Abstract
Background
Diagnostics are increasingly shifting to patients’ home environment, facilitated by new digital technologies. Digital diagnostics (diagnostic services enabled by digital technologies) can be a tool to better respond to the challenges faced by primary care systems while aligning with patients’ and healthcare professionals’ needs. However, it needs to be clarified how to determine the success of these interventions.
Objectives
We aim to provide practical guidance to facilitate the adequate development and implementation of digital diagnostics.
Strategy
Here, we propose the quadruple aim (better patient experiences, health outcomes and professional satisfaction at lower costs) as a framework to determine the contribution of digital diagnostics in primary care. Using this framework, we critically analyse the advantages and challenges of digital diagnostics in primary care using scientific literature and relevant casuistry.
Results
Two use cases address the development process and implementation in the Netherlands: a patient portal for reporting laboratory results and digital diagnostics as part of hybrid care, respectively. The third use case addresses digital diagnostics for sexually transmitted diseases from an international perspective.
Conclusions
We conclude that although evidence is gathering, the often-expected value of digital diagnostics needs adequate scientific evidence. We propose striving for evidence-based ‘responsible digital diagnostics’ (sustainable, ethically acceptable, and socially desirable digital diagnostics). Finally, we provide a set of conditions necessary to achieve it. The analysis and actionable guidance provided can improve the chance of success of digital diagnostics interventions and overall, the positive impact of this rapidly developing field.
KEY MESSAGES
Digital diagnostics is defined as diagnostic services facilitated by digital technologies, which partially or wholly replace healthcare professionals.
Responsible digital diagnostics is technically robust, lawful, and ethical.
Digital diagnostics tools should generate comprehensive evidence regarding the quadruple aim (better patient experiences, health outcomes and professional satisfaction at lower costs).
Introduction
Healthcare worldwide faces a heavier workload due to ageing populations, rising multimorbidity, increased complexity of healthcare, and personnel shortages. Primary care bears the full brunt of this pressure because it’s usually the first port of call [Citation1,Citation2]. Also, the number of laboratory tests is unnecessarily rising because of this pressure [Citation3].
Healthcare needs transformation, ensuring access and continuity even in limited access to traditional face-to-face services. Advocates of digital health (Box 1) propose that digital technologies can facilitate this transformation, improving care from a quadruple-aim perspective (better patient experiences, health outcomes and professional satisfaction at lower costs) [Citation4].
Box 1 Working definition of digital health.
This article addresses digital health as ‘health services and information delivered or enhanced through the Internet and related technologies’ [Citation44]. Its complexity can be brought down to three domains [Citation45]:
consumer-driven and consumer-controlled technologies (e.g. wearables and apps),
digital tools for health stakeholders to interact with each other (e.g. telemedicine and messaging systems),
technologies that improve health and health services through data (e.g. data management systems and repositories).
Additionally, WHO defines eHealth even broadly as ‘the cost-effective and secure use of information and communications technologies in support of health and health-related fields, including health-care services, health surveillance, health literature, and health education, knowledge and research’ [Citation46].
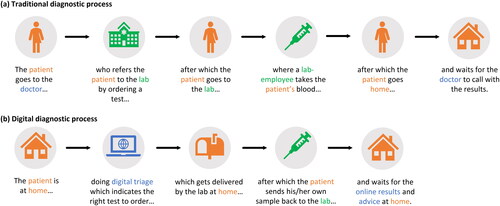
In primary care settings, for example, the digital capture of questions (digital triage) can be coupled to diagnostic services at home, which can support daily practice. This new process is called ‘digital diagnostics’ (Box 2 and ). This patient-driven approach is attractive because it can offer ‘the right care at the right time’ with direct access to triage, laboratory tests, and results with partial (remote and asynchronous) or no direct support from healthcare professionals (HCPs).
Box 2 Working definition of digital diagnostics.
