid history is challenging. We examined his school records and could not find any reference to a specific predisposition or training before the injury that could explain these skills. At the same time, we obtained a list of video games that A.V. played most often, namely “Megaman,” “Final Fantasy,” “Zelda,” “Metroid,” and “Twisted Metal”. Out of these titles, “Final Fantasy,” “Zelda” and “Twisted Metal” are role play games that entail navigation through different, often complex, maps and using visual clues to unlock features or new environments. This background is an additional element that sets A.V. apart from the other amnesic patients listed in .
Navigation is an aspect of A.V.’s adaptation in which neither the phone, nor A.V.’s mother, seem to play any significant role. Further studies under controlled experimental conditions (Aghajan et al., Citation2017; Bohbot et al., Citation2017; Maguire et al., Citation2006; Rivest et al., Citation2018) will be needed in order to resolve the question and contextualize our field observations with respect to the existing scientific literature.
The fact that A.V.’s retrograde memory is largely preserved (see below), may also play a role in A.V.’s navigation abilities. He moved three times since he became amnesic, which means he had to learn new routes each time that he moved. Notably, most of his travel is local and typically within the area where he lived before his brain injury. A.V. is not significantly impaired in remembering facts and events that happened before the injury, making him an outlier among other MTL patients. H.M., who also became amnesic when he was relatively young (age 27), had a temporally graded retrograde amnesia for the 11 years prior to the MTL surgery (Corkin, Citation1984). The other amnesic cases with HSE etiology and bilateral MTL lesions all exhibited varying degrees of retrograde amnesia, some spanning several decades (e.g., patient B (Damasio et al., Citation1985). and patient E.P. (Stefanacci et al., Citation2000)) and others showing a temporally-graded retrograde memory impairment similar to H.M. (e.g., Roger (Feinstein et al., Citation2010)). It is difficult to determine which factors contributed to the relative preservation of A.V.'s retrograde memory. Previous studies suggest that the extent of damage to the lateral temporal neocortex and temporal white matter (Insausti et al., Citation2013) may account for individual patterns of retrograde memory loss that are observed in different patients. A.V.’s brain injury occurred relatively early in life, and it created an uneven lesion with sparing of his left lateral temporal cortex, factors which may have contributed to the relative preservation of remote, pre-HSE memories.
Another unanticipated finding in the analysis of A.V.’s smartphone data pertains to camera and photo apps, which rank visibly low in the scale of the most used apps (). Our expectation was that, as an amnesic, he would rely on the camera for storing new autobiographical memories. When we first met A.V., we observed him taking pictures and recording short videos with a digital pocket camera, a habit he may have acquired before smartphones were equipped with high-quality lenses or sophisticated imaging software. Subsequently, we learned that he uses pocket cameras only when he travels and on special occasions, which explains why he had a pocket camera when he visited the laboratory in San Diego. In his day-to-day life, A.V. does not appear to be motivated to chronicle his experiences visually, perhaps because he leads a highly ordered life with predictable routines. He has nevertheless collected many photos and videos that he regularly backs up on digital storage that make up for the lack of biological autobiographical memories post-HSE. It would be interesting to evaluate A.V.’s use and the eventual benefits of more recent and highly targeted rehabilitation applications that developed from the early “lifelogging” concept and implementations (Bell & Gemmell, Citation2009; Doherty et al., Citation2012; Heersmink, Citation2017; Martin et al., Citation2022).
A.V.’s performance on neuropsychological tests, combined with field observations in his natural environment and the analysis of his smartphone usage reveal a coping strategy that leverages surviving neural structures combined with digital services, such as online calendars, notifications, and text messaging. Observations and data from our study corroborates the utility of portable digital devices and similar technology as cognitive support for people with memory impairment that is associated with aging (Benge et al., Citation2018), traumatic brain injury (Evald, Citation2015; Ferguson et al., Citation2015; Rispoli et al., Citation2014), and progressive neurological disorders like Alzheimer’s disease (Armstrong et al., Citation2010). A.V. demonstrates how smartphone use can have tangible effects on cognition in everyday life (Martin et al., Citation2022; Wilmer et al., Citation2017).
