ABSTRACT
Introduction
Cognitive activity questionnaires could provide insight into neurocognitive reserve. The Lifetime Cognitive Activities Questionnaire (LCAQ) assesses cognitive activities at four stages of life. The Modified Current Cognitive Activities Questionnaire (CCAQ) assesses current cognitive activities. We examined the construct validity, internal consistency, test-retest reliability, and stability of these questionnaires throughout the Brain in Motion (BIM) study and their relationship with cognitive performance.
Methods
The LCAQ, Montreal Cognitive Assessment (MoCA), and neuropsychological battery were administered at the initial pre-intervention and six-year follow-up. The CCAQ was administered at five timepoints. Construct validity of the CCAQ/LCAQ was assessed using proxies of cognitive engagement (educational attainment and the North American Adult Reading Test [NAART]). Cronbach alpha analysis determined internal consistency. LCAQ reliability was established by comparing the pre-intervention and six-year follow-up. CCAQ reliability was determined by comparing both pre-intervention assessments, correlations throughout BIM determined stability. A multiple linear regression investigated the associations between cognitive engagement and cognitive domains derived from a principal component analysis.
Results
MoCA scores at the initial pre-intervention (27.49 ± 1.46) and six-year follow up (26.53 ± 2.08). The LCAQ and CCAQ correlated with educational attainment and the NAART. The LCAQ (n = 266) produced an alpha of 0.90 (20 items). The CCAQ (n = 261) resulted in an alpha of 0.71 (25 items). LCAQ scores (n = 94) at the initial pre-intervention and six-year follow-up were correlated. CCAQ (n = 94) scores at the initial pre-intervention correlated with scores at all five other timepoints. The multiple linear regression revealed associations between the CCAQ and verbal memory/attention. The NAART was associated with processing speed, concept formation, and verbal memory/attention.
Conclusions
In the absence of cognitive decline, these questionnaires exhibit significant construct validity, internal consistency, test-retest reliability, and the CCAQ displayed stability. The NAART and CCAQ were associated with neuropsychological performance. Our findings support future use of these questionnaires and exemplify the neuroprotective role of cognitive engagement.
Introduction
Cognitive reserve, maintenance, and compensation
Cognitive reserve is defined as a cumulative improvement in neural resources that mitigates the effects of neural decline with increased age or age-related diseases (Cabeza et al., Citation2018). This benefit is due to an individual’s ability to make flexible and efficient use of the brain’s structural and functional resources during various tasks (Stern et al., Citation2019). The original concept of reserve is divided into two aspects, brain and cognitive reserve. Brain reserve refers to the anatomical traits of the brain, such as the number of neurons, synapses, and abundance of brain hardware that provides redundancy to cope with injury before cognitive function is impaired. Cognitive reserve refers to the development of strong, adaptable connections and associations between neuronal networks to actively mitigate the effects of disease or aging on cognitive function, through brain plasticity influenced by life experience and environmental factors (Stern et al., Citation2019). The cognitive reserve model has evolved into an umbrella term of neurocognitive reserve as brain and cognitive reserve are complex and not mutually exclusive. The neurocognitive model has three components: reserve, compensation, and maintenance. Reserve refers to the accumulation of resources over one’s lifetime, such that, they have a greater capacity before deficits arise. Compensation is the ability to mobilize these resources in response to cognitive demands, and maintenance refers to the preservation of these resources (Cabeza et al., Citation2018). Neurocognitive reserve has been assessed through proxy measures such as years of education and literacy attainment or premorbid intelligence quotient (IQ) through the North American Reading Test (NAART) and their impact on cognitive performance (Blair & Spreen, Citation1989; MacPherson et al., Citation2017; Soldan et al., Citation2020; Tucker & Stern, Citation2011). However, the validation of reserve using such proxies is unclear and may be due to multiple fluid factors for which convergent and discriminant validity are seldomly reported. Multiple proxy frameworks have been proposed encompassing executive function, processing resources, complex mental activity, and general intelligence, however, none of these models have been effectively characterized to represent the concept of neurocognitive reserve (Satz et al., Citation2011). Regardless, these proxy measures have independently demonstrated a relationship to buffer neurocognitive change despite pathological insult, representing the underlying concept of neurocognitive reserve, maintenance, and compensation. This substantiates the role that proxies of neurocognitive reserve play in resisting typical and pathological neurocognitive changes in older adults at risk for cognitive deficits (Hertzog et al., Citation2008). Individuals with a higher neurocognitive reserve measured through traditional proxies such as educational attainment, verbal IQ, occupational attainment, and participation in leisure activities defined as unsupervised activities of daily living and enjoyment that has a cognitively demanding component (functional resources), and brain reserve (structural resources), display better clinical cognitive outcomes when examined for any level of pathology (e.g., β-amyloid) (Tucker & Stern, Citation2014). The positive relationship between clinical outcomes and cognitive engagement, despite other factors, demonstrates that cognitive activity may play a role in building neurocognitive reserve over a person’s lifetime and be a sensitive predictor of clinical outcomes, irrespective of pathology (Eskes et al., Citation2010; Tucker & Stern, Citation2014). Neurocognitive reserve is thereby modifiable and dependent on environmental factors such as years of education (Tucker & Stern, Citation2014) and leisure cognitive activities performed during adulthood (Reed et al., Citation2011; Tyndall et al., Citation2018). Landau and colleagues reported that higher lifetime cognitive activity was significantly associated with lower cortical carbon 11–labeled Pittsburgh Compound B ([11C]PiB), used to image fibrillar forms of β-amyloid protein, an important pathophysiological hallmark of Alzheimer’s Disease. Specifically, when comparing current and prior cognitive activities, only prior activities had a significant association to [11C]PiB uptake. The lower levels of [11C]PiB in those who reported greater lifetime cognitive activity suggests that increased cognitive activity can play a role in reducing β-amyloid aggregation before Alzheimer’s or dementia onset (Landau et al., Citation2012). Friedland and colleagues assessed different non-occupational activities associated with intellectual, passive, and physical activities. Friedland found evidence for the protective benefit of intellectual or cognitive activities in preventing the development of Alzheimer’s disease (Friedland et al., Citation2001). The ability to modify neurocognitive reserve and maintenance through engagement in cognitive activities could be a potential strategy to resist preclinical signs of cognitive decline associated with aging and/or pathology as seen in Alzheimer’s disease and related dementias.
Aging, and neurocognitive performance
Typical age-related changes in cognition include a decline of fluid intelligence, as it becomes more difficult to process new information, problem solve, or manipulate one’s environment, and is associated with declines in executive function, processing speed, psychomotor abilities, and memory (Harada et al., Citation2013). In preclinical stages (Mild Cognitive Impairment, MCI) and further Alzheimer’s disease and related dementias, these typical neurocognitive changes are accelerated, particularly for episodic and verbal memory, executive functioning, visuospatial skills, attention, perceptual speed, and word finding ability (Bäckman et al., Citation2005; Linn et al., Citation1995; Small et al., Citation2000). Bäckman and colleagues highlighted that the global cognitive scores, measured through the Mini-Mental State Examination (MMSE), may be the most sensitive in detecting preclinical cognitive deficits with the largest effect size (Cohens d of 1.19) (Bäckman et al., Citation2005). In the present study, we use a similar screening tool, the Montreal Cognitive Assessment (MoCA) as a measurement of global cognition. This may reflect preclinical changes and is used in clinical practice to screen for MCI that may later progress into a dementia. The MoCA is a 30-point test that assesses short term memory recall, visuospatial abilities, executive function, fluency, attention, concentration, working memory, language, and place/time orientation. A cutoff score of 26, or a score of 25 or below, was determined to identify individuals who experienced MCI with 90% sensitivity and an 87% specificity (Nasreddine et al., Citation2005). Cutoff scores have also been established when correcting for age and education for the MoCA (Malek-Ahmadi et al., Citation2015). The MoCA is particularly sensitive to verbal memory and processing speed cognitive domains commonly impacted by aging, however, this results in the underrepresentation of other cognitive domains. The MoCA is perhaps more applicable to those with higher levels of education and lacks the ability to determine the severity and domain attribution of specific cognitive deficits, requiring a more comprehensive neuropsychological battery to confirm these results. However, the MoCA is an easily accessible, widely used tool with impressive sensitivity and specificity metrics to flag an individual for MCI or dementia. In healthy controls, there is the possibility of a ceiling effect, however, this effect is much less than with other screening tools like the MMSE (18.1 vs 71.4% respectively (Trzepacz et al., Citation2015)).
