Abstract
Background: Information on the incidence and prevalence of diseases is a core indicator for public health. There are several ways to estimate morbidity in a population (e.g., surveys, healthcare registers). In this paper, we focus on one particular source: general practice based registers. Dutch general practice is a potentially valid source because nearly all non-institutionalized inhabitants are registered with a general practitioner (GP), and the GP fulfils the role as “gatekeeper”. However, there are some unexplained differences among morbidity estimations calculated from the data of various general practice registration networks (GPRNs). Objective: To describe and categorize factors that may explain the differences in morbidity rates from different GPRNs, and to provide an overview of these factors in Dutch GPRNs. Results: Four categories of factors are distinguished: “healthcare system”, “methodological characteristics”, “general practitioner”, and “patient”. The overview of 11 Dutch GPRNs reveals considerable differences in factors.
Conclusion: Differences in morbidity estimation depend on factors in the four categories. Most attention is dedicated to the factors in the “methodology characteristics” category, mainly because these factors can be directly influenced by the GPRN.
Introduction
Morbidity rates are core indicators of public health and the healthcare needs of a population; therefore, valid information on incidence and prevalence rates of diseases is important Citation[1]. There are several ways to estimate morbidity rates in a population, such as health interview surveys, health examination surveys, and healthcare registers, of which general practice based registers are an example Citation[1]. Compared to morbidity rates estimated from health interviews, an important advantage of morbidity rates estimated from care-based data is that health problems are diagnosed by a physician.
In the Netherlands, and other countries such as the UK, nearly all non-institutionalized inhabitants are registered with a general practitioner (GP) Citation[2–4]. Additionally, Dutch GPs fulfil the role as “gatekeeper”: when patients seek medical care from a medical specialist, they have to be referred by their GP and, after consultation, the medical specialist reports back to the patient's GP Citation[5]. GPs have contact with patients suffering from diseases in various stages of their disease and with all patient groups without selection regarding age, gender, socio-economic status, or ethnicity Citation[6–9]. This makes Dutch general practice a potentially valid source of information on morbidity.
Many GPs keep an electronic medical record (EMR), primarily for direct patient care Citation[10]. When several GPs collaborate in the collection of patient information (e.g., using a uniform data collection method, and the same registration rules and classification system) and, gather their information from separate EMRs into a central database, a general practice registration network (GPRN) is established Citation[9], Citation[11], Citation[12]. Besides estimating morbidity figures, GPRNs can be used for a variety of purposes: they can act as an index for selecting patients with certain characteristics for research, for research into the course of illnesses, healthcare utilization, or quality of care, and for education or management Citation[5].
In the Netherlands, there are multiple continuously recording GPRNs. The Continuous Morbidity Registration (CMR) Nijmegen, the oldest Dutch GPRN, dates back to 1967 Citation[9]. Since then, many other continuously recording GPRNs have been established, and several continuously operating GPRNs still exist today.
Gijsen and Poos Citation[13] demonstrated how data from GPRNs can be used to estimate morbidity in the Dutch population. They also showed that these estimations differ among various Dutch GPRNs. An example of these differences, the prevalence rates of rheumatoid arthritis, calculated from data from five different GPRNs, are presented in Box 1 Citation[14].
To increase the utility of GPRN data for morbidity estimations in the Netherlands, a research project has been set up. The first part of this project is to gain more insight into differences in morbidity estimations among GPRNs. In this article, we describe and categorize several factors that may explain the differences in morbidity rates as calculated from data provided by Dutch GPRNs. In addition, we give an overview of several Dutch GPRNs and consider their dissimilarities as a first step towards explaining these differences.