We define digital diagnostics based on the definition of eHealth: ‘diagnostic services facilitated by digital technologies.’ As diagnostic services provide information necessary for healthcare professionals and patients to make decisions, it is found in two digital health domains: consumer-driven technologies and digital tools to interact. Finally, the definition of WHO adds two relevant aspects, which we consider important for evaluating digital diagnostics, cost-effectiveness and technical robustness.
The core principle of digital diagnostics is that the traditional role of healthcare professionals (HCPs) is partially or wholly replaced or facilitated by digital systems () and therefore, its implementation is flexible. Digital diagnostics can support pathways that are entirely digital or blended. For example, the advice that leads to a test being ordered online may have been discussed by the patient and HCP during an in-person appointment; after triaging and advice online, the patient may go on-site for sampling or the results can be discussed during an in-person appointment with a HCP.
Figure 1. Simplified representation of the diagnostic process, the traditional vs the digital. Traditional digital diagnostics (a) requires patients to physically visit their physician and the diagnostic laboratory premises and go back home to wait for the results and instructions for follow-up when necessary. In the case of digital diagnostics (b), patients are provided with the right online tools to determine if a test is necessary. When necessary, a request is placed online, the sample can be taken at home and sent to the lab, and the results can be received at home, for example, through a patient portal. (a) Traditional diagnostic process. (b) Digital diagnostic process.
Digital diagnostics is a rapidly developing field of growing importance in the primary care landscape. As in any young field, it is still unclear how to determine the success of these interventions. Here, we propose the quadruple aim (better patient experiences, health outcomes and professional satisfaction at lower costs) as a tool to determine the contribution of digital diagnostics in primary care [Citation1]. We also aim to establish, based on our experience and the experience of others, practical guidance which can facilitate the adequate development and implementation of digital diagnostics. For these purposes, we critically analyse the advantages and challenges of digital diagnostics in primary care, first in general and then in the context of three use cases. The use cases were chosen based on our own experience and represent areas where digital diagnostics is already having an important impact in Europe. Finally, we introduce the concept of ‘responsible digital diagnostics’ and derive a set of conditions necessary to achieve it.
The focus of this article is digital diagnostics as a direct-to-patient service. In literature, it can also be referred to as ‘direct-to-consumer testing’, ‘direct-access testing’, ‘patient-authorised testing’, or ‘consumer-initiated testing’. However, this article does not focus on genetic testing nor recreational direct-to-customer testing [Citation5] but on facilitating the provision of (primary) healthcare via digital means.
Analysis of advantages of digital diagnostics
Digital diagnostics can lead to positive effects in the quadruple aim’s domains: improved patient experience, better patient outcomes, improved provider satisfaction and optimisation of healthcare resources.
From the healthcare system’s perspective, digital diagnostics can avoid unnecessary medical consultations and shorten the time to diagnostics, optimising the use of resources [Citation6]. Resource optimisation can alleviate pressure caused by staff shortages, improving HCPs’ work experience. Digital diagnostics can also enhance the referral behaviour of doctors regarding laboratory tests [Citation3]. Additionally, avoiding unnecessary travelling contributes to ecologic sustainability [Citation7].
Digital diagnostics can be a tool to make tests more easily accessible since it is not restricted by opening hours or availability of personnel, as traditional diagnostics is. It can also improve access to diagnostics in the face of insufficient infrastructure and medical staff, as it happens more acutely in low-resource settings in high-, middle- and low-income countries [Citation6,Citation8]. Additionally, saving patients from travelling and visiting (crowded) healthcare facilities can be particularly valuable for frail or immunocompromised individuals and patients with mobility issues. Digital diagnostics may also have a positive impact on populations struggling with barriers to seeking and accessing healthcare related to (gender) inequity issues [Citation9] or social stigma [Citation10]. Promoting access has indeed a strong connection with reducing health inequalities [Citation11]. Moreover, improving access may shorten the time to diagnosis, improving patient experience and outcomes.
