Abstract
Objective: To evaluate the quality of management of oral anticoagulation among patients on oral anticoagulation for atrial fibrillation, and to verify the relation between patient performance and the risk of an event due to therapy. Methods: In a retrospective cross-sectional study involving 66 general practices, international normalized ratio (INR) values obtained over a 6-mo period were analysed. All INR values were determined by a single clinical laboratory, and additional medical information was provided by GPs. Results: 395 patients were included in the study, with a mean age of 74±9.6 y. In total, 3111 INR values were obtained. The mean number of tests/month per patient was 2.7±4.3. A total of 49 728 d of therapy was evaluated. Fifty-three per cent of the day values were within 0.5 INR units of the target (and 69% within 0.75 INR units of the target). The incidence rate for major bleeding was 4.4/100 patient years (and 2.9/100 patient years for thromboembolic events). There was a significant relation between patient performance and the presence of an event (p=0.017), with an odds ratio of 2.8 (95% CI 1.3–6.3). Conclusion: The quality of oral anticoagulation in patients with atrial fibrillation is suboptimal. This is significantly related to an increased risk of haemorrhagic events.
Introduction
Recent years have seen an increase in interest in the management of oral anticoagulation. Principally, this has been driven by the increasing numbers of patients receiving oral anticoagulation as a result of trials demonstrating the effectiveness of this treatment in preventing strokes in patients with atrial fibrillation (AF) Citation[1], Citation[2]. However, the risk of therapy with oral anticoagulants is high because of the following factors: the narrow therapeutic range of the anticoagulants, patient characteristics, and the variable quality of care depending on the management system.
Deviation from the therapeutic range is an important cause of treatment failure, including both thrombosis and haemorrhage. Cannegieter et al. found an increasing risk of haemorrhage or thrombosis when the international normalized ratio (INR) was above or below the therapeutic range of 2.5–4.9 Citation[3]. Van der Meer et al. found that the risk of major bleeding increased by 42% for each one-point increase in the INR Citation[4]. According to the third edition of the guidelines on oral anticoagulation, the target INR for patients with AF is 2.5 Citation[5]. The following quality criteria are defined: 50% of the INR results of the patient population should be within the range of 2–3 (and 80% within the INR range of 1.75–3.25). Several studies found an increase of bleeding with age and the dependence of bleeding frequency on the type of coumarin derivative used Citation[4], Citation[6–8]. The quality of supervision of the anticoagulation therapy by the physician is the third risk factor determining the occurrence of adverse events. In the Netherlands, the follow-up of the therapy is performed in anticoagulation clinics Citation[9]. The UK has a mixed system, the anticoagulant dosing being prescribed by the anticoagulation clinic rather than by GPs Citation[10]. In Germany, more than 90% of orally anticoagulated patients are controlled by the GP Citation[11]. As in Germany, the management of oral anticoagulation in Belgium is mainly performed by the GP. A venous blood sample is taken by the GP at the patient's home or in the office. Blood analysis is performed by an external laboratory. After obtaining the INR, the patient receives information from the GP regarding the dosage for the following days or weeks. The dosing and next date for INR control is based on the GP's clinical judgement.
A retrospective study of 15 GPs in Belgium showed 58% of the results within the target INR interval of 2–3 (target INR 2.5), and 68.38% within 1.75–3.25 Citation[12]. The aim of this cross-sectional study was to analyse the quality of the management of oral anticoagulation in patients with AF, and to verify the relation between patient performance and risk of haemorrhagic or thromboembolic events.
Methods
Population
All 255 GPs who send blood samples for analysis to the clinical laboratory at the Medical Centre for GPs in Tessenderlo (the northern part of the province of Limburg and the western part of the province of Antwerp, action rate 100 km) were invited to participate in the study. In total, 96 GPs (response rate of 38%) were enrolled in the study (71 males and 25 females). No significant difference in gender, age and type of practice was found between the participating GPs and the non-participating GPs. Of the 96 participating GPs, 52 were working in group practice and 44 were working in single practice. The mean number of years after graduation for the GPs was 18 y. Nine per cent of the GPs had been educated in oral anticoagulation within the year before the study. Eighty-one per cent graduated at the University of Leuven, 9% at the University of Antwerp, 6% at the University of Gent and 3% at the University of Brussels. Three sessions were organized to train the GPs in filling in patient anticoagulation files. During these sessions, the aim of the study and the definition of events were explained. The group practices counted for one entity; this resulted in 66 GP practices being included in the study.