Factors influencing morbidity estimates from GPRNs
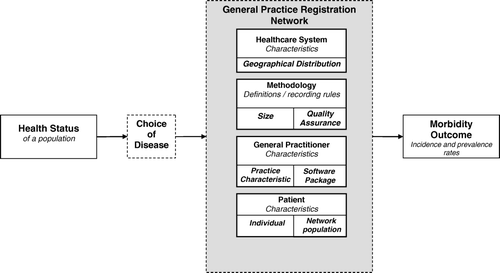
The factors described in this section potentially influence morbidity estimates calculated from GPRNs. The categories of factors are based on different levels, such as country, region, practice, and doctor, as described by Marinus Citation[15]. These levels relate to different sources of variation, which we translated into the GPRN situation and to which added the “patient” level as an additional source of variation Citation[15]. Explanations for these factors are based on findings from previously published studies. It is important to realize that the occurrence of diseases in the population determines morbidity, but that the different factors described here influence the estimation of that morbidity. The categories and factors are presented in .
Within a GPRN, we distinguish four categories of factors: “healthcare system”, “methodological characteristics of the network”, “general practitioner”, and “patient”. The factors and sub-factors are shown as independent, but they are often interrelated.
The healthcare system refers to the levels of the country and regions. The healthcare system defines the accessibility of GPs and other healthcare professionals in a specific country and the rules or laws to which GPs have to comply Citation[3], Citation[16]. If medical specialists or other healthcare providers are directly accessible and do not report information to the patient's GP, the completeness of the information from general practice based data about morbidity will be reduced Citation[5]. Most healthcare system related characteristics are identical for an entire country, but within a country regional differences also exist. Examples of regional differences are the distance between the general practice and the nearest hospital, the organization of GP out-of-hours services, and cooperation with other healthcare facilities Citation[17]. The geographical spread of the GPRN is also an important factor Citation[18].
The second category of factors includes the methodological characteristics of the GPRN. The operating definitions and registration rules affect the validity and reliability of the data for estimating morbidity rates Citation[13], Citation[19], Citation[20]. A large range of factors concerning definitions and rules are important: Which morbidity data are included in the GPRN database (only chronic conditions or also acute, minor health problems)? Which classification system or diagnostic criteria, if any, are used to record the morbidity information? What are the operational definitions of incidence and prevalence to determine morbidity? How are all patients with a specific disease counted in the GPRN (numerator)? Are data from all contact with the patient taken into account or only from face-to-face contact? Is all information received by a GP on morbidity taken into account, such as information from medical specialists? Citation[21], Citation[22]. Another important methodological aspect is size, i.e., the sampling size of the total population of interest (in our case, the total Dutch population). Sampling size influences the power of the estimations Citation[23].
The methodological characteristics of a GPRN are strongly influenced by the main purpose of the GPRN. As Knottnerus Citation[24] comments: “a diversity of objectives inevitably brings diversity of methods and systems”. The definitions and registration rules of a GPRN are derived from its purpose Citation[19].
In addition, “quality assurance” is an essential methodological factor; it determines, for example, GPs’ compliance with the rules and therefore also influences the validity and reliability of the data Citation[9]. Key issues here are the application of minimum quality criteria, systematic checking of the data, and feedback to GPs about data quality, all of which are incentives for providing high-quality data Citation[23], Citation[25].
The third category of factors regards the “general practitioner” and refers to the influence of GP characteristics within a GPRN on morbidity figures. Marinus Citation[26] studied this factor and concluded that morbidity rates vary considerably among GPs. Research also showed that this variation depends on the disease under study Citation[27]. Less variation in morbidity rates between GPs is found regarding diseases that are easy to recognize or have clear diagnostic criteria, such as herpes zoster or diabetes mellitus Citation[8], Citation[15], Citation[28–30].
The factor “general practitioner” also contains practice characteristics. These characteristics include, for example, the number of GPs working in a practice, whether GPs work in a healthcare centre or in separate practices, the intensity of cooperation among GPs, and the employment of other personnel such as practice assistants and practice nurses Citation[29]. Less variation in contact frequency is found among GPs within a practice compared to that among practices Citation[29].
