ABSTRACT
Introduction
Among the cognitive difficulties shown by myotonic dystrophy type 1 (DM1) patients, visuoconstructional impairment – specifically measured with the Rey-Osterrieth Complex Figure Test (RCFT) – is particularly notable. This study aimed to analyze the performance of DM1 patients and healthy controls (HC) in the RCFT, using different correction systems in order to explore the cognitive processes underlying the poor performance and its associations with other signs and symptoms.
Methods
Data from 66 DM1 patients and 68 HC were included in this study. All participants had a comprehensive neuropsychological assessment, including the RCFT, which was scored using both the traditional Osterrieth and the Boston Qualitative Scoring System (BQSS) procedures. ANCOVA and Spearman’s correlation analyses were conducted.
Results
DM1 Patients obtained significantly poorer scores than HC on the RCFT using both correction systems. Regarding BQSS, patients performed worse than HC in both main indexes (Copy Presence Accuracy-CPA and Organization-ORG), and specifically on scores of Configural accuracy, Planning, and Perseveration. Both main indexes – but especially CPA – showed significant and strong correlations with several clinical and cognitive variables.
Conclusions
Both visuoconstruction and organizational impairments underlie the poor RCFT performance in DM1. Moreover, visuoconstruction ability appears to be sensitive to the clinical hallmarks of DM1 patients. The RCFT is proposed as a gold standard in DM1 assessment and the merits of using alternative scoring systems are discussed.
1. Introduction
Myotonic Dystrophy type 1 (DM1) or Steinert’s Disease, is a hereditary and multisystemic disease with Central Nervous System (CNS) affectation. Regarding the cognitive abilities of DM1 patients, they usually perform significantly worse than healthy controls (HC) in most cognitive domains (visuoconstruction, executive functioning, attention and processing speed etc.) The.
Although the exact cognitive profile of DM1 patients is still under debate, the visuoperception/visuoconstruction domain has been found to be the most affected, suggesting that these abilities could be considered a hallmark of the DM1 cognitive profile (Gliem et al., Citation2019; Labayru et al., Citation2020; Okkersen et al., Citation2017; Sistiaga et al., Citation2010). In addition, these visuoperceptive/visuoconstructional abilities have been suggested as reliable predictors of CNS-related deterioration and have been consistently associated with other cognitive abilities and brain alterations (Baldanzi et al., Citation2016; Cabada et al., Citation2020; Labayru et al., Citation2020, Citation2022). Visuoconstruction has also been associated with DM1 clinical outcomes, such as CTG (cytosine thymine guanine) expansion size and muscular impairment rating scale (MIRS) (Labayru et al., Citation2020).
In DM1, visuoconstruction has been analyzed by tests such as the Block design subtest of the Wechsler Adult Intelligence Scale, but predominantly with the Rey-Osterrieth Complex Figure Test (RCFT). Use of the latter has consistently revealed impairments in DM1 patients, and according to a relatively recent meta-analysis, this test has demonstrated considerable effects in DM1 (Okkersen et al., Citation2017) and is considered one of the most sensitive tools for evaluating DM1 patients’ cognitive profile and deterioration, showing sensitivity to cognitive impairments that develop over (Labayru et al., Citation2020).
Beyond visuoperceptive/visuoconstructional abilities, correct performance in the RCFT also demands an adequate organization and integration of visual information. Therefore, there is an interest in elucidating whether the visuoconstruction load or the complexity and organizational component of the task is defective in DM1 (Labayru et al., Citation2020). In this regard, and considering the complexity of the figure, it could be of interest to explore alternative scoring systems for RCFT that provide qualitative information about the figure productions.
Aside from the traditional correction system proposed by Osterrieth (Osterrieth, Citation1944), other alternative correction systems have been developed, such as the Boston Qualitative Scoring System (BQSS). One of the advantages of the latter is that provides qualitative information regarding the respondent’s strategy and organizational approach, thus capturing why patients underperform.
This study aims to reveal the cognitive processes underlying performance in RCFT. Authors hypothesize that DM1 will perform significantly worse than HC in RCFT (depicted by both correction systems) and that RCFT performance will correlate to clinical and cognitive variables in patients. For this purpose, this study analyses 1) the performance of DM1 patients and HC in the RCFT, using both the traditional and BQSS correction systems, and 2) the associations between RCFT performance and clinical and neuropsychological variables in DM1 population.
