Abstract
Background: In 1993–1997, we described a high incidence of wheezing in children living near the iron, steel, and coke factory of Călăraşi (Romania). In 1998, the factory was closed. Objective: To investigate the influence of closing the factory on the incidence of wheezing in children living near the factory. Methods: We used this natural experiment to compare wheezing occurrence in children below age 2 in an area near the factory and in a village 10 km from the factory (Roseţi). We studied three birth cohorts: those 2 years old before the closing of the factory (group 1), those born before the closing and returning 2 after the closing (group 2), those born after the closing (group 3). Results: The relative risk (95% CI) of having at least one episode of wheezing during the first life-year was 0.51 (0.30–0.85) in group 3 versus group 1, and 0.95 (0.64–1.40) for group 2 versus group 1. After adjusting for possible confounders, the incidence dropped in Călăraşi (odds ratio [OR] 0.38, 95% CI 0.19–0.76), while it increased in Roseţi (OR 8.36, 95% CI 1.84–38.0). Results for the 2-year incidence were similar.
Conclusion: Industrial air pollution by the factory was the main risk factor for wheezing during the first life-years of the children of Călăraşi. Closing the factory resulted in a significant decrease in wheezing incidence rates, which still remained higher than in a nearby village.
Introduction
Air pollution standards are concentrations over a given period that are considered to be acceptable Citation[1]. Since 1997, the WHO Air Quality Guidelines have been promoted Citation[2]. SILAQ (Sofia Initiative on Local Air Quality) countries have adopted high standards for air quality. Romania and some other eastern European countries apply weaker standards. Concentrations often exceed standards by several times, particularly in Bulgaria, Romania, and Slovakia Citation[3]. In Romania, until 1998, the average concentration in 24 hours exceeded the maximum permissible values with a frequency of over 25% in 20 cities; Călăraşi was the 10th among these Citation[4], Citation[5]. The prevalence of asthma in Romania increased from 5% in 1994–1995 to 7% in 2000–2001. In Romania, about 1 in 20 schoolchildren are expected to have asthma, whether formally diagnosed or not. In Bucharest, about 10% of schoolchildren have asthma Citation[6].
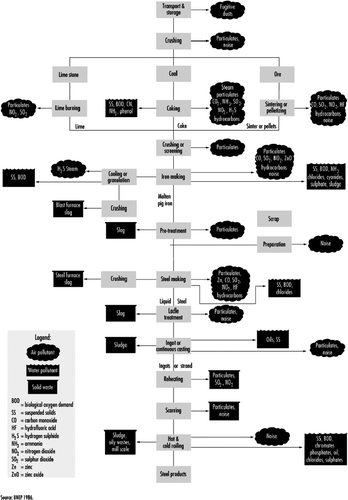
In this study, we examined the relationship between air pollution and the incidence of respiratory symptoms in children. Our study took place in Călăraşi and studied children living near the local iron, steel, and coke factory. Emitted pollutants of ferrous metallurgy were sulphurous anhydride, carbon oxide, nitrogen oxides, sulphur dioxide, hydrocarbon, H2S, NH3, chlorides, cyanides, and sulphate. Iron production results in slag and dust, and steel production in metal dust (iron particles, zinc, and other metals) Citation[7–9] (). In coke production, the resulting residues are ammonia, phenol, cyanide, hydrogen sulphide, oil (K143, K144), lime, and sludge Citation[9]. Ferrous metallurgy is the most important emitter of CO and NO2, and the second most important for solid substances Citation[8], Citation[9].
Figure 1. Flow chart of pollutants and wastes generated by different processes. Adapted from UNEP and IISI 1997, and an unpublished article by Jerry Spiegel.
CO results in nausea, dizziness, or headache Citation[9], and SO2 aggravates existing lung disease, constricts the airways, and causes new attacks in asthmatic patients, resulting in wheezing, shortness of breath, and coughing Citation[10], Citation[11]. Combined with water, SO2 converts to sulphur, which is highly irritating to the sensitive mucosal surface of the respiratory tract Citation[11]. NO2 is a respiratory irritant in its own right, causing respiratory problems and irritating the nose and throat, especially in people with asthma. It also increases the susceptibility for respiratory infections Citation[10], Citation[11]. NO2 has an essential role in the production of ozone Citation[12]. Ozone increases respiratory discomfort and disease, and reduces lung function Citation[10], Citation[13]. Suspended particles (total suspended particles [TSP], particles under 10 microns [PM10]) result in the worsening of respiratory symptoms and the impairment of lung function. People with an existing respiratory disease, the elderly, and children are most at risk Citation[10], Citation[11], Citation[14].
