Abstract
It is reported that elevated visfatin level is associated with gestational diabetes mellitus (GDM). However, the relationship between visfatin level and GDM remains controversial. The aim of our study was to systematically review available literature linking visfatin to GDM for a comprehensive understanding of the relationship between circulating visfatin level and GDM in human. PubMed, The Cochrane Library and Web of Science were searched for studies published up to July 2020. Standard mean difference with 95% confidence interval was calculated to evaluate the relationship between visfatin level and GDM using the Review Manager 5.3 and Stata 12.0. The evidence indicated that no significant difference was observed in the level of circulating visfatin between the women with GDM and normal glucose tolerance, suggesting circulating visfatin level is not independently related to GDM. Nevertheless, visfatin is involved in the development of GDM in obese women.
Introduction
Gestational diabetes mellitus (GDM) is defined as abnormal glucose metabolism that is first discovered or diagnosed during pregnancy (Alberti and Zimmet Citation1998). As most patients with GDM usually do not show obvious clinical manifestations, GDM screening has become a routine prenatal examination using a glucose tolerance test (OGTT) during the second trimester of pregnancy. The prevalence of GDM has been markedly rising over the past few decades. It is reported that GDM has affected up to 14% of pregnant women, depending on different screening methods, diagnostic criteria and the population screened (Jiwani et al. Citation2012). With the global increasing prevalence of GDM, more and more attention has been attracted to the effects of GDM on mothers and their offspring, especially in the developing countries such as China, India and Africa (Tutino et al. Citation2014). Various risk factors, including genetic, social, environmental and psychological, contribute to the development of GDM. In addition to the higher risk of maternal postpartum type 2 diabetes mellitus (T2DM), GDM is related to an increased frequency of potential adverse outcomes and long-term metabolic dysregulation in offspring (Metzger et al. Citation2008; Damm Citation2009; Catalano Citation2014; Page et al. Citation2014). Undoubtedly, GDM has become an important public health problem worldwide. However, the related pathological mechanisms underlying GDM are still not understood.
Recent reports have suggested that adipokines (e.g. adiponectin, leptin, and visfatin), secreted by the adipose tissue, are involved in various physiological and pathological processes, including insulin sensitivity, energy expenditure, glucose and lipid metabolism, inflammatory activity, homeostasis, and cardiovascular function (Koerner et al. Citation2005; Bluher Citation2012; Fasshauer and Bluher Citation2015). Visfatin, also named pre-B cell colony-enhancing factor (PBEF)/nicotinamide phosphoribosyltransferase (Nampt), is a protein with a molecular weight of 52 kDa coded by a gene of 34.7 kb located on chromosome 7q22.2 that is transcribed in a 2.4 kb long mRNA (Morgan et al. Citation2008; Sitticharoon et al. Citation2014). Visfatin is secreted predominantly by the adipose tissue, but it is also synthesised in other tissues such as the skeletal muscle, liver, immune cells, cardiomyocytes, and brain. Visfatin exhibits intrinsic NAMPT activity, with its intracellular form (iNAMPT) regulating the intracellular levels of oxidised nicotinamide adenine dinucleotide (NAD) and its extracellular form acting as a cytokine in response to inflammatory stimuli or cellular stress (Grolla et al. Citation2016). It is involved in the synthesis of NAD, and it has been associated with obesity development, insulin secretion, lipid profile, and inflammation, among others. Visfatin was initially reported as an insulin-mimetic adipokine, which binds to and activates the insulin receptor, promotes adipogenesis, stimulates glucose uptake in vitro, and exerts glucose-lowering effects in mice in vivo (Fukuhara et al. Citation2005). However, subsequent studies were unable to reproduce these hypoglycaemic effects in humans (Fukuhara et al. Citation2007). Recently, increasing evidence points to a detrimental role for visfatin in the glycolipid metabolism and the pathogenesis of related diseases. An elevated visfatin level was observed in both T1DM and T2DM (Lopez-Bermejo et al. Citation2006). The main pathogenesis of GDM is insufficient insulin secretion from pancreatic β-cells to compensate for physiological insulin resistance. Hence, several studies have been conducted to explore the relationship between visfatin and GDM, while the results are controversial. Previous studies have suggested either increased (Souvannavong-Vilivong et al. Citation2019), comparable (Boyadzhieva et al. Citation2013), or decreased (Chan et al. Citation2006) visfatin levels in GDM women compared to those healthy pregnant controls.
In order to provide a more comprehensive estimation of the association between circulating visfatin level and GDM, we performed a systematic review and meta-analysis of related studies aiming for getting a more persuasive conclusion.