2. Methodology
2.1. Participants
The data of this study were retrospectively analyzed from the DM1 follow-up cohort attending the Neurology Department of the Donostia University Hospital (Gipuzkoa, Spain) (Labayru et al., Citation2020). Data from 66 non-pediatric DM1 patients (36 women) and 68 HC (41 women) were analyzed in this study. All participants in the mentioned cohort were older than 18 years, had a molecular confirmation of the disease, and had no other neurological or psychiatric disorders or a history of drug or alcohol abuse. HC met the same inclusion/exclusion criteria, with the exception of molecular confirmation of the disease.
Concerning the equivalence of both groups, DM1 patients and HC were equivalent in sex and age (χ2 (1) = 0.45; p = 0.501 and t(132) = 0.43; p = 0.667; d = 0.08, respectively). However, there were small but significant differences in years of education (t(132) = 2.09; p = 0.039; d=0.36). Therefore, this variable was controlled in subsequent analyses.
All participants signed an informed consent form, and the study received the approval of the Ethics Committee for Clinical Investigation of the Health Department of Gipuzkoa (DMRM-2017-01).
2.2. Assessment
2.2.1. Clinical and genetic data
Data on muscular impairment (gathered through the Muscular Impairment Rating Scale, MIRS, (Mathieu et al., Citation2001)), phenotype, CTG expansion size, and inheritance pattern were obtained through medical records and a research database.
2.2.2. Neuropsychological assessment
All participants had a comprehensive neuropsychological assessment (see ), along with the RCFT scored through the traditional correction system (Osterrieth, Citation1944). In addition, for this study, a trained neuropsychologist scored the copy of all the drawings using the BQSS, while remaining blind to the participant’s condition Stern et al. (Citation1999). Data on the planning of the drawings were available, as the neuropsychologist responsible for evaluating RCFT performance recorded a stepwise flowchart.
Table 1. Tests employed for the IQ and cognitive domains.
BQSS follows a process-based approach for correcting the RCFT, and unlike the traditional scoring system that only measures presence, localization, and accuracy of the elements of the figure, BQSS provides detailed information about participant performance on this task, including both visuoconstructive and executive variables.
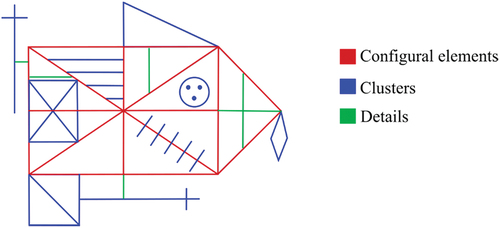
This scoring approach involves dividing the figure into three sets of elements that are hierarchically arranged in terms of structural importance: configural elements, clusters, and details (see ). There are six configural elements, which are considered fundamental to the structure of the figure. The clusters are nine important secondary elements that are comprised of one or more shapes or line segments that appear to form a coherent whole. Finally, there are six elements related to details, consisting of single line segments. Considering these sets of elements, BQSS provides scores for the 17 qualitative scales: Configural/Cluster/Detail Presence, Configural/Cluster Accuracy, Cluster/Detail Placement, Fragmentation, Planning, Neatness, Vertical/Horizontal Expansion, Reduction, Rotation, Perseveration, Confabulation, and Asymmetry.
From these scales, two main indexes are calculated: Copy Presence Accuracy (CPA), and Organization (ORG). The former measures visuoperceptual accuracy and visuoconstructional ability (arithmetic mean of Configural/Cluster/Detail Presence, Configural/Cluster Accuracy, Cluster/Detail Placement scores), while the latter measures organizational skills (arithmetic sum of Fragmentation and Planning scores).
Additionally, an IQ estimation (Sattler & Ryan, Citation2001) and the following cognitive domains were calculated: attention/processing speed, executive functions, visuoconstruction, memory, and language. Information about the tests employed for each cognitive domain are described in the following Table ().
With respect to the standardized scores, the scores for all neuropsychological tests were based on normative data from the Spanish population, as provided in each test manual. T scores were obtained for the traditional RCFT scoring system and the main indexes of the BQSS (CPA and ORG), and percentiles for the BQSS subdomains.
