ABSTRACT
Introduction
22q11.2 deletion syndrome (22qDS) has been associated with varying levels of social impairments, and with atypical visual scanning of faces. The present study explored whether self-reported sensitivity to eye contact might be related to these phenomena.
Method
Individuals with confirmed 22qDS were interviewed about their experience and possible discomfort with eye contact. In cases where individuals expresesed discomfort, they were subsequently asked about coping mechanisms used to deal with this discomfort. In addition to self-reported eye contact discomfort, gaze to emotional faces was examined using eye tracking.
Results
In the subgroup of individuals who reported discomfort during eye contact, eye tracking results revealed a lower amount of gaze in the eyes of neutral faces, as well as the absence of the typical left visual field (LVF) bias, indicative of alterations in hemispheric lateralization. This subgroup also scored lower on a measure of everyday functioning.
Conclusions
Our results show that, by simply asking individuals with this social and communicative disorder about eye gaze discomfort, we may better understand the specific challenges that they experience. Moreover, information gained from such first-person reports together with eye-tracking measures further informs about the integrity of their face processing system, as well as about the nature and degree of impairment in this population.
Introduction
Chromosome 22q11.2 deletion syndrome (22q11.2.2.2DS or 22qDS for short) is caused by a deletion on the long arm of chromosome 22. It is a relatively common disorder, affecting 1 in 3000–6000 live births worldwide (Botto et al., Citation2003; McDonald McGinn et al., Citation2015). Phenotypically, individuals with 22qDS show a wide range of symptoms, with some individuals presenting no or relatively mild psychiatric impairments, while others exhibit comorbid neurodevelopmental disorders (Gillberg, Citation2010; Wallin et al., Citation2023), including an increased prevalence of autism spectrum disorders (ASD), and of psychotic disorders (e.g., schizophrenia; Cohen et al., Citation1999; Niklasson et al., Citation2001; Provenzani et al., Citation2022; Vorstman et al., Citation2006). The average IQ in this population is approx. 71, with a normal distribution around this mean (Niklasson & Gillberg, Citation2010). The genetic uniformity, along with diverse cognitive and psychiatric phenotypes of 22qDS, represents a unique model for neurodevelopmental research.
Like individuals with ASD, schizophrenia, social anxiety, and ADHD, individuals with 22qDS present impairments in social skills (Shashi et al., Citation2012). Clinical experience and research findings using eye tracking (Campbell et al., Citation2010; Glaser et al., Citation2010) further confirm that individuals with 22qDS display atypical scanning patterns of other people’s faces, marked – at a group level – by reduced visual exploration of the eye region, potentially resulting in negative effects on social functioning and emotional processing (Franchini et al., Citation2016). The presumed significance of appropriate visual exploration of faces and a specific preference for eye gaze is crucial in establishing a “typical” social brain, wherein lateralization is commonly considered a significant component (Grossmann & Johnson, Citation2007; Meng et al., Citation2012). At the same time, previous research has evidenced a substantial individual variability in eye gaze patterns in 22qDS; indeed, in the eye tracking group comparison by Campbell et al. (Citation2010), the largest standard deviation for eye gaze was seen in the 22qDS group. Moreover, current research has revealed that, when asked, people with intellectual and communication disorders often have the capacity to provide important first-hand information on the experienced quality of the eye gaze discomfort, as well as their coping mechanisms (Andréen et al., Citation2021; Ridley et al., Citation2022) This demonstrates that triangulating of methods, by combining qualitative and quantitative experimental information, can increase the validity and credibility of data collected and may assist in a better understanding of reduced eye gaze in 22qDS.
Here, we suggest that differences in self-reported eye contact discomfort may provide a way of parsing current neurodevelopmental and psychiatric heterogeneity among individuals sharing 22qDS, thus providing a potential marker for a particular set of behavioral/psychiatric impairments and vulnerabilities.
The aims of the present research were three-fold. First, we wanted to characterize individual differences in self-reported eye contact sensitivity in a sample of adults with 22qDS and to explore qualitative features of any discomfort, including contexts modulating its expression and the individuals’ ways of coping. This aim was addressed in a clinical interview about participants’ experience with eye contact.