Measures of cognitive activity
A series of questionnaires have been developed to assess cognitive activities in both recent and historical contexts. Measuring neurocognitive reserve is a challenge, but systematic reviews indicate that lifetime experience questionnaires, such as the Lifetime of Experiences Questionnaire (LEQ) and Cognitive Reserve Scale (CRS), appear to be the most thorough instruments in assessing how environmental factors may impact neurocognitive reserve. These questionnaires measure nonspecific mental activities over an individual’s lifetime. The LEQ is lengthy and not appropriate for general use, whereas the CRS is shorter and could be used with a larger population. These tools are considered thorough, as they not only assess cognitive activity cross sectionally but also consider lifetime cognitive activities, extending beyond occupational or educational determinants of neurocognitive reserve. However, no singular tool has demonstrated proficiency in determining one’s neurocognitive reserve (Kartschmit et al., Citation2019; León et al., Citation2016; Valenzuela & Sachdev, Citation2007). In the present study, we examine the psychometric properties of the Lifetime Cognitive Activities Questionnaire (LCAQ) and the modified Current Cognitive Activities Questionnaire (CCAQ) over an extended interval, potentially identifying another valid and reliable set of tools to measure cognitive activity.
The CCAQ
The modified Current Cognitive Activities Questionnaire (CCAQ) (Salthouse et al., Citation2002; Wilson et al., Citation1999) assesses current engagement in cognitive activities with 25 questions, where responders rate the frequency of their engagement in a variety of cognitive activities using a 5-point Likert scale: (5) Every day or almost every day; 4) Several times a week; 3) Several times a month; 2) Several times a year; 1) Once a year or less. This questionnaire encapsulates daily cognitive activities, such as playing strategic games of checkers or chess or participating in musical or artistic activities (items can be found in supplementary table S4). The items on the CCAQ were chosen as they were rated as cognitively demanding tasks when participants were asked to rate the cognitive demands of a specific activity (Salthouse et al., Citation2002; Wilson et al., Citation1999). Individuals report their activities over the past week, month, or year, capturing type, frequency, duration, and intensity (Gill et al., Citation2015). Wilson et al. (Citation1999) determined the construct validity of the CCAQ where the CCAQ had positive correlations with educational attainment, and the association between involvement in cognitive activities and cognitive performance assessed by neuropsychological tests in the domains of memory (delayed recall), perceptual speed, and verbal fluency (Wilson et al., Citation1999). Wilson further examined associations with individuals’ scores on the MMSE (Folstein et al., Citation1975). This analysis revealed that individuals with greater frequency and intensity of cognitive activities also performed better on neuropsychological tests and the MMSE (Wilson et al., Citation1999). Eskes et al. (Citation2010) revealed that the number of cognitive activities reported on the CCAQ, but not their frequency, significantly predicted overall cognitive performance, attention, and executive function in a cohort of 42 healthy post-menopausal women (Eskes et al., Citation2010). This study highlighted that the diversity of cognitive activities may be more beneficial to maintain cognitive performance than the amount of time spent.
The LCAQ
The LCAQ originally developed by Wilson and colleagues, assessed cognitive activities over an individual’s lifetime, whereas most other assessments in the literature primarily focus on current cognitive activities. The primary aim of this questionnaire is to assess an individual’s cumulative experience with cognitively demanding activities across their lifetime (Wilson et al., Citation2003), at four specific stages of life: i) Childhood (age 6); ii) Teens (age 12); iii) Young Adult (age 18) and iv) Adulthood (age 40+). For each stage of life, a series of questions; 20 items in total, 3–6 questions for each stage, (items on the LCAQ can be found in supplementary table S5) assess each participant’s engagement in a variety of cognitive activities, and responders are required to rate the frequency of their engagement on a 5-point Likert scale: 5) Every day or almost every day; 4) Several times a week; 3) Several times a month; 2) Several times a year; 1) Once a year or less. Various studies assessed the importance of engaging in cognitive activities at specific periods through self-reported scales (Friedland et al., Citation2001) and showed that the frequency of cognitive activity in childhood (Ceci & Williams, Citation1997), middle age (Kohn & Schooler, Citation1978; Miller et al., Citation1985), and old age (Surber et al., Citation1984), is related to better cognitive function in that “intellectually engaging activities buffer against longitudinally measured cognitive decline” (Hultsch et al., Citation1999). A composite score computed for the LCAQ which was modestly associated with an individual’s level of education and representative of their cognitive abilities, irrespective of socioeconomic status. This provided an indication of construct validity using a traditional proxy of neurocognitive reserve (Wilson et al., Citation2003). In the original paper, Wilson and colleagues assessed the psychometric properties of the LCAQ by measuring its’ internal consistency and test-retest reliability with a 4-week interval between assessments. The authors reported that there were no statistically significant differences between the first and second administration of the test (t21 = 1.59 p = 0.13), and a Cronbach alpha of 0.88, indicating a high degree of consistency between items in the questionnaire (Wilson et al., Citation2003). However, this study was limited by a small sample of 23 participants, and a brief (four-week) interval between the initial assessment and the retest. Carryover effects could have potentially confounded the results as the follow-up assessment was scored based on recall of the answers that were provided at the previous administration. A longer time interval between test conditions could yield different findings.
Purpose
Both the LCAQ and CCAQ have been used in the Brain in Motion (BIM) study, a longitudinal single cohort study, assessing the effectiveness of a six-month aerobic exercise intervention on cerebrovascular regulation and cognitive functioning in sedentary, middle age and older adults (Guadagni et al., Citation2020; Tyndall et al., Citation2013, Citation2018). These tools incorporated in the study design of the BIM study assess self-reported changes in cognitive activity engagement, at different stages in a participant’s life, and have been proposed to be linked to favorable cognitive outcomes for older adults. The LCAQ and first 10 items of the CCAQ included common activities and those with minimal physical, social, or economic barriers such as reading, playing strategic games like checkers, and visiting a library. This consideration ensures that both the CCAQ and LCAQ are applicable to persons with diverse cultural and socioeconomic backgrounds (Wilson et al., Citation1999). However, the psychometric properties and association with cognitive performance have not been established (Jackson et al., Citation2016; Tyndall et al., Citation2013, Citation2018). In this present study, we aim to examine the psychometric properties of both the LCAQ and CCAQ questionnaires and investigate whether engagement in cognitive activities is an effective strategy to maintain cognitive health as people age. Specifically, we will test construct validity of the LCAQ and CCAQ as measurements of cognitive activity using proxy measures such as years of education and the NAART, establish their inter-item reliability (internal consistency), test-retest reliability/dependability, and the stability of responses on the CCAQ when administered multiple times over the course of the BIM study. We also explore the association of lifetime and current cognitive activity on various domains of cognitive performance. All of these analyses were stratified by sex to identify any sex differences regarding cognitive activity and by extension the protective effects of neurocognitive reserve on neuropsychological performance. To our knowledge, this is the first study to evaluate the construct validity, reliability/dependability, and stability of these questionnaires with such a considerable period of time of up to six years between test administrations. This research stresses the importance of lifelong cognitive engagement and its’ contribution to neurocognitive reserve reflected by improved performance on the neuropsychological battery.
Methods
Participants
The BIM study consisted of overall healthy, but underactive, English speaking, middle-aged and older adults between 55 to 80 years of age. Participants were recruited through media, posters, and advertisements. To be enrolled in the study, individuals had to be able to ambulate up and down at least 20 stairs and have a body mass index (BMI) less than 35 kg/m2 to ensure no comorbidities associated with obesity (Tyndall et al., Citation2013). Participants were evaluated and cleared to participate in physical activity by their family physician, and indicated no history of cardiovascular disease, obstructive airway disease, neurological disorders, no recent trauma or surgery within the past six months, and no smoking in the last 12 months (Tyndall et al., Citation2013). They also completed the Montreal Cognitive Assessment (MoCA) as an index of global cognition and to screen for cognitive dysfunction. An absence of cognitive impairment was required to participate, as indicated by a score ≥ 24 on the MoCA (Tyndall et al., Citation2013).
Neuropsychological battery
The BIM study included a battery of neuropsychological tests that examine global cognition with the MoCA. Executive function was assessed through the Delis-Kaplan Executive Function System (D-KEFS) color-word (CW), card sorting (CS), and verbal fluency tasks. Written and oral processing speed was assessed with the Symbol Digit Modalities Test. Declarative memory was derived from the Buschke Selective Reminding Test (SRT), and figurative memory through The Medical College of Georgia Complex Figure Test (MCG). Finally, attention was assessed using the Auditory Consonant Trigram Test (ACT). A more detailed description of the tests included in the neuropsychological battery can be found in the supplementary material (Buschke, Citation1973; Delis et al., Citation2001; Ingram et al., Citation1997 ; Shura et al., Citation2016 ; Smith, Citation1979 ; Strauss et al., Citation2006).