It is well known that tobacco-smoke exposure increases the prevalence of asthma, wheezing, and chronic bronchitis Citation[15], Citation[16]. The development of the atopic phenotype requires environmental factors. Genetic factors do not change during a lifetime, so any increase in prevalence is largely due to an increase in exposure to allergenic and non-specific environmental factors Citation[17]. However, gene–environment interactions may be present. A significant association between family history of allergy/asthma and smoking has been identified Citation[18]. Mould exposure has been associated with several effects on health Citation[19]. Observational studies have shown a 30–50% reduction in childhood asthma and a lower incidence of wheezing Citation[20] with exclusive breastfeeding for 3 months Citation[21], Citation[22]. Another study, however, showed that breastfeeding in infancy might also be related to a higher prevalence of asthma during early adolescence Citation[23]. During the first 2 years of life, the immune system is sensitive to environment- and lifestyle-related stress Citation[24].
In a previous study (1993–1997), the 5-year incidence of at least one episode of wheezing in children less than 10 years old and living within 3 km of the iron and steel factory of Călăraşi was around 42.9% Citation[25]. The 2-year incidence (November 1994–October 1996) in children under 2 years was 41%. After May 1997, new filters were used in the factory, and the factory stopped production in October 1998, resulting in a significant decrease in air pollution.
Roseţi is a village 10 km east of Călăraşi, without any industrial air pollution. The 2-year incidence of at least one episode of wheezing in children under 2 years old and born between 1 November 1994 and 30 October 1996 is 1.9%. Călăraşi and Roseţi lie by the arm of the Danube, in southeast Romania (). The studied area of Călăraşi was in the west of the city, southeast of the factory (). The wind direction there is mainly N-NW.
Figure 2. Map of Călăraşi County: the city, Călăraşi, and the village, Roseţi, are indicated by large dots.
We used a natural experiment in Călăraşi to study the relationship between the occurrence of wheezing in children between 0 and 2 years and the change in industrial pollution from the factory. We took into consideration the presence of other risk factors such as the smoking habits of parents, the presence of mould in houses, a family history of allergy, and the duration of breastfeeding, and compared the results with the evolution in the Roseţi population, where no apparent environmental changes took place. We hypothesized that the introduction of new filters and the closing of the factory would result in a decrease in the difference of wheezing incidence between Călăraşi and Roseţi.
Methods
Design
We designed a prospective cohort study in order to compare the occurrence of wheezing in children younger than 2 years in two regions: one area within a diameter of 3 km in the main wind direction near the iron and steel factory gate in Călăraşi, and the other in the village of Roseţi, situated 10 km east of Călăraşi. After the iron and steel factory reduced and finally stopped its activity, this turned into a natural experiment, as factory-related pollution disappeared in Călăraşi.
The factory stopped production in October 1998, which led us to a year-of-birth categorization of three groups. The first birth cohort consisted of children born from 1 November 1994 to 31 October 1996. They were all over the age of 2 when the factory was closed. The second birth cohort consisted of children born between 1 November 1996 and 31 October 1998. They were born before the factory was closed, and the 2-year study period included the factory closure. The third birth cohort consisted of children born after the factory stopped all activities (1 November 1998–31 October 2000). We compared all children of these cohorts living in the Călăraşi area with children of the same birth years living in Roseţi.
Patients
The children from Călăraşi lived in flats or in small houses. There was central heating for those living in flats and stoves for those living in houses. Traffic there was not very extensive. The children of Roseţi lived in small houses heated by stoves. Traffic was less in Roseţi compared to Călăraşi. The climate of the two places is the same, as are the customs of the people (same cooking habits, same lifestyle, etc.). It is a custom in both Călăraşi and Roseţi to cover whole floors with carpets and sometimes to decorate the walls with them.
Both groups of children under study were cared for by one family physician each. Both physicians graduated from the same university and in the same year. The list size was 324 children below the age of 2 for the Călăraşi practice and 550 children in Roseţi.
