In a recent article entitled “Neurotensin protects pancreatic beta cells from apoptosis” Coppola et al. (Citation2008) show that neurotensin (NT) protects insulin-producing cells (β-TC3, INS-1E) against IL-1β and staurosporine (STS)-induced cell death.
NT responses are known to be mediated through three receptors. Two NT receptors (NTSR1 and NTSR2) belong to the family of G-protein coupled receptors (GPCRs). The third NT receptor, NTSR3, also called sortilin, belongs to the family of the receptors related to the yeast sorting receptor Vps10p. All three receptors are expressed in these cells, although NTSR2 expressed in β-TC3 has a different molecular weight (MW) from that in INS-1E cells, which in turn has a different MW from that expected for NTSR2. According to the authors “discrepancies are the consequences of distinct phospholipids environments leading to different solubilities in detergent”. However, they provide no references to support this assumption and, in addition, specificity controls, i.e. pre-adsorption of the serum by its immunogenic peptide, that would strengthen this point, were not done.
The NTSR1 antagonist SR48692 did not reverse the protective action of NT on IL-1β induced cell death while the NTSR2 agonist, levocabastine, had the same protective action as NT, thus implicating NTSR2 in this protection. NT in IL-1β treated cells reduced caspase-3 activity. Levocabastine had the same effect as NT on caspase-3, while SR48692 had no effect; the NTSR3 propeptide released from the precursor form of NTSR3, and described as an antagonist of NT, also had no effect (Munck Petersen et al., Citation1999). Paradoxically, equimolar amounts of NTSR3 propeptide were used in this study, while at least 10-fold higher concentrations are needed to effectively antagonize NT, thus no conclusion can be drawn as to its NT-antagonizing/caspase-3-activating effect.
No data is given as to the percent of β-TC3 and INS-1E cell death induced by STS, as determined by Hoechst 33342 staining. Some effect of NT on the STS-induced caspase-3 activity was seen after 8 hours, but the effect was not followed even for 24 hours.
In Figure 5 (of Coppola et al., 2008) some indirect evidence is given suggesting that the PI-3 kinase pathway is involved in the NT effect against STS-induced cell death (individual effects of the kinase inhibitors are not shown). Unfortunately the study provides little information on the mechanisms involved in the NT protection against IL-1β induced cell death, and it would have been more probing that the authors explored further this effect instead of studying also the STS-induced effect. Cytokine-induced cell death of pancreatic beta cells implicated nitric oxide (NO) and prostaglandin E2 production, due to the up-regulation of the inducible form of NO synthase and cyclo-oxygenase-2, JAK/STAT activation and NF-κB activation, and it would be of interest to evaluate whether NT interferes with any of these pathways (Eizirik et al., Citation2008).
For unexplained reasons, IL-1β and STS are added in cell cultures in serum-free medium. However, in all recent articles IL-1β and STS are added to pancreatic cell lines in culture in serum-supplemented medium (Ortis et al., Citation2006; Tejedo et al., Citation2004). It is well known that β-TC3 and INS-1E are clonal insulin-producing cells that depend on serum for their survival. So, the reported 2 to 5% cell death for the control cells seems low for these conditions.
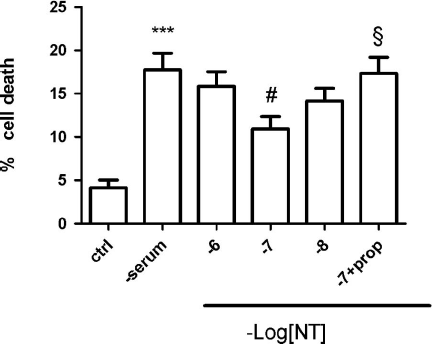
As shown in the , there is a very significant cell death of β-TC3 cells grown to about 70% confluency in RPMI-1640, 10% FCS, 2 mM glutamine, penicillin (100 U/ml) streptomycin (100 μg/ml) and followed by a 48 hour serum deprivation, as measured with the Hoechst 33342 dye. Addition of 0.1 μM NT significantly protected against cell death and this effect was also significantly reversed by the addition of 1 μM NTSR3 propeptide, thus implicating sortilin in mediating the NT protective effect against cell death by serum deprivation. Additional experiments should be performed in primary beta cells to clarify the role of NT for beta cell survival.