Study phases
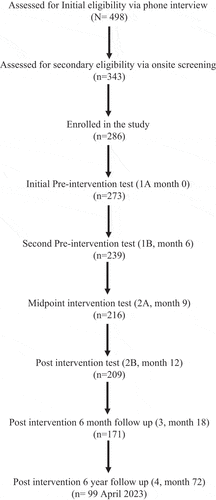
Participants were assessed at an initial pre-intervention baseline and instructed not to alter their exercise routine for the following six months, when a second pre-intervention baseline assessment was conducted. After the second pre-intervention assessment, participants started the six-month aerobic exercise intervention and were assessed halfway through and immediately after the intervention (midpoint and post-intervention, respectively) (Guadagni et al., Citation2020). Participants returned for follow up assessments 6 months after the completion of the exercise program, and again, six years later (Guadagni et al., Citation2020). Please refer to for the BIM study flow chart. The assessment of lifetime and current cognitive activities is the specific focus in this study, but various other measurements of physiological and cognitive parameters were recorded in the BIM study and results are published elsewhere (Gill et al., Citation2015; Guadagni et al., Citation2020; Hall et al., Citation2019; Rytz et al., Citation2020; Tyndall et al., Citation2013, Citation2018).
LCAQ administration
Participants completed the LCAQ at the initial pre-intervention assessment and at the six-year follow up (). This questionnaire was administered verbally by experienced researchers, and each question was read in its’ entirety, together with all five available answers: “every day or almost every day, several times a week, several times a month, several times a year, once a year or less”. They were required to rate their engagement in common cognitive activities at age 6 (three items), age 12 (six items), age 18 (six items) and age 40 (five items). A full list of the items on the LCAQ can be found in supplementary table S5. An additional evaluation of current activities was assessed with five questions (Wilson et al., Citation2003). Each specific stage of life was assessed at the initial pre-intervention assessment and at the six-year follow up, where each item was averaged, with a final score out of five, at each phase, indicating the frequency of engagement in cognitive activities at various times in their lives.
CCAQ administration
The CCAQ was assessed at five different time points; initial and secondary pre-intervention assessments, post-intervention, and at both the six month and six-year follow up appointments (). The purpose was to assess current cognitive activity levels, as revealed by questionnaire responses. The CCAQ was administered and each participant’s engagement in various activities over the last six months was rated on a scale from 1 to 5. The scores estimated the frequency of engagement in each activity listed in the CCAQ. Subjects were allowed to comment about various other activities that are not listed, such as teaching classes, writing, baking, singing, video editing, and social activities that they deemed to be cognitively demanding (Eskes et al., Citation2010). However, this information was not included in the present analysis. All items of the CCAQ were averaged, resulting in a total score out of 5 to indicate the relative frequency that individuals participate in cognitive activities. Missing items from the LCAQ and CCAQ were not included in the calculation of mean scores or data analysis. A full list of the items on the CCAQ can be found in supplementary table S4.
Construct Validity
Construct validity of the LCAQ and CCAQ was determined via Pearson correlation analysis with both the NAART and years of education at the initial pre-intervention timepoint.
Reliability and dependability
Cronbach’s alpha was used to determine the internal (inter-item) consistency of responses on both the LCAQ and CCAQ questionnaires. This analysis is generally used to assess the internal consistency of questionnaire items and the results were corroborated using McDonald’s omega, see supplementary material (Hayes & Coutts, Citation2020; Heo et al., Citation2015; Kalkbrenner, Citation2023; Putnick & Bornstein, Citation2016). Test-retest reliability for the LCAQ was assessed by a Pearson correlation analysis between the initial pre-intervention score, and those collected at the six-year follow up, investigating whether retrospective scoring of one’s lifetime activities is reproducible regardless of the time of administration. Reliability of the CCAQ was assessed using Pearson’s correlation at the two pre-intervention timepoints ().
Stability
The stability of current cognitive activities in older adults was examined through Pearson’s correlational analysis and a repeated measure analysis of variance (RM-ANOVA) between the initial pre-intervention, post intervention, 6-month follow up and six-year follow up. Correlational and RM-ANOVA analysis determined if there was a significant variation in CCAQ scores across phases of the BIM study. This investigated whether cognitive engagement, as measured by the CCAQ remains stable, despite various life events, the passage of time (~6 years) and participation in the exercise intervention.
Neuropsychological performance
Principal component analysis (PCA) was used to reduce the number of neuropsychological outcomes analyzed (Guadagni et al., Citation2020). The neuropsychological battery test scores at the initial pre-intervention included 18 different tasks. We used the Kaiser criterion (eigenvalue greater than 1), and a Monte Carlo simulation to determine the number of components to be retained (Dziuban & Shirkey, Citation1974; Longman et al., Citation1989). Factors were subjected to varimax rotation, and the rotated components were assigned a cognitive domain description based on the tests that they incorporated. Scores on each cognitive domain were used as dependent variables in separate multiple linear regression models. Scores on the LCAQ, CCAQ and traditional markers of neurocognitive reserve (NAART and years of education) were the independent variables in separate models. These models evaluated the relationship between cognitive activity and neuropsychological performance at the pre-intervention assessment.
An exploratory correlational analysis was also performed to assess the association between scores on both the LCAQ and CCAQ, and MoCA scores at the initial pre-intervention and the six-year follow up assessment to evaluate longitudinal changes in global cognition.
Correlational analyses were conducted in the whole sample first, and then stratified analyses (Pearson correlations and independent sample t-tests) were used to assess sex differences. Sex was included in the multiple regression models as an independent predictor.
The data was analyzed with the software IBM SPSS Statistics for Mac, version 26.0 (IBM, Armonk, NY, USA). All the analyses were two-tailed, underwent a Bonferroni correction for multiple comparisons, effect size was estimated using Cohen’s d and statistical significance was set at p < 0.05.
Results
The participants’ demographic characteristics for the initial pre-intervention assessment (n = 273) and for the six-year follow up (n = 94) are reported in . The two assessments were separated by a mean (SD) of 2330 (458) days or 6.39 (1.26) years.
Table 1. Participant’s’ characteristics.
Construct validity of cognitive activity
At the pre-intervention timepoint, bivariate Pearson correlations were performed between proxy measures of cognitive engagement. Years of education and the NAART were highly correlated r260 = 0.43, p < 0.001, 95% CI [0.32, 0.52]. Correlations between educational attainment and the LCAQ were significant for all stages of life: Childhood, r265 = 0.18, p = 0.003, 95% CI [0.06, 0.30]; Teen, r265 = 0.31, p < 0.001, 95% CI [0.20, 0.41]; Young adult, r265 = 0.37, p < 0.001, 95% CI [0.26, 0.47]; Adult, r265 = 0.24, p < 0.001, 95% CI [0.12, 0.35]. Correlations between the CCAQ and educational attainment were also significant, r261 = 0.27, p < 0.001 (supplementary table S1).
When conducting sex specific analysis, correlations for males and females between both the CCAQ and LCAQ with the NAART were significant (p < 0.05), (supplementary table S2).
Sex specific analysis exhibited significant correlations between education and the LCAQ with childhood r143 = 0.19 p = 0.023, teenage r143 = 0.29 p < 0.001, and young adult stages of life r143 = 0.30 p < 0.001 for females, with an absence of significance for the adult stage of life r143 = 0.14 p = 0.11. Males had significant correlations between the LCAQ and years of education for each stage of life during the initial pre-intervention assessment all p < 0.05. Females and males had significant correlations between the CCAQ and years of education, all p < 0.05 (supplementary tables S1 and S2.)
LCAQ reliability
The Cronbach alpha calculation for the responses on the LCAQ was based on 266 participants that provided complete data at the initial pre-intervention assessment. The alpha for the internal consistency analysis was 0.895 for the 20-item LCAQ scale, inter-item correlations ranged from 0.06–0.72. When stratifying by sex, the Cronbach alpha analysis revealed results similar to the entire sample (LCAQ Cronbach alpha for males = 0.90, females = 0.90). These results were confirmed with McDonalds omega analysis (see supplementary material).
The Pearson correlation analysis revealed substantial test-retest reliability as correlations between corresponding LCAQ scores at different phases of life (Child, Teen, Young Adult, and Adult) when comparing the first pre-intervention assessment to scores at the 6 year follow up assessment: Childhood, r93 = 0.76, 95% CI [0.66,0.83]; Teen, r92 = 0.83, 95% CI [0.76,0.89]; Young adult, r93 = 0.73, 95% CI [0.61,0.81]; Adult, r93 = 0.75, 95% CI [0.65, 0.83], all p < 0.001 (). When stratifying by sex, the test-retest reliability results were similar to the entire sample ().