The software package used to record patient information, the actual EMR, is also a factor that can explain differences between GPRNs. For example, previous research showed some unexpected differences in consultation rates related to these information systems, even after adjustment for explaining factors Citation[29].
The category of factors related to the “patient” is divided into individual patient characteristics (“case mix”) and the GPRN population as a whole. Patients differ from each other in many aspects, such as age, gender, socio-economic status, ethnic origin, and lifestyle Citation[31]. These aspects determine the probability of contracting a disease and whether a person seeks help and contacts his or her GP Citation[32]. Furthermore, the representativeness of the population of all practices participating in the GPRN, compared to the population of interest, is important for the generalizability of the results Citation[19].
GPRNs in the Netherlands
A first step towards understanding the differences in morbidity estimations among various GPRNs is to review these GPRNs with respect to the factors from the four categories. The GPRNs described in this article meet two criteria: they continuously collect data concerning all morbidity presented in general practice, and they are part of a long-term project. Eleven Dutch GPRNs fulfilled these criteria; the abbreviation and full name of each GPRN is presented in Box 2.
The authors from RIVM created a list of GPRN characteristics, which includes different aspects of the main categories of factors. Using the available background information in books, reports, and articles, they completed this list for each GPRN. The network coordinators of each GPRN checked and completed the list. shows the characteristics of the 11 GPRNs.
Table I. Outline of 11 Dutch general practice registration networks (GPRNs).
Table II. Box 1. Prevalence rates of rheumatoid arthritis from five general practice registration networks (GPRNs) in the Netherlands. The National Institute for Public Health and the Environment uses information about diseases derived from GPRNs for the estimation of morbidity rates presented in the National Public Health Compass Citation[14]. Prevalence‡ rheumatoid arthritis (per 1000 patients)
Table III. Box 2. Ten Dutch general practice registration networks.
All the studied GPRNs function within the Dutch healthcare system, so little difference is expected in terms of the healthcare system. The only differences may occur with respect to geographical differences, as only LINH and IPCI operate nationally.
Methodological characteristics, however, do vary between GPRNs. The sizes of the GPRNs range from 13 500 to 600 000 registered patients, with the number of GPs and practices ranging between 8 and 362 and 3 and 80, respectively.
The main goals of the GPRNs can be divided into two objectives, but providing input for and conducting scientific research are common aims. One objective is to generate information about general practice in general; the other objective regards to supply a sampling framework.
The method used to establish the epidemiological numerator depends on several characteristics, such as the type of network, the recording rules of the GPRN, the classification system used, and the software package used. In the Netherlands, there are two main network types: “contact” and “problem list” based GPRNs. “Problem list” based GPRNs only contain information about the health problems of a patient that are permanent, chronic (duration longer than 6 months), or recurrent Citation[18]. HAG-net-AMC and RNH are “problem list” based GPRNs and consequently count the number of diseases recorded on the “problem list” to establish the numerator. “Contact” based GPRNs store information about all patients’ health complaints and diagnoses from all contact with the practice in their database. Information from several contacts is structured into “episodes” for a specific disease. Such “episodes” are assigned by the GP. To establish a numerator, all episodes are counted. CMR-N, HNU, RNG, and Trans count all “episodes” for a specific disease. ANH-VUmc, RNUH-LEO, and SMILE extract information from both methods (problem list and episode construction by GPs) into their database. LINH is a “contact” based GPRN, which constructs episodes afterwards using EPICON, a computerized algorithm which links separate contacts into one “episode” Citation[33].
The most commonly used classification system for classifying diseases is ICPC-1. Other classifications in use are ICPC-2 and E-list. All GPs in the included GPRNs record information in an electronic medical record software system, but they vary with regard to the software package used. A GPRN usually utilizes only one or two software packages.