Through digital diagnostics, patients can gain access to high-quality health information, learn to manage their health, maintain adherence to medical treatment and adopt healthier lifestyles [Citation12]. Regarding the doctor-patient relationship, the proximity of diagnostic services to patients offers the possibility of shortening the information gap between patients and HCPs, increasing patients’ autonomy. This facilitates a more equitable doctor-patient relationship where the HCP can coach the empowered patient [Citation13].
Analysis of challenges of digital diagnostics
Digital diagnostics entails changes for patients, HCPs and healthcare organisations. Such reorganisation of healthcare comes with relevant challenges, addressed in this section.
Safety, right to information, transparency, and comprehensibility
We address safety as a broad concept that includes physical and psychological integrity, data security and privacy [Citation5]. All steps of digital diagnostics () must be carefully evaluated to ensure safety:
triage systems and laboratory tests must be based on scientifically validated medical and quality guidelines,
the apps and/or platforms that support digital diagnostics services must be validated and continuously evaluated,
logistics must be secure, trackable, and reliable, ensuring the integrity of the test kits and samples while protecting patient privacy.
Safety also depends on communicating the relevant information to the patient in a comprehensible, transparent and accessible way. Patients should be informed about the basis of the triage system (e.g. medical guidelines, algorithms, AI systems), scientific validation and certification. In the case of automated decision-making, patients must be explicitly notified and be able to refuse with equal access to care, in line with current EU regulations [Citation14]. The goal is to enable patients to interpret the information and understand the risks and benefits so that they can take appropriate actions [Citation1]. Physicians must be equally informed to support their patients. The fear of digital diagnostics causing psychological distress can be avoided by offering high-quality information explaining the tests and results (use cases 1 and 3). We recommend designing the digital diagnostics platform in such a way that results can be also delivered through direct contact with HCP if desired by the patient or HCP (post-test consultations).
Equitable access and (digital) health literacy
There needs to be more research on the intersection of digital diagnostics and equity. However, as a digital health application, digital diagnostics can either increase inequality and broaden the digital divide or be a tool to achieve equitable access to healthcare [Citation15]. In this context, its inclusion in healthcare insurance packages is key. Underserved and vulnerable populations and groups with low (digital) health literacy should not be left behind, especially because digital diagnostics may lower barriers to accessing care (use case 3). In the Netherlands, organisations are working to provide accessible information, for example, using animations (KIJKsluiter) or working together with users with low (eHealth) literacy (Pharos). Tailoring digital diagnostics to different patient groups can be a solution, which may be challenging from technical, design, security, and practical perspectives. When this is impossible, or when groups object to digital diagnostics, access to regular face-to-face healthcare must remain a possibility.
Providing the correct information and support to patients for sampling is a key to success. Otherwise, letting the sampling be carried out by HCPs or carers at home could be a feasible solution. For HCPs, it may sometimes be clear that patients can use the digital diagnostics pathway independently. If that is not the case, a doctor-patient dialogue (and carers when applicable) is necessary to determine the optimal solution for the patient.
Over-testing
A pending risk of digital diagnostics is over-testing, an inefficient use of resources that generates unnecessary psychological, physical, and economic burdens for patients [Citation16]. Validated triage systems, based on established guidelines, appropriate patient information and human oversight (use case 2) can help avoid this issue. Continuous research (e.g. data analysis, validation and surveillance) is necessary to guarantee the value of the service.
Integration with the workflow and cost-effectiveness
HCPs report reservations and challenges about incorporating the digital component in healthcare pathways, such as suboptimal integration with the current workflow, (perceived) increased workload, and decreased satisfaction of HCPs [Citation17]. Despite patients’ positive experiences, physicians report negative experiences such as faltering technology, uncomfortable new communication channels, insufficient digital skills, or lack of confidence in data privacy measures [Citation18–20]. This underlines the importance of designing and implementing digital diagnostics in co-creation with HCPs and careful incorporation in their workflows. For solid blending of digital and regular care, quality models can support the integration of digital health into regular care [Citation21].