Methods
Our review followed the Meta-analysis of Observational Studies in Epidemiology guidelines (Stroup et al. Citation2000). The data were presented according to the recommendations of the PRISMA statement (Moher et al. Citation2009).
Search strategy
To study the association between circulating visfatin level and GDM, a comprehensive systematic literature search was conducted with the use of PubMed, The Cochrane Library and Web of Science up to July 2020. The search strategy included key terms that were summarised as follows: (1) gestational diabetes, pregnancy diabetes, maternal diabetes, gestational diabetes mellitus, or GDM. (2) nicotinamide phosphoribosyltransferase, pre-B cell colony enhancing factor, visfatin, NAMPT Protein, NAmPRTase, NMN Pyrophosphorylase, or PBEF. In addition, reference lists of the original studies and review articles were considered and manually searched.
Inclusion and exclusion criteria
An initial screening was based on the titles and abstracts of published papers, and the full texts were then reviewed carefully during the second screening. Studies were considered eligible if they met the following criteria: (1) the study design was an observational study; (2) the study reported the association of visfatin level with GDM; (3) GDM as the outcome and the control were women with normal glucose tolerance (NGT); (4) all participants did not have a previous history of diabetes or present pregnant complications; (5) full-text articles were published in English. Studies were excluded if they were (1) available only as abstracts, review studies, case reports, expert comment, or editor opinion; (2) experimentation on animals or in vitro; (3) predefined outcome data required for analyses were lacking.
Data extraction and quality evaluation
All of the literature searches were reviewed independently by two authors and relevant data were extracted from each paper with the use of standardised data collection forms. If there was a discrepancy, a discussion was carried out to reach an agreement. If a consensus could not be reached, a third experienced investigator was consulted. The following information about each study was recorded: first author, year of publication, country of the study, GDM criteria, sample source, assay method of visfatin, sample size of the case and control group, mean and standard deviation (SD) (part of the data were converted) of visfatin level, trimester of visfatin level measurement, and mean of age and body mass index (BMI) of GDM women.
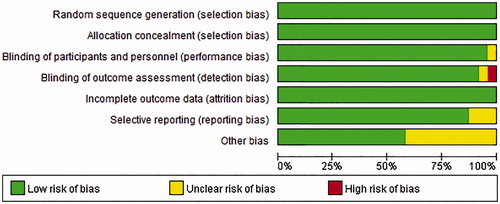
The individual study quality was assessed according to the Cochrane collaboration’s tool for risk of bias, which contains random sequence generation, allocation concealment, blindness, incomplete outcome data, selective outcome reporting, and other biases.
Statistical analysis
Standard mean difference (SMD) and 95% confidence interval (95% CI) were calculated to assess the differences in visfatin levels between groups. Significance levels were determined by Z test. Forest plots were used to demonstrate effect sizes and their CI. Heterogeneity amongst the included studies was assessed by Cochran’s Q statistics and I2 statistics. According to heterogeneity inspection results, the corresponding pooled method was chosen: if I2 > 50%, the random effect model was used; while I2 ≤ 50%, the fixed effect model was adapted. We also did subgroup analyses to explore the potential source of heterogeneity if heterogeneity across studies was statistically significant. Potential publication bias was evaluated using Begg’s test and Egger’s test. Sensitivity analyses were carried out by sequentially omitting one single study each time to test the robustness of uncertainty in the meta-analysis. All data were analysed with Review Manager (RevMan 5.3) statistical software provided by The Cochrane Collaboration and Stata 12.0 (Stata Corp, College Station, TX, USA). The significance level was set as .05, except the Cochran’s Q test for heterogeneity as 0.1.
Results
Literature search
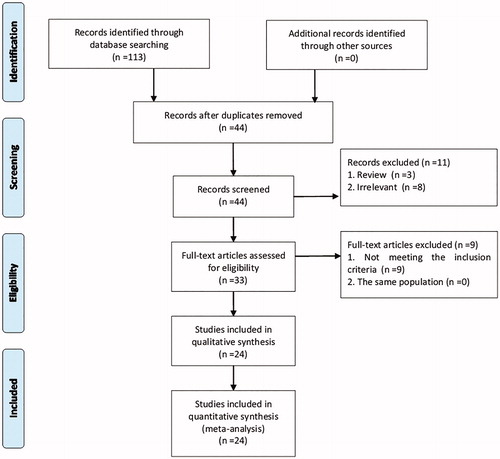
A flow diagram of the included and excluded studies was shown in . According to the search strategy, 113 citations were identified from the three databases and a manual search. After removing the duplicates (n = 69), two reviewers screened the titles and abstracts of potentially relevant studies (n = 44) independently. Finally, a total of 26 studies that were published in 24 articles were included in the current meta-analysis (Chan et al. Citation2006; Telejko et al. Citation2009; Abell et al. Citation2017; Souvannavong-Vilivong et al. Citation2019).