A software package sometimes forces the GP or the GPRN to choose a certain method. GPRNs use different operational definitions of episodes. CMR-N includes all the information a GP has about a patient in determining morbidity. SMILE, RNG, and LINH include data about all GP–patient contact, including indications for prescriptions. For “contact” based GPRN databases, the completeness of the numerator depends on what information is recorded. “Contact” based GPRNs vary substantially in this respect. Data from face-to-face contact with the GP and home visits during weekdays are usually recorded, as well as data from telephone contact. Data regarding contact during out-of-hours services are recorded the least. Data regarding contact with practice nurses and assistants are recorded when this is important for patient care, but these entries are often incomplete.
Nine GPRNs check for misclassifications and impossible or illogical data combinations after data extraction from the practices. HAG-net-AMC, HNU, IPCI, LINH, and RNUH-LEO also monitor data completeness.
To ensure a reliable and valid registration of diseases, different methods are used: training of GPs, explicit documentation, and meetings among GPs concerning registration difficulties and consensus procedures. Ten out of the 11 registrations give feedback to GPs about their recording performance.
The epidemiological denominator indicates the total population at risk for all practices participating in the GPRN. It is possible that the composition of the population with respect to socio-economic status, ethnicity, level of urbanization, etc. differs among the GPRNs. Moreover, several GPRNs are located in limited regions of the country, and it is well known that the health status of the population differs among regions Citation[34]. For all GPRNs, the population's age and gender distribution is known. Eight out of 10 GPRNs also record family characteristics, such as household size. CMR-N, RNH, and SMILE also include socio-economic status indicators, such as education and occupation. Most GPRNs include the numerical part of the zip codes of the addresses of their population, from which socio-economic status can be roughly estimated Citation[35].
Discussion
In this article, several factors that may explain the differences in morbidity estimates from various GPRNs are described. Four main categories of factors are distinguished. In future research, we will investigate the influence of these different factors on morbidity estimations. In addition, an overview is given of 11 Dutch GPRNs, which reveals considerable differences among GPRNs. In this article, most attention is dedicated to the factors in the “methodological characteristics” category. One reason for this is that these factors can be directly influenced by the GPRNs, unlike for example the healthcare system or patient factors.
Using the differences in estimations of the prevalence of rheumatoid arthritis (RA) among five GPRNs (Box 1) and the variation of factors among these GPRNs, we can identify several possible explanations. RNH and RNUH-LEO show relatively high estimations, which may be explained by the fact that both GPRNs are “problem list” based networks. A diagnosis on the “problem list” remains in the database until the patient is cured or until the disease is no longer relevant for the patient's care, whereas the contact-based databases LINH and Trans only count prevalent cases of RA when contact related to RA has taken place in a particular year. Another difference is that CMR-N uses the E-list for classification of RA, in contrast with the other networks, which use ICPC. In the ICPC classification, the code for RA also contains other rheumatoid disorders such as ankylosing spondylitis, whereas the E-list code only includes RA. However, this was not reflected by a lower estimation of RA in the CMR-N. At this point, the only conclusion is that explaining the differences is complex.
The other categories of factors that might explain the differences may not be influenced by the GPRN, but they cannot be ignored. The geographical areas covered by the Dutch GPRNs vary. Because some GPRNs act regionally instead of nationally, a part of the variation in morbidity rates is probably based on real differences, as the health status of the population is not equally distributed across the country Citation[34].
The composition of the practice and GP and patient characteristics in relation to the entire population of interest determine the representativeness of the GPRN population. In addition to adjusting for gender and age of the GPRN population, one could adjust for socio-economic status. Direct measurements of socio-economic status, such as education, are preferred to indirect measures such as zip codes.
In further research, we want to study the influence of the factors described in this article. It would be particularly interesting to establish which factors affect the validity of the estimations of morbidity figures. However, we do not expect that the factors presented here will explain all variance in morbidity figures, because the process of diagnosing is known to be a complex interaction between knowledge, the wishes of the patient, the GP's opinion, and other factors Citation[29].