In conclusion, patient A.V. sustained extensive bilateral MTL lesions that led to one of the most severe forms of anterograde amnesia on record. He was nonetheless able to overcome the consequences of his brain injury to such a degree that with his mother’s help and oversight he eventually managed to live in his own house, to drive alone navigating without the aid of maps, and to work multiple jobs that require different skills and levels of responsibilities. In all respects, A.V. accomplished a level of autonomy that other amnesic patients with equivalent MTL damage never achieved. Our study indicates that A.V.’s success depends on multiple factors that include the knowledgeable and unrelenting caregiving by his mother, highly-preserved intellectual functioning, intact procedural memory, and on the discriminatory use of his smartphone which is supported by a rich pre-morbid technical skillset. As such, the case of patient A.V. is significant not only for advancing memory research, but also for the opportunity to investigate the powerful interplay between digital technology and human cognition in health and disease.
Author contributions
J.A., R.K., L.H-A., J.S.F. contributed to the study design and reviewed the manuscript. J.A., R.K. and J.S.F wrote the main manuscript text. J.A. prepared all the figures. J.A. acquired, analyzed, and interpreted neuroimaging data. R.K., L.H-A., J.S.F. administrated and provided interpretation of neuropsychological testing.
Supplemental Material
Download MS Word (376.4 KB)Acknowledgments
We are deeply indebted to A.V. for making this research study possible. We also thank A.V.’s mother for checking the factual accuracy of this manuscript with respect to A.V.’s behavior and case history. Her active participation greatly improved our understanding of A.V.’s condition and the factors that contributed to his remarkable adaptation.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/13803395.2023.2254911
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Aghajan, Z. M., Schuette, P., Fields, T. A., Tran, M. E., Siddiqui, S. M., Hasulak, N. R., & Stern, J. (2017). Theta oscillations in the human medial temporal lobe during real-world ambulatory movement. Current Biology, 27(24), 3743–3751. https://doi.org/10.1016/j.cub.2017.10.062
- Annese, J., Schenker-Ahmed, N. M., Bartsch, H., Maechler, P., Sheh, C., Thomas, N., Frosch, M. P., & Corkin, S. (2014). Postmortem examination of patient H.M.’s brain based on histological sectioning and digital 3D reconstruction. Nature Communications, 5(1), 3122. https://doi.org/10.1038/ncomms4122
- Annie, A. (2019). State of mobile report https://www.appannie.com/en/insights/market-data/the-state-of-mobile-2019/
- Armstrong, N., Nugent, C., Moore, G., & Finlay, D. (2010). Using smartphones to address the needs of persons with Alzheimer’s disease. annals of telecommunications-annales des télécommunications, 65(9–10), 485–495. https://doi.org/10.1007/s12243-010-0165-3
- Baddeley, A. (2012). Working memory: Theories, models, and controversies. Annual Review of Psychology, 63(1), 1–29. https://doi.org/10.1146/annurev-psych-120710-100422
- Bell, C. G., & Gemmell, J. (2009). Total Recall: How the E-memory Revolution Will Change Everything. United States: Dutton.
- Benge, J. F., Dinh, K. L., Logue, E., Phenis, R., Dasse, M. N., & Scullin, M. K. (2018). The smartphone in the memory clinic: A study of patient and care partner’s utilisation habits. Neuropsychological Rehabilitation, 30(1), 1–15. https://doi.org/10.1080/09602011.2018.1459307
- Bohbot, V. D., Copara, M. S., Gotman, J., & Ekstrom, A. D. (2017). Low-frequency theta oscillations in the human hippocampus during real-world and virtual navigation. Nature Communications, 8(1), 14415. https://doi.org/10.1038/ncomms14415
- Bohbot, V. D., & Corkin, S. (2007). Posterior parahippocampal place learning in HM. Hippocampus, 17(9), 863–872. https://doi.org/10.1002/hipo.20313
- Comscore. (2018). Global digital future in focus. Retrieved from https://www.comscore.com/Insights/Presentations-and-Whitepapers/2018/Global-Digital-Future-in-Focus-2018
- Corkin, S. (1984). Lasting consequences of bilateral medial temporal lobectomy: Clinical course and experimental findings in H.M. Seminars in Neurology, 4(2), 249–259. https://doi.org/10.1055/s-2008-1041556
- Corkin, S. (2002). What’s new with the amnesic patient H.M? Nature Reviews Neuroscience, 3(2), 153. https://doi.org/10.1038/nrn726
- Damasio, A., Damasio, H., & Tranel, D. (2013). Persistence of feelings and sentience after bilateral damage of the insula. Cerebral Cortex (New York, N.Y. : 1991), 23(4), 833–846. https://doi.org/10.1093/cercor/bhs077
- Damasio, A. R., Eslinger, P. J., Damasio, H., Van Hoesen, G. W., & Cornell, S. (1985). Multimodal amnesic syndrome following bilateral temporal and basal forebrain damage. Archives of Neurology, 42(3), 252–259. https://doi.org/10.1001/archneur.1985.04060030070012
- Damasio, A., & Van Hoesen, G. (1985). The limbic system and the localisation of herpes simplex encephalitis. Journal of Neurology, Neurosurgery & Psychiatry, 48(4), 297–301. https://doi.org/10.1136/jnnp.48.4.297
- DiPaola, M., Caltagirone, C., Fadda, L., Sabatini, U., Serra, L., & Carlesimo, G. A. (2008). Hippocampal atrophy is the critical brain change in patients with hypoxic amnesia. Hippocampus, 18(7), 719–728. https://doi.org/10.1002/hipo.20432
- Dittrich, L. (2017). Patient H.M.: A story of memory, madness, and family secrets. Random House Trade Paperbacks.