Characteristics and quality assessment of study
All included studies published from 2006 to 2020 were designed as observational studies involving 1060 GDM patients and 1302 healthy pregnant women. The characteristics of the studies included in the present meta-analysis were shown in . Of the 24 included studies, half of the studies were conducted in Asia, including Turkey, China, Saudi Arabia and Thailand, and the other half in the Caucasian region, including Australia, Bulgaria, Iran, United Kingdom, Slovenia and Poland. The sample size of these studies ranged from 10 to 300. GDM was usually diagnosed during the 24–28 weeks of gestation using appropriate criteria, including Carpenter and Coustan criteria (C&C), World Health Organisation criteria (WHO), American Diabetes Association criteria (ADA), Australasian Diabetes in Pregnancy Society (ADIPS), International Association of Diabetes and Pregnancy study groups (IADPSG), National Diabetes Data Group (NDDG), and the 4th Workshop Conference of Gestational Diabetes (4th WCGD). In addition, except for one study conducted in Australia (Haider et al. Citation2007) that did not report the BMI of GDM patients, 17 of the included studies involved in the GDM women with BMI < 30 kg/m2, and the rest with BMI ≥ 30 kg/m2. Plasma or serum samples for visfatin measurement were collected in the first, second or third trimester of gestation through enzyme-linked immunosorbent assay (ELISA) or enzyme immune assay (EIA) assay.
Table 1. Characteristics of studies included in the meta-analysis.
The assessment of the quality of the included studies was shown in .
Overall meta-analysis
As indicated in , the overall levels of circulating visfatin in GDM patients were not significantly different from that in the healthy controls (SMD = 0.1; 95% CI: −0.28–0.48, p = .61). The SMDs from the individual studies were analysed using random-effects models, as the heterogeneity was considered significant (p < .00001, I2 = 94%).
Figure 3. Forest plot of circulating visfatin level in GDM or healthy pregnant women. The random effect model (Inverse Variance method) was applied.
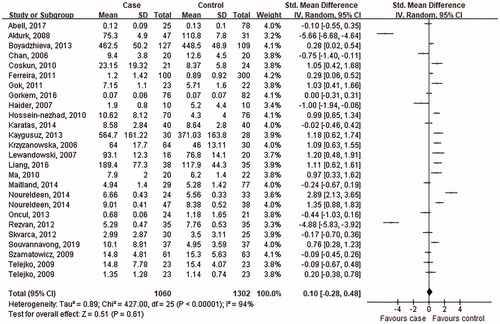
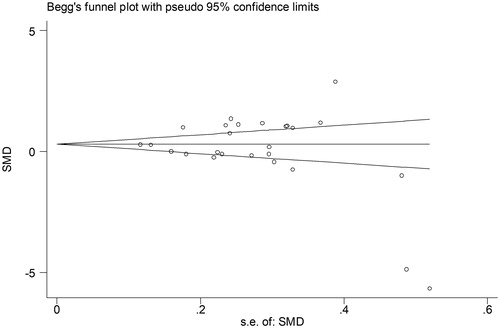
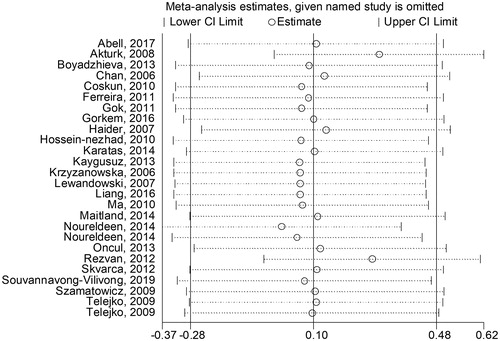
No significant publication bias was found in our meta-analysis as indicated in (Begg’s test: p = .454; Egger’s test: p = .302). Sensitivity analysis was performed to explore potential sources of heterogeneity and assess relevant changes on the combined results. As suggested in , the estimates effects showed no obvious relevant changes in the combined results by study removals, indicating the findings were robust against study deletions.
Subgroup analysis
To further investigate the source of heterogeneity and obtain thorough information from this meta-analysis, subgroup analysis was performed. Subgroup analysis was conducted based on geographic site, measurement trimester, measurement method, sample source, and mean age and BMI of GDM women. The comprehensive results were shown in , and .
Figure 6. Subgroup analysis of visfatin levels in GDM or healthy pregnant women based on average BMI. The random effect model (Inverse Variance method) was applied.