Patients
A list of all patients from the co-operating GPs who had had more than one INR determination during the 6-mo study period was generated by the laboratory computer. This list of patient names was sent to the general practitioner, who was asked to fill in an anticoagulation file for each patient. Only patients anticoagulated for atrial fibrillation were included. The patients were entered into the study as soon as they were in a steady state after the initial start up of therapy. This initial start-up period was considered to be 28 d for all oral anticoagulants, starting doses and schemes. Patients who needed to discontinue the anticoagulation therapy for a surgical procedure during the registration period were dropped from the study. All lethal/severe/minor bleedings, thromboembolic complications or hospitalizations during this period had to be declared.
Study design
The study consists of a retrospective analysis of all INR determinations that were requested by the co-operating general practitioners. The primary outcome measure was quality of anticoagulation management, defined as the percentage of time that INR values were within their target ranges. This was calculated according to Rosendaal's algorithms, assuming a linear increase or decrease between two consecutive INR determinations. This percentage was calculated on the group and on the patient level. The latter implies the possibility of classifying the patients as poor (<50% of INR day values within INR range of 2–3; <80% within INR range of 1.75–3.25) or good (>50% within INR range of 2–3; >80% within INR range of 1.75–3.25) performers. Secondary outcome measures were the number of thromboembolic complications and the number of haemorrhages. The complications were classified according to severity, and were defined according to the European Atrial Fibrillation Trial (20).
Approval was obtained from the ethics committee of the Catholic University Hospital Leuven, and signed informed consent was obtained from each general practitioner before the start of the study. In accordance with Belgian law, the study was reported to the Belgian Commission for the Protection of Privacy, controlling for the private manipulation of patients data (name and address) in the study.
Analysis
In the descriptive analysis, the mean and interquartile range were calculated. Linear mixed models were used to model the percentage INR within the target range as a function of different covariates and factors. This means that, in order to take into account the dependency between patients of the same GP, the GP was added to the model as a random effect. The relation between patient performance and the presence of an event was analysed with a random-effect logistic regression model (with GP as the random effect). The alpha level was set at 5%. All analyses were performed with the statistical package SAS (version 8.2), using the procedure PROC MIXED for the linear mixed model and PROC NLMIXED for the random-effect logistic regression.
Results
Patient characteristics
Three hundred and ninety-five patients were included in the study: 51% were males (n=200) and 49% were females (n=195). The mean age of the patients was 74 y (interquartile range (IQR) 68–80 y). The risk factors for stroke and the occurrence of thromboembolic complications or bleedings were as follows: 239 patients had hypertension, 69 diabetes mellitus, 100 a prior stroke or transient ischaemic attack, 61 peripheral vascular disease, 144 congestive heart failure, 54 a previous myocardial infarction, 98 a valve disease, 19 a history of malignancy, and 45 were smokers. Three hundred and thirty-six patients were anticoagulated using phenprocoumon, 39 used acenocoumarol, 14 used warfarin, and in six patients the anticoagulant was unknown. The mean number of patients per GP practice was 7 (IQR 3–8).
Proportion of time that INR values were within their target ranges
The average follow-up per patient, including patients on short- and long-term anticoagulation, was 4 mo (IQR 3.2–5.3 mo). A total of 3111 INR values were obtained, with a mean number of 8 INR values/patient (IQR 5–10). The mean number of tests/month per patient was 2.7 (IQR 1.3–2.4).
Percentage of time in range and patient performance
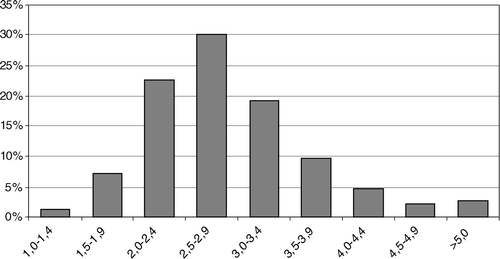
A total of 49 728 d of therapy were counted. Fifty-three per cent (26 555 d of therapy) were within the INR range of 2–3 (and 69% were within the INR range of 1.75–3.25) (). shows the distribution of the INRs: 8.7% were below the target range and 38.5% were above the target range; 2.8% of the patient days gave an INR > 5. For an INR range of 2–3, 213 were more than 50% within this range (good performers) and 182 patients were less than 50% within this range (poor performers). For an INR range of 1.75–3.25, 294 were more than 80% within this range (good performers) and 101 patients were less than 80% within this range (poor performers).