Second, based on the findings from self-reported discomfort, we examined how differences in eye contact sensitivity relate to objective measures of gaze patterns during the presentation of images of faces. Initially, we explored the amount of spontaneous gaze to the eye region, hypothesizing that those who report discomfort would also gaze less at the eye region. Additionally, we explored the amount of gaze spontaneously allocated to the left versus the right side of the face, i.e., the so-called LVF bias – which reflects the brain’s right-sided specialization for faces (Gilbert & Bakan, Citation1973; Harrison & Strother, Citation2021). Eyecontact avoidance has indeed been hypothesized to have adverse effects on the development of neural circuits in face processing systems (Hadjikhani et al., Citation2018), and we therefore wanted to explore whether eye gaze discomfort would align with altered lateralization, which would bring new evidence about the underlying mechanisms of face processing development.
Finally, we considered how reported eye contact sensitivity related to neurodevelopmental and psychiatric outcomes, specifically (social) anxiety, frequency of ASD, ADHD, psychosis, everyday global functioning, and IQ.
Methods
Ethics statement
This study has received ethical approval by the Regional Ethical Approval Board in Gothenburg, Sweden (reference: 487–16). All participants received information about the study and provided written consent before the start of the study.
Participants
The participant sample consisted of 27 individuals (15 females) aged between 18 and 50 years (M = 28.43, SD = 7.23) who are part of an original cohort of 100 children identified to have 22qDS in 2007. These participants are also taking part in a longitudinal study aimed at examining the relationship between 22qDS and autism, ADHD, behavioral problems, and intellectual disability (Niklasson & Gillberg, Citation2010). Data presented here were collected by an experienced child and adolescent psychiatrist and a clinical professor of psychology as part of the follow-up study in adulthood (more details can be found in Wallin et al., Citation2023).
Instruments
Eye contact interview
As part of the clinical examination, a brief 3-item questionnaire was administered in interview to determine how the individual experiences eye contact with other people. If the participant answered “no” when asked “do you find it uncomfortable to maintain eye contact with others,” the interview stopped. If the participant answered “yes,” follow-up questions asked how they usually handle eye contact, with the possibility to choose some given examples (e.g., “I try to look at other parts of the face for example a person’s nose or their mouth”), or inviting them to describe their own strategy of handling eye contact. The participants were also asked whether or not eye contact felt particularly uncomfortable with some, or with all people, specifying whether discomfort was more pronounced (1) with family members, (2) with strangers, or (3) with people who show a lot of emotion (e.g., people who are crying or are visibly angry). The questionnaire was initially developed by the authors based on clinical and research experience with people on the autism spectrum and/or with (social) anxiety (Landberg, Åsberg Johnels, Galazka & Hadjikhani, submitted; Andréen et al., Citation2021) and was considered suitable for exploring the experience of eye contact in 22q11.2 deletion in view of the overlaps in functioning between these conditions.
Face stimuli for eye tracking
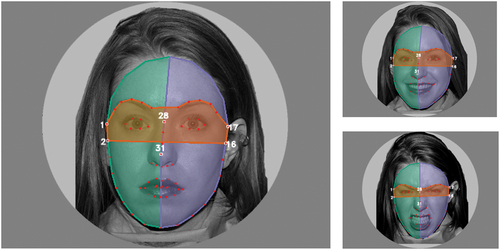
The face stimuli for eye tracking were 24 black-and-white circularly cropped photographs depicting angry, happy, and neutral faces (eight photographs each − four female and four male faces), created from the NimStim set of facial expressions (Tottenham et al., Citation2009), presented against a gray background. Images were shown in random order, with each stimulus shown once. Adhering to the NimStim dataset requirements, only pictures of model #1 are shown in the present article for illustration (see ).
Figure 1. An example of the presented stimuli of angry (lower right), happy (upper right), and neutral (lower right) facial expressions with automatic generation of areas of interest. For each presented image, the eye area (orange) and the left (green) and right (blue) facial areas were automatically determined. Facial landmarks used for defining AOI boundaries are displayed as red points.
Presence of autism, psychosis, social anxiety, and ADHD
Clinical evaluation and diagnostic decision according to DSM-5 criteria were performed by a clinically experienced child and adolescent psychiatrist in collaboration with an experienced clinical psychologist. The diagnostic decisions were based on all available data, including the Structured Clinical Interview for DSM-5 Axis I Disorders (M.I.N.I., Sheehan et al., Citation1998), which is a diagnostic interview for the main psychiatric disorders in accordance with DSM-5 and ICD-10.