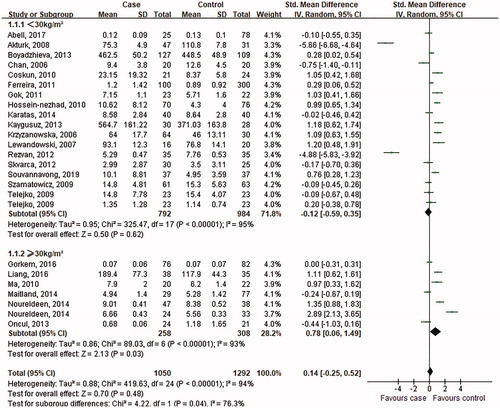
Figure 7. Subgroup analysis of visfatin levels in GDM or healthy pregnant women based on measurement trimester. The random effect model (Inverse Variance method) was applied.
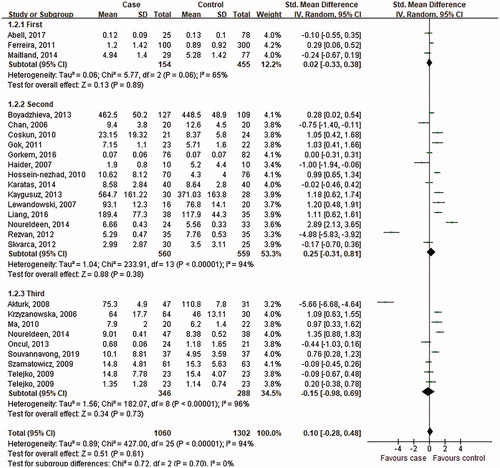
Table 2. Subgroup meta-analysis of the included studies.
As indicated in , in the subgroup analysis depending on BMI of GDM women, the random effect model was chosen to do the pooled analysis because significant heterogeneity was observed (BMI < 30 kg/m2: p < .00001, I2 = 95%; BMI ≥30 kg/m2: p < .00001, I2 = 93%). For women with BMI ≥30 kg/m2, the circulating visfatin levels increased in the GDM women (SMD = 0.78; 95% CI: 0.06–1.49, p = .03); however, for women with BMI <30 kg/m2, the difference was not significant (SMD = −0.12; 95% CI: −0.59 − 0.35, p = .62).
When stratifying by the measurement trimester of visfatin, these studies were classified as the first, second and third trimester. Three studies conducted in the first trimester, fourteen carried out in the second, and the rest in the third presented a conclusion of heterogeneity (first: p = .06, I2 = 65%; second: <.001, I2 = 94%; third: <.001, I2 = 96%), then the random effect model was chosen to do the pooled analysis. The results in revealed that the visfatin level exhibited no significant difference between GDM women and control subjects in the first trimester (SMD = 0.02, 95% CI: −0.33–0.38, p = .89), second trimester (SMD = 0.25, 95% CI: −0.31–0.81, p = .38), or third trimester (SMD = −0.15; 95% CI: −0.98–0.69, p = .73).
Additionally, subgroup analysis was also carried out based on geographic site, average age of GDM women, sample and assay method of visfatin. When stratifying by geographic site, these studies were classified as the Caucasian group and the Asian group (). No significant difference was observed in the visfatin level between the GDM group and NGT participants in different regions (Asian: SMD = 0.31; 95% CI: −0.37–0.99, p = .37; Caucasian: SMD = −0.1; 95% CI: −0.55–0.34, p = .65). Moreover, the studies were classified to two subgroups according to the average age of GDM women. The results indicated no obvious changes in the visfatin level among women with GDM regardless of age (<30 years: SMD = 0.67; 95% CI: −0.13–1.47, p = .1; ≥ 30 years: SMD = 0.01; 95% CI: −0.41–0.44, p = .95). Additionally, with regard to visfatin assay methods, the subgroup analysis demonstrated that there was no significant statistical difference in the level of visfatin between the GDM women and corresponding controls (ELISA: SMD = 0.23; 95% CI: −0.2–0.66, p = .29; EIA: SMD = −0.29; 95% CI: −1.16–0.59, p = .53). Furthermore, in the subgroup analysis of sample source of visfatin, the difference of plasma visfatin level between the GDM participants and controls was not statistically significant (SMD = −0.81; 95% CI: −2.01–0.39, p = .18); on the contrary, the difference was more significant in the serum visfatin level (SMD = 0.4; 95% CI: 0.03–0.77, p = .03) ().