Table 2. Mean LCAQ scores at each stage of life, at the initial pre-intervention assessment and at the post-intervention 6-year follow up.
Table 3. Mean LCAQ scores stratified by sex at each stage of life, at the initial pre-intervention assessment (n = 123 male, n = 143 female) and at the post-intervention 6-year follow (n = 46 male, n = 48 female).
CCAQ reliability
The Cronbach alpha analysis for responses on the CCAQ was conducted on complete data of 261 participants at the initial assessment. The alpha was 0.71 for 25 items. Inter-item correlations ranged from −0.15 to 0.52. When stratifying by sex, the Cronbach alpha analysis showed results similar to the entire sample (CCAQ Cronbach alpha Males = 0.76, Females = 0.62). These results were confirmed with McDonalds omega analysis (see supplementary material).
The Pearson correlations between CCAQ scores collected at the two pre-intervention assessments 6 months apart indicated a strong correlation between the initial CCAQ scores and the second pre-intervention assessment, r226 = 0.81, 95% CI [0.75,0.85] p < 0.001 (). When stratifying by sex, the test-retest reliability results were similar to the entire sample ().
Table 4. Mean CCAQ scores at each phase of the Brain in Motion study.
Table 5. Mean CCAQ scores at each phase of the Brain in Motion study stratified by sex.
CCAQ stability
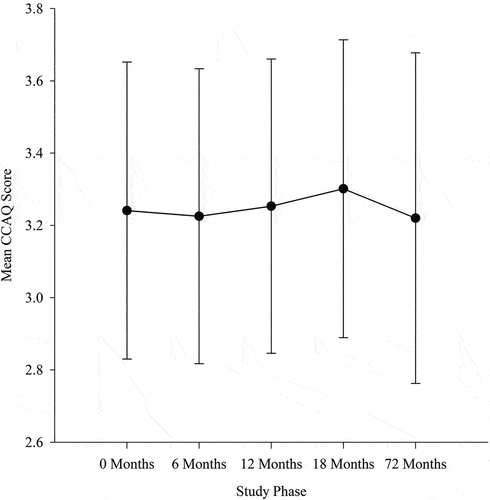
Pearson correlational analysis indicated a strong correlation between the initial CCAQ scores and the post-intervention r200 = 0.82, 95% CI [0.77,0.86], six-month follow up, r175 = 0.80, 95% CI [0.74,0.85], and six-year follow up, r92 = 0.73, 95% CI [0.62,0.81], all p < 0.001 (). The RM ANOVA on the CCAQ scores indicated no statistically significant differences in mean scores between phases, F4,324 = 2.369 p = 0.053, ηp2 = 0.028. Specific results from the RM ANOVA were as follows: initial and second pre-intervention stages M ± SD = 3.24 ± 0.41 and M ± SD = 3.23 ± 0.41, F1,81 = 0.276 p = 0.601, ηp2 = 0.003), post-intervention, (M ± SD = 3.25 ± 0.41 assessment F1,81 = 0.235 p = 0.629, ηp2 = 0. 003, 6 month follow up M ± SD = 3.30 ± 0.41, F1,81 = 4.596 p = 0.035, ηp2 = 0.054, six-year follow up M ± SD = 3.22 ± 0.46, F1,81 = 0.358 p = 0.551, ηp2 = 0.004 ().
Sex differences in responses on the CCAQ
An independent samples t-test on the CCAQ scores at different stages revealed significant sex differences. Females had higher scores at the initial pre-intervention timepoint (t259= −3.46, p < 0.001, d = −0.43; (males M ± SD = 3.18 ± 0.44, females M ± SD = 3.35 ± 0.35); at the second pre-intervention (t230= −3.43, p < 0.001, d = −0.45; males M ± SD = 3.18 ± 0.43, females M ± SD = 3.36 ± 0.37); at post-intervention (t204= −2.72, p < 0.05, d = −0.38; males M ± SD = 3.22 ± 0.44, females M ± SD = 3.37 ± 0.38); and at the 6-month follow up assessment (t179= −3.09, p < 0.05, d = −0.46; males M ± SD = 3.23 ± 0.45, females M ± SD = 3.42 ± 0.35). There were no statistically significant sex differences in CCAQ scores at the 6-year follow up ().
Cognitive activity and domain specific cognitive performance
Cognitive outcomes were appropriate for PCA dimensionality reduction and the Kaiser-Meyer-Olkin measure of sample adequacy in the PCA on different cognitive outcomes was 0.80, (above the recommended threshold of 0.6) and the Bartlett test of sphericity was significant (χ2 [153] = 2,895.99, p < 0.001). Results of the PCA and Monte Carlo simulation indicated that 4 components explained 64.87% of the variance in the dataset, while inclusion of 5 components explained 70.75% of the variability and still reduced the dimensionality of the cognitive dataset. Increasing the number of components from 4 to 5 allowed for increased interpretability of the data into the following domains: processing speed, concept formation, verbal memory/attention, fluency, and figural memory. Rotated component loading factors are reported in the supplementary material table S3.
Multiple linear regression models were used to assess if the traditional markers of neurocognitive reserve, as well as the CCAQ and the LCAQ (independent variables), can predict performance on domain specific cognitive performance (dependent variables). The full models for concept formation, verbal memory/attention, fluency, and the MoCA were significant (). The NAART was associated with processing speed (β = −0.22 t = −2.92 p = 0.004), concept formation (β = 0.24 t = 3.19 p = 0.002), and verbal memory/attention (β = 0.15 t = 2.10 p = 0.04) domains. Educational attainment and scores on the LCAQ stages of life were not associated with cognitive domains or the MoCA. The CCAQ was associated with the verbal memory/attention domain (β = 0.19 t = 2.33 p = 0.02). Sex was associated with verbal memory/attention (β = −0.21 t = −3.22 p = 0.001), and fluency (β = −0.24 t = −3.81 p < 0.001) domains, and the MoCA (β = −0.15 t = −2.33 p = 0.02) ().
Table 6. Model summary from the multiple linear regression analysis at the initial pre-intervention assessment.
Table 7. Coefficient summary from the multiple linear regression analysis at the initial pre-intervention assessment.
Cognitive activity and longitudinal global cognition (MoCA)
The exploratory analysis revealed significant correlations between the CCAQ and MoCA scores at the initial preintervention assessment (r260 = 0.15, p = 0.015). In contrast, the LCAQ scores at any stage of life, did not significantly correlate with the MoCA scores at the initial assessment (p > 0.05). The correlation between CCAQ and MoCA scores at the six-year follow-up were non-significant (p > 0.05). Similarly, no correlation was found between the LCAQ at any stage of life and MoCA scores at the six-year follow-up (p > 0.05). A paired t-test between MoCA scores at the initial pre-intervention (27.68 ± 1.36) and at the six-year follow-up (26.60 ± 2.06), indicated a significant difference in MoCA scores at pre-intervention and the six-year follow-up (t78 = 5.24, p < 0.001, d = 0.53).
When stratifying by sex, females showed significant correlations between the CCAQ and MoCA scores at the pre-intervention phase (r139 = 0.17, p = 0.05), there was no significant relationships between the LCAQ and MoCA performance and no relationships between either the CCAQ or LCAQ and the MoCA at the 6-year follow up. Males had no significant correlations between CCAQ/LCAQ and MoCA scores, at the initial pre-intervention or at the 6-year follow up.
Discussion
Construct validity
In this study, we assessed the psychometric properties of the Lifetime Cognitive Activity Questionnaire (LCAQ), and of the Modified Current Cogntive Activity Questionnaire (CCAQ) in the context of the Brain in Motion (BIM) study. Participants were assessed at multiple points with a considerable amount of time between assessments. Construct validity analysis from the Pearson correlations revealed significant relationships between both proxy measures of cognitive engagement and scores on the CCAQ and LCAQ at the pre-intervention assessment. The CCAQ demonstrated significant associations to neurocognitive performance. This analysis reveals that both the lifetime and current cognitive activities are associated with expected correlates of cognitive engagement, which may be contributing factors related to neurocognitive reserve. Intercorrelations between the CCAQ, LCAQ, educational attainment, the NAART, and neurocognitive performance demonstrates that these proxy measures are convergent, possibly cumulative and contribute to the underlying concept of neurocognitive reserve. Additionally, it demonstrates how neurocognitive reserve may be a product of a multidimensional concept of accumulated experiences, as suggested by Satz and colleagues (Satz et al., Citation2011). This shows that individuals with greater educational attainment and verbal intellectual abilities (traditional markers of neurocognitive reserve and crystalized abilities) are more likely to engage in intellectually stimulating activities in current times and throughout their lifetime. This has also been shown in the literature as individuals with more access and experience (educational attainment) will have greater performance on tests of crystalized intelligence, justifying our use of education and premorbid IQ as expected correlates of cognitive engagement (Ackerman, Citation2014). In comparison, those who do not perform well on a non-academic reading test or have limited educational attainment due to socioeconomic or other barriers in early life, may not engage in cognitive activities as readily and thereby are more at risk for cognitive deficit.