Disease definition
We studied the incidence of at least one period of wheezing in children between birth and their second birthday. Wheezing as a symptom and/or the presence of sibilants were criteria for defining the patients. Only those who showed wheezing detected by the doctor were considered. We did not use information provided by the parents.
Data collection
For all children born between 1 November 1994 and 31 October 2000, the date of diagnosis of every episode of wheezing within the first 2 years of life was registered as well as parents’ smoking habits, the presence or absence of a family history of allergy, and the presence of mould on the walls of the family home.
Data concerning the children were collected from doctors’ files. All parents were asked if one of them smoked. The presence of mould on the walls was identified during a house visit and inspection of each of the houses. Family history of allergy was checked in medical files, as families tend to stay in the same municipalities and therefore keep the same doctor. For health surveillance, both the GP and the nurse saw each child at least 24 times during the first year of life and at least four times during the second year of life, even if there were no health problems. In case of health problems, patients and parents consulted the GP. If a child was hospitalized, the GP was always informed by mail and the patients had to contact the GP for drug prescriptions. Data concerning babies’ nutrition were taken from the children's files, in which the GP had to register all details concerning feeding.
For each year, we calculated an infection index as the quotient of the number of respiratory infections reported and the number of patients of each doctor. This was both a marker of the number of infections, which may provoke the occurrence of wheezing, and an indicator of the stability of the process of diagnosis and registration.
Statistical analyses
For both Călăraşi and Roseţi, we compared the incidence of at least one period of wheezing in the second and third birth cohort with the incidence of at least one period of wheezing in the first birth cohort (2→1, 3→1), using relative risks (RR) and their 95% confidence intervals (95% CI). We also performed multiple logistic regression analysis. At least one episode of wheezing until the age of 1 (and 2) was the dependent variable. The municipality and the birth cohort were the main independent variables, while smoking habits of the parents, family history of allergy, the presence of mould on walls, and the number of months (less or more than 12 months) each baby received breastfeeding were included as covariables. In particular, we examined the interaction between municipality and period. All analyses were performed using SPSS for Windows version 10 (SPSS Inc., Chicago, Ill., USA) and Epi Info 6.4 (CDC, Atlanta, Ga., USA).
Results
We followed 851 children: 314 in Călăraşi and 537 in Roseti. Birth numbers were relatively stable throughout the study period in Călăraşi, with an increasing trend in Roseţi (). In Călăraşi, the infection index was stable throughout the study period. In Roseti, an increase over the last 3 years was identified, possibly indicating either an increased awareness of respiratory disorders by patients, physician, or both, or improved registration (). The background characteristics of the children were stable over the three periods for each area studied (). Most measurements were available for all 851 children. Information concerning family history of allergy was not available for six children, as they were orphans and in family care.
Table I. Background characteristics of the children in Călăraşi and Roseţi.
Table II. Infection index throughout the study period.
Bivariate analysis
In Călăraşi, 33% of the children (n = 33) in birth cohort 1, 31.3% (n = 35) in birth cohort 2, and 16.7% (n = 17) in birth cohort 3 had at least one episode of wheezing in their first year of life. The RR (95% CI) for children from birth cohort group 3 versus group 1 was 0.51 (0.30–0.85), for group 2 versus group 1 it was 0.95 (0.64–1.40), and for group 3 versus group 2 it was 0.53 (0.32–0.89) ().
Table III. First-year and 2-year incidences of wheezing according to birth cohort and municipality (bivariate analysis).
In Roseţi, 1.3% of the children (n = 2) in birth cohort 1, 1.1% of the children (n=2) in birth cohort 2, and 8% (n = 16) in birth cohort 3 had at least one episode of wheezing. For Roseţi, the RR (95% CI) for group 3 versus group 1 was 6.83 (1.48–27.25), for cohort 2 versus cohort 1 it was 0.89 (0.13–6.27), and for cohort 3 versus cohort 2 it was 8.68 (2.03–37.19) ().
We did the same analysis using at least one episode of wheezing until the age of 2 as the outcome measure (). The results were similar.