Discussion
This systematic review and meta-analysis found that circulating visfatin levels in GDM patients were not significantly different from that in the healthy controls. Nevertheless, subgroup analysis showed that visfatin level was significantly higher in the obesity GDM participants than the normal controls. The changes of visfatin level in GDM patients had been already suggested by other authors, and a meta-analysis was made in 2018 (Zhang et al. Citation2018), which indicated no significant changes of visfatin level among women with GDM from 24 observational studies. Similarly, our meta-analysis of 26 independent observational studies published in 24 articles provided strong evidence that circulating visfatin levels in GDM women were equivalent to the healthy pregnant controls. However, compared with previous meta-analysis (Zhang et al. Citation2018), our meta-analysis conducted more comprehensive and thorough analysis and proposed more convincing and detailed results.
GDM is defined as any degree of glucose intolerance with onset or first recognition during pregnancy (Alberti and Zimmet Citation1998). In the second and third trimester, due to the physiological insulin resistance, maternal insulin secretion is elevated to maintain blood glucose levels. Impairment of pancreatic β-cell function or ompensatory increases of insulin secretion or both leads to GDM (Catalano Citation2014). However, the precise mechanisms of insulin resistance underlying GDM remain unknown. It is likely as a result of up-regulation of insulin antagonist hormones during pregnancy. Given the prevalence of GDM, a growing body of studies has been conducted to investigate the physiological and pathological mechanisms of GDM both in animal and human (Todoric et al. Citation2013; Ameri et al. Citation2015; Wu et al. Citation2015; Giacobbe et al. Citation2016; Retnakaran et al. Citation2016). As an important adipocytokines, several studies have explored the role of visfatin in humans, particularly in the context of non-alcoholic fatty liver disease, obesity, DM and insulin resistance. Previous studies have primarily focussed on the relationship between visfatin and T2DM, and the focus shifts to GDM in recent years. However, the evidence about the relationship between visfatin and GDM is conflicting.
In the current meta-analysis, 26 independent observational studies published in 24 articles involving 2362 patients were included. In these studies, 12 studies indicated higher visfatin level in the GDM women, 6 studies showed higher visfatin level among healthy pregnant women, and 8 studies did not identify significant difference. 12 studies claimed that patients with GDM showed significantly increased visfatin level compared with healthy subjects (Coskun et al. Citation2010; Hossein-Nezhad et al. Citation2010; Ma et al. Citation2010; Gok et al. Citation2011; Kaygusuz et al. Citation2013; Noureldeen et al. Citation2014; Souvannavong-Vilivong et al. Citation2019). Coskun et al. (Citation2010) revealed that patients with GDM had significantly higher levels of serum visfatin in the second trimester. This trend was furtherly confirmed at different gestational stages, including the first trimester (Ferreira et al. Citation2011), the second trimester (Lewandowski et al. Citation2007; Hossein-Nezhad et al. Citation2010; Gok et al. Citation2011; Kaygusuz et al. Citation2013; Noureldeen et al. Citation2014; Liang et al. Citation2016), and the third trimester (Krzyzanowska et al. Citation2006; Ma et al. Citation2010; Souvannavong-Vilivong et al. Citation2019). In addition, elevated visfatin level was observed not only in GDM patients, but also in pre-GDM women (Coskun et al. Citation2010). Meanwhile, visfatin increased during pregnancy and 2 weeks after delivery in women with GDM (Krzyzanowska et al. Citation2006), and then reduced within 6 to 10 weeks after delivery (Gok et al. Citation2011). A significant gradual increase of insulin resistance during pregnancy may partly contribute to the phenomenon mentioned above. The alteration of visfatin has been reported to be influenced by multiple factors, such as age, sex, duration of diabetes and BMI, as well as environmental and genetic factors. It is indicated that visfatin concentration increases during physiological pregnancy. This increase is further enhanced by obesity and obesity-related pathologies (Zahorska-Markiewicz et al. Citation2007). And obesity is one of the major factors leading to the development of GDM. Consistently, it is demonstrated that visfatin level was significantly higher in the obesity GDM participants than the normal controls in this meta-analysis. Taken together, these results indicate that increased circulating visfatin in GDM women, which has insulin-mimetic effect, might be associated with a compensatory mechanism that improves the impaired insulin function during pregnancy, especially in obese GDM women.
Circulating visfatin levels are regulated by insulin and glucose levels, and enhanced in various conditions, including insulin resistance, obesity and diabetes (Pfutzner et al. Citation2006). In the state of GDM, characterised by chronic hyperglycaemia or insulin resistance, individuals are more susceptible to various stimuli-mediated oxidative stress and inflammation, which may lead to the increased level of visfatin, especially in the second and third trimester (Morgan et al. Citation2008). Hence, it may be speculated that an insufficiency of visfatin may play a role in the pathogenesis of GDM. Consistently, six studies suggested that circulating visfatin concentrations are lower in GDM patients than that in the NGT subjects (Chan et al. Citation2006; Haider et al. Citation2007; Akturk et al. Citation2008; Telejko et al. Citation2009; Rezvan et al. Citation2012; Oncul et al. Citation2013). Moreover, the trend was indicated not only in maternal serum, but also in cord blood serum with GDM (Oncul et al. Citation2013), suggesting a potential effect of GMD on offspring. Meanwhile, this change was not correlated with dietary intake of pregnant women (Rezvan et al. Citation2012). Taken together, the evidence mentioned above suggests that an insufficiency of visfatin may play a role in the pathogenesis of GDM. However, it is not yet known whether the low visfatin status contributes to disease aetiology, or may be a consequence of a disease that aggravates the condition further.