Measures of FSIQ, social phobia, and GAF
To estimate Full Scale IQ (FSIQ), the Wechsler Adult Intelligence Scales (WAIS-IV, Wechsler, Citation2008), eight-subtest form was used (Bulzacka et al., Citation2016). Social phobia was examined using the Fear Survey Schedule-III (FSS-III; Wolpe & Lang, Citation1964) that was completed by 25 participants. This particular instrument covers five different fear factors in which the respondents are asked to perform intensity ratings on a 5-point-scale to determine the degree of their fear in the presence of 76 different situations/objects. The instrument validity for anxiety disorders has been demonstrated in a variety of contexts (Arrindell et al., Citation1984; Blankstein et al., Citation1993). Here, we considered the social subscale, which evaluates the average reaction on 16 items specific to social phobias. Finally, the Global Assessment of Functioning (GAF) scale (American Psychiatric Association, Citation2000) was used to quantify the severity of psychiatric symptoms and overall functioning of an individual within social, occupational, and psychological domains. This particular assessment is scored between 0 and 100 by a clinician, with high scores reflecting superior functioning and low scores denoting significant impairments. Scores between 60 and 51 indicate moderate difficulties in social functioning (e.g., few friends and conflicts with coworkers), while scores between 50 and 41 reflect more serious impairments in social, occupational, or school functioning (e.g., no friends and inability to maintain employment).
Procedure
Participants’ eye movements were recorded using Tobii T120 tracker at 120 Hz. Eye tracking acquisition was conducted in a silent testing room devoid of natural light, where participants sat approximately 60 cm away from the computer screen. Following the standard 9-point calibration, participants were instructed to look freely at the images presented on the screen. Each of the 24 stimuli images was presented once in random order for 6 s, followed by a 15 s fixation cross. Eye tracking data were exported from iMotions version 6.4 and analyzed with Python 3.8.5 scripts developed for this project. Statistical analyses and visualizations were performed in R version 4.1 with ggstatsplot package (Patil, Citation2021) and SPSS version 28. Finally, due to the small sample size and presence of possible outliers, nonparametric tests are reported throughout.
Automatic generation of areas of interest, classification of gaze, and data reduction
To account for a slight variation of presented images (different actors, slight head tilting, change within the facial features due to expressed emotions), an automatic generation of areas of interest (AOIs) for classification of gaze was used. Using a custom Python script each image was processed as follows: first, a rectangle enclosing the face was approximated by a convolutional neural network, followed by the detection of 68 facial landmarks using an ensemble of regression trees (Kazemi & Sullivan, Citation2014), which was trained on the 300-W dataset (Sagonas et al., Citation2016) as implemented in Dlib (King, Citation2009).
Three areas of interest were defined as polygons determined by the locations of the landmarks. First, the eye area (see the red area in ) included the eyebrow landmarks and the two upper cheek landmarks (see numbers 1, 2, 16, and 17 in ). To include the forehead, a half-ellipse was estimated. The major axis reached between the top cheek landmarks (see numbers 1 and 17 in ) on each side. The upper half of the ellipse’s minor axis was two times the length of the nose bridge (see numbers 28 to 31 in ). The facial area was then split using the face’s left and right sides (see green [left] and blue [right] areas in ).
To classify if the gaze coordinates were within the specified AOIs, a point-in-polygon problem was set up and solved by using the ray tracing method ensuring higher precision of areas than classical rectangular ones. Missing data, including eye blinks, were classified as outside the screen. All AOIs, of the 24 presented images, were visualized using OpenCV (Bradski, Citation2000) and were visually inspected. The percentage of gaze within each area was calculated as a proportion of total data points whose coordinates are within the specified area divided by the total number of data points during the entire stimulus presentation averaged for each participant. The code used in our analysis is available on GitHub at the URL https://github.com/thoraxmax/automaticAOIs.git.
From the calculated proportions, trials with less than 25% of recorded gaze on the screen were removed from further analysis (11.15%). Following this exclusion, on average, participants looked at the screen 84.53% of the time (range: 25.38–100%) and the amount of gaze on the screen did not differ between the groups based on their reported sensitivity to eye contact (Mann–Whitney U = 118, p = .18).
Results
How many individuals with 22q11 report discomfort with direct eye contact and how do they deal with it?
Following the clinical interview on eye gaze discomfort, 27 participants were subdivided into two groups. Little less than half of the participants (12/27) reported problems with eye contact, while 15 reported no discomfort during eye contact. For 9 of the 12 participants who reported discomfort with eye contact, the clinician confirmed their report with her own clinical impression of reduced eye contact, describing it as “fleeing,” “evasive,” and characterized as “scanning rather than fixating.” For the other three individuals who reported discomfort, reduced eye contact was not obvious to the clinician.