Reliability
The Cronbach alpha, for both the LCAQ and CCAQ, exceeded the generally accepted minimum value of 0.7 for a reliable scale, with values of 0.89 and 0.71, respectively. McDonalds omega analysis was utilized to ensure that the Cronbach alpha did not underestimate the internal consistency of the questionnaires. This analysis revealed similar relationships for the LCAQ and CCAQ, both exceeding the minimum value of 0.7, which indicates that they are both adequately internally reliable. The similarity between the Cronbach alpha and McDonalds omega indicates that the items on the LCAQ and CCAQ meet the tau-equivalence assumption, with the items on the questionnaires measuring the same latent variable on the same scale with equivalent precision. However, there may be differences in the amount of error produced by each item (Graham, Citation2006; Hayes & Coutts, Citation2020; Kalkbrenner, Citation2023; Putnick & Bornstein, Citation2016). The similarity in both omega and alpha analysis results confirms that the assumption of tau-equivalence is satisfied and indicates that each item on the CCAQ and LCAQ equally and precisely measures the underlying construct of current cognitive activity and lifetime cognitive activity respectively.
All items of the LCAQ were positively intercorrelated. Interestingly, both negative and positive item intercorrelations existed for the CCAQ. The unanimous positive intercorrelations for the LCAQ is consistent with our expectations as it is generally designed to measure cognitive activity focused on engagement in reading, writing and strategic games and the items for each age epoch were similar. In contrast, the CCAQ’s lower inter-item reliability could be due to the broad range of activities represented in the CCAQ, encompassing both cognitive and leisure activity which may require less cognitive demands (ex: activities of daily living) rather than exclusively investigating cognitively focused tasks. For example, we found negative correlations between reading the newspaper and writing in a journal, preparing meals, supervising other people, or participating in musical or artistic activities. The reduced inter-item reliability of the CCAQ could reflect a trade-off of what people can do in their spare time; for example, if a person is routinely reading the daily newspaper, they would not devote as much time to other activities. Diversity of cognitive engagement can also be measured with the CCAQ which may better reflect this trade off phenomenon.
The Pearson correlation analysis, assessing test-retest reliability of responses on the LCAQ over a six-year gap, demonstrated a very strong correlation between scores assessed at the initial pre-intervention phase and scores at the six-year follow-up, with correlation coefficients ranging from 0.81 to 0.85 at various phases of life. The strong correlation indicates that the LCAQ scores are reliable over time, producing consistent results, despite the six-year period between assessments. Such a result indicates that retrospective ratings of childhood and young adult activity are reliable, and do not merely reflect current perceptions or biases. CCAQ scores followed a similar trend, with strong correlations between scores at every phase of the BIM study, ranging from 0.79 to 0.84. Particularly, the correlation between the initial and second pre-intervention assessments demonstrates the test-retest reliability of the CCAQ r43 = 0.81, 95% CI [0.68,0.89]. The strong test-retest reliability of these scales will allow future studies to confidently use both the LCAQ and CCAQ scales as assessment tools to determine individuals’ historical and current engagement in cognitive activities. Similar results have been found by Landau and colleagues where the frequency of cognitively stimulating activities was measured, throughout an individual’s lifespan, using the LCAQ (Landau et al., Citation2012). The authors performed a test-retest reliability analysis with a 1-year gap between administrations on 41 cognitively normal adults and found a correlation of r = 0.81. They did not assess the inter-item reliability of the scale.
CCAQ stability
Strong correlations between responses on the CCAQ at each phase demonstrates that it is a stable and consistent measure of participants’ cognitive activity levels throughout the Brain in Motion study and did not decline despite a significant passage of time, life events, the time commitment associated with the aerobic exercise intervention and the participant’s increasing age (Tyndall et al., Citation2018). One might speculate that an educated population (15 ± 2.4 years of education) may be aware of the benefits of having the proper tools and training in early life and participating in cognitively demanding tasks throughout their lifetime (Ackerman, Citation2014). A closer look at the repeated assessments with the CCAQ (), demonstrates a significant increase in the mean CCAQ scores at the 6-month follow-up, but similar scores at all other intervention stages. There was a slight increase after the intervention, a greater increase at 6 months following the intervention, and then a decline at the 6-year follow-up where scores on the CCAQ were similar to those collected at the pre-intervention phases. The immediate post-intervention changes are encouraging and may indicate the importance of an exercise intervention to counterbalance the natural decline in cognitive activity associated with advanced age, and possibly promote cognitive engagement. The level of cognitive activity may increase after the exercise intervention due to a possible preference of physical activity combined with a cognitively stimulating component of strategy. Individuals may choose group activities vs isolated tasks to enhance the cognitive load to overlap with their physical activity. Engagement in physical activity could induce an increase in the CCAQ response as physical activities are often associated with socialization or engagement in strategic games. Overall, the strong correlations and similarities across phases emphasize the stability of the CCAQ despite the passage of time, life events, the aerobic exercise intervention time commitment, and increasing age. It also suggests that physical activity may play a role in increasing cognitive engagement in older adults.
Cross-sectional investigation of cognitive engagement and neurocognitive performance
To investigate the relationship between lifetime and current cognitive activities with cognitive performance, a PCA analysis was performed to make domain-specific cognitive scores and a multiple linear regression model was created. The NAART and education were included in these models to assess the relationship between traditional markers of neurocognitive reserve on cognitive performance. We found that the NAART, but not education, had significant contributions to performance on the various cognitive domains assessed. IQ may be a better indicator of a traditional marker of neurocognitive reserve than educational attainment. The NAART had significant contributions to predictions of processing speed and verbal memory/attention cognitive domains. This is expected, as premorbid verbal intelligence is not confined to childhood, teenage or young adult years like educational attainment may be, and could have a more profound impact on current cognitive function. However, the positive correlation between the NAART and educational attainment indicate that verbal intelligence is likely dependent on early life influences such as education. These models found that current cognitive activities, assessed with the CCAQ, had significant contributions to the prediction of verbal memory/attention cognitive domain, however, the LCAQ had no significant relationships. This suggests that current cognitive activities (CCAQ), and verbal intellectual ability (e.g., reading skills) assessed with the NAART may be a more importnat marker of an individuals cognitive performance compared to lifetime cognitive activity engagement and educational attainment. Previous investigations have focused on the association between crystalized abilities and cognitive engagement as a result of early life social stratification, socioeconomic status and a culmination of skills that attributed to greater educational attainment (Ackerman, Citation2014). The results of the current study confirm these findings and extend it to include verbal memory and attention domains, which encompass fluid abilities such as sustained attention which often becomes impaired as people age.
Sex played a significant role in predicting verbal memory/attention, fluency, and performance on the MoCA, suggesting that there could be fundamental sex or gender differences in how neurocognitive reserve affects domain performance. The sex-specific results suggested that females had greater scores in the verbal memory/attention, and fluency, domains of cognitive function and in the MoCA as compared to males. This could be due to the fact that females also had greater scores on the CCAQ compared to males throughout the BIM study, suggesting that females are engaging in more cognitively stimulating activities than males. This may confer a neurocognitive benefit, resulting in better performance on the various domain-specific tests, however, the interaction between sex and cognitive engagement is beyond the scope of this manuscript.
Cognitive or mental activities have been previously correlated with both fluid and crystalized tasks and overall cognitive abilities (Ackerman, Citation2014; Christensen & Mackinnon, Citation1993). There is substantial evidence, from cross-sectional and longitudinal studies, that patterns and amount of cognitive activities performed in midlife are associated with improved cognitive function and a decreased risk of dementia as one ages. Using the LCAQ and CCAQ, researchers have demonstrated that cognitively inactive individuals were 2.6 times more likely to develop Alzheimer’s disease than those who were engaged in more cognitive activities (Wilson et al., Citation2007). Thus, it is important to assess the cumulative effects of engagement in cognitive activities over a lifetime and not limit the assessment to the current time. Previous work has highlighted that the amount and diversity of cognitive activities has implications on improving the working memory of older adults (Luo et al., Citation2023), and overall cognitive performance, attention, executive function (Eskes et al., Citation2010), and perceptual speed (Bielak et al., Citation2014). All of these studies align with the results of our study and justify the measurement of cognitive engagement as a potential buffer for future age-related and pathological neurocognitive deficits that may be particularly salient in this study population, as all BIM study participants were cognitively intact. However, preclinical deficits may appear, and future cognitive performance may be impacted by the lifetime accumulation of neurocognitive reserve via cognitive activity engagement. Therefore, the measurement of both lifetime and current cognitive activity may serve as a prognostic and predictive measurement of future neurocognitive deficits in older adults.
