Abstract
Objective: In 1999, the World Health Organization (WHO) published new diagnostic criteria for diabetes mellitus (DM). The cut-off value of the fasting plasma glucose concentration was lowered from 7.8 to 7.0 mmol/l. The WHO criteria were used to validate the diagnosis made by the general practitioner, and to compare the diagnostic validity of diabetes mellitus in different countries. Methods: We retrospectively analysed 2556 newly diagnosed diabetics. Incidence was calculated according to the 1999 WHO criteria. Data were collected in general practice networks in five European countries or regions (Belgium, England, the Netherlands, Portugal, Spain). Results: According to the WHO criteria, 82% of the cases were valid diagnoses. Compared to the total group, in Spain, significantly more diagnoses were in agreement with the WHO criteria, whereas this number was significantly lower in England and Portugal. From the patients whose diagnosis was not in agreement with the WHO criteria, significantly more were women than men.
Conclusion: By using the WHO diagnostic criteria, the international standard, as a validation tool, we show that the diagnoses of diabetes mellitus made in primary care are valid. Furthermore, we show that these diagnoses are comparable between countries. Therefore, information from general practice registration networks is a valuable and valid source for international comparisons.
Introduction
The number of people with diabetes mellitus (DM) is expected to rise by 42% in industrialized countries between 1995 and 2025 Citation[1]. DM is a major public health problem worldwide. Current lifestyles characterized by decreasing physical activity and changing eating habits lead to an increased prevalence of obesity and increased risk of DM. According to the World Health Organization (WHO), surveillance is an urgent priority for (inter)national health authorities to prevent and control this epidemic Citation[2]. This stresses the need for internationally comparable figures on the occurrence of DM. A potential complication of international comparisons, however, is the difference of validity of the data between countries. Since DM is often diagnosed in primary care, data from general practitioners (GPs) could be a potential information source for the surveillance of DM. We have previously shown that GP networks are an appropriate surveillance tool Citation[3]. In 1980, the WHO introduced criteria for the diagnosis of DM and updated these in 1985 Citation[4], Citation[5]. As it became apparent that many diabetics remained undiagnosed in the community, the WHO decided to further revise the criteria Citation[6]. Microvascular complications of diabetes were found in 10–20% of patients at the time of diagnosis, some of whom had not yet shown evidence of hyperglycaemia Citation[7]. Also, the fasting cut-off plasma glucose value of 7.8 mmol/l does not detect all individuals who demonstrate a 2-h post-load value of ≥11.1 mmol/l in an oral glucose tolerance test (OGTT) Citation[8]. This latter value is associated with an increased risk of developing microvascular complications Citation[9]. Therefore, the WHO recommended in 1999 a reduction of the fasting plasma glucose concentration from 7.8 to 7.0 mmol/l. This cut-off value more adequately represents the upper end of the range 2 h after an OGTT Citation[10]. The new cut-off value is equally arbitrary as the previous ones; the risk of complications gradually increases with the fasting glucose level. Previous studies have shown that the application of the more recent diagnostic criteria leads to increased DM prevalence rates, as expected Citation[11–13].
The present study is concerned with incident cases of DM in the year 2000. Incidence was determined in general practice registration networks in five European countries or regions (Belgium, England, the Netherlands, Portugal, and Basque Country, Castilla and Léon, and Valencia in Spain). These networks were established to function as an early warning system for communicable diseases (e.g. by participating in the European Influenza Surveillance Scheme) and to monitor the health of the population for chronic diseases, mainly on a national basis—their primary aim is not to perform research Citation[3], Citation[14]. Diagnostic guidelines for diabetes existed at the time of the study in Belgium, the Netherlands and Spain, and these were based on the latest WHO criteria Citation[15]. Our objective was to show that these networks are also a valuable source for surveillance of a chronic disease like DM. We studied the validity of the diagnosis in DM patients newly diagnosed in 2000 by their GP by applying the WHO diagnostic criteria as an external “gold standard”.