Acknowledgements
This article has been made possible by cooperation of the 11 GPRNs. In this respect, we would like to thank W. M. Boon (ANH VUmc), C. van Boven (Transition Project), H. J. Brouwer, (HAG-net-AMC), H. J. M. van den Hoogen (CMR Nijmegen) and J. van der Lei (IPCI) for their input.
References
- Hoogenveen R, Westert G, Dijkgraaf M, Schellevis F, de Bakker D. Disease prevalence estimations based on contact registrations in general practice. Stat Med 2002; 21: 2271–85
- van Weel C, Weel Baumgarten van E, Mold J. The importance of longitudinal studies in family medicine: experiences of two practice-based research networks. J Am Board Fam Med 2006; 19: 69–74
- Fleming DM, Schellevis FG, Van Casteren V. The prevalence of known diabetes in eight European countries. Eur J Public Health 2004; 14: 10–4
- van Weel C. Longitudinal research and data collection in primary care. Ann Fam Med 2005;3 Suppl 1:S46–51.
- Okkes IM, Polderman GO, Fryer GE, Yamada T, Bujak M, Oskam SK, et al. The role of family practice in different health care systems. A comparison of reasons for encounter, diagnoses and interventions in primary care populations in the Netherlands, Japan, Poland and the United States. J Fam Pract 2002; 51: 72–82
- Metsemakers JF, Knottnerus JA, van Schendel GJ, Kocken RJ, Limonard CB. Unlocking patients’ records in general practice for research, medical education and quality assurance: the Registration Network Family Practices. Int J Biomed Comput 1996; 42: 43–50
- Verheij RA, van der Zee J. Chapter 30. Collecting information in general practice: ‘just by pressing a single button?. Morbidity, performance and quality in primary care Dutch general practice on stage, GP Westert, L Jabaaij, FG Schellevis. Radcliffe Publishing, Oxford 2006; 265–72
- Oliemans AP. Morbiditeit in de huisartspraktijk. Stenfert Kroese, Leiden 1969; 1–257
- van Weel C. Validating long term morbidity recording. J Epidemiol Community Health 1995; 49 Suppl 1: 29–32
- de Jongh H, Kole H, Metsemakers JFM, Peerden H, Smit C, Stroucken J, et al. Richtlijn adequate dossiervorming met het Elektronisch Medisch Dossier. NHG-Publicatie automatisering. Nederlands Huisartsen Genootschap, Urecht 2004
- Majeed A. Sources, uses, strengths and limitations of data collected in primary care in England. Health Stat Q 2004; 21: 5–14
- Hart HE, van der Wouden JC, Hoppener P, van Schendel GJ, Knottnerus JA. General practice registration networks in the Netherlands: a brief report. J Am Med Inform Assoc 1999; 6: 173–5
- Gijsen R, Poos R. Using registries in general practice to estimate countrywide morbidity in The Netherlands. Public Health 2006; 120: 923–36
- Poos MJJC, Gijsen R. Reumatoide arthritis. Omvang van het probleem. Achtergronden en details bij cijfers uit huisartsregistraties. National Public Health Compass (Nationaal Kompas Volksgezondheid), part of the Dutch Public Health Status and Forecasts, version 3.11.1 Bilthoven: RIVM; 8 November, 2007. URL: www.nationaalkompas.nl.
- Marinus AMF. Inter-doktervariatie in de huisartspraktijk. S.n. 1993. Universiteit van Amsterdam.
- Cardol M, Schellevis FG, Spreeuwenberg P, van de Lisdonk EH. Changes in patients’ attitudes towards the management of minor ailments. Br J Gen Pract 2005; 55: 516–21
- Huisartsenzorg. Volksgezondheid Toekomst Verkenning, Nationale Atlas Volksgezondheid. Bilthoven: RIVM, ( www.zorgatlas.nl) versie 3.13; 17 April, 2008.