Previous studies have reported the changes of visfatin levels in GDM, including elevation and decrease. In addition, eight studies reported that there was no significant correlation between visfatin and GDM (Szamatowicz et al. Citation2009; Skvarca et al. Citation2012; Boyadzhieva et al. Citation2013; Karatas et al. Citation2014; Maitland et al. Citation2014; Noureldeen et al. Citation2014; Gorkem et al. Citation2016; Abell et al. Citation2017). The current systematic review and meta-analysis similarly concluded no association between circulating visfatin concentrations and GDM. Difference in trimester, assay method, diagnostic criteria, age and BMI, or even racial differences may partly contribute to this discrepancy. Potential mechanisms are not clear because of the insufficient studies. Further related studies should be conducted.
The current meta-analysis showed that the pooled value of Mean [95% CI] was not statistically significant, revealing equivalent circulating levels of visfatin in GDM women and controls. However, according to the overall forest plot in , substantial heterogeneity (I2 = 94%) was observed among the studies. Accordingly, subgroup analysis and sensitivity analysis were performed to elucidate the possible confounding factors and investigate the source of heterogeneity. Subgroup analysis was depended on geographic site, sample source, measurement trimester, measurement method, and mean of age and BMI of GDM women. Previous research indicated that the circulating visfatin level is associated with the BMI (Berndt et al. Citation2005; Zahorska-Markiewicz et al. Citation2007). For the 7 original studies (from 6 articles), in which the level of BMI in GDM women was higher than 30 kg/m2, subgroup analysis indicated that visfatin levels were elevated in GDM patients with BMI ≥30 kg/m2, while not in women with BMI <30 kg/m2. The higher tendency of insulin resistance and increasing level of lipid profiles in obesity patients may contribute to the above results. However, the trend was not available in the other subgroup analysis, including age, sampling time during pregnancy, race, and assay method. Additionally, the result of subgroup analysis based on sample source demonstrated that the serum visfatin level was higher among GDM participants than the controls. Considering that the detection methods are similar, different components in serum and plasma may partly contribute to the above result. Additionally, sensitivity analysis was performed by excluding articles one by one before reanalysing statistically. The results showed that the final meta-analysis results were stable with omittance of any study, which provided more credibility to our interpretation of results.
Giving the acute adverse outcomes and long-term effects on pregnant women and offspring, GDM is drawing increasing attention in recent years. The topic is therefore of relevance from the public health perspective and the present meta-analysis can contribute to clarify some of the pathophysiological mechanisms of GDM by providing statistical assessment. However, there are some limitations to this meta-analysis. First, the publication bias cannot be avoided absolutely, as only published studies in English in the selected databases were included. Second, the measurement methods of visfatin were not consistent among included studies, which may affect the accuracy of circulating visfatin concentration. Third, we have no access to get the original data of the included literature, so we cannot guarantee the accuracy of the data. Therefore, the results should be interpreted with caution.
Conclusion
In conclusion, the meta-analysis of all published observational studies on visfatin and GDM revealed equivalent circulating levels of visfatin in patients with GDM, suggesting circulating visfatin level is not independently related to GDM.
Nevertheless, visfatin participates in the development of GDM in obese women. Considering that GDM usually has no obvious symptoms, visfatin might be a potential predictor for assessing GDM with obesity. This result could help clinical staff to instruct women with GDM to prevent the development and progression of GDM. The regulation and metabolism of visfatin remained unclear in human. For further studies, well-designed epidemiological studies with large sample sizes and strict stratification of potential confounding factors should be performed. It will be meaningful and interesting to explore the potential role of visfatin in GDM prediction and therapeutics.
Author contributions
YK Jiang and FX Gong designed the research; YK Jiang and HY Deng performed the research; FX Gong and HY Deng analysed the data; FX Gong and ZY Qiao wrote the paper; HY Deng and ZY Qiao provided critical feedback and revised the manuscript. All authors read and approved the final manuscript.
Disclosure statement
The authors declare that they have no conflict of interest.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author, FX Gong. The data are not publicly available due to the containing information that could compromise the privacy of research participants.