Material and methods
In many European countries and regions, general practice surveillance networks collect information to monitor the health of the population. We identified networks collecting data on a continuous basis in a representative population. The present study was carried out as an addition to the routine activities of the networks in five European countries or regions (Belgium, England, the Netherlands, Portugal, and Basque Country, Castilla and Léon, and Valencia in Spain). Results from the three Spanish regions were combined. Whereas the English network routinely registers all diagnoses presented to the GP, all others monitor an annually variable selection of diseases Citation[3]. The study was a retrospective, cross-sectional survey covering the calendar year 2000. In those practices in which a patient list of all diabetics was routinely available (England, the Netherlands and Portugal), patients were recruited from these lists. In these countries, a list of diabetics was made at the end of the year, and all patients who were diagnosed during the preceding 12 months were labelled as newly diagnosed cases. The GPs completed the registration forms on these cases retrospectively. In the remaining countries (Belgium and Spain), patients were recruited at the time of their first contact for DM between 1 January 2000 and 31 December 2000. Results from all practices were aggregated at the national level to provide a framework for comparison. The number of participating practices (in parentheses, the percentage of all practices in the respective network) was 146 (92%) in Belgium, 36 (10%) in England and Wales, 57 (88%) in the Netherlands, 171 (100%) in Portugal, and 294 (100%) in Spain. At the time of this study, English GPs were asked to participate in a large number of studies. The impact of these requests on the daily workload of the GP has presumably resulted in a low participation in the English network. Since practices vary in the number of GPs, the population covered by the network in each country varied less than these figures suggest.
The data collection was identical in all countries. GPs were asked to complete a questionnaire for each newly diagnosed diabetic, including information on age and gender of the patient, and date and basis of diagnosis. Questions on the basis of the diagnosis included: presence of classical symptoms (e.g. thirst, polyuria, unexplained weight loss and urogenital candida) and details of the blood test on which the diagnosis was finally based. Details of the blood test consisted of status of the patient (fasting, random, post-prandial), the type of specimen (blood, plasma, unknown) and the final test result in mmol/l. The operationalization of the diagnostic criteria is shown in . The 1999 WHO criteria distinguish between the type of specimen. The cut-off values which were used for the analyses were 6.1 mmol/l for fasting and 11.1 mmol/l for all other samples of whole blood. The concentrations for serum, plasma or samples of unknown source were 7.0 mmol/l and 12.2 mmol/l, respectively. We used the most conservative measure for all samples of unknown origin or unknown patient status (i.e. the cut-off value of 12.2 mmol/l).
Table I. Classification of diabetes according to WHO 1999 diagnostic criteria.
Data were examined separately by country, gender and age group. Agreement with the WHO criteria was calculated as the percentage of the total number of included patients. Incidence rates were calculated based on the populations under surveillance derived from the registration lists of patients in the practices surveyed (England, the Netherlands, Portugal, Spain) or by estimation of the population from the frequency of consultations (Belgium) Citation[16]. Statistical analysis was performed in SPSS 11.5 using the Kruskal-Wallis test for statistical significance.
Results
All 2556 patients who received a diagnosis of “diabetes mellitus” from their GP were included in the analysis. In the vast majority of cases (94.5%), results of a blood test were available (). In 5.5% of all cases, information obtained on the type of blood test that was performed or on the glucose concentration was not in an analysable format. We therefore labelled this group as “no blood test result available”, although we know that the test had been undertaken. The group for which results were available (n=2415) was analysed for agreement with the WHO criteria. The vast majority (82%) of patients diagnosed with DM by their GP had glucose levels matching the WHO criteria. Significantly more were found in Spain (90%), and fewer in England (69%) and Portugal (74%). According to the available information, 13% of the newly diagnosed cases were false positives, matching neither of the WHO criteria, ranging from 6.8% in Portugal to 27% in England. Of these, 89% had glucose values lower than those required to diagnose diabetes but higher than the “normal” reference range which is suggestive of impaired glucose tolerance.
Table II. Incidence of diabetes mellitus according to 1999 WHO criteria. Figures are percentage of subjects.