Furthermore, all cognitive tests scores were converted to T scores and the mean T values were computed for each cognitive domain.
2.3. Statistical analysis
The collected data were analyzed using the SPSS statistical package (Version 27).
Descriptive analyses were conducted to examine the demographic data and the clinical, genetic, and cognitive performance of the participants.
RCFT performance of DM1 patients and HC was compared using ANCOVA analyses, controlling for years of education. The analyses were conducted using both RCFT correction systems. ANCOVA analyses were also conducted to compare the performance in the cognitive domains. Hedges’ g was calculated to indicate effect sizes, which were interpreted as small (.20), medium (.50) or large (.80) (Cohen, Citation1988).
Additionally, Spearman’s correlation analyses were performed between BQSS scores and clinical, genetic, and cognitive variables, but only in DM1 population. The effect sizes were interpreted as small (<0.19), medium (0.20–0.29), or large (≥0.3) (López-Martín & Ardura, Citation2023).
3. Results
3.1. Descriptive data
Of the DM1 sample, 53% of the patients were classified as adult-onset DM1, 30.3% juvenile-onset, and 16.7% late-onset. Concerning inheritance pattern, 79% had a paternal inheritance, 19.4% maternal, and one patient had both maternal and paternal.
The demographic, clinical, and genetic data of DM1 patients are presented in the following table ().
Table 2. Demographic, clinical, and genetic data of the participants.
3.2. Comparisons between DM1 and HC
A comparison of DM1 patients and HC using both scoring systems and controlling for years of education confirmed the difficulties shown by DM1 patients in this task. Results of the intergroup comparisons are displayed in .
Table 3. Comparisons between DM1 patients and HC in RCFT.
Using the traditional scoring system, DM1 patients showed significantly worse performance compared to HC in the copy and delayed memory of the figure (p = 0.001; Hedges’ g = 0.81; p = 0.020; Hedges’ g = 0.49, respectively). Moreover, patients needed significantly more time copying the figure (p = 0.020; Hedges’ g = 0.46). The effect sizes ranged from medium to large.
When the BQSS scoring system was applied, DM1 patients showed poorer performance than HC in the two main indexes (Copy Presence Accuracy and Organization). These differences were statistically significant with medium effect sizes (p = 0.018, Hedges’ g = 0.60; p = 0.002; Hedges’ g = 0.58, respectively). Specifically, significant differences were found in the subdomains of Configural Accuracy, Planning, and Perseveration, with medium to large effect sizes (p = 0.001, Hedges’ g = 0.71; p < 0.001; Hedges’ g = 0.75; p = 0.47; Hedges’ g = 0.48, respectively).
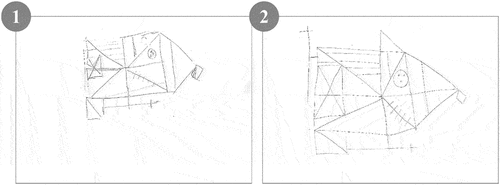
Examples of two different performances according to the two main indexes of BQSS are shown in ; the first patient obtained a low score on CPA but a normal score in ORG, while the opposite was observed for the second patient. Further information on the stepwise flowchart of the patients’ drawings is provided in Supplementary Data (Supplementary Figures S1 and S2).
Figure 2. Examples of patients’ figures of the RCFT.
In addition, when comparing the performance of DM1 patients and HC in the cognitive domains, ANCOVA analyses revealed significant differences with large effect sizes for the domains of attention/processing speed, executive functions, and visuoconstruction (p = 0.001, Hedges’ g = 0.77; p = 0.001; Hedges’ g = 0.74; p = 0.001; Hedges’ g = 0.93, respectively), but not for memory and language (p = 0.199, Hedges’g = −0.03; p = 0.084; Hedges’ g = 0.46, respectively). Notably, differences in the visuoconstruction domain showed the largest effect size (see Supplementary Table S1).
3.3. Associations between RCFT and demographic, clinical, genetic, and cognitive variables
The results of the Spearman’s correlation analysis are displayed in .
Figure 3. Spearman’s correlations between the BQSS main indexes and demographic, clinical, genetic, and cognitive data.