- Doherty, A. R., Pauly-Takacs, K., Caprani, N., Gurrin, C., Moulin, C. J., O’Connor, N. E., & Smeaton, A. F. (2012). Experiences of aiding autobiographical memory using the SenseCam. Human–Computer Interaction, 27(1–2), 151–174. https://doi.org/10.1080/07370024.2012.656050
- Duff, M. C., Wszalek, T., Tranel, D., & Cohen, N. J. (2008). Successful life outcome and management of real-world memory demands despite profound anterograde amnesia. Journal of Clinical and Experimental Neuropsychology, 30(8), 931–945. https://doi.org/10.1080/13803390801894681
- Evald, L. (2015). Prospective memory rehabilitation using smartphones in patients with TBI: What do participants report? Neuropsychological Rehabilitation, 25(2), 283–297. https://doi.org/10.1080/09602011.2014.970557
- Feinstein, J. S., Rudrauf, D., Khalsa, S. S., Cassell, M. D., Bruss, J., Grabowski, T. J., & Tranel, D. (2010). Bilateral limbic system destruction in man. Journal of Clinical and Experimental Neuropsychology, 32(1), 88–106. https://doi.org/10.1080/13803390903066873
- Ferguson, S., Friedland, D., & Woodberry, E. (2015). Smartphone technology: Gentle reminders of everyday tasks for those with prospective memory difficulties post-brain injury. Brain Injury, 29(5), 583–591. https://doi.org/10.3109/02699052.2014.1002109
- Glisky, E. L., Schacter, D. L., & Tulving, E. (1986). Computer learning by memory-impaired patients: Acquisition and retention of complex knowledge. Neuropsychologia, 24(3), 313–328. https://doi.org/10.1016/0028-3932(86)90017-5
- Gordon Hayman, C. A., Macdonald, C. A., & Tulving, E. (1993). The role of repetition and associative interference in new semantic learning in amnesia: A case Experiment. Journal of Cognitive Neuroscience, 5(4), 375–389. https://doi.org/10.1162/jocn.1993.5.4.375
- Heersmink, R. (2017). Distributed selves: Personal identity and extended memory systems. Synthese, 194(8), 3135–3151. https://doi.org/10.1007/s11229-016-1102-4
- Hierons, R., Janota, I., & Corsellis, J. (1978). The late effects of necrotizing encephalitis of the temporal lobes and limbic areas: A clinico-pathological study of 10 cases. Psychological Medicine, 8(1), 21–42. https://doi.org/10.1017/S0033291700006607
- Insausti, R., Annese, J., Amaral, D. G., & Squire, L. R. (2013). Human amnesia and the medial temporal lobe illuminated by neuropsychological and neurohistological findings for patient EP. Proceedings of the National Academy of Sciences, 110(21), E1953–E1962. https://doi.org/10.1073/pnas.1306244110
- Johnson, V. E., Stewart, W., & Smith, D. H. (2013). Axonal pathology in traumatic brain injury. Experimental Neurology, 246, 35–43. https://doi.org/10.1016/j.expneurol.2012.01.013
- Kapur, N., Barker, S., Burrows, E., Ellison, D., Brice, J., Illis, L., & Loates, M. (1994). Herpes simplex encephalitis: Long term magnetic resonance imaging and neuropsychological profile. Journal of Neurology, Neurosurgery & Psychiatry, 57(11), 1334–1342. https://doi.org/10.1136/jnnp.57.11.1334
- Kemp, S. (2020). Digital 2020: Global digital overview. https://datareportal.com/reports/digital-2020-global-digital-overview
- Maguire, E. A., Nannery, R., & Spiers, H. J. (2006). Navigation around London by a taxi driver with bilateral hippocampal lesions. Brain A Journal of Neurology, 129(11), 2894–2907. https://doi.org/10.1093/brain/awl286
- Martin, C. B., Hong, B., Newsome, R. N., Savel, K., Meade, M. E., Xia, A., & Barense, M. D. (2022). A smartphone intervention that enhances real-world memory and promotes differentiation of hippocampal activity in older adults. Proceedings of the National Academy of Sciences, 119(51), e2214285119. https://doi.org/10.1073/pnas.2214285119
- Milner, B. (1962). Les troubles de la memoire accompagnant des lesions hippocampiques bilaterales. In P. Passouant (Ed.), Physiologie de l’Hippocampe (Vol. 390, pp. 257–272). Centre National de la Recherche.