Of the 12 individuals who expressed discomfort with eye contact, one did not comment on strategies. Eleven individuals reported employing one or more strategies to cope, usually forcing themselves to make eye contact despite discomfort (4/11), or avoiding looking at the face altogether by looking elsewhere (e.g., the floor; 5/11) or using both strategies (2/11). Three out of 11 stated that the discomfort was experienced when interacting with everyone, 5/11 reported that discomfort was felt most often when interacting with people they did not know, and 3/11 reported discomfort with strangers and people who are expressing intense emotions such as anger or when crying.
How does reported eye contact discomfort relate to measured eye gazing patterns to face stimuli during eye tracking?
In terms of the measured amount of eye gaze during the eye tracking assessment, group differences were observed mainly for stimuli with a neutral expression, with those who reported discomfort looking significantly less at the eye region of the pictures (see ). Gaze behaviors for the other emotional expressions were not statistically different between groups, although numerically the values trended in the same direction as for neutral faces. presents all variables of interest, including demographics, in the two subgroups.
Table 1. Demographic, clinical, and eye gaze characteristics of the participant sample.
LVF bias
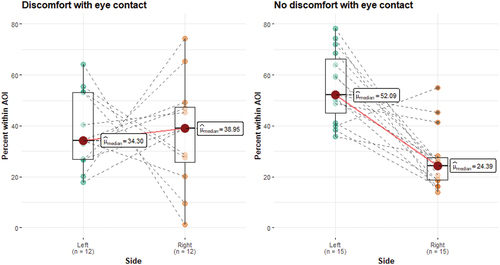
In terms of LVF bias, a Wilcoxon signed-rank test of the percentage of gaze on the left versus the right side of the presented face showed that in the group who reported no discomfort with eye contact, there was clear evidence of the typical LVF bias, W = 116.0, p < .001, rrb = 0.93, CI95% (0.80, 0.98) meaning that they looked considerably more to the left side of the face stimuli (from the viewer's perspective). The same pattern was, however, not present in the group who reported having discomfort during eye contact. In this group, there was no bias in looking at either side of the face, W = 40.0, p = .97, rrb = 0.03, CI95% (−0.55, 0.58). See . Finally, it is important to mention handedness in this group. Confirmed hand dominance was available for only 17 participants of which all of them were right-handed. Of these, five expressed discomfort with eye contact, of which two individuals showed evidence of LVF bias. Of the 12 right-handed participants who expressed no problem with eye contact, 8 showed evidence of LVF bias.
Figure 2. The percentage of gaze within the left- and the right- half of the face (from the observer’s perspective) in the group of individuals with 22q11.2 deletion based on self-reported discomfort with eye contact.
How does reported eye gaze discomfort relate to neurodevelopmental and everyday functioning?
Finally, we considered how reported eye contact sensitivity related to neurodevelopmental and everyday functioning (). Those participants who reported discomfort with eye contact and those who reported no discomfort with eye contact did not differ on IQ, with both subgroups scoring characteristically low ([Ms = 73], Mann–Whitney U = 97.5, p = .719). Similarly, the two subgroups did not differ on the social subscale of the Fear Survey Schedule-III (Mann–Whitney U = 46.0, p = .095). Significant group differences, however, were evident when considering the level of everyday functioning on the GAF (p = .032), with those reporting difficulties with eye contact scoring lower in this regard.
In terms of comorbid diagnoses, for the participants who reported discomfort with eye contact, four were also diagnosed with autism, two with psychosis, eight social anxiety disorder, and four met diagnostic criteria for ADHD. Of those who reported no discomfort with eye contact, four were diagnosed with autism, four with psychosis, 14 social anxiety disorder, and six with ADHD. Due to the low sample size, we refrained from performing formal statistical analysis, but descriptive patterns suggest no unique diagnostic patterns based on eye contact sensitivity.
Discussion
The current study explored the nature and correlates of self-reported eye gaze discomfort in young adults with 22qDS. We found that almost half of the interviewed individuals reported feeling discomfort when making eye contact with others. Further qualifying their discomfort, these individuals were able to recognize their use of specific strategies in coping with it and, in this context, acknowledged that the discomfort pertained mostly to those with whom they were not familiar. We also found that the subjective level of discomfort was related to objective measures of gaze, with a comparatively lower percentage of looking at the eyes, at least when observing faces whose facial expression is neutral, i.e., more ambiguous faces (Mobach et al., Citation2022; Nudelman et al., Citation2022; Schurgin et al., Citation2014; Tottenham et al., Citation2013). By pairing subjective descriptions with objective measures of eye contact sensitivity, the present study moves beyond one quantitative facet of eye contact, and considers the quality of eye contact, which indeed appears to be an informative aspect among various neurodivergent groups (Ridley et al., Citation2022). This also lends further support to the viability of self-reported assessments within clinical populations, also in those with social, intellectual, and communication disorders (Ridley et al., Citation2022; Shipman et al., Citation2011).