In children with smoking parents, 14% of the children had at least one episode of wheezing by the age of 1. In children with non-smoking parents, this was 13% (p=0.74). In children having a positive family history of allergy, 20% had at least one episode of wheezing by the age of 1. In children without a family history of allergy, this was 11% (p=0.003). In children who were breastfed for at least 12 months, 7% had at least one episode of wheezing. For those with at least 6 months of breastfeeding, this was 9%, and for children with less then 6 months of breastfeeding, 24% (chi-square for trend, p<0.001). The effect of breastfeeding was most pronounced in Roseţi, the village without apparent air pollution: the RR of at least one episode of wheezing in children with versus without breastfeeding for 12 months was 0.33 (0.13–0.80), versus 0.76 (0.56–1.04) in Călăraşi. The presence of mould on the walls was associated with at least one episode of wheezing in the first year of life in 10% of cases. In children living in houses without mould, this was 14% (p=0.10). These results were largely similar when using the first 2 years of life as the follow-up period.
Multivariate analysis
The odds ratio (OR) of first-year wheezing incidence in birth cohort 2 compared to birth cohort 1 was non-significant in both municipalities, adjusted for smoking habits of the parents, family history of allergy, at least 1 year of breastfeeding, and presence of mould on the walls (). For birth cohort 3, however—the children born after the closing of the factory—there was a large and significant decrease in Călăraşi (OR 0.38) and an increase in Roseţi (OR 8.36). Testing for interaction between municipality and birth cohorts revealed p < 0.001. The model also revealed a significant independent and inverse relationship with at least 12 months of breastfeeding (OR 0.60, 95% CI 0.37–0.98). There was no significant independent relationship with a family history of allergy, smoking habits of the parents, or mould on the walls. We found similar results when using the first 2 years as the follow-up period ().
Table IV. Adjusted odds ratios of the relationship between birth cohort and first-year and 2-year incidence of wheezing in children in Călăraşi and Roseţi, resulting from multiple logistic regression analysis.
Discussion
We found a significant decrease in the incidence of wheezing in small children after the factory stopped its activities. The decrease was robust after controlling for a number of possible confounders. We additionally found a significant relationship with breastfeeding of the children, especially in the region without apparent air pollution. No significant relationship could be identified with a family history of allergy, smoking habits of the parents, or presence of mould on the walls.
Our study results are reinforced by the fact that the initially designed cohort study turned into a natural experiment after the closing of the factory, by careful registration over the years by the same physicians, and by the robustness after adjusting for the main possible confounders. The infection index that was separately calculated proved to be stable throughout the study period in Călăraşi. It indicated the absence of a secular trend in the emergence of respiratory infections (that may provoke wheezing episodes) and a stable diagnostic and registration process. In Roseţi, there was a sudden increase during the last 3 years, related to a parallel increase in wheezing incidence. As we have no reason to believe that this may result from a sudden increase in infection rates, it probably indicates an increased diagnostic awareness of respiratory disorders, improved registration, or both, perhaps provoked by the ongoing study. If this is the case, the effect we describe in this report would in reality be even stronger. Some factors are difficult to quantify and to include in statistical models. They may, however, influence the care-seeking pattern of patients and therefore our outcome measures. Firstly, the factory closing had some influence on jobs and incomes. In the first few years after closure, workers were given a compensatory fee, and some were given new work placements—some in other cities or other countries (one reason for the loss of some children during follow-up). Over the years, other modern and less-pollutant industries, including part of the steel factory, developed and a large part of the original workforce now works there. In the transition period, economic difficulties may have led to less frequent consultations with the GP, although no data are available and local GPs did not have that impression. The fact that we used “at least one episode within a period of 2 years” as our main outcome measure decreases the likelihood of important bias by this phenomenon, but we cannot totally exclude it. Secondly, the Romanian healthcare system is also going through a major transition, and the effect of this on the likelihood of the diagnosis of wheezing is difficult to estimate. We have no reason, however, to suppose that this influence, if present, would be selectively different between Călăraşi and Roseţi.