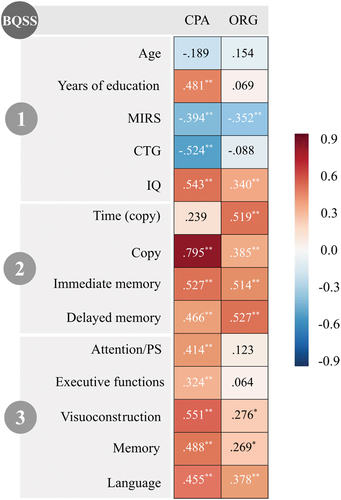
Regarding the correlations between the BQSS main indexes and demographic, clinical, and genetic variables, the CPA index score showed statistically significant and large correlations with years of education (ρ = 0.481), muscular impairment (ρ=-0.394), CTG expansion (ρ=-0.524), and the IQ (ρ = 0.543) of DM1 patients. The ORG index score only showed a significant correlation with patients’ muscular impairment (ρ = 0.352) and IQ level (ρ = 0.340) (large effect size).
When considering both RCFT scoring systems, strong and significant positive correlations were found between them. While CPA index showed high correlation with the RCFT copy (ρ = 0.795), ORG index was highly correlated with the time employed to copy the figure (ρ = 0.519).
Regarding the correlation between each of the CPA and ORG indexes and different cognitive domains, it can be noted in that the former showed a significant positive correlation with all cognitive domains (ρ>0.3 in all cases) (large effect size), and the latter showed significant correlations with visuoconstruction (ρ = 0.276), memory (ρ = 0.269), and language domains (ρ = 0.378) (with medium to large effect sizes).
3.4. Ad hoc analyses
Previous analyses demonstrated deficits in both the visuoconstructional (CPA) and organizational components of BQSS in DM1 patients. Given these findings, further analyses were considered to explore the processes underlying the underperformance in RCFT, and to delve into the potential mediating effect of CPA over ORG, as well as inversely.
To address this, two separate ANCOVA analyses were conducted to explore the independent effects of CPA and ORG: 1) ANCOVA analysis to account for differences between DM1 and HC in CPA, while controlling the effect of ORG, and 2) another ANCOVA analysis to account for differences between DM1 and HC in ORG, while controlling the effect of CPA.
The statistical analyses revealed significant differences between DM1 patients and HC, both in CPA and ORG, showing poorer performance in patients. Specifically, after controlling for the effect of ORG, differences in CPA persisted (F (1) = 6.57; p = 0.011). Similarly, after controlling for the effect of CPA, significant differences in ORG persisted (F (1) = 6.27; p = 0.014).
4. Discussion
This is the first study to qualitatively examine the performance of DM1 patients on the RCFT. As previously reported in other studies (Gliem et al., Citation2019; Labayru et al., Citation2020; Okkersen et al., Citation2017; Sistiaga et al., Citation2010), in this study, patients performed significantly worse than HC on this visuoconstructive task, regardless of the correction system. In fact, this is the cognitive domain predominately altered in this sample (see Supplementary Table S1).
Various cognitive functions are involved in completing the RCFT, and the BQSS allowed us to unravel the complex cognitive mechanisms underlying this consistently poor performance in DM1. Considering its general indexes, DM1 patients presented poor visuoperceptual accuracy and visuoconstructional ability (CPA), along with poor organizational skills (ORG), in comparison to HC. Specifically, patients showed difficulties with the quality of the production of fundamental/structural elements of the figure (Configural Accuracy) and with the overall integrity and the order in which elements were drawn and placed (Planning). Moreover, patients tended to inappropriately repeat elements of the figure (Perseveration).
Nonetheless, according to the correlation analyses, the visuoconstructive load (as proxied by CPA score) appears to have a greater effect on performance than the organizational component of the task. Indeed, CPA was shown to be sensitive to all clinical, genetic, and cognitive variables, while the Organization score showed fewer correlations. Thus, visuoconstruction ability is not only cognitive hallmark of the disease, but it is also closely related to the clinical features of patients.