Overall, it appears that current cognitive activities and verbal intelligence may have a more important role in determining cognitive performance than other associated indices of neurocognitive reserve. This highlights the interplay and modifiability of neurocognitive reserve, compensation, and maintenance through engagement in intellectually stimulating activities and how this may contribute to improved cognitive performance. However, it is important to consider that there may be other physiological factors at play, outpacing the protective effects of neurocognitive reserve in maintaining cognitive performance in older adults.
Longitudinal cognitive engagement and neurocognitive performance
Examining scores on both the LCAQ and CCAQ, longitudinally, allowed investigation into their association with MoCA scores, at the initial assessment and at the six-year follow-up. CCAQ scores correlated with MoCA scores at the initial assessment, but not at the six-year follow up. The lack of a relationship between LCAQ and MoCA scores might suggest that during midlife (), current cognitive activity may be more important for overall cognitive functioning than lifetime cognitive activities. This proposes that the CCAQ is a more important determinant of cognitive performance and is consistent with the cross-sectional regression performed at the initial pre-intervention, as it had significant associations with verbal memory/attention whereas the LCAQ had no significant findings. In contrast, at the six-year follow-up, neither current or lifetime cognitive activities had significant correlations with MoCA scores, which may suggest that with increasing age (70.8 years), neither lifetime nor current cognitive activities alone are enough to combat the age-related decline in cognitive function. These results should be interpreted with caution due to the nature of the MoCA cognitive screening tool as it has significant limitations and is not a definitive indicator of cognitive performance. A more comprehensive neuropsychological battery is required to confirm these findings.
The absence of a relationship between the LCAQ and the MoCA differ from the significant correlations between the LCAQ and the MMSE scores reported by Wilson and colleagues (Wilson et al., Citation2003). The contrasting findings between the LCAQ and MoCA could be due to differences between the MMSE and MoCA in detecting cognitive decline. Education-stratified MoCA scores reported by Rossetti et al. (Citation2011) show a decline of 0.40 points on the MoCA score over a 5-year time period for a similar cohort (Rossetti et al., Citation2011). Our sample revealed a significant difference between the initial pre intervention and 6-year follow up examined through a paired samples t-test (t98 = 5.24 p < 0.001), with a 0.96 drop in mean scores over 6 years. The 6-year decline in MoCA score, observed in our study, was larger than the one reported in the normative sample (Rossetti et al., Citation2011). The greater change in MoCA could explain its’ dissociation with the LCAQ and indicate that there are more factors other than cognitive activity involved in determining cognitive performance in later stages of life.
Limitations
There are a few important limitations in this study that should be addressed. First, the BIM cohort is composed of predominantly healthy but sedentary, highly educated, Caucasian individuals with an interest in their health and how physical activity can improve their quality of life. Second, this cohort primarily consisted of retired individuals of higher socioeconomic status, with both the time and means to engage in a variety of activities to improve their cognitive function. Thus, results may differ for those individuals of lower socioeconomic status with limited means, opportunities and time to engage in various activities. Expanding the sample population to include more clinical, sedentary, or active individuals with diverse education status may yield different results. There is also a possibility of carryover or contamination effects of the LCAQ and CCAQ, inflating the estimates of their reliability, but these effects are expected to be minimal due to the large time gap between assessments. Self-reported questionnaires are also subject to the inherit bias to overreport activities, and thus absolute engagement may be overestimated by the LCAQ and CCAQ (Bielak & Gow, Citation2023; Salthouse, Citation2006). The validation analysis of self-reported activities in proxies of cognitive engagement is limited and would have benefitted from a more direct measurement such as self-report diaries. This was infeasible in this case, given the longitudinal nature of the study. The results between the LCAQ and CCAQ should be cautiously interpreted, as the MoCA is prone to ceiling effects in healthy populations, emphasizes verbal memory and processing speed cognitive domains and may be inappropriate for a highly educated cohort such as the one presented. The MoCA also lacks the ability to determine the severity and domain attribution of these cognitive deficits and requires a more comprehensive neuropsychological battery to confirm these results. However, the MoCA has impressive sensitivity and specificity metrics to flag an individual who may have MCI or dementia and is bolstered by the relationships derived from the neuropsychological battery in the cross-sectional analysis. The longitudinal correlations with the MoCA are exploratory in nature which will prompt investigative research to examine the longitudinal effects of cognitive activities on future cognitive abilities.
Conclusion
This study supports the importance and use of the LCAQ and CCAQ questionnaires to assess cognitive engagement. The findings support the concept of neurocognitive reserve but focuses on the construct validity, reliable assessment, and stability of cognitive engagement assessed through the LCAQ and CCAQ. Our results reveal that current cognitive activities have significant implications on the cognitive function of older adults and how it affects their global cognitive performance during midlife. It could have a reduced importance in older adults when other determinants of cognition overwhelm the beneficial effects of neurocognitive reserve. The high-test retest reliability and inter-item consistency indicated by these questionnaires allows researchers to use the LCAQ and CCAQ to assess how previous and current cognitive activities and other intercorrelated factors such as educational attainment and verbal intelligence converge to build neurocognitive reserve and thereby impact cognitive function in older populations. This research is particularly relevant in relation to dementia and Alzheimer’s disease, or in more diverse fields, such as concussion or stroke, where previous cognitive activities may have consequential effects on clinical outcomes.
Future directions
The LCAQ and CCAQ specifically measure the frequency of cognitively demanding activities that are a part of daily living. The LCAQ and CCAQ could also be used to assess the diversity of cognitive activities, instead of the frequency, and the relationship to overall cognition. For a more comprehensive assessment of cognitive health, other questionnaires should be used to encompass other determinants of cognitive function, such as physical fitness, which also has a considerable impact on cerebrovascular health (Tyndall et al., Citation2018). Future studies should analyze associations between engagement in both physical and cognitive activities with the rate of age-related cognitive decline. A recent study by Cheval et al. (Citation2020), attempted to investigate the relationship between physical and cognitive activity on age-related cognitive decline by correlating cognitive resources and physical activity levels in older adults. The authors assessed declarative memory, verbal fluency, and educational attainment as indicators of cognitive abilities, and revealed that a decrease in physical fitness precedes the decline in cognitive functioning (Cheval et al., Citation2020). However, this study was limited due to the lack of objective measures of physical activity and the participants’ fitness status. Instead, the authors simply assessed activity levels by frequency and energy expenditure (i.e., low vs. moderate). A future study should use the LCAQ or CCAQ to assess engagement in cognitive activity in combination with physiological measurements of fitness status (VO2max). This would provide a more conclusive picture of the determinants of cognitive decline, common in the aging population, and possibly act as a measure of neurocognitive reserve similar to the previously mentioned LEQ and CRS instruments used to assess neurocognitive reserve (Kartschmit et al., Citation2019; León et al., Citation2016; Valenzuela & Sachdev, Citation2007).
Acknowlegements
We want to thank all members of the Brain in Motion team and all study participants.
Supplemental Material
Download MS Word (52.5 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/13803395.2023.2272979.