The 2-year wheezing incidence during the first two periods in Călăraşi was similar to 2-year incidence rates (37%) described in the Netherlands. In the third period, rates were lower, and in Roseţi the rates were very low compared to the Dutch rates for all periods Citation[26]. In previous studies, an association was described between air pollution and the number of consultations or hospital admissions for asthma and other respiratory diseases. Hajat and Cholab Citation[27], studying the effects of air pollution in London, found a significant association of childhood consultations for asthma and lower respiratory disease with NO2, CO, and SO2 as the most important pollutants. In adults, the only consistent association was with PM10 or SO2 Citation[27–33]. Hospitalization of children with wheezing, respiratory disease, pneumonia, or asthma was associated with the presence of air pollution in general Citation[34–36]. Chronic exposure to TSP and SO2 was significantly associated with wheezing, asthma, and bronchitis Citation[28], Citation[37–41]. Air pollution in the steel cities of New South Wales, Australia, influenced children's health at lower-than-expected levels Citation[42]. The presence of a relationship between ozone concentration and the occurrence of respiratory diseases was either absent Citation[43] or strongly present Citation[44]. In Călăraşi, SO2 pollution and to a lesser extent PM10 dropped after the closure of the factory. For the rural region around Roseţi, only a PM10 concentration was registered. There was only a minimal decrease over time, and the difference with Călăraşi remained important ().
Table V. Evolution of air pollution in Călăraşi and Roseţi.
In the bivariate analysis, we found an association between family history of allergy and the presence of wheezing, which was not confirmed during the multivariate analysis. In previous studies, too, the incidence of asthma and wheezy bronchitis was strongly associated with the presence of atopic disease Citation[43]. Rhodes et al., studying for 22 years a birth cohort with a genetic risk of atopy, found that 16% of subjects developed sensitivity to common allergens by the age of 2 and were showing broncho-hyperresponsiveness (BHR) by mid-school age Citation[45]. Interaction between genetic and environmental factors and early allergic sensitization increase the risk for asthma Citation[46], Citation[47].
A strong association has been described between wheezing and passive smoking. Exposure to tobacco smoke carries a high risk for childhood asthma Citation[48]. Passive smoking increases BHR Citation[49]. In this study, we found no association between smoking parents and wheezing. This probably happened because most of the people in the region are used to smoking outside and almost never smoke in the presence of small children.
Our study shows that industrial air pollution affects children's health, and that its containment has a direct short-term preventive effect. This information may also be important for similar situations in other regions in central and eastern Europe. A follow-up study evaluating the effect at primary-school age is currently underway. According to our data, industrial pollution caused by the Călăraşi iron and steel factory was the main risk factor for the emergence of wheezing during the first years of life of neighbouring children. Consequently, closing the factory resulted in a significant decrease in wheezing incidence rates, which still remained higher, however, compared to the control village.
Acknowledgements
The authors thank J. Degryse, I. Kant, B. Nemery, and P. Portegijs for comments on previous versions of the manuscript, and M. Devis, B. Schouten, and M. Brinkman for logistic help and language editing.
References
- Anonymous. Air Quality Standards-UK National Air Quality Department for Environment Food and Rural Affairs ( This website is prepared and hosted by AEA Energy & Environment, on behalf of the UK Department for Environment, Food & Rural Affairs and the Devolved Administrations; http://www.airquality.co.uk/archive/standards.php#std.
- Anonymous. Management of pollution and of natural resources. Chapter 6 Air Pollution—Uzbekistan.
- Anonymous. Sofia Initiative on Local Air Quality ( SILAQ conference report). http://www.mem.dk/aarhus-conference/issues/lead-out/localair.htm.
- Anonymous. Report from PHARE Country Visit (an attachment to the report from the PHARE Country visit) Romania 8–11 December 1998 An Extract from “Environment Protection Strategy” published by the Ministry of Waters, Forests and Environment protection http://www.chmi.cz/uoco/isko/ptl/99ptl/a5-rom.html.
- Anonymous. Hot spots of air pollution – Critical areas in Romania with regard to deterioration of the environmental quality. State of the environment in Romania 2000; Critical areas in Romania with regard to the deterioration of environmental quality Data provided by MAPM) http://enrin.grida.no/htmls/romania/soe2000/eng/cap10/index.htm.
- Trifan A. Informare-educare-comunicare. Medical Life 2006; 48: 1
- Viliam-Demko, Jozel-Skalsky. Experience Learned from Authorized Measurements of Air Pollution Sources in Production and Processing of Metals and Refractories –Imackozey, ( EKO-TERM SERVIS sro. KOSCE, Kukiciova, Slovak Repiblic).
- Anonymous. Analysis of existing state of environment and environmental policy in Russian Federation.