Figure 1. Neurotensin protects β-TC3 pancreatic cells against serum deprivation-induced cell death. Cells were cultured in serum-free medium for 48 hours. NT was added at the concentrations indicated at 0 hour and 24 hours of starvation. The NTSR3 propeptide (prop) was added at 1 μM. Controls were in presence of serum. After 48 hours viable cells were determined using the Hoechst 33342 dye. Means ± SEM are from five independent experiments. ***p < 0.001 as compared to control; #,§p < 0.05 as compared to – serum and 10−7 M NT respectively, by Student's t-test.
Sortilin together with p75NTR was shown to mediate neuronal cell death by proneurotrophins and excess of NT reversed this effect (Nykjaer et al., Citation2004; Teng et al., Citation2005). However, the pathway involved in sortilin mediated cell death is not yet known. Several evidences link cell death due to serum deprivation to a pathway independent of caspase activation, via mitochondrial pro-apoptotic factors such as AIF (Joza et al., Citation2001; Pandey et al., Citation2003), while cytokine-induced and STS-induced apoptosis in pancreatic cells are caspase dependent.
In summary, different death stimuli in pancreatic cells may involve different NT receptors and be mediated via different pathways.
Acknowledgement
The author is chargée de recherche at the French CNRS (Centre National de la Recherche Scientifique).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- T Coppola, S Beraud-Dufour, A Antoine, J-P Vincent, and A Mazella. (2008). Neurotensin protects pancreatic beta cells from apoptosis. Int J Biochem Cell Biol 40:2296–2302.
- D L Eizirik, F Moore, D Flamez, and F Ortis. (2008). Use of a systems biology approach to understand pancreatic β-cell death in Type 1 diabetes. Biochem Soc Trans 36:321–327.
- N Joza, S A Susin, E Daugas, W L Stanford, S K Cho, C YJ Li, T Sasaki, A J Elia, H YM Cheng, L Ravagnan, K F Ferri, and et al (2001). Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature 410:549–554.
- C Munck Petersen, M S Nielsen, C Jacobsen, J Tauris, L Jacobsen, J Gliemann, S K Moestrup, and P Madsen. (1999). Propeptide cleavage conditions sortilin/neurotensin receptor-3 for ligand binding. EMBO J 18:595–604.
- A Nykjaer, R Lee, K K Teng, P Jansen, P Madsen, M S Nielsen, C Jacobsen, M Kliemannel, E Schwarz, T E Willnow, B L Hempstead, and C M Petersen. (2004). Sortilin is essential for proNGF-induced neuronal cell death. Nature 427:843–848.
- F Ortis, A K Cardozo, D Crispim, J Störling, T Mandrup-Poulsen, and D L Eizirik. (2006). Cytokine-induced pro-apoptotic gene expression in insulin-producing cells is related to rapid, sustained, and non-oscillatory nuclear factor-κB activation. Mol Endocrinol 20 (8):1867–1879.
- S Pandey, C Lopez, and A Jammu. (2003). Oxidative stress and activation of proteasome protease during serum deprivation-induced apoptosis in rat hepatoma cells; inhibition of cell death by melatonin. Apoptosis 8:497–508.
- J R Tejedo, G M Cahuana, R Ramírez, M Esbert, J Jiménez, F Sobrino, and F J Bedoya. (2004). Nitric oxide triggers the phosphatidylinositol 3-kinase/Akt survival pathway in insulin-producing RINm5F cells by arousing Src to activate insulin receptor substrate-1. Endocrinol 145:2319–2327.
- H K Teng, K K Teng, R Lee, S Wright, S Tevar, R D Almeida, P Kermani, R Torkin, Z Y Chen, F S Lee, R T Kraemer, A Nykjaer, and B L Hempstead. (2005). ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J Neurosci 25:5455–5463.