As argued previously, digital diagnostics should allow HCPs to assist patients if they require help. The challenge is to use different strategies for different patient groups: solely digital, hybrid and traditional face-to-face (use cases 1 and 2). The suitable model can only be found through co-creation and inclusiveness when designing, testing, and implementing digital diagnostics.
Cost-effectiveness evaluations are generally challenging and not yet widely carried out [Citation22]. Evidence of the cost-effectiveness of digital diagnostics is also scarce but is decisive for investments in this type of innovation, inclusion in insurance packages, and finally, its sustainability.
Legal compliance and data security
Privacy and data safety and security must be assessed by experts, as established by European regulation [Citation14,Citation23,Citation24]. Data Protection Impact Assessments (DPIA), Data Management Plans (DMP), and security assessments are important tools to comply with data security standards. Additionally, contractual frameworks that include data privacy, safety, and security aspects should be in place. However, legal challenges remain. For example, the European Medical Device Regulation (MDR) provides a definition of ‘medical device’ but digital diagnostics may not perfectly fall under this definition [Citation23]. In the case of a patient results portal (use case 1) and Homelab (use case 2), no final diagnosis is offered to the patients, which remains the physician’s responsibility.
Use cases of digital diagnostics
Digital diagnostics has been implemented for various objectives, for example, genetic and hormone testing, hepatitis C, sexually transmitted diseases (STD), communication of tuberculosis test results and laboratory results in general, and more recently for COVID-19 testing [Citation6,Citation10,Citation25–29]. This section will present three relevant use cases: two implementation examples and an overview of STD digital diagnostics.
Use case 1: Patient portals for reporting laboratory results
Patient portals are web-based platforms that offer healthcare information, such as laboratory results, directly to patients. However, there is fear of causing distress to patients. This can negatively affect patients’ health and generate unnecessary burdens for HCPs [Citation30,Citation31]. This risk can be minimised by ensuring that information is accessible, adequate, relevant, and easy to interpret. Success is improved when all end-users are involved early on, from the design phase [Citation32].
In the Netherlands, patient portals are increasingly common. One example is a portal used by a diagnostics company and connected to primary care. After carrying out the diagnostic test, patients can log into the website of their primary care practice (in line with EU and national data security standards) to access their laboratory results. Patients can see the tests arranged by date, each test result is accompanied by a traffic light-colour system that indicates normal or abnormal results and an explanation [Citation30].
The portal was aligned with primary care services and co-created with family physicians (FP), patients and communication experts [Citation21,Citation33]. High-quality and patient-tailored information was designed in a user-friendly environment. Patients experienced the portal positively for usability, motivation, and confidence to act. Our research suggests that it is necessary to tailor patient portals to different groups, for example, according to education level, age, or disease [Citation21]. Additionally, it is important to include people with low (digital) health literacy and unique needs in the target group. Patient-centred research is needed for the permanent improvement of this system. The system has also been implemented in Norway, Portugal and Switzerland.
Use case 2: A primary care digital diagnostics portal, an example of hybrid care
Nowadays, a combination of digital and in-person care provision is often regarded as the most efficient approach. High-quality hybrid healthcare must meet the patient’s needs with the digital component seamlessly integrated into healthcare processes, often causing a transformation of workflows [Citation34–36].
An example of hybrid care is Homelab, an online patient service integrated into the digital environment of FPs in the Netherlands [Citation37]. Frequently requested standard tests are offered through Homelab (e.g. for fatigue or STD). Patients can log in securely to Homelab using a national identity management system. First, patients must answer questions in a validated digital triage tool, which directs to the advised diagnostic tests. Patients can request the tests directly online. A FP reviews the request, and if authorised, the test will be automatically reimbursed by the patient’s healthcare insurance. When a diagnostic test result is abnormal, it is possible to consult the FP in person or via (e)consultation. The test results are available online for patients and their FPs through the primary care information system. The FP validation step was implemented to avoid the misuse of Homelab by patients, and it was product of a co-creation process with FPs and patients [Citation38]. The system provides a good balance between patient autonomy and FP oversight. Homelab has been adapted to be used in Norway, Portugal and Switzerland.