References
- Abell, S.K., et al., 2017. The association between dysregulated adipocytokines in early pregnancy and development of gestational diabetes. Diabetes/metabolism research and reviews, 33 (8), e2926.
- Akturk, M., et al., 2008. Visfatin concentration is decreased in women with gestational diabetes mellitus in the third trimester. Journal of endocrinological investigation, 31 (7), 610–613.
- Alberti, K.G. and Zimmet, P.Z., WHO Consultation, 1998. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabetic medicine, 15 (7), 539–553.
- Ameri, P., et al., 2015. Impaired increase of plasma abscisic Acid in response to oral glucose load in type 2 diabetes and in gestational diabetes. Plos one, 10 (2), e115992.
- Berndt, J., et al., 2005. Plasma visfatin concentrations and fat depot-specific mRNA expression in humans. Diabetes, 54 (10), 2911–2916.
- Bluher, M., 2012. Clinical relevance of adipokines. Diabetes & metabolism journal, 36, 317–327.
- Boyadzhieva, M., et al., 2013. Adipocytokines during pregnancy and postpartum in women with gestational diabetes and healthy controls. Journal of endocrinological investigation, 36, 944–949.
- Catalano, P.M., 2014. Trying to understand gestational diabetes. Diabetic medicine: a journal of the British Diabetic Association, 31 (3), 273–281.
- Chan, T.F., et al., 2006. Decreased plasma visfatin concentrations in women with gestational diabetes mellitus. Journal of the Society for Gynecologic Investigation, 13 (5), 364–367.
- Coskun, A., et al., 2010. Plasma visfatin levels in pregnant women with normal glucose tolerance, gestational diabetes and pre-gestational diabetes mellitus. The journal of maternal-fetal & neonatal medicine, 23 (9), 1014–1018.
- Damm, P., 2009. Future risk of diabetes in mother and child after gestational diabetes mellitus. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics, 104 Suppl 1, S25–S26.
- Fasshauer, M. and Bluher, M., 2015. Adipokines in health and disease. Trends in pharmacological sciences, 36 (7), 461–470.
- Ferreira, A.F., et al., 2011. Maternal serum visfatin at 11-13 weeks of gestation in gestational diabetes mellitus. Clinical chemistry, 57 (4), 609–613.
- Fukuhara, A., et al., 2005. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science (New York, N.Y.), 307 (5708), 426–430.
- Fukuhara, A., et al., 2007. Retraction. Science (New York, N.Y.), 318 (5850), 565.
- Giacobbe, A., et al., 2016. Association between maternal serum high mobility group box 1 levels and pregnancy complicated by gestational diabetes mellitus. Nutrition, metabolism, and cardiovascular diseases: NMCD, 26 (5), 414–418.
- Gok, D.E., et al., 2011. The role of visfatin in the pathogenesis of gestational diabetes mellitus. Journal of endocrinological investigation, 34 (1), 3–7.
- Gorkem, U., et al., 2016. Are adipokines associated with gestational diabetes mellitus? Journal of the Turkish German Gynecological Association, 17 (4), 186–190.
- Grolla, A.A., et al., 2016. Extracellular nicotinamide phosphoribosyltransferase, a new cancer metabokine. British journal of pharmacology, 173 (14), 2182–2194.
- Haider, D.G., et al., 2007. Visfatin response to glucose is reduced in women with gestational diabetes mellitus. Diabetes care, 30 (7), 1889–1891.
- Hossein-Nezhad, A., et al., 2010. Resistin, adiponectin and visfatin; can adipocytokines predict gestational diabetes mellitus and early post partum metabolic syndrome. Iranian journal of diabetes and lipid disorders, 9, 1–8.
- Jiwani, A., et al., 2012. Gestational diabetes mellitus: results from a survey of country prevalence and practices. The journal of maternal-fetal & neonatal medicine, 25 (6), 600–610.
- Karatas, A., et al., 2014. Relationship of maternal serum resistin and visfatin levels with gestational diabetes mellitus. Gynecological endocrinology, 30 (5), 355–358.
- Kaygusuz, I., et al., 2013. Serum levels of visfatin and possible interaction with iron parameters in gestational diabetes mellitus. Gynecologic and obstetric investigation, 75 (3), 203–209.
- Koerner, A., Kratzsch, J., and Kiess, W., 2005. Adipocytokines: leptin-the classical, resistin-the controversical, adiponectin-the promising, and more to come. Best practice & research. Clinical endocrinology & metabolism, 19 (4), 525–546.
- Krzyzanowska, K., et al., 2006. Increased visfatin concentrations in women with gestational diabetes mellitus. Clinical science, 110 (5), 605–609.