- EPA. Iron and Steel Industry. Note book section III b. Row Material Inputs and Pollution Out Puts. ( EPA Office of Compliance Sector Notebook Project.) Profile of the Iron and Steel Industry September 1995 Office of Compliance Office of Enforcement and Compliance Assurance U.S. Environmental Protection Agency 401 M St., SW (MC 2221-A) Washington, DC 20460.
- Air Monitoring Bureau. Pollutants and their health effects. New Jersey: New Jersey Department of Environmental Protection, Air Monitoring Bureau; Application for Investigation under the Ontario Environmental Bill of Rights.
- Schwella D. Air pollution and health in urban areas. Rev Environ Health 2000; 15: 13–42
- Puget Sound Clean Air Agency-Working togather for clean air-What is Air Pollution. About our common pollutants-“The Dirty Six”.
- Introduction in Environmental Studies. Es.110 (Youth Environmental Sanity side).
- Pope CA, 3rd. Respiratory hospital admission associated with pollution in Utah, Salt Lake, Cache Valleys. Arch Environ Health 1991; 46: 90–7
- Gergen PJ, Fowler JA, Maurer KR, Davis WW, Overpeck MD. The burden of environmental tobacco smoke on respiratory health of children 2 months through 5 years of age in the United States: Third National health and Nutrition Examination Survey, 1988 to 1994. Pediatrics1998; 101. Available at: www.pediatrics.org/cgi/content/full/101/2/e8.
- Vor Mutis E. Environmental factors influencing the development of progression of pediatric asthma. Allergy Clin Immunol 2002; 109(Suppl 6)S525–32
- Marin A, Esevsre JL, Botey J. De la dermatitis atopica al asma. Allergol Imunopathol (Madr) 1998; 26: 114–9
- Singh D, Arora V, Sobti-Praven C. Chronic recurrent cough in rural children in Ludhiana, Punja. Indian Pediatr 2002; 39: 23–9
- Muller A, Lehmann I, Seiffart A, Borte M, Herbath O. Increased incidence of allergic sensitization and respiratory disease due to mould exposure: results of Leipzig Allergy Children (LARS). Int J Hyg Environ Health 2002; 204: 363–5
- Mellis CM. Is asthma prevention possible with dietary manipulation?. Med J Aust 2000; 177(Suppl 6)S78–80
- Oddy WH, Peat JK, de Klerk NHJ. The risk of asthma increased if exclusive breastfeeding was stopped before 4 months. Asthma Clin Immunol 2002; 110: 65–7
- Burr ML. Infant feeding, wheezing and allergy: a prospective study. Arch Dis in Childhood 1993; 68: 724–8
- Takemura J, Sacurai Y, Honjo S, Kusakari A, Hara T, Gigu M, et al. Relation between breastfeeding and the prevalence of asthma: the Tokorozawa Childhood Asthma and Pollinosis Study. Am J Epidemiol 2001; 154: 115–9
- Steerenberg PA, van Amsterdam C, Vandebriel RJ, Vos JG, van Bree L, Van Loveren H. Environmental and lifestyle factors may act in concert to increase the prevalence of respiratory allergy including asthma. Clin Exp Allergy 1999; 29: 1304–8
- Câra AC, Buntinx F. Incidence of episodes of wheezy bronchitis in children living near the iron and steel factory from Cãlãrasi. Arch Publ Health 2005; accepted for publication.