Use case 3: The case of digital diagnostics and sexually transmitted diseases (STD)
STD testing is one of digital diagnostics’ most promising application areas, showing positive effects at individual and population health levels. Acceptability, usage, and follow-up rates are high [Citation10,Citation39]. Results suggest that STD digital diagnostics may overcome shame-related issues about being tested [Citation29]. Constant evaluation should prevent adverse effects on patients (e.g. psychological, social, or behavioural). Failing to follow medical guidelines as well as align with patients’ needs can cause anxiety, insufficient follow-up, suboptimal treatment, and undesired or no changes in sexual behaviour [Citation10]. Additionally, because STD prevalence intersects with race, class, and gender inequities, equitable access remains challenging [Citation40].
Although evidence of HCP satisfaction has not been specifically reported for STD digital diagnostics, avoiding unnecessary consultations reduces the workload and eases administrative burdens, leading to higher job satisfaction [Citation41]. However, more research is needed in this area, including the evaluation of over-testing.
There are strong indications that STD digital diagnostics can be cost-effective. Home-based STD tests showed lower or similar prices compared with clinic-based testing. Additionally, direct access to STD testing through digital diagnostics lowers patients’ testing threshold, reduces direct and indirect costs (e.g. transportation, childcare costs, missed work), increases follow-up, and reduces unnecessary primary care consultations [Citation10].
In summary, evidence is gathering that STD digital diagnostics can reduce overall costs and improve population health, patient experience, and team well-being, but more research is needed.
Responsible digital diagnostics
Using the three use cases, we elaborated on the advantages (benefits) and challenges (risks) of digital diagnostics, and provided practical recommendations to design, and implement digital diagnostics responsibly. Aligned with the definition of trustworthiness for AI systems and Responsible Research and Innovation [Citation42,Citation43], we state that responsible digital diagnostics need to be technically robust (including ecological and economical sustainability), lawful, and ethical (and therefore socially desirable). To move in this direction, seven conditions were derived from our analysis (Box 3).
Box 3 Conditions for ‘responsible digital diagnostics.’
Seven concise and practical requirements for ensuring that digital diagnostics systems are technically and socially robust (including ecological and economic sustainability), lawful, and ethical (and therefore socially desirable), as derived from the analysis of the use cases.
Involvement of all end-users starting at the design phase (co-create).
Exploration and incorporation of the ethical perspective of the technology in the context of implementation.
Compliance.
Solid digital triage with medical guidelines as a basis.
Pre-test information and test results are communicated in a patient-friendly and understandable way.
Integration of digital diagnostics into regular care, including possibilities of intervention by HCPs in every step, depending on the necessities of the patient.
Evidence of positive impact on workload and job satisfaction of healthcare professionals.
Establishment of a continuous plan of evaluation according to the quadruple aim (reduce costs, improve population health and patient experience, and increase team well-being).
Conclusion
In this article, we have presented the case of digital diagnostics, moving away from recreational testing and instead presenting it as an option to improve access to medical diagnostics as part of regular care. Although there are initiatives to shift diagnostics to patients’ home environment, our analysis shows that scientific evidence is promising but often insufficient.
The field of digital diagnostics should strive to provide scientific evidence to guarantee the quality of hardware, software and medical materials, and the digital or blended healthcare as a whole. Embracing responsible digital diagnostics will contribute to the success and desirable societal impact of these new technologies. By deriving seven concise practical conditions, based on real use cases, this publication provides a way forward to innovate in digital diagnostics responsibly.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper. EPTK was previously Chief Innovation Officer with Unilabs. Currently, none of the authors are employees of Unilabs or another laboratory company and there are no personal or economic interests related to the use cases presented in this article. The authors are scientific experts on eHealth, they have for example, carried out substantial scientific research about digital diagnostics and developed programmes with a digital approach to diagnostic tests. In the case of EPTK, she has combined jobs in the scientific world with leadership roles in diagnostic centres (Saltro & Unilabs).