- Milner, B. (1965). Memory disturbance after bilateral hippocampal lesions. Vanostrand.
- Moser, E. I., Kropff, E., & Moser, M.-B. (2008). Place cells, grid cells, and the brain’s spatial representation system. Annual Review of Neuroscience, 31(1), 69–89. https://doi.org/10.1146/annurev.neuro.31.061307.090723
- O’keefe, J., & Nadel, L. (1978). The hippocampus as a cognitive map. Clarendon Press.
- Rispoli, M., Machalicek, W., & Lang, R. (2014). Assistive technology for people with acquired brain injury. In Assistive technologies for people with diverse abilities (pp. 21–52). Springer New York. https://doi.org/10.1007/978-1-4899-8029-8_2
- Rivest, J., Svoboda, E., McCarthy, J., & Moscovitch, M. (2018). A case study of topographical disorientation: Behavioural intervention for achieving independent navigation. Neuropsychological Rehabilitation, 28(5), 797–817. https://doi.org/10.1080/09602011.2016.1160833
- Rosenbaum, R. S., Kohler, S., Schacter, D. L., Moscovitch, M., Westmacott, R., Black, S. E., Gao, F., & Tulving, E. (2005). The case of K.C.: Contributions of a memory-impaired person to memory theory. Neuropsychologia, 43(7), 989–1021. https://doi.org/10.1016/j.neuropsychologia.2004.10.007
- Schapiro, A. C., Gregory, E., Landau, B., McCloskey, M., & Turk-Browne, N. B. (2014). The necessity of the medial temporal lobe for statistical learning. Journal of Cognitive Neuroscience, 26(8), 1736–1747. https://doi.org/10.1162/jocn_a_00578
- Scoville, W. B., & Milner, B. (1957). Loss of recent memory after bilateral hippocampal lesions. Journal of Neurology, Neurosurgery, and Psychiatry, 20(1), 11. https://doi.org/10.1136/jnnp.20.1.11
- Shaw, C.-M., & Alvord, J. E. (1997). Neuropathology of the limbic system. Neuroimaging Clinics of North America, 7(1), 101–142.
- Sköldenberg, B., Alestig, K., Burman, L., Forkman, A., Lövgren, K., Norrby, R., Stiernstedt, G., Forsgren, M., Bergstrom, T., & Dahlqvist, E. (1984). Acyclovir versus vidarabine in herpes simplex encephalitis: Randomised multicentre study in consecutive Swedish patients. The Lancet, 324(8405), 707–711. https://doi.org/10.1016/S0140-6736(84)92623-0
- Squire, L. R. (2009). The legacy of patient H.M. for neuroscience. Neuron, 61(1), 6–9. https://doi.org/10.1016/j.neuron.2008.12.023
- Stapleton, S., Adams, M., & Atterton, L. (2007). A mobile phone as a memory aid for individuals with traumatic brain injury: A preliminary investigation. Brain Injury, 21(4), 401–411. https://doi.org/10.1080/02699050701252030
- Stefanacci, L., Buffalo, E. A., Schmolck, H., & Squire, L. R. (2000). Profound amnesia after damage to the medial temporal lobe: A neuroanatomical and neuropsychological profile of patient EP. Journal of Neuroscience, 20(18), 7024–7036. https://doi.org/10.1523/JNEUROSCI.20-18-07024.2000
- Svoboda, E., & Richards, B. (2009). Compensating for anterograde amnesia: A new training method that capitalizes on emerging smartphone technologies. Journal of the International Neuropsychological Society, 15(4), 629–638. https://doi.org/10.1017/S1355617709090791
- Talairach, J., & Tournoux, P. (1988). Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system: An approach to cerebral imaging (1st ed.). Thieme.