Patient characteristics are described in . We did not observe any relationship between age and matching the WHO criteria, or among false positives, or the group for which no blood test result was available. Among the false positives were relatively more females than average (p<0.05; ). Classical symptoms were present in about 25% of all patients. This proportion was significantly lower in both groups for which blood test results were available. Data on symptoms were not available in almost half of the patients in whom no blood test was available.
Table III. Age and gender, and symptom status of diagnosed diabetics. Agreement with 1999 WHO criteria is shown as percentage of subjects.
We calculated the incidence rate of DM using only cases according to the 1999 criteria, and compared these values to the incidence rate of DM using all cases recorded by the GPs (). The mean incidence rate, as reported by GPs in five countries, was 3.21 per 1000 persons per year, and ranged from 2.13 per 1000 in England to 5.70 per 1000 in Belgium. When applying the WHO criteria, the incidence varied from 1.47 per 1000 in England to 4.59 per 1000 in Belgium. Significant differences in the ratio between men and women were only observed in England. This statistical difference was observed in both the GP-diagnosed group as well as in the group matching the WHO criteria, with the incidence rate being higher in males ().
Figure 1. Female/male ratio of incidence of diabetes by country. Diagnosis according to GP, to 1985 WHO criteria and to 1999 WHO criteria. * p<0.05, males versus females.
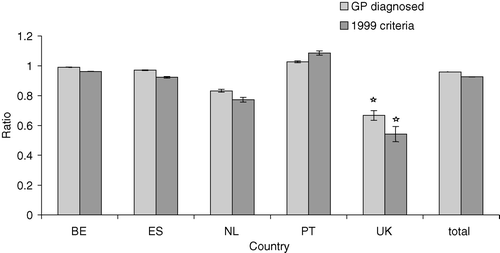
Table IV. Incidence of diabetes. Total incidence reported by GPs and according to 1999 WHO criteria. Ninety-five per cent confidence intervals are shown in parentheses.
Discussion
In this paper we assessed the validity of the GP diagnosis for DM by comparing clinical diagnostic information with an internationally, widely accepted “gold standard”: the WHO diagnostic criteria. We showed that the number of false positives is limited (and mainly concerns persons with impaired glucose regulation), that the majority of diagnoses (82%) accords with the diagnostic criteria, and that the differences between countries were limited. The overall comparability in agreement with international criteria suggests that data from general practice registration networks can be used for international comparisons. The majority of cases in the false-positive group had glucose values suggesting some impairment of glucose metabolism. In the 1999 guideline, the WHO states that impaired glucose tolerance is a stage in the natural history of disordered carbohydrate metabolism Citation[9]. These individuals are likely to be at increased risk of developing diabetes and/or cardiovascular disease, and are perhaps not truly misdiagnosed Citation[17]. It was shown previously that the percentage of false-positive diabetes diagnoses in Dutch general practice is low Citation[18], Citation[19]. We have confirmed this in a larger sample, and extended it to other European countries.
Our data were obtained in general practice networks in various European countries and regions. All networks were set up with the purpose of surveillance in primary care rather than research Citation[3]. The request for additional data might therefore have caused a lower participation grade among GPs in some countries. The provision of health services differs between the participating countries. In some, the GP functions as a gate keeper (e.g. in the Netherlands, and in England and Wales). In others, direct access to specialists is possible (e.g. in Belgium), which might lead to diagnoses more frequently made by medical specialists instead of GPs. However, the lowest incidence rate observed in England and Wales does not indicate an underestimation of DM in general-practice-based registration networks.
In general, the differences between the various countries with respect to the differing diagnostic criteria were small. At the time of this study, the Belgian, Dutch and Spanish guidelines included the 1999 WHO criteria, whereas the guidelines in England were concerned only with standards of foot care and did not specify glucose levels. In Portugal, guidelines were not implemented until 2002. This perhaps explains some of the variation found between countries.