References
- van der Kleij RMJJ, Kasteleyn MJ, Meijer E, et al. SERIES: eHealth in primary care. Part 1: concepts, conditions and challenges. Eur J Gen Pract. 2019;25(4):179–189. doi:10.1080/13814788.2019.1658190.
- Goyal DK, Mansab F, Naasan AP, et al. Restricted access to the NHS during the COVID-19 pandemic: is it time to move away from the rationed clinical response? Lancet Reg Health Eur. 2021;8:100201. doi:10.1016/j.lanepe.2021.100201.
- Duddy C, Wong G. Efficiency over thoroughness in laboratory testing decision making in primary care: findings from a realist review. BJGP Open. 2021;5(2):bjgpopen20X101146. doi:10.3399/bjgpopen20X101146.
- Bodenheimer T, Sinsky C. From triple to quadruple aim: care of the patient requires care of the provider. Ann Fam Med. 2014;12(6):573–576. doi:10.1370/afm.1713.
- Lippi G, Favaloro EJ, Plebani M. Direct-to-consumer testing: more risks than opportunities: direct-to-consumer testing. Int J Clin Pract. 2011;65(12):1221–1229. doi:10.1111/j.1742-1241.2011.02774.x.
- Babirye D, Shete PB, Farr K, et al. Feasibility of a short message service (SMS) intervention to deliver tuberculosis testing results in peri-urban and rural Uganda. J Clin Tuberc Other Mycobact Dis. 2019;16:100110. doi:10.1016/j.jctube.2019.100110.
- Tennison I, Roschnik S, Ashby B, et al. Health care’s response to climate change: a carbon footprint assessment of the NHS in England. Lancet Planet Health. 2021;5(2):e84–92–e92. doi:10.1016/S2542-5196(20)30271-0.
- Rakers M, van de Vijver S, Bossio P, et al. SERIES: eHealth in primary care. Part 6: eHealth in low-resource primary care settings. Learning about eHealth contributions and barriers across high- middle- and low-income countries. Eur J Gen Pract. 2023;1:2241987. doi:10.1080/13814788.2023.2241987.
- Fleming KA, Horton S, Wilson ML, et al. The lancet commission on diagnostics: transforming access to diagnostics. Lancet. 2021;398(10315):1997–2050. doi:10.1016/S0140-6736(21)00673-5.
- Versluis A, Schnoor K, Chavannes NH, et al. Direct access for patients to diagnostic testing and results using eHealth: systematic review on eHealth and diagnostics. J Med Internet Res. 2022;24(1):e29303. doi:10.2196/29303.
- Shadmi E, Wong WC, Kinder K, et al. Primary care priorities in addressing health equity: summary of the WONCA 2013 health equity workshop. Int J Equity Health. 2014;13(1):104. doi:10.1186/s12939-014-0104-4.
- Kumar RB, Goren ND, Stark DE, et al. Automated integration of continuous glucose monitor data in the electronic health record using consumer technology. J Am Med Inform Assoc. 2016;23(3):532–537. doi:10.1093/jamia/ocv206.
- Penedo FJ, Oswald LB, Kronenfeld JP, et al. The increasing value of eHealth in the delivery of patient-centred cancer care. Lancet Oncol. 2020;21(5):e240–251–e251. doi:10.1016/S1470-2045(20)30021-8.
- Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the protection of natural persons with regard to the processing of personal data and on the free movement of such data, and repealing Directive 95/46/EC (General Data Protection Regulation) (Text with EEA relevance). (OJ L 119 04.05; 2016, p. 1, ELI. [cited 2023 Oct 11]. Available from: http://data.europa.eu/eli/reg/2016/679/oj.
- World Health Organization. Global diffusion of eHealth: making universal health coverage achievable: report of the third global survey on eHealth. Geneva: World Health Organization; 2016 [cited 2023 Oct 11]. Available from: https://iris.who.int/bitstream/handle/10665/252529/9789241511780-eng.pdf;jsessionid=5863887BBE11DBC278E094FDB4511B2C?sequence=1.