In addition to gaining novel insights into how people with 22qDS experience eye gaze, the present findings revealed that these experiences are reflected in other face scanning patterns. Specifically, individuals who reported discomfort with eye contact did not exhibit the expected LVF bias. LVF bias relates to the brain’s right-sided lateralization for face processing. Given this, the present findings lend support for an association between eye gaze discomfort and weakened lateralization of the face processing system. While previous studies confirm atypical visual scanning patterns in individuals with 22qDS (Campbell et al., Citation2010; Franchini et al., Citation2016; McCabe et al., Citation2011), to our knowledge, this is the first report confirming atypical lateralization which relates to eye gaze sensitivity.
Of course, important work remains to be done in order to establish causality and developmental mechanisms underlying the observed associations in the current study. Given recent demonstrations of alterations in the LVF bias in other clinical groups, including dyslexia (Åsberg Johnels et al., Citation2022), social anxiety (Löwenberg et al., Citation2020) autism (Masulli et al., Citation2022), and depression (Masulli et al., Citation2022), it is of particular importance for future work to explore the underlying mechanisms of the association between eye gaze discomfort and weakened LVF bias. Interestingly, previous findings as well as theoretical considerations on face perception, suggest that appropriate attention to faces and eyes, especially during early development, promotes neural face-processing specialization, which may further improve social functioning (Johnson, Citation2000; Klin, Citation2008; Nelson, Citation2002; Schultz, Citation2005; Vacas et al., Citation2022). In addition, an idea that has been proposed within research on autism is that over-sensitivity to faces in general and eye contact specifically, may have cascading consequences, since it might result in lowered face time, atypical brain organization (Hadjikhani et al., Citation2018) and lower social and emotional functioning – a pattern that seems consistent with the observations in the present study of adults with 22qDS as well.
While novel in several regards, some important limitations to this study need to be noted. The first limitation concerns the relatively small sample size. Acknowledging this, we utilized non-parametric tests when examining gaze differences between the two subgroups. Although the sample size in our study was comparable to several other studies with the same population examining social processing (Campbell et al., Citation2010; Leleu et al., Citation2016; McCabe et al., Citation2011), the low statistical power increases the likelihood of type 2 error and underscores the importance of replication in larger samples, and preferably, by independent research groups. Second, although the present study took steps to ensure high precision in examining gaze data as well as subdivision according to individual reports, future research could benefit from an even more multidimensional analysis of eye gaze discomfort, including the use of more detailed interview analysis on the experience with eye contact, data on physiological (e.g., stress) reactivity, video analysis of real-life face-to-face interaction and/or proxy reports of eye gaze avoidance (e.g., through parental retrospective interviews). Finally, including other measures of lateralization would further probe to what extent our observations are specific to faces or more general abilities (e.g., language and hand dominance).
Despite these limitations, our novel results suggest that by simply asking individuals about eye gaze discomfort, we can not only gain insight into the nature and coping strategies of the individual, but that such information can also help us identify a subgroup of people with 22qDS who show distinct alterations in face processing lateralization, and who present more difficulties in everyday life.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- American Psychiatric Association. (2000). The diagnostic and statistic manual of mental disorders (4th ed., text rev.).
- Andréen, L., Galazka, M., Hadjikhani, N., Jeuris, S., Masulli, P., & Johnels, J. Å. (2021). Developing tolerance to eye contact in autism: A feasibility study with adults using behavioral, interview, and psychophysiological data. Psychology of Language & Communication, 25(1), 240–263. https://doi.org/10.2478/plc-2021-0011
- Arrindell, W. A., Emmelkamp, P. M., & van der Ende, J. (1984). Phobic dimensions: I. Reliability and generalizability across samples, gender and nations: The fear survey schedule (FSS-III) and the fear questionnaire (FQ). Advances in Behaviour Research and Therapy, 6(4), 207–253. https://doi.org/10.1016/0146-6402(84)90001-8
- Blankstein, K. R., Flett, G. L., Hewitt, P. L., & Eng, A. (1993). Dimensions of perfectionism and irrational fears: An examination with the fear survey schedule. Personality & Individual Differences, 15(3), 323–328. https://doi.org/10.1016/0191-8869(93)90223-P
- Botto, L. D., May, K., Fernhoff, P. M., Correa, A., Coleman, K., Rasmussen, S. A., Merritt, R. K., O'Leary, L. A., Wong, L., Elixson, E. M., Mahle, W. T., & Campbell, R. M. (2003). A population-based study of the 22q11.2 deletion: Phenotype, incidence, and contribution to major birth defects in the population. Pediatrics, 112(1), 101–107. https://doi.org/10.1542/peds.112.1.101
- Bradski, G. (2000). The openCV library. Dobb’s Journal: Software Tools for the Professional Programmer, 25(11), 120–123. Retrieved from: elibrary.ru.