- Schönberger HJAM, Dompeling E, Knottnerus JA, Maas T, Muris JW, van Weel C, van Schayck CP, et al. The Prevasc study: the clinical effect of a multifaceted educational intervention to prevent childhood asthma. Eur Resp J 2005; 25: 1–11
- Hajat S, Haines A, Goubet SA, Atkinson RW, Anderson HR. Association of air pollution with daily GP consultations for asthma and other lower respiratory conditions in London. Thorax 1999; 54: 597–605
- David A, Kegel E, Rudnai P, Sarkanay E, Kertesz M. Correlation between air pollution and respiratory morbidity in children in Dorog. Orvosi Hetilap 1990; 131: 513–7
- Perslagen G, Rylander E, Norberg S, Eviksson M, Nordvall SL. Air pollution involving nitrogen dioxide exposure and wheezing bronchitis in children. Int J Epidemiol 1995; 24: 1147–53
- Pless-Mulloli T, Howel D, King A, Stone I, Merefield J, Bessell J, et al. Living near open coal mining sites and children's respiratory health. Occup Environ Med 2000; 57: 145–51
- Hajat S, Anderson HR, Atkinson RW, Haines A. Effect of air pollution on general practitioner consultations for upper respiratory diseases in London. Occup Environ Med 2002; 59: 294–9
- Atkinson RW, Brenner SA, Anderson HR, Strachan DP, Bland JM, de Leon AP. Short term association between emergency hospital admission for respiratory and cardiovascular diseases and outdoor air pollution in London. Arch Environ Health 1999; 54: 398–411
- Sanchez J, Romien I, Ruiz S, Pino O, Gutierez M. Acute effects of the breathing of industrial waste and of sulfur dioxide on the respiratory health of children living in the industrial area of Puchuncavi. Rev Panam Salud Publica 1999; 6: 384–91
- Michellozzi P, Forastiere F, Perucci CA, Fusco D, Barca A, Spadia T. Acute effects of air pollution in Rome. Ann Ist Super Sanita 2000; 36: 297–304
- Atkinson RW, Anderson HR, Sunyer J, Ayres J, Baccini M, Vonk JM, Boumghar A, Forastiere F, Forsberg B, Touloumi G, Schwartz J, Katsouyanni K. Acute effects of particulate air pollution on respiratory admission: results from APHEA 2 projects. Air Pollution and Health: a European Approach. Am J Respir Crit Care Med, Volume 164, Number 10, November 2001, 1860–1866.
- Hruba F, Fubianova E, Koppova K, Vandenberg JJ. Childhood respiratory symptoms, hospital admission, and long term exposure to airborne particulate matter. J Expo Anal Environ Epidemiol 2001; 11: 33–40
- Petroeschevsky A, Simpson RW, Thalis L, Rutherford S. Association between outdoor air pollution and hospital admission in Busbane, Australia. Arch Environ Health 2001; 56: 37–52
- Schvartz J. Particulate air pollution and chronic respiratory diseases. Environ Res 1993; 62: 7–13
- Nicolai T. Air pollution and respiratory diseases in children: what is the clinically relevant impact?. Pediatr Pulmonol Suppl 1999; 18: 9–13
- Hebourth O, Futz G, Krumbiegel P, Diez V, Frank U, Richter. Effect of sulphur dioxide and particulate pollutant on bronchitis in children—a risk analysis. Environ Toxicol 2001; 16: 269–76
- Thompson AJ., Patterson CC. Acute Asthma Exacerbation and Air Pollutions in children Living in Belfast, Northen Ireland. Arch Environ Health 2001; 56: 234–41
- Lewis PR, Hensley MJ, Wlodarezyk J, Toneguzzi RC, Westley-Wise VJ, Dunn T, et al. Outdoor air pollution and children's respiratory symptoms in the steel cities of New South Wales. Med J Aust 1998; 169: 459–63
- Anderson HR, Pottier AC, Strachan DP. Asthma from birth to age 23: incidence and relation to prior and concurrent atopic diseases. Thorax 1992; 47: 537–42
- Buchdahl R, Parker A, Stebbings T, Babiker A. Association between air pollution and acute childhood wheezing episodes: prospective observational study. Br Med J (Clin Res Ed) 1996; 312: 661–5
- Rhodes H, Thomas P, Sporik R, Holgate ST, Cogswell JJ. A birth cohort study on subjects at risk of atopy: twenty-two-year follow-up of wheeze and atopic status. Am J Respir Crit Care Med 2002; 165: 176–80
- Yang KD. Childhood asthma: aspects of global environment, genetics and management. Changgeng Yi Xue Za Zhi 2000; 23: 641–61
- Dutau C. De la bronchiolitis a l'asthme. Arch Pediatr 2002; 9(Suppl 3)372s–6s
- Heraud MC, Herbelin-Wagner ML. Facteurs de risque: environment, tabac. Arch Pediatr 2002; 9(Suppl 3)377s–83s
- Janson C, Chinn S, Jarvis D, Zock JP, Toren K, Burney P. Effect of passive smoking on respiratory symptoms, bronchial responsiveness, lung function and total serum Ig in the European Community. Respiratory Health Survey: a cross-sectional study. Lancet 2001; 358: 2103–9