- Greenberg J, Green JB. Over-testing: why more is not better. Am J Med. 2014;127(5):362–363. doi:10.1016/j.amjmed.2013.10.024.
- Versluis A, van Luenen S, Meijer E, et al. SERIES: eHealth in primary care. Part 4: addressing the challenges of implementation. Eur J Gen Pract. 2020;26(1):140–145. doi:10.1080/13814788.2020.1826431.
- de Wilt T, Versluis A, Goedhart A, et al. General practitioners attitude towards the use of eHealth and online testing in primary care. Clin EHealth. 2020;3:16–22. doi:10.1016/j.ceh.2020.02.002.
- Dutch National Institute for Public Health and the Environment, Ministry of Health, Welfare and Sport. E-health monitor 2021. Stand van zaken digitale zorg [State of affairs of digital health]; 2021. [cited 2023 Oct 11]. Available from: https://www.rivm.nl/sites/default/files/2022-11/E-healthmonitor-2021-Stand-van-zaken-digitale-zorg.pdf.
- Dijkstra A, Heida A, Van Rheenen PF. Exploring the challenges of implementing a web-based telemonitoring strategy for teenagers with inflammatory bowel disease: empirical case study. J Med Internet Res. 2019;21(3):e11761. doi:10.2196/11761.
- Tossaint-Schoenmakers R, Kasteleyn M, Goedhart A, et al. The impact of patient characteristics on their attitudes toward an online patient portal for communicating laboratory test results: real-world study. JMIR Form Res. 2021;5(12):e25498. doi:10.2196/25498.
- van der Pol S, Rojas Garcia P, Antoñanzas Villar F, et al. Health-economic analyses of diagnostics: guidance on design and reporting. PharmacoEconomics. 2021;39(12):1355–1363. doi:10.1007/s40273-021-01104-8.
- Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on medical devices, amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and repealing Council Directives 90/385/EEC and 93/42/EEC (Text with EEA relevance). (OJ L 117 05.05); 2017, p. 1, ELI [cited 2023 Oct 11]. Available from: http://data.europa.eu/eli/reg/2017/745/oj.
- Regulation (EU) 2017/746 of the European Parliament and of the Council of 5 April 2017 on in vitro diagnostic medical devices and repealing Directive 98/79/EC and Commission Decision 2010/227/EU (Text with EEA relevance). (OJ L 117, 5.5); 2017, p. 176. ELI [cited 2023 Oct 11]. Available from: http://data.europa.eu/eli/reg/2017/746/oj.
- Kalokairinou L, Zettler PJ, Nagappan A, et al. The promise of direct-to-consumer COVID-19 testing: ethical and regulatory issues. J Law Biosci. 2020;7(1):lsaa069. doi:10.1093/jlb/lsaa069.
- Yang YT, Zettler PJ. Food and drug administration’s regulatory shift on direct-to-consumer genetic tests for cancer risk: direct-to-consumer genetic tests. Cancer. 2019;125(1):12–14. doi:10.1002/cncr.31773.
- Kyweluk MA. Quantifying fertility? Direct-to-consumer ovarian reserve testing and the new (in)fertility pipeline. Soc Sci Med. 2020;245:112697. doi:10.1016/j.socscimed.2019.112697.
- Layton JB, Kim Y, Alexander GC, et al. Association between direct-to-consumer advertising and testosterone testing and initiation in the United States, 2009-2013. J Am Med Assoc. 2017;317(11):1159–1166. doi:10.1001/jama.2016.21041.
- Gilbert M, Salway T, Haag D, et al. Use of GetCheckedOnline, a comprehensive web-based testing service for sexually transmitted and blood-borne infections. J Med Internet Res. 2017;19(3):e81. doi:10.2196/jmir.7097.
- Struikman B, Bol N, Goedhart A, et al. Features of a patient portal for blood test results and patient health engagement: web-based pre-post experiment. J Med Internet Res. 2020;22(7):e15798. doi:10.2196/15798.