- Lewandowski, K.C., et al., 2007. Elevated serum levels of visfatin in gestational diabetes: a comparative study across various degrees of glucose tolerance. Diabetologia, 50 (5), 1033–1037.
- Liang, Z., et al., 2016. Correlations of serum visfatin and metabolisms of glucose and lipid in women with gestational diabetes mellitus. Journal of diabetes investigation, 7 (2), 247–252.
- Lopez-Bermejo, A., et al., 2006. Serum visfatin increases with progressive beta-cell deterioration. Diabetes, 55 (10), 2871–2875.
- Ma, Y., et al., 2010. The changes of visfatin in serum and its expression in fat and placental tissue in pregnant women with gestational diabetes. Diabetes research and clinical practice, 90 (1), 60–65.
- Maitland, R.A., et al., UPBEAT trial consortium, 2014. Prediction of gestational diabetes in obese pregnant women from the UK Pregnancies Better Eating and Activity (UPBEAT) pilot trial. Diabetic medicine: a journal of the British Diabetic Association, 31 (8), 963–970.
- Metzger, B.E., et al., HAPO Study Cooperative Research Group, 2008. Hyperglycemia and adverse pregnancy outcomes. The New England journal of medicine, 358 (19), 1991–2002.
- Moher, D., et al., PRISMA Group, 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS medicine, 6 (7), e1000097.
- Morgan, S.A., Bringolf, J.B., and Seidel, E.R., 2008. Visfatin expression is elevated in normal human pregnancy. Peptides, 29 (8), 1382–1389.
- Noureldeen, A.F., et al., 2014. Maternal leptin, adiponectin, resistin, visfatin and tumor necrosis factor-alpha in normal and gestational diabetes. Indian journal of clinical biochemistry: IJCB, 29 (4), 462–470.
- Oncul, M., et al., 2013. Maternal and cord blood apelin, resistin and visfatin levels in gestational diabetes mellitus. Minerva medica, 104 (5), 527–535.
- Page, K.A., et al., 2014. Gestational diabetes mellitus, maternal obesity, and adiposity in offspring. Journal of pediatrics, 164 (4), 807–810.
- Pfutzner, A., et al., 2006. The release of the adipocytokine visfatin is regulated by glucose and insulin. Diabetologia, 49, 1909–1914. Diabetologia 2006;49:2795, 2796
- Retnakaran, R., et al., 2016. Evaluation of Circulating Determinants of Beta-Cell Function in Women With and Without Gestational Diabetes. The journal of clinical endocrinology & metabolism, 101 (7), 2683–2691.
- Rezvan, N., et al., 2012. Serum visfatin concentrations in gestational diabetes mellitus and normal pregnancy. Archives of gynecology and obstetrics, 285 (5), 1257–1262.
- Sitticharoon, C., et al., 2014. Interactions between adiponectin, visfatin, and omentin in subcutaneous and visceral adipose tissues and serum, and correlations with clinical and peripheral metabolic factors. Peptides, 62, 164–175.
- Skvarca, A., et al., 2012. Adipocytokines and insulin resistance across various degrees of glucose tolerance in pregnancy. The journal of international medical research, 40 (2), 583–589.
- Souvannavong-Vilivong, X., et al., 2019. Placental expressions and serum levels of adiponectin, visfatin, and omentin in GDM. Acta diabetologica, 56 (10), 1121–1131.
- Stroup, D.F., et al., 2000. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA, 283 (15), 2008–2012.
- Szamatowicz, J., et al., 2009. Serum visfatin concentration is elevated in pregnant women irrespectively of the presence of gestational diabetes. Ginekologia Polska, 80 (1), 14–18.
- Telejko, B., et al., 2009. Visfatin in gestational diabetes: serum level and mRNA expression in fat and placental tissue. Diabetes research and clinical practice, 84 (1), 68–75.
- Todoric, J., et al., 2013. Relationship of pentraxin 3 with insulin sensitivity in gestational diabetes. European journal of clinical investigation, 43 (4), 341–349.
- Tutino, G.E., et al., 2014. Diabetes and pregnancy: perspectives from Asia. Diabetic medicine: a journal of the British Diabetic Association, 31 (3), 302–318.
- Wu, H., et al., 2015. High-fat diet induced insulin resistance in pregnant rats through pancreatic pax6 signaling pathway. International journal of clinical and experimental pathology, 8, 5196–5202.
- Zahorska-Markiewicz, B., et al., 2007. Serum concentration of visfatin in obese women. Metabolism: clinical and experimental, 56 (8), 1131–1134.
- Zhang, W., et al., 2018. Association between circulating visfatin and gestational diabetes mellitus: a systematic review and meta-analysis. Acta diabetologica, 55 (11), 1113–1120.