Table I. Percentage of time in target range and patient performance.
Relation between the percentage of time in range and co-variables
Simple models (without correction for other influences) showed no significant correlation between the percentage of time in the 2–3 INR range and gender (p=0.13), the age of the patients (p=0.06), the type of GP practice (single or group practice) (p=0.99), the anticoagulant used (phenprocoumon, acenocoumarol, warfarin) (p=0.43), the frequency of tests/patient (p=0.92), or the number of patients on oral anticoagulation/practice (p=0.14). A significant relation between the percentage of time in the 1.75–3.25 INR range and age was found (p = 0.049). The variability between GP practices was 22%.
Event rates
shows the number of thromboembolic and haemorrhagic events. The incidence rate of minor bleeding was 14 per 100 patient years. There were six major bleeding events, representing an incidence rate of 4.4 per 100 patient years. There were four thromboembolic events, representing an incidence rate of 2.9 per 100 patient years. In the logistic regression analysis, no significant relation between the presence of an event and hypertension, diabetes mellitus, prior stroke or transient ischaemic attack, congestive heart failure, myocardial infarction, valve disease, smoking habit, or a history of malignancy was found. In a random-effect logistic regression model, no significant relation between the presence of an event and patient performance within the 2–3 INR range was found (p=0.056). A significant relation between the presence of an event and patient performance within the 1.75–3.25 INR range was found (p=0.017). The odds ratio for patient performance (poor versus good) and an event was 2.85 (95% CI 1.3–6.3). The relation between patient performance and thromboembolic events was not significant (p=0.9), but the relation between patient performance and haemorrhagic events was significant (p=0.02), with an odds ratio of 5.16 (SE 2.92).
Table II. Number of thromboembolic and haemorrhagic events.
Discussion
In a retrospective cross-sectional study involving 66 GP practices, the INR values obtained over a 6-mo period were analysed. Fifty-three per cent of the day values were within 0.5 INR units of the target (69% within 0.75 INR units of the target). Analysing the distribution of the INRs, it appears that the suboptimal results are due to the fact that patients were over-anticoagulated: 38.5% of the INRs were above the target range (2.8% of the INRs were >5). In 101 of the 395 patients, the INR day values were <80% within the 1.75–3.25 INR range (poor performers). A significant relation between the presence of an event and patient performance (good versus poor) was found, with an odds ratio for a haemorrhagic event of 5.16.
The strength of this study is that all the INR analyses were performed in one single clinical laboratory. A check for possible inclusion bias of the participating GPs (response rate of 38% for all 255 GPs) showed no significant difference in distribution (p=0.7) of the INRs between patients from participating and patients from non-participating GPs. This study is limited by being a retrospective cross-sectional study of general practice case records. Therefore, the dates of the events were often missing and a survival analysis could not be performed because no time-to-event was available. The analyses to look for a relation between patient performance and the presence of an event are therefore only exploratory. Collection of the events occurred on a voluntary basis and could have been incomplete, especially regarding minor events. In addition, no reliable information about concomitant medication was available. An additional strength of study is the fact that we first made a list of all the INR analyses performed in the lab during the study period and sent the names of the corresponding patients to the GPs, so no patients on oral anticoagulation were missed and the INRs were reliable.
The percentage of time within 0.5 INR of the target range was 53%, as proposed in British guidelines Citation[5]. In the literature, this percentage ranges from 53.2–86.3% Citation[13–17]. A comparison between the British guidelines and our results within the 1.75–3.25 INR range shows that the patients were 11% of the time less within the range than proposed by the guidelines (69% vs 80%) Citation[5]. This study shows the importance of the latter quality criterion; it was in this group that a significant relation between poor performers (patients who were <80% of their time within this range) and the occurrence of a haemorrhagic event was found. A high frequency of testing was found in our study, namely 2.7 per patient per month. However, no significant relation between the percentage of time in the 2–3 INR range and the frequency of tests/patient was found. This implies that it is acceptable to test the INR in stable patients monthly to every 6 wk Citation[5]. The high frequency of testing could be explained by GPs’ fear of anticoagulation therapy, namely excessive INRs and events due to therapy. Education on the guidelines of oral anticoagulation could increase the knowledge and self-confidence of GPs about this therapy, and thus reduce the number of tests.