- Irizarry T, DeVito Dabbs A, Curran CR. Patient portals and patient engagement: a state of the science review. J Med Internet Res. 2015;17(6):e148. doi:10.2196/jmir.4255.
- van Gemert-Pijnen JE, Nijland N, van Limburg M, et al. A holistic framework to improve the uptake and impact of eHealth technologies. J Med Internet Res. 2011;13(4):e111. doi:10.2196/jmir.1672.
- Talboom-Kamp E, Tossaint-Schoenmakers R, Goedhart A, et al. Patients’ attitudes toward an online patient portal for communicating laboratory test results: real-world study using the eHealth impact questionnaire. JMIR Form Res. 2020;4(3):e17060. doi:10.2196/17060.
- Reiners F, Sturm J, Bouw LJW, et al. Sociodemographic factors influencing the use of eHealth in people with chronic diseases. Int J Environ Res Public Health. 2019;16(4):645. doi:10.3390/ijerph16040645.
- Dutch National Institute for Public Health and the Environment. Ministry of Health, Welfare and Sport. E-Health Monitor 2021. Ervaringen uit het zorgveld [Experiences from the healthcare field]; 2022 [cited 2023 Oct 11]. Available from: https://www.rivm.nl/documenten/e-healthmonitor-2021-ervaringen-uit-zorgveld (Dutch).
- Greenhalgh T, Wherton J, Shaw S, et al. Video consultations for covid-19. BMJ. 2020;368:m998. doi:10.1136/bmj.m998.
- Unilabs. Homelab; 2023 [cited 2023 Oct 11]. Available from: https://www.homelab.nu/informatie/over-homelab.
- Schnoor K, Versluis A, Chavannes NH, et al. The usability of homelab, a digital self-service at a Dutch general practice, for diagnostic tests: pilot study with a questionnaire. JMIR Form Res. 2023;7:e42151. doi:10.2196/42151.
- Exten C, Pinto CN, Gaynor AM, et al. Direct-to-Consumer sexually transmitted infection testing services: a position statement from the American sexually transmitted diseases association. Sex Transm Dis. 2021;48(11):e155–159–e159. doi:10.1097/OLQ.0000000000001475.
- Adimora AA, Ramirez C, Poteat T, et al. HIV and women in the USA: what we know and where to go from here. Lancet. 2021;397(10279):1107–1115. doi:10.1016/S0140-6736(21)00396-2.
- Győrffy Z, Radó N, Mesko B. Digitally engaged physicians about the digital health transition. PLOS One. 2020;15(9):e0238658. doi:10.1371/journal.pone.0238658.
- High-Level Expert Group on Artificial Intelligence. Ethics guidelines for trustworthy AI. European Commission; 2019 [cited 2023 Oct 11]. Available from: https://digital-strategy.ec.europa.eu/en/library/ethics-guidelines-trustworthy-ai.
- European Commission. Directorate General for Research and Innovation. Responsible research and innovation: Europe’s ability to respond to societal challenges. LU: Publications Office; 2012 [cited 2023 Oct 11]. Available from: https://op.europa.eu/en/publication-detail/-/publication/bb29bbce-34b9-4da3-b67d-c9f717ce7c58/language-en.
- Eysenbach G. What is e-health? J Med Internet Res. 2001;3(2):e20. doi:10.2196/jmir.3.2.e20.
- Shaw T, McGregor D, Brunner M, et al. What is eHealth (6)? development of a conceptual model for eHealth: qualitative study with key informants. J Med Internet Res. 2017;19(10):e324. doi:10.2196/jmir.8106.
- World Health. Organization. Regional office for the Western Pacific. Regional action agenda on harnessing e-health for improved health service delivery in the Western Pacific; 2019 [cited 2023 Oct 11]. Available from: https://apps.who.int/iris/handle/10665/330700. License: CC BY-NC-SA 3.0 IGO.
