ABSTRACT
Background
Fabry disease (FD) is an X-linked lysosomal disease, in which diagnosis is often established several years after onset of symptoms. Ocular manifestations can occur in childhood and be a clue to earlier diagnosis. The aim was to report ocular outcome and visual quality of life (QoL) in patients with FD.
Material and methods
FD-patients recruited from Karolinska University Hospital underwent ophthalmological examinations including best corrected visual acuity (BCVA), refraction, biomicroscopy, optical coherence tomography, keratometry, review of medical records and QoL Inventories. A total severity score (TSS), as estimated via Fabry Stabilization Index, was calculated.
Results
Twenty-six FD-patients (16 men) mean age 36.4 years (range 5.0–63.5 years) were included. BCVA was median 1.0 (range 0.5–1.6). Conjunctival blood vessel tortuosity occurred in 15/26 patients, chemosis in 2/26 patients, cornea verticillata in 23/26 patients, lens opacities in 19/26 patients, and tortuous or dilated retinal vessels in 20/25 patients. Group-wise comparisons of adult patients showed no differences regarding age, TSS, or ocular parameters. Overall, TSS was correlated to age (r = 0.53, p = 0.02). A linear regression model showed that age and sex explained 38% of the variance in TSS. Keratometry did not reveal corneal ectasia in any of the 12 patients examined. VFQ 25 in 15 patients showed a high median composite score, 93.6 (range: 78.1–100).
Conclusions
BCVA in FD-patients was good despite corneal and lens pathology. Ocular variables did not show an association with TSS in adult patients. Corneal or lens opacities should also lead to a suspicion of FD in children.
Introduction
Fabry disease (FD) is a rare lysosomal storage disorder with an estimated prevalence of 1:40,000 to 1:117,000 world-wide (Citation1). It is caused by pathogenic variants in the X-chromosomal (×q22.1) GLA gene encoding α-galactosidase A, necessary for metabolism of globotriaosylceramide (Gb3). Deficiency results in the progressive accumulation of Gb3 and related compounds in cells throughout the body. Many patients develop pain crisis (acroparasthesias), hypohidrosis, and skin lesions (angiokeratomas) during childhood, and later in life, gradual accumulation can result in end-stage renal disease, hypertrophic cardiomyopathy, and cerebrovascular disease (Citation2). However, there is a wide spectrum of disease manifestations, ranging from the classical severe phenotype to atypical late-onset forms.
More than 900 pathogenic variants in the GLA gene have been reported with variable phenotypes (Human Gene Mutation Database, http://www.hgmd.org). This, in addition to the non-specific symptoms, can