- Plat AW, te Wierik MJ, Kroon AA, Schouten HJ, van den Akker M, van Schayck CP, et al. Regional differences in cardiovascular risk factor profile cannot fully explain differences in cardiovascular morbidity in the Netherlands: a comparison of two urban areas. Neth J Med 2005; 63: 309–15
- Schellevis F, Westert G, de Bakker D, Foets M, vd Velden J. Kritisch lezen van informatie uit grote registratiebestanden. Huisarts Wet 1999; 42: 591–601
- van der Meer V, de Waal MWM, Timmers AP, de Bock GH, Springer MP. Hoe up-to-date is het medisch dossier? Een onderzoek op vijf lokaties. Huisarts Wet 2001; 44: 194–7
- de Lusignan S, van Weel C. The use of routinely collected computer data for research in primary care: opportunities and challenges. Fam Pract 2006; 23: 253–63
- van Weel C, de Grauw W. Family practices registration networks contributed to primary care research. J Clin Epidemiol 2006; 59: 779–83
- Deckers JG, Paget WJ, Schellevis FG, Fleming DM. European primary care surveillance networks: their structure and operation. Fam Pract 2006; 23: 151–8
- Knottnerus JA. Registreren van morbiditeit in de huisartsgeneeskunde, over diversiteit van doelstellingen en vereisten. Huisarts Wet 1994; 37: 136–41
- Porcheret M, Hughes R, Evans D, Jordan K, Whitehurst T, Ogden H, et al. Data quality of general practice electronic health records: the impact of a program of assessments, feedback, and training. J Am Med Inform Assoc 2004; 11: 78–86
- Marinus AMF. Inter-doktervariatie in het Transitieproject. Huisarts Wet 1990; 33: 4–8
- Brouwer HJ, van Weert HC, Vintges MMQ, Bindels PJE. De betekenis van interpraktijkvariatie in registratienetwerken. Huisarts Wet 2000; 43: 426–9
- Thiru K, Hassey A, Sullivan F. Systematic review of scope and quality of electronic patient record data in primary care. BMJ. 2003; 326: 1070
- Cardol M, van Dijk L, de Jong JD, de Bakker DH, Westert GP. Huisartsenzorg: Wat doet de poortwachter? Tweede Nationale studie naar ziekten en verrichtingen in de huisartspraktijk. Nivel, Utrecht 2004
- Fleming DM, Bartelds A, Chapman RS, Cross KW. The consistency of shingles and its significance for health monitoring. Eur J Epidemiol 2004; 19: 1113–8
- Westert GP, Schellevis FG, de Bakker DH, Groenewegen PP, Bensing JM, van de Zee J. Monitoring health inequalities through general practice: the Second Dutch National Survey of General Practice. Eur J Public Health 2005; 15: 59–65
- van Lindert H, Droomers M, Westert GP. Een kwestie van verschil: verschillen in zelf-gerapporteerde leefstijl, gezondheid en zorggebruik. Overzicht van bestaand onderzoek naar verschillen in Nederland. Tweede Nationale Studie naar ziekten en verrichtingen in de huisartspraktijk. Utrecht: Nivel; 2004.
- Biermans MCJ, de Bakker DH, Verheij RA, Gravestein JV, van der Linden MW, de Vries Robbé PF. Development of a case-based system for grouping diagnoses in general practice. Int J Med Inform 2007; doi: 10.1016/j.ijmedinf.2007.08.002.
- Ziekten en aandoeningen. Gezondheidsproblemen. Volksgezondheid Toekomst Verkenning, Nationale Atlas Volksgezondheid. Bilthoven: RIVM, ( www.zorgatlas.nl) versie 3.13; 17 April, 2008.
- Rangorde naar sociale status van postcodegebieden in Nederland. 26 April, 2007 [ cited 2 June 2007]; Available from URL: www.scp.nl/onderzoek/statusscores/index.shtml.