- Bulzacka, E., Meyers, J. E., Boyer, L., Le Gloahec, T., Fond, G., Szöke, A., L Eboyer, M., & Schürhoff, F. (2016). WAIS-IV seven-subtest short form: Validity and clinical use in schizophrenia. Archives of Clinical Neuropsychology, 31(8), 915–925. https://doi.org/10.1093/arclin/acw063
- Campbell, L., McCabe, K., Leadbeater, K., Schall, U., Loughland, C., & Rich, D. (2010). Visual scanning of faces in 22q11.2 deletion syndrome: Attention to the mouth or the eyes? Psychiatry Research, 177(1–2), 211–215. https://doi.org/10.1016/j.psychres.2009.06.007
- Cohen, E., Chow, E. W., Weksberg, R., & Bassett, A. S. (1999). Phenotype of adults with the 22q11 deletion syndrome: A review. American Journal of Medical Genetics, 86(4), 359–365. https://doi.org/10.1002/(SICI)1096-8628(19991008)86:4<359:AID-AJMG10>3.0.CO;2-V
- Franchini, M., Schaer, M., Glaser, B., Kott‐Radecka, M., Debanné, M., Schneider, M., Menghetti, S., Sander, D., & Eliez, S. (2016). Visual processing of emotional dynamic faces in 22q11. 2 deletion syndrome. Journal of Intellectual Disability Research, 60(4), 308–321. https://doi.org/10.1111/jir.12250
- Gilbert, C., & Bakan, P. (1973). Visual asymmetry in perception of faces. Neuropsychologia, 11(3), 355–362. https://doi.org/10.1016/0028-3932(73)90049-3
- Gillberg, C. (2010). The ESSENCE in child psychiatry: Early symptomatic syndromes eliciting neurodevelopmental clinical examinations. Research in Developmental Disabilities, 31(6), 1543–1551. https://doi.org/10.1016/j.ridd.2010.06.002
- Glaser, B., Debbane, M., Ottet, M. C., Vuilleumier, P., Zesiger, P., Antonarakis, S. E., & Eliez, S. (2010). Eye gaze during face processing in children and adolescents with 22q11.2 deletion syndrome. Journal of American Academy of Child Adolescent Psychiatry, 49(7), 665–674. https://doi.org/10.1016/j.jaac.2010.04.004
- Grossmann, T., & Johnson, M. H. (2007). The development of the social brain in human infancy. European Journal of Neuroscience, 25(4), 909–919. https://doi.org/10.1111/j.1460-9568.2007.05379.x
- Hadjikhani, N., Åsberg Johnels, J., Lassalle, A., Zürcher, N. R., Hippolyte, L., Gillberg, C., Lemonnier, E., & Ben-Ari, Y. (2018). Bumetanide for autism: More eye contact, less amygdala activation. Scientific Reports, 8(1), 1–8. https://doi.org/10.1038/s41598-018-21958-x
- Harrison, M. T., & Strother, L. (2021). Does face-selective cortex show a left visual field bias for centrally-viewed faces? Neuropsychologia, 159, 107956. https://doi.org/10.1016/j.neuropsychologia.2021.107956
- Johnels, J. Å., Galazka, M. A., Sundqvist, M., & Hadjikhani, N. (2022). Left visual field bias during face perception aligns with individual differences in reading skills and is absent in dyslexia. The British Journal of Educational Psychology. https://doi.org/10.1111/bjep.12559
- Johnson, M. H. (2000). Cortical specialization for higher cognitive functions: Beyond the maturational model. Brain and Cognition, 42(1), 124–127. https://doi.org/10.1006/brcg.1999.1180
- Kazemi, V., & Sullivan, J. (2014, June 24–27). One millisecond face alignment with an ensemble of regression trees. In Proceedings of the IEEE conference on computer vision and pattern recognition. Columbus, Ohio, USA, (pp. 1867–1874).