Accordingly, the literature has shown that visuoconstruction ability (and more specifically RCFT) has been considered a sensitive way to monitor DM1 progression, since it has consistently been associated with CNS-related deterioration (both cognitive and brain impairment), and the clinical symptoms in DM1 (Baldanzi et al., Citation2016; Cabada et al., Citation2020; Gliem et al., Citation2019; Labayru et al., Citation2020, Citation2020, Citation2022; Okkersen et al., Citation2017; Sistiaga et al., Citation2010). Moreover, in other pathologies, such as Lewy Body Dementia (Liu et al., Citation2022; Tiraboschi et al., Citation2006), impaired visuoconstruction is notably the main cognitive difficulty, and has been put forward as an early cognitive marker of the disease. The search for overlapping cognitive profiles between DM1 and other clinical conditions may provide the opportunity to explore common brain damage (involved brain areas or connectivity patterns) and underlying pathological mechanisms, leading to a deeper understanding of the etiology of the disease.
However, the results of this study do not allow us to rule out the possibility that DM1 patients’ executive dysfunction impacts their poor RCFT performance, since this was also captured when a process-based approach such as the BQSS was employed. But as previously mentioned, the results suggest that the visuoconstructive ability (CPA) may be a better marker of this clinical population.
The findings of this study could help to better interpret the RCFT score in DM1, considering that both visuoconstructive and organizational impairment could impact the final score. In fact, when controlling for ORG, significant differences between DM1 patients and HC persisted in CPA; similarly, when controlling for CPA, significant ORG differences remained. These findings might suggest that both cognitive abilities have an independent effect over the performance on RCFT.
However, having clarified the cognitive processes underlying poor RCFT performance in DM1 patients using the BQSS, any further contribution of this scoring system to the assessment of this population might be questionable. One limitation of this correction system is that it is time-consuming and might not be suitable for evaluating large samples in relatively short time periods (i.e., clinical trials). The results support the notion that performance on the visuoconstructive component of the task (CPA) is a better indicator of the features of this clinical population. Thus, it might be argued that this component is already covered by the traditional correction system proposed by Osterrieth, which can be scored in a shorter period.
This study was not free of limitations. Regarding the sample size, it is important to note that this was a retrospective study where all eligible participants were included. While larger samples are generally desirable, it is worth considering that the sample size of this study is reasonable when taking into account the context of DM1 literature and the fact that DM1 is a rare disorder. Another challenge in this study involves the BQSS, a complex qualitative scoring tool. To address the complexity of this scoring system, all the participants’ figures were scored by the same neuropsychologist, who was blind to the participants’ condition.
An additional possible limitation of this study is that only the RCFT copy has been scored with the BQSS, and not the memory of the figure (immediate and delayed). Thus, it was not possible to calculate all the BQSS indexes, that is, those related to memory. However, memory is not among the main impaired domains in DM1, as evidenced in this sample (see Supplementary Table S1).
Taken together, the findings of this study suggest that impaired visuoconstruction could be a cognitive marker of DM1, and thus, RCFT should be included as a relevant measure in the neuropsychological assessment of these patients and considered as an outcome measure for future clinical trials. In addition, RCFT was closely related to the muscular, genetic, and cognitive profile of patients, which suggests the importance of considering its use as a predictor of disease status/progression.
In summary, this study has helped to shed light on the cognitive processes underlying the poor performance of RCFT in DM1. The findings indicated that both the visuoperceptive/visuoconstruction load and the organizational skills of the task were defective in DM1, and thus both could explain the consistent underperformance. A recent study by this research group and other studies in DM1 found that executive functions were closely related to patients’ daily functioning (Muslemani et al., Citation2022; Tremblay et al., Citation2021; Van Heugten et al., Citation2018). However, further research is needed to address the implications of visuoconstructional deficits for the daily life and functionality of DM1 patients.
Supplemental Material
Download MS Word (920.8 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author, [G.L.], upon reasonable request.