A number of methodological issues need to be addressed with regard to the data collection. Firstly, although data collection varied between countries, in the end all participating GPs provided detailed information as requested on nearly all newly diagnosed patients. All practices had been participating in GP networks for some time and were therefore familiar with this kind of data collection. Secondly, we have previously described these networks in more detail and shown that the patient lists used by the networks are representative, in general at least for age and sex. Thirdly, all countries in this study, except Belgium, have a list system in general practice. Therefore, incidence rates can be easily calculated based on patient lists. Since all data we presented are based on patients actually visiting their GP in the year of the study, and not on disease registers or health interview surveys, we feel it is more likely that variation in practice population would influence the DM incidence rates within a country rather than between countries. Fourthly, the frequency of testing glucose levels by GPs could have influenced the outcome of this study. We know for instance that in Belgium an active screening programme was being implemented. Also, financial incentives for participation in the network vary from country to country. However, the GP registration networks have a low turnover rate, as we have shown elsewhere Citation[3]. Therefore, GPs were already familiar with the registration of diagnoses, and we do not think it is likely that financial incentives would be associated with the differences observed between countries.
We reported previously on the prevalence of DM in these primary care networks Citation[20]. The high incidence rate found in Belgium is in accordance with the high prevalence we observed in the same population; both may have been influenced by high levels of public awareness and an active screening programme in that country. However, it has been shown that socio-economic deprivation rather than detection through screening was responsible for the variation in DM prevalence in general practice Citation[21]. Recently, it was also shown that the familiarity of the GP with the patient influences the stage at which DM is diagnosed Citation[22]. The incidence we observed in England and the Netherlands is comparable to previously published reports Citation[23–25]. For Belgium, Wens et al. Citation[26] reported an incidence of 231 per 100 000 inhabitants, which is lower than our finding. A possible explanation for this discrepancy is the earlier timing of that study which, amongst others, led to the application of earlier WHO criteria rather than the 1999 criteria. One could argue that the increase of the number of diabetic patients leads to increased workload for GPs. However, by diagnosing the disease at an earlier stage, as was the aim of the new criteria, the number of complications is also expected to decrease, leading to a lower workload for GPs. An important observation we made is the wide variation in endorsement of international diagnostic guidelines at a national level. Timely endorsement of international criteria at a national level is an important issue to be considered by all GP organizations.
We have demonstrated the validity of the diagnosis of DM made in general practice using WHO diagnostic criteria. Knowing that the diagnoses made by GPs are valid allows for wider use of data from general practice networks, for instance for international comparisons and public health surveillance. Many other chronic diseases like diabetes are initially diagnosed and, to a large extent, managed in primary care, e.g. asthma, low back pain and depression. For these, general practice networks are a potential tool for monitoring of public health. Timely endorsement of accepted international diagnostic criteria by appropriate national advisory bodies should be encouraged.
This study was funded by the European Commission, Directorate General Health and Consumer Protection, as part of the Health Monitoring Programme (project number 1998/IND/1021). Opinions expressed in this paper are exclusively those of the authors. We gratefully acknowledge the contribution of the sentinel GPs and their network coordinators (A. Bartelds, V. van Casteren, I. Falcao, A. Ross, T. Vega Alfonso, O. Zurriaga).
References
- Narayan KM, Gregg EW, Fagot-Campagna A, Engelgau MM, Vinicor F. Diabetes—a common, growing, serious, costly, and potentially preventable public health problem. Diabetes Res Clin Pract 2000; 50: S77–84
- King H, Aubert RE, Herman WH. Global burden of diabetes, 1995–2025: prevalence, numerical estimates, and projections. Diabetes Care 1998; 21: 1414–31
- Deckers JG, Paget WJ, Schellevis FG, Fleming DM. European primary care surveillance networks: their structure and operation. Fam Pract 2006; 23: 151–8
- WHO Expert Committee on Diabetes Mellitus. Second report. WHO Technical Report Series 1980;646:1–80.
- World Health Organization. Diabetes mellitus: report of a WHO Study Group. WHO Technical Report Series 1985;727:1–104.
- World Health Organization. Definition, diagnosis and classification of diabetes mellitus and its complications: report of a WHO consultation. Part 1: diagnosis and classification of diabetes mellitus. Geneva: WHO; 1999.