- King, D. E. (2009). Dlib-ml: A machine learning toolkit. The Journal of Machine Learning Research, 10, 1755–1758. https://dl.acm.org/doi/10.5555/1577069.1755843
- Klin, A. (2008). In the eye of the beholden: Tracking developmental psychopathology. Journal of the American Academy of Child & Adolescent Psychiatry, 47(4), 362–363. https://doi.org/10.1097/CHI.0b013e3181648dd1
- Leleu, A., Saucourt, G., Rigard, C., Chesnoy, G., Baudouin, J., Rossi, M., Edery, P., Franck, N., & Demily, C. (2016). Facial emotion perception by intensity in children and adolescents with 22q11.2 deletion syndrome. European Child & Adolescent Psychiatry, 25(2016), 297–310. https://doi.org/10.1007/s00787-015-0741-1
- Löwenberg, E. B., Aili, F., Serlachius, E., Högström, J., & Kleberg, J. L. (2020). Reduced left visual field bias for faces in adolescents with social anxiety disorder. Cognitive Neuropsychiatry, 25(6), 421–434. https://doi.org/10.1080/13546805.2020.1832456
- Masulli, P., Galazka, M., Eberhard, D., Johnels, J. Å., Gillberg, C., Billstedt, E., Hadjikhani, N., & Andersen, T. S. (2022). Data-driven analysis of gaze patterns in face perception: Methodological and clinical contributions. Cortex, 147, 9–23. https://doi.org/10.1016/j.cortex.2021.11.011
- McCabe, K., Rich, D., Loughland, C. M., Schall, U., & Campbell, L. E. (2011). Visual scanpath abnormalities in 22q11. 2 deletion syndrome: Is this a face specific deficit? Psychiatry Research, 189(2), 292–298. https://doi.org/10.1016/j.psychres.2011.06.012
- McDonald McGinn, D. M., Sullivan, K. E., Marino, B., Philip, N., Swillen, A., Vorstman, J. A., Zackai, E. H., Emanuel, B. S., Vermeesch, J. R., Morrow, B. E., Scambler, P. J., & Bassett, A. S. (2015). 22q11. 2 deletion syndrome. Nature Reviews Disease Primers, 1(1), 15071. https://doi.org/10.1038/nrdp.2015.71
- Meng, M., Cherian, T., Singal, G., & Sinha, P. (2012). Lateralization of face processing in the human brain. , 279, 2052–2061. https://doi.org/10.1098/rspb.2011.1784
- Mobach, L., Rinck, M., Becker, E. S., Carl, T., Klein, A. M., Rapee, R. M., & Hudson, J. L. (2022). Facing uncertainty: Interpretation of ambiguous emotional faces in childhood social anxiety disorder. Journal of Clinical Child & Adolescent Psychology, 51(6), 955–969. https://doi.org/10.1080/15374416.2022.2070850
- Niklasson, L., & Gillberg, C. (2010). The neuropsychology of 22q11 deletion syndrome. A neuropsychiatric study of 100 individuals. Research in Developmental Disabilities, 31(1), 185–194. https://doi.org/10.1016/j.ridd.2009.09.001
- Niklasson, L., Rasmussen, P., Oskarsdóttir, S., & Gillberg, C. (2001). Neuropsychiatric disorders in the 22q11 deletion syndrome. Genetics in Medicine: Official Journal of the American College of Medical Genetics, 3(1), 79–84. https://doi.org/10.1097/00125817-200101000-00017
- Nudelman, M. F., Portugal, L. C., Mocaiber, I., David, I. A., Rodolpho, B. S., Pereira, M. G., & Oliveira, L. D. (2022). Long-term influence of incidental emotions on the emotional judgment of neutral faces. Frontiers in Psychology, 12, 5881. https://doi.org/10.3389/fpsyg.2021.772916
- Patil, I. (2021). Visualizations with statistical details: The’ggstatsplot’ approach. Journal of Open Source Software, 6(61), 3167. https://doi.org/10.21105/joss.03167
- Provenzani, U., Damiani, S., Bersano, I., Singh, S., Moschillo, A., Accinni, T., Brondino, N., Oliver, D., & Fusar-Poli, P. (2022). Prevalence and incidence of psychotic disorders in 22q11.2 deletion syndrome: A meta-analysis. International Review of Psychiatry, 34(7–8), 1–13. https://doi.org/10.1080/09540261.2022.2123273