Additional information
Funding
References
- Ackerman, P. L. (2014). Adolescent and adult intellectual development. Current Directions in Psychological Science, 23(4), 246–251. https://doi.org/10.1177/0963721414534960
- Bäckman, L., Jones, S., Berger, A. K., Laukka, E. J., & Small, B. J. (2005). Cognitive impairment in preclinical Alzheimer’s disease: A meta-analysis. Neuropsychology, 19(4), 520–531. https://doi.org/10.1037/0894-4105.19.4.520
- Bielak, A. A. M., Gerstorf, D., Anstey, K. J., & Luszcz, M. A. (2014). Longitudinal associations between activity and cognition vary by age, activity type, and cognitive domain. Psychology and Aging, 29(4), 863–872. https://doi.org/10.1037/a0036960
- Bielak, A. A. M., & Gow, A. J. (2023). A Decade Later on how to “use it” so we don’t “lose it”: An update on the unanswered questions about the influence of activity participation on cognitive performance in older age. Gerontology, 69(3), 336–355. https://doi.org/10.1159/000524666
- Blair, J. R., & Spreen, O. (1989). Predicting premorbid IQ: A revision of the national adult reading test. Clinical Neuropsychologist, 3(2), 129–136. https://doi.org/10.1080/13854048908403285
- Buschke, H. (1973). Selective reminding for analysis of memory and learning. Journal of Verbal Learning & Verbal Behavior, 12(5), 543–550. https://doi.org/10.1016/S0022-5371(73)80034-9
- Cabeza, R., Albert, M., Belleville, S., Craik, F. I. M., Duarte, A., Grady, C. L., Lindenberger, U., Nyberg, L., Park, D. C., Reuter-Lorenz, P. A., Rugg, M. D., Steffener, J., & Rajah, M. N. (2018). Maintenance, reserve and compensation: The cognitive neuroscience of healthy ageing. Nature Reviews Neuroscience, 19(11), 701–710. https://doi.org/10.1038/s41583-018-0068-2
- Ceci, S. J., & Williams, W. M. (1997). Schooling, intelligence, and income. American Psychologist, 52(10), 1051–1058. https://doi.org/10.1037/0003-066X.52.10.1051
- Cheval, B., Orsholits, D., Sieber, S., Courvoisier, D., Cullati, S., & Boisgontier, M. P. (2020). Relationship between decline in cognitive resources and physical activity. Health Psychology: Official Journal of the Division of Health Psychology, American Psychological Association, 39(6), 519–528. https://doi.org/10.1037/hea0000857
- Christensen, H., & Mackinnon, A. (1993). The association between mental, social and physical activity and cognitive performance in young and old subjects. Age and Ageing, 22(3), 175–182. https://doi.org/10.1093/ageing/22.3.175
- Delis, D. C., Kaplan, E., & Kramer, J. H. (2001). Delis-Kaplan Executive Function System. https://doi.org/10.1037/t15082-000
- Dziuban, C. D., & Shirkey, E. C. (1974). When is a correlation matrix appropriate for factor analysis? Some decision rules. Psychological Bulletin, 81(6), 358–361. https://doi.org/10.1037/h0036316
- Eskes, G. A., Longman, S., Brown, A. D., McMorris, C. A., Langdon, K. D., Hogan, D. B., & Poulin, M. (2010). Contribution of physical fitness, cerebrovascular reserve and cognitive stimulation to cognitive function in post-menopausal women. Frontiers in Aging Neuroscience, 2, 137. https://doi.org/10.3389/fnagi.2010.00137
- Folstein, M. F., Folstein, S. E., & McHugh, P. R. (1975). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–198. https://doi.org/10.1016/0022-3956(75)90026-6
- Friedland, R. P., Fritsch, T., Smyth, K. A., Koss, E., Lerner, A. J., Chen, C. H., Petot, G. J., & Debanne, S. M. (2001). Patients with Alzheimer’s disease have reduced activities in midlife compared with healthy control-group members. Proceedings of the National Academy of Sciences of the United States of America, 98(6), 3440–3445. https://doi.org/10.1073/pnas.061002998
- Gill, S. J., Friedenreich, C. M., Sajobi, T. T., Longman, R. S., Drogos, L. L., Davenport, M. H., Tyndall, A. V., Eskes, G. A., Hogan, D. B., Hill, M. D., Parboosingh, J. S., Wilson, B. J., & Poulin, M. J. (2015). Association between lifetime physical activity and cognitive functioning in middle-aged and older community dwelling adults: Results from the Brain in motion study. Journal of the International Neuropsychological Society: JINS, 21(10), 816–830. https://doi.org/10.1017/s1355617715000880
- Graham, J. M. (2006). Congeneric and (essentially) tau-equivalent estimates of score reliability: What they are and how to use them. Educational and Psychological Measurement, 66(6), 930–944. https://doi.org/10.1177/0013164406288165
- Guadagni, V., Drogos, L. L., Tyndall, A. V., Davenport, M. H., Anderson, T. J., Eskes, G. A., Longman, R. S., Hill, M. D., Hogan, D. B., & Poulin, M. J. (2020). Aerobic exercise improves cognition and cerebrovascular regulation in older adults. Neurology, 94(21), e2245–e2257. https://doi.org/10.1212/wnl.0000000000009478
- Hall, S. E., Lawal, O. A., Clark, C. M., Tyndall, A. V., Hill, M. D., Sajobi, T. T., & Poulin, M. J. (2019). Novel approach to characterize heterogeneity in an aerobic exercise intervention. Medicine & Science in Sports & Exercise, 51(7), 1506–1516. https://doi.org/10.1249/mss.0000000000001909
- Harada, C. N., Natelson Love, M. C., & Triebel, K. L. (2013). Normal cognitive aging. Clinics in Geriatric Medicine, 29(4), 737–752. https://doi.org/10.1016/j.cger.2013.07.002
- Hayes, A. F., & Coutts, J. J. (2020). Use omega rather than Cronbach’s alpha for estimating reliability. But …. Communication Methods and Measures, 14(1), 1–24. https://doi.org/10.1080/19312458.2020.1718629
- Heo, M., Kim, N., & Faith, M. S. (2015). Statistical power as a function of cronbach alpha of instrument questionnaire items. BMC Medical Research Methodology, 15(1), 86. https://doi.org/10.1186/s12874-015-0070-6
- Hertzog, C., Kramer, A. F., Wilson, R. S., & Lindenberger, U. (2008). Enrichment effects on adult cognitive development: Can the functional capacity of older adults be preserved and enhanced? Psychological Science in the Public Interest: A Journal of the American Psychological Society, 9(1), 1–65. https://doi.org/10.1111/j.1539-6053.2009.01034.x
- Hultsch, D. F., Hertzog, C., Small, B. J., & Dixon, R. A. (1999). Use it or lose it: Engaged lifestyle as a buffer of cognitive decline in aging? Psychology and Aging, 14(2), 245–263. https://doi.org/10.1037/0882-7974.14.2.245
- Ingram, F., Soukup, V. M., & Ingram, P. T. (1997). The Medical College of Georgia complex Figures: Reliability and preliminary normative data using an intentional learning paradigm in older adults. Neuropsychiatry, Neuropsychology, and Behavioral Neurology, 10(2), 144–146.