- Klein R. Hyperglycemia and microvascular and macrovascular disease in diabetes. Diabetes Care 1995; 18: 258–68
- Lamb EJ, Day AP. New diagnostic criteria for diabetes mellitus; are we any further forward?. Ann Clin Biochem 2000; 37: 588–92
- Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabet Med 2003; 15: 539–53
- Engelgau MM, Thompson TJ, Herman WH, Boyle JP, Aubert RE, Kenny SJ, et al. Comparison of fasting and 2-hour glucose and HbA1c levels for diagnosing diabetes: diagnostic criteria and performance revisited. Diabetes Care 1997; 20: 785–91
- Gabir MM, Hanson RL, Dabelea D, Imperatore G, Roumain J, Bennett PH, et al. The 1997 American Diabetes Association and 1999 World Health Organization Criteria for hyperglycemia in the diagnosis and prediction of diabetes. Diabetes Care 2000; 23: 1108–12
- DECODE Study Group. Will new diagnostic criteria for diabetes mellitus change phenotype of patients with diabetes? Reanalysis of European epidemiological data. Br Med J 1998;317:371–5.
- Shaw JE, de Courten M, Boyko EJ, Zimmet PZ. Impact of new diagnostic criteria for diabetes on different populations. Diabetes Care 1999; 22: 762–6
- Fleming DM, Schellevis FG, Paget WJ. Health monitoring in sentinel practice networks: the contribution of primary care. Eur J Public Health 2003; 13: 80–4
- Donker GA, Fleming DM, Schellevis FG, Spreeuwenberg P. Differences in treatment regimes, consultation frequency and referral patterns of diabetes mellitus in general practice in five European countries. Fam Practice 2003; 21: 364–9
- Lobet MP, Stroobant A, Mertens R, Van Casteren V, Walckiers D, Masuy-Stroobant G, et al. Tool for validation of the network of sentinel general practitioners in the Belgian health care system. Int J Epidemiol 1987; 16: 612–8
- DECODE study group. Age- and sex-specific prevalences of diabetes and impaired glucose regulation in 13 European cohorts. Diabetes Care 2003;26:61–9.
- Schellevis FG, van de Lisdonk E, van der Velden J, van Eijk JThM, van Weel C. Validity of diagnoses of chronic diseases in general practice: the application of diagnostic criteria. J Clin Epidemiol 1993; 46: 461–8
- van Weel C. Validating long term morbidity recording. J Epidemiol Community Health 1995; 49(Suppl 1)29–32
- Fleming DM, Schellevis FG, van Casteren V. The prevalence of known diabetes in eight European countries. Eur J Public Health 2004; 14: 10–4
- Whitford DL, Griffin SJ, Prevost AT. Influences on the variation in prevalence of type 2 diabetes between general practices: practice, patient or socioeconomic factors?. Br J Gen Pract 2003; 53: 9–14
- Drivsholm T, de Fine Olivarius N. General practitioners may diagnose type 2 diabetes mellitus at an early disease stage in patients they know well. Fam Pract 2006; 23: 192–7
- Gatling W, Guzder RN, Turnbull JC, Budd S, Mullee MA. The Poole Diabetes Study: how many cases of type 2 diabetes are diagnosed each year during normal health care in a defined community?. Diabetes Res Clin Pract 2001; 53: 107–12
- Vanderpump MP, Tunbridge WM, French JM, Appleton D, Bates D, Rodgers H, et al. The incidence of diabetes mellitus in an English community: a 20-year follow-up of the Whickham Survey. Diabet Med 1996; 13: 741–7
- Ubink-Veltmaat LJ, Bilo HJG, Groenier KH, Houweling ST, Rischen RO, Meyboom-de Jong B. Prevalence, incidence and mortality of type 2 diabetes mellitus revisited: a prospective population-based study in the Netherlands (ZODIAC-1). Eur J Epidemiol 2003; 18: 793–800
- Wens J, Van Casteren V, Vermeire E, Van Royen P, Pas L, Denekens J. Diagnosis and treatment of type 2 diabetes in three Belgian regions. Registration via a network of sentinel general practices. Eur J Epidemiol 2001; 17: 743–50