- Ridley, E., Arnott, B., Riby, D. M., Burt, D. M., Hanley, M., & Leekam, S. R. (2022). The quality of everyday eye contact in Williams syndrome: Insights from cross-syndrome comparisons. American Journal on Intellectual and Developmental Disabilities, 127(4), 293–312. https://doi.org/10.1352/1944-7558-127.4.293
- Sagonas, C., Antonakos, E., Tzimiropoulos, G., Zafeiriou, S., & Pantic, M. 2016. 300 faces in-the-wild challenge: Database and results. Image and Vision Computing, 47, 3–18. https://doi.org/10.1016/j.imavis.2016.01.002
- Schultz, R. T. (2005). Developmental deficits in social perception in autism: The role of the amygdala and fusiform face area. International Journal of Developmental Neuroscience, 23(2–3), 125–141. https://doi.org/10.1016/j.ijdevneu.2004.12.012
- Schurgin, M. W., Nelson, J., Iida, S., Ohira, H., Chiao, J. Y., & Franconeri, S. L. (2014). Eyemovements during emotion recognition in faces. Journal of Vision, 14(13), 14–14. https://doi.org/10.1167/14.13.14
- Shashi, V., Veerapandiyan, A., Schoch, K., Kwapil, T., Keshavan, M., Ip, E., & Hooper, S. (2012). Social skills and associated psychopathology in children with chromosome 22q11. 2 deletion syndrome: Implications for interventions. Journal of Intellectual Disability Research, 56(9), 865–878. https://doi.org/10.1111/j.1365-2788.2011.01477.x
- Sheehan, D. V., Lecrubier, Y., Sheehan, K. H., Amorim, P., Janavs, J., Weiller, E., & Dunbar, G. C. (1998). The Mini-International Neuropsychiatric interview (MINI): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry, 59(20), 22–33.
- Shipman, D. L., Sheldrick, R. C., & Perrin, E. C. (2011). Quality of life in adolescents with autism spectrum disorders: Reliability and validity of self-reports. Journal of Developmental & Behavioral PediatricsThe Mini-International Neuropsychiatric Interview, 32(2), 85–89. https://doi.org/10.1097/DBP.0b013e318203e558
- Tottenham, N., Phuong, J., Flannery, J., Gabard-Durnam, L., & Goff, B. (2013). A negativity bias for ambiguous facial-expression valence during childhood: Converging evidence from behavior and facial corrugator muscle responses. Emotion, 13(1), 92–103. https://doi.org/10.1037/a0029431
- Tottenham, N., Tanaka, J. W., Leon, A. C., McCarry, T., Nurse, M., Hare, T. A., Marcus, D., Westerlund, A., Casey, B., & Nelson, C. (2009). The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research, 168(3), 242–249. https://doi.org/10.1016/j.psychres.2008.05.006
- Vacas, J., Antolí, A., Sánchez-Raya, A., Pérez-Dueñas, C., & Cuadrado, F. (2022). Social attention and autism in early childhood: Evidence on behavioral markers based on visual scanning of emotional faces with eye-tracking methodology. Research in Autism Spectrum Disorders, 93, 101930. https://doi.org/10.1016/j.rasd.2022.101930
- Vorstman, J. A. S., Morcus, M. E. J., Duijff, S. N., Klaassen, P. W. J., Heineman de Boer, J. A., Beemer, F. A., van Engeland, H., Kahn, R. S., & van Engeland, H. (2006). The 22q11.2 deletion in children. The Journal of the American Academy of Child & Adolescent Psychiatry, 45(9), 1104–1113. https://doi.org/10.1097/01.chi.0000228131.56956.c1
- Wallin, L., Gillberg, C., Fernell, E., Gillberg, C., & Billstedt, E. (2023). Neurodevelopmental and other psychiatric disorders in 22q11. 2 deletionsyndrome from childhood to adult age: Prospective longitudinal study of 100 individuals. In American Journal of Medical genetics part C: Seminars in Medical genetics. John Wiley & Sons, Inc. https://doi.org/10.1002/ajmg.c.32052
- Wechsler, D. (2008). Wechsler adult intelligence scale–fourth edition (WAIS–IV). NCS Pearson.
- Wolpe, J., & Lang, P. J. (1964). A fear survey schedule for use in behaviour therapy. Behaviour Research and Therapy, 2(1), 27–30. https://doi.org/10.1016/0005-7967(64)90051-8