Supplemental data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/13803395.2023.2274623
Additional information
Funding
References
- Baldanzi, S., Cecchi, P., Fabbri, S., Pesaresi, I., Simoncini, C., Angelini, C., Bonuccelli, U., Cosottini, M., & Siciliano, G. (2016). Relationship between neuropsychological impairment and grey and white matter changes in adult-onset myotonic dystrophy type 1. NeuroImage: Clinical, 12, 190–197. https://doi.org/10.1016/j.nicl.2016.06.011
- Cabada, T., Díaz, J., Iridoy, M., López, P., Jericó, I., Lecumberri, P., Remirez, B., Seijas, R., & Gomez, M. (2020). Longitudinal study in patients with myotonic dystrophy type 1: Correlation of brain MRI abnormalities with cognitive performances. Neuroradiology, 63(7), 1019–1029. https://doi.org/10.1007/s00234-020-02611-9
- Casals-Coll, M., Sánchez-Benavides, G., Quintana, M., Manero, R. M., Rognoni, T., Calvo, L., Palomo, R., Aranciva, F., Tamayo, F., & Peña-Casanova, J. (2013). Estudios normativos españoles en población adulta joven (proyecto NEURONORMA jóvenes): Normas para los test de fluencia verbal. Neurología, 28(1), 33–40. https://doi.org/10.1016/j.nrl.2012.02.010
- Cohen, J. (1988). Statistical Power analysis for the behavioral sciences (2nd ed). Academic press.
- Gliem, C., Minnerop, M., Roeske, S., Gärtner, H., Schoene-Bake, J.-C., Adler, S., Witt, J.-A., Hoffstaedter, F., Schneider-Gold, C., Betz, R., Helmstaedter, C., Tittgemeyer, M., Amunts, K., Klockgether, T., Weber, B., & Kornblum, C. (2019). Myotonic dystrophy research: Imaging result files (DTI, VBM) from a 5-year longitudinal follow-up study. PLOSone, ii, 1–21. https://doi.org/10.5281/zenodo.2556355
- Golden, C. J. (2001). STROOP. Color and word test (3rd ed.). TEA Ediciones.
- Labayru, G., Aliri, J., Zulaica, M., López de Munain, A., & Sistiaga, A. (2020). Age-related cognitive decline in myotonic dystrophy type 1: An 11-year longitudinal follow-up study. Journal of Neuropsychology, 14(1), 121–134. https://doi.org/10.1111/jnp.12192
- Labayru, G., Camino, B., Jimenez-Marin, A., Garmendia, J., Villanua, J., Zulaica, M., Cortes, J. M., López de Munain, A., & Sistiaga, A. (2022). White matter integrity changes and neurocognitive functioning in adult-late onset DM1: A follow-up DTI study. Scientific Reports, 12(1), 3988. https://doi.org/10.1038/s41598-022-07820-1
- Labayru, G., Jimenez-Marin, A., Fernández, E., Villanua, J., Zulaica, M., Cortes, J. M., Díez, I., Sepulcre, J., Munain, A. L. D., & Sistiaga, A. (2020). Neurodegeneration trajectory in pediatric and adult/late DM1: A follow-up MRI study across a decade. Annals of Clinical & Translational Neurology, 7(10), 1802–1815. https://doi.org/10.1002/acn3.51163
- Lezak, M., Howieson, H., & Loring, D. (2004). Neuropsychological assessment (4th ed.). Oxford University Press.
- Liu, S., Liu, C., Wang, X., Lu, H., & Ji, Y. (2022). Cognitive profile in mild cognitive impairment with Lewy bodies. Singapore Medical Journal, 64(8), 487–492. https://doi.org/10.11622/smedj.2022085
- López-Martín, E., & Ardura, D. (2023). El tamaño del efecto en la publicación científica. Educación XX1, 26(1). https://doi.org/10.5944/educxx1.36276
- Mathieu, J., Boivin, H., Meunier, D., Gaudreault, M., & Bégin, P. (2001). Assessment of a disease-specific muscular impairment rating scale in myotonic dystrophy. Neurology, 56(3), 336–340. https://doi.org/10.1212/WNL.56.3.336
- Miller, E. N. (1990). CalCAP: California computerized assessment package. Norland Software.
- Muslemani, S., Gagnon, C., & Gallais, B. (2022). Instrumental activities of daily living in adults with the DM1 childhood phenotype: Going beyond motor impairments. Neuromuscular Disorders: NMD, 32(4), 313–320. https://doi.org/10.1016/j.nmd.2022.02.004
- Okkersen, K., Buskes, M., Groenewoud, J., Kessels, R. P. C., Knoop, H., Engelen van, B., & Raaphorst, J. (2017). The cognitive profile of myotonic dystrophy type 1: A systematic review and meta-analysis. Cortex, 95, 143–155. https://doi.org/10.1016/j.cortex.2017.08.008
- Osterrieth, P. A. (1944). Le test de copie d’une figure complexe; contribution à l’étude de la perception et de la mémoire [Test of copying a complex figure; contribution to the study of perception and memory]. Archives de Psychologie, 30, 206–356. https://psycnet.apa.org/record/1946-02126-001.