A study in the Netherlands within the anticoagulation clinics compared phenprocoumon versus acenocoumarol Citation[6]. The authors concluded that phenprocoumon leads to better quality regarding the percentage in range; no difference in occurrence of major bleedings was found. We could not confirm this conclusion. No significant correlation between the percentage of time in the 2–3 INR range and the anticoagulant used (phenprocoumon, acenocoumarol, warfarin) was found. However, in our study, only a small percentage of patients were anticoagulated with acenocoumarol (10%) and warfarin (3.5%).
There was great variability in the percentage in range between GP practices, although the quality of management of oral anticoagulation was not significantly different in single or group practices, and the quality of the management did not depend on the number of anticoagulant patients per GP practice. These results support the conclusion of a previous published study that there is no evidence that single-practice GPs are clinically underperforming Citation[18].
The incidence rate of major bleeding was 4.4 per 100 patient years, and patients were only included after the first month of therapy. The incidence rate for major bleeding was higher when comparing our data with data from a meta-analysis of 33 studies. In this study, the incidence rate for patients after the first 3 mo of therapy was 2.7 per 100 patient years (95% CI 2.71–2.77) Citation[19]. It is proven that the risk of haemorrhage increases when the INR is above the therapeutic range of 2.5–4.9 Citation[3], Citation[4]. In the European Atrial Fibrillation Trial, the incidence rate of haemorrhagic events was 3 per 100 patient years for the overall group. This incidence rate rose to 50 per 100 patient years when the INR was above 5 Citation[20]. In that study, 2.6% of the INRs were >5, compared to 2.8% in our study. However, the overall incidence rate for bleeding was significantly higher in our study (3 per 100 patient years versus 4.4 per 100 patient years). This could be explained by the higher age of our population, known to be a risk factor for a haemorrhagic event Citation[21]. A significant relation between age and the occurrence of an event was shown, with an odds ratio of 1.033 (95% CI 1.007–1.061) for every additional year in age Citation[22]. The incidence rate for thrombotic events was not substantially different from those (ranging from 3.5–3.9 per 100 patient years) in two available observational studies Citation[17], Citation[21]. Remarkably, in our study population, no significant relation between (history of) hypertension and the presence of bleeding was found. Possible explanations may be that the study sample was too small or that only patients with adequately controlled hypertension were enrolled.
Comparing these results with results in the literature, we conclude that the quality of monitoring oral anticoagulation by GPs in Belgium is suboptimal, as reflected by the distribution of INRs within the 1.75–3.25 INR range and the number of bleedings. A significant relation between poor performance and the occurrence of a haemorrhagic event was found.
This study is part of an intervention study pointing out the importance of a new model of care supporting GPs (multifaceted education and point-of-care testing) in their management of patients with oral anticoagulation Citation[23]. This model of care is proven to be effective but still has to be propagated in general practice, and has to be financially supported by the Belgian government.
This study would not have been possible without the efforts of biologist F. Frenay, of all collaborating GPs, and of the laboratory of the Diagnostic Centre of Tessenderlo, to whom we are grateful for their contribution.
References
- Benavente O, Hart R, Koudstaal P, Laupacis A, McBride R. Oral anticoagulants for preventing stroke in patients with non-valvular atrial fibrillation and no previous history of stroke or transient ischemic attacks. Cochrane Database Syst Rev 2000; 2: CD001927
- Evans A, Kalra L. Are the results of randomized controlled trials on anticoagulation in patients with atrial fibrillation generalizable to clinical practice?. Arch Intern Med 2001; 161: 1443–7
- Cannegieter SC, Rosendaal FR, Wintzen AR, van der Meer FJ, Vandenbroucke JP, Briet E. Optimal oral anticoagulant therapy in patients with mechanical heart valves. N Engl J Med 1995; 333: 11–7
- van der Meer FJ, Rosendaal FR, Vandenbroucke JP, Briet E. Bleeding complications in oral anticoagulant therapy. An analysis of risk factors. Arch Intern Med 1993; 153: 1557–62
- Guidelines on oral anticoagulation. third edition. Br J Haematol 1998;101:374–87.