- Tranel, D., Damasio, H., & Damasio, A. R. (2000). Amnesia caused by herpes simplex encephalitis, infarctions in basal forebrain, and anoxia/ischemia (Vol. 2, 2nd ed.). Elsevier.
- Tranel, D., & Jones, R. D. (2006). Knowing “what” and knowing “when”. Journal of Clinical and Experimental Neuropsychology, 28(1), 43–66. https://doi.org/10.1080/13803390490919344
- Tyler, K. L. (2004). Herpes simplex virus infections of the central nervous system: Encephalitis and meningitis, including Mollaret’s. Herpes Cambridge, 11, 57A–64A.
- Volpe, B. T., & Hirst, W. (1983). The Characterization of an amnesic Syndrome following Hypoxic Ischemic injury. Archives of Neurology, 40(7), 436–440. https://doi.org/10.1001/archneur.1983.04050070066017
- Warren, D. E., Duff, M. C., Magnotta, V., Capizzano, A. A., Cassell, M. D., & Tranel, D. (2012). Long-term neuropsychological, neuroanatomical, and life outcome in hippocampal amnesia. The Clinical Neuropsychologist, 26(2), 335–369. https://doi.org/10.1080/13854046.2012.655781
- Wegner, D. M. (1986). Transactive memory: A contemporary analysis of the group mind. In B. Mullen & G. R. Goethals (Eds.), Theories of group behavior (pp. 185–208). Springer-Verlag. https://doi.org/10.1007/978-1-4612-4634-3_9
- Wegner, D. M., Erber, R., & Raymond, P. (1991). Transactive memory in close relationships. Journal of Personality and Social Psychology, 61(6), 923–929. https://doi.org/10.1037/0022-3514.61.6.923
- West, G. L., Patai, Z. E., Coutrot, A., Hornberger, M., Bohbot, V. D., & Spiers, H. J. (2023). Landmark-dependent navigation strategy declines across the human life-span: Evidence from over 37,000 participants. Journal of Cognitive Neuroscience, 35(3), 452–467. https://doi.org/10.1162/jocn_a_01956
- Whitley, R. J., Alford, C. A., Hirsch, M. S., Schooley, R. T., Luby, J. P., Aoki, F. Y., & Group*, N. C. A. S. (1986). Vidarabine versus acyclovir therapy in herpes simplex encephalitis. The New England Journal of Medicine, 314(3), 144–149. https://doi.org/10.1056/NEJM198601163140303
- Wilmer, H. H., Sherman, L. E., & Chein, J. M. (2017). Smartphones and cognition: A review of research exploring the links between mobile technology habits and cognitive functioning. Frontiers in Psychology, 8, 605–605. https://doi.org/10.3389/fpsyg.2017.00605
- Wilson, B. A., Baddeley, A. D., & Kapur, N. (1995). Dense amnesia in a professional musician following herpes simplex virus encephalitis. Journal of Clinical and Experimental Neuropsychology, 17(5), 668–681. https://doi.org/10.1080/01688639508405157
- Wilson, B. A., Kopelman, M., & Kapur, N. (2008). Prominent and persistent loss of past awareness in amnesia: Delusion, impaired consciousness or coping strategy? Neuropsychological Rehabilitation, 18(5–6), 527–540. https://doi.org/10.1080/09602010802141889
- Wilson, B. A., & Wearing, D. (1995). Prisoner of consciousness: A state of just awakening following herpes simplex encephalitis. In R. Campbell, and E. A. Conway (Eds.), Broken memories: Case studies in memory impairment (pp. 14–30). Blackwell Publishing.
- Zola-Morgan, S., Squire, L. R., & Amaral, D. G. (1986). Human amnesia and the medial temporal region: Enduring memory impairment following a bilateral lesion limited to field CA1 of the hippocampus. Journal of Neuroscience, 6(10), 2950–2967. https://doi.org/10.1523/JNEUROSCI.06-10-02950.1986