- Pena-Casanova, J., Quinones-Ubeda, S., Gramunt-Fombuena, N., Aguilar, M., Casas, L., Molinuevo, J. L., Robles, A., Rodriguez, D., Barquero, M. S., Antunez, C., Martinez-Parra, C., Frank-Garcia, A., Fernandez, M., Molano, A., Alfonso, V., Sol, J. M., Blesa, R., & for the NEURONORMA Study Team. (2009). Spanish multicenter normative studies (NEURONORMA project): Norms for Boston naming Test and Token Test. Archives of Clinical Neuropsychology, 24(4), 343–354. https://doi.org/10.1093/arclin/acp039
- Pena-Casanova, J., Quinones-Ubeda, S., Quintana-Aparicio, M., Aguilar, M., Badenes, D., Molinuevo, J. L., Torner, L., Robles, A., Barquero, M. S., Villanueva, C., Antunez, C., Martinez-Parra, C., Frank-Garcia, A., Sanz, A., Fernandez, M., Alfonso, V., Sol, J. M., Blesa, R., & for the NEURONORMA Study Team. (2009). Spanish multicenter normative studies (NEURONORMA project): Norms for verbal span, visuospatial span, letter and number sequencing, trail making Test, and symbol digit modalities Test. Archives of Clinical Neuropsychology, 24(4), 321–341. https://doi.org/10.1093/arclin/acp038
- Sattler, J. M., & Ryan, J. J. (2001). Wechsler Adult Intelligence Scale-III (WAIS-III): Description. In I. I. A. I. M. Sattler (Ed.), Assessment of children. Cognitive applications (4th ed., pp. 375–414). Sattler Publisher, Inc.
- Sistiaga, A., Urreta, I., Jodar, M., Cobo, A. M., Emparanza, J., Otaegui, D., Poza, J. J., Merino, J. J., Imaz, H., Martí-Mass, J. F., & Munain, A. L. D. (2010). Cognitive/Personality pattern and triplet expansion size in adult myotonic dystrophy type 1 (DM1): CTG repeats, cognition and personality in DM1. Psychological Medicine, 40(3), 487–495. https://doi.org/10.1017/S0033291709990602
- Stern, R. A., Javorsky, D. J., Singer, E. A., Harris, N. G. S., Somerville, J. A., & Duke, L. M., y Kaplan, E. (1999). BQSS: The Boston Qualitative scoring system for the Rey-Osterrieth Complex Figure. Psychological Assessment Resources Inc.).
- Tiraboschi, P., Salmon, D. P., Hansen, L. A., Hofstetter, R. C., Thal, L. J., & Corey-Bloom, J. (2006). What best differentiates Lewy body from Alzheimer’s disease in early-stage dementia? Brain A Journal of Neurology, 129(3), 729–735. https://doi.org/10.1093/brain/awh725
- Tremblay, M., Muslemani, S., Côté, I., Gagnon, C., Fortin, J., & Gallais, B. (2021). Accomplishment of instrumental activities of daily living and its relationship with cognitive functions in adults with myotonic dystrophy type 1 childhood phenotype: An exploratory study. BMC Psychology, 9(1), 56. https://doi.org/10.1186/s40359-021-00562-1
- Tulsky, D. S., Chiaravalloti, N. D., Palmer, B. W., & Chelune, G. J. (2003). The Wechsler memory Scale. In En clinical interpretation of the WAIS-III and WMS-III (Third ed., pp. 93–139). Elsevier. https://doi.org/10.1016/B978-012703570-3/50007-9
- Van Heugten, C., Meuleman, S., Hellebrekers, D., Kruitwagen van Reenen, E., & Visser-Meily, J. (2018). Participation and the role of neuropsychological functioning in myotonic dystrophy type 1. Journal of Neuromuscular Diseases, 5(2), 205–214. https://doi.org/10.3233/JND-170246
- Wechsler, D. (1999). WAIS-III: Escala de Inteligencia de Wechsler para Adultos III. TEA Ediciones.