- Gadisseur AP, van der Meer FJ, Adriaansen HJ, Fihn SD, Rosendaal FR. Therapeutic quality control of oral anticoagulant therapy comparing the short-acting acenocoumarol and the long-acting phenprocoumon. Br J Haematol 2002; 117: 940–6
- Fihn SD, Gadisseur AA, Pasterkamp E, van der Meer FJ, Breukink-Engbers WG, Geven-Boere LM, et al. Comparison of control and stability of oral anticoagulant therapy using acenocoumarol versus phenprocoumon. Thromb Haemost 2003; 90: 260–6
- Wittkowsky AK. Warfarin and other coumarin derivatives: pharmacokinetics, pharmacodynamics, and drug interactions. Semin Vasc Med 2003; 3: 221–30
- Breukink-Engbers WG. Monitoring therapy with anticoagulants in the Netherlands. Semin Thromb Hemost 1999; 25: 37–42
- Rose PE, Fitzmaurice D. New approaches to the delivery of anticoagulant services. Blood Rev 1998; 12: 84–90
- Morsdorf S, Leipnitz G, Pindur G, Schenk JF, Erdlenbruch W, Krischek B, et al. Improved therapeutic safety of oral anticoagulant therapy in Germany: the Saarland model. Semin Thromb Hemost 1999; 25: 49–55
- Mermans D. Anticoagulatie in de huisartsenpraktijk, een evaluatie. Huisarts Nu 2001; 2: 4–9
- Malik AK, Taylor AJ. Can warfarin randomized trials be reproduced in'real life'? Adherence to warfarin guidelines for intensity of anticoagulation in a university-based warfarin clinic. South Med J 2000; 93: 58–61
- Taylor FC, Gaminara E, Cohen H, Ramsay M, Miller D. Evaluation of a nurse specialist anticoagulant service. Clin Lab Haem 1997; 19: 267–72
- Poller L, Shiach CR, MacCallum PK, Johansen AM, Munster AM, Magalhaes A, et al. Multicentre randomised study of computerised anticoagulant dosage. European Concerted Action on Anticoagulation. Lancet 1998; 352: 1505–9
- Pengo V, Barbero F, Banzato A, Garelli E, Noventa F, Biasiolo A, et al. A comparison of a moderate with moderate-high intensity oral anticoagulant treatment in patients with mechanical heart valve prostheses. Thromb Haemost 1997; 77: 839–44
- Fitzmaurice DA, Hobbs FD, Murray ET, Holder RL, Allan TF, Rose PE. Oral anticoagulation management in primary care with the use of computerized decision support and near-patient testing: a randomized, controlled trial. Arch Intern Med 2000; 160: 2343–8
- Hippisley-Cox J, Pringle M, Coupland C, Hammersley V, Wilson A. Do single handed practices offer poorer care? Cross sectional survey of processes and outcomes. Br Med J 2001; 323: 320–3
- Linkins LA, Choi PT, Douketis JD. Clinical impact of bleeding in patients taking oral anticoagulant therapy for venous thromboembolism: a meta-analysis. Ann Intern Med 2003; 139: 893–900
- The European Atrial Fibrillation Trial Study Group. Optimal oral anticoagulant therapy in patients with nonrheumatic atrial fibrillation and recent cerebral ischemia. N Engl J Med 1995; 333: 5–10
- Palareti G, Manotti C, D'Angelo A, Pengo V, Erba N, Moia M, et al. Thrombotic events during oral anticoagulant treatment: results of the inception-cohort, prospective, collaborative ISCOAT study: ISCOAT study group (Italian Study on Complications of Oral Anticoagulant Therapy). Thromb Haemost 1997; 78: 1438–43
- Claes N, Buntinx F, Vijgen J, Arnout J, Vermylen J, Fieuws S, et al. Quality assessment of oral anticoagulation in Belgium, as practiced by a group of general practitioners. Acta Cardiol 2005; 60: 247–52
- Claes N, Buntinx F, Vijgen J, Arnout J, Vermylen J, Fieuws S, et al. The Belgian Improvement Study on Oral Anticoagulation Therapy: a randomized clinical trial. Eur Heart J 2005; 26: 2159–65