- Jackson, P. A., Pialoux, V., Corbett, D., Drogos, L., Erickson, K. I., Eskes, G. A., & Poulin, M. J. (2016). Promoting brain health through exercise and diet in older adults: A physiological perspective. The Journal of Physiology, 594(16), 4485–4498. https://doi.org/10.1113/jp271270
- Kalkbrenner, M. T. (2023). Alpha, omega, and H internal consistency reliability estimates: Reviewing these options and when to use them. Counseling Outcome Research and Evaluation, 14(1), 77–88. https://doi.org/10.1080/21501378.2021.1940118
- Kartschmit, N., Mikolajczyk, R., Schubert, T., & Lacruz, M. E. (2019). Measuring cognitive reserve (CR) - a systematic review of measurement properties of CR questionnaires for the adult population. PLoS One, 14(8), e0219851. https://doi.org/10.1371/journal.pone.0219851
- Kohn, M. L., & Schooler, C. (1978). The reciprocal effects of the substantive complexity of work and intellectual flexibility: A longitudinal assessment. American Journal of Sociology, 84(1), 24–52. https://doi.org/10.1086/226739
- Landau, S. M., Marks, S. M., Mormino, E. C., Rabinovici, G. D., Oh, H., O’Neil, J. P., Wilson, R. S., & Jagust, W. J. (2012). Association of lifetime cognitive engagement and low β-amyloid deposition. Archives of Neurology, 69(5), 623–629. https://doi.org/10.1001/archneurol.2011.2748
- León, I., García-García, J., & Roldán-Tapia, L. (2016). Cognitive reserve scale and ageing. Anales de Psicología, 32(1), 218–223. https://doi.org/10.6018/analesps.32.1.182331
- Linn, R. T., Wolf, P. A., Bachman, D. L., Knoefel, J. E., Cobb, J. L., Belanger, A. J., Kaplan, E. F., & D’Agostino, R. B. (1995). The ‘preclinical phase’ of probable Alzheimer’s disease. A 13-year prospective study of the framingham cohort. Archives of Neurology, 52(5), 485–490. https://doi.org/10.1001/archneur.1995.00540290075020
- Longman, R. S., Cota, A. A., Holden, R. R., & Fekken, G. C. (1989). A regression equation for the parallel analysis criterion in Principal components analysis: Mean and 95th percentile eigenvalues. Multivariate Behavioral Research, 24(1), 59–69. https://doi.org/10.1207/s15327906mbr2401_4
- Luo, M., Moulder, R. G., Breitfelder, L. K., & Röcke, C. (2023). Daily activity diversity and daily working memory in community-dwelling older adults. Neuropsychology, 37(2), 181–193. https://doi.org/10.1037/neu0000878
- MacPherson, S. E., Healy, C., Allerhand, M., Spanò, B., Tudor-Sfetea, C., White, M., Smirni, D., Shallice, T., Chan, E., Bozzali, M., & Cipolotti, L. (2017). Cognitive reserve and cognitive performance of patients with focal frontal lesions. Neuropsychologia, 96, 19–28. https://doi.org/10.1016/j.neuropsychologia.2016.12.028
- Malek-Ahmadi, M., Powell, J. J., Belden, C. M., O’Connor, K., Evans, L., Coon, D. W., & Nieri, W. (2015). Age- and education-adjusted normative data for the Montreal cognitive assessment (MoCA) in older adults age 70-99. Neuropsychology, Development, and Cognition Section B, Aging, Neuropsychology and Cognition, 22(6), 755–761. https://doi.org/10.1080/13825585.2015.1041449
- Miller, J., Slomczynski, K. M., & Kohn, M. L. (1985). Continuity of learning-generalization: The effect of job on Men’s Intellective Process in the United States and Poland. The American Journal of Sociology, 91(3), 593–615. https://doi.org/10.1086/228315
- Nasreddine, Z. S., Phillips, N. A., Bédirian, V., Charbonneau, S., Whitehead, V., Collin, I., Cummings, J. L., & Chertkow, H. (2005). The montreal cognitive assessment, MoCA: A brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society, 53(4), 695–699. https://doi.org/10.1111/j.1532-5415.2005.53221.x
- Putnick, D. L., & Bornstein, M. H. (2016). Measurement invariance conventions and reporting: The state of the art and future directions for psychological research. Developmental Review, 41, 71–90. https://doi.org/10.1016/j.dr.2016.06.004
- Reed, B. R., Dowling, M., Tomaszewski Farias, S., Sonnen, J., Strauss, M., Schneider, J. A., Bennett, D. A., & Mungas, D. (2011). Cognitive activities during adulthood are more important than education in building reserve. Journal of the International Neuropsychological Society, 17(4), 615–624. https://doi.org/10.1017/S1355617711000014
- Rossetti, H. C., Lacritz, L. H., Cullum, C. M., & Weiner, M. F. (2011). Normative data for the Montreal cognitive assessment (MoCA) in a population-based sample. Neurology, 77(13), 1272–1275. https://doi.org/10.1212/WNL.0b013e318230208a
- Rytz, C. L., Pialoux, V., Mura, M., Martin, A., Hogan, D. B., Hill, M. D., & Poulin, M. J. (2020). Impact of aerobic exercise, sex, and metabolic syndrome on markers of oxidative stress: Results from the Brain in Motion study. Journal of Applied Physiology (1985), 128(4), 748–756. https://doi.org/10.1152/japplphysiol.00667.2019
- Salthouse, T. A. (2006). Mental exercise and mental Aging: Evaluating the validity of the “use it or lose it” hypothesis. Perspectives on Psychological Science: A Journal of the Association for Psychological Science, 1(1), 68–87. https://doi.org/10.1111/j.1745-6916.2006.00005.x
- Salthouse, T. A., Berish, D. E., & Miles, J. D. (2002). The role of cognitive stimulation on the relations between age and cognitive functioning. Psychology and Aging, 17(4), 548–557. https://doi.org/10.1037/0882-7974.17.4.548
- Satz, P., Cole, M. A., Hardy, D. J., & Rassovsky, Y. (2011). Brain and cognitive reserve: Mediator(s) and construct validity, a critique. Journal of Clinical and Experimental Neuropsychology, 33(1), 121–130. https://doi.org/10.1080/13803395.2010.493151
- Shura, R. D., Rowland, J. A., & Miskey, H. M. (2016). Auditory consonant trigrams: A psychometric update. Archives of Clinical Neuropsychology, 31(1), 47–57. https://doi.org/10.1093/arclin/acv083
- Small, B. J., Fratiglioni, L., Viitanen, M., Winblad, B., & Bäckman, L. (2000). The course of cognitive impairment in preclinical Alzheimer disease: Three- and 6-year follow-up of a population-based sample. Archives of Neurology (Chicago), 57(6), 839–844. https://doi.org/10.1001/archneur.57.6.839
- Smith, A. (1979). Symbol digit modalities test manual (Western psychological services, los angeles). BD Schwartz Al/Schizophrenia Res, 211(19), 549–586.
- Soldan, A., Pettigrew, C., & Albert, M. (2020). Cognitive reserve from the perspective of preclinical alzheimer disease: 2020 update. Clinics in Geriatric Medicine, 36(2), 247–263. https://doi.org/10.1016/j.cger.2019.11.006
- Stern, Y., Barnes, C. A., Grady, C., Jones, R. N., & Raz, N. (2019). Brain reserve, cognitive reserve, compensation, and maintenance: Operationalization, validity, and mechanisms of cognitive resilience. Neurobiology of Aging, 83, 124–129. https://doi.org/10.1016/j.neurobiolaging.2019.03.022
- Strauss, E., Sherman, E. M., & Spreen, O. (2006). A compendium of neuropsychological tests: Administration, norms, and commentary. American chemical society.
- Surber, J. R., Kowalski, A. H., & Peña-Páez, A. (1984). Effects of aging on the recall of extended expository prose. Experimental Aging Research, 10(1), 25–28. https://doi.org/10.1080/03610738408258537
- Trzepacz, P. T., Hochstetler, H., Wang, S., Walker, B., & Saykin, A. J. (2015). Relationship between the Montreal cognitive assessment and mini-mental state examination for assessment of mild cognitive impairment in older adults. BMC Geriatrics, 15(1), 107. https://doi.org/10.1186/s12877-015-0103-3
- Tucker, A. M., & Stern, Y. (2011). Cognitive reserve in aging. Current Alzheimer Research, 8(4), 354–360. https://doi.org/10.2174/156720511795745320
- Tucker, A. M., & Stern, Y. (2014). Cognitive Reserve and the Aging Brain. In: Nair, A. K., Sabbagh, M. N. (Eds.), Geriatric Neurology (1st ed., pp. 118–125). https://doi.org/10.1002/9781118730676.ch5
- Tyndall, A. V., Clark, C. M., Anderson, T. J., Hogan, D. B., Hill, M. D., Longman, R. S., & Poulin, M. J. (2018). Protective effects of exercise on cognition and Brain Health in older adults. Exercise and Sport Sciences Reviews, 46(4), 215–223. https://doi.org/10.1249/jes.0000000000000161
- Tyndall, A. V., Davenport, M. H., Wilson, B. J., Burek, G. M., Arsenault-Lapierre, G., Haley, E., Eskes, G. A., Friedenreich, C. M., Hill, M. D., Hogan, D. B., Longman, R. S., Anderson, T. J., Leigh, R., Smith, E. E., & Poulin, M. J. (2013). The brain-in-motion study: Effect of a 6-month aerobic exercise intervention on cerebrovascular regulation and cognitive function in older adults. BMC Geriatrics, 13(1), 21. https://doi.org/10.1186/1471-2318-13-21
- Valenzuela, M. J., & Sachdev, P. (2007). Assessment of complex mental activity across the lifespan: Development of the lifetime of experiences questionnaire (LEQ). Psychological Medicine, 37(7), 1015–1025. https://doi.org/10.1017/s003329170600938x
- Wilson, R., Barnes, L., & Bennett, D. (2003). Assessment of lifetime participation in cognitively stimulating activities. Journal of Clinical and Experimental Neuropsychology, 25(5), 634–642. https://doi.org/10.1076/jcen.25.5.634.14572
- Wilson, R. S., Bennett, D. A., Beckett, L. A., Morris, M. C., Gilley, D. W., Bienias, J. L., Scherr, P. A., & Evans, D. A. (1999). Cognitive activity in older persons from a geographically defined population. The Journals of Gerontology Series B, Psychological Sciences and Social Sciences, 54(3), 155–160. https://doi.org/10.1093/geronb/54b.3.p155
- Wilson, R. S., Scherr, P. A., Schneider, J. A., Tang, Y., & Bennett, D. A. (2007). Relation of cognitive activity to risk of developing Alzheimer disease. Neurology, 69(20), 1911–1920. https://doi.org/10.1212/01.wnl.0000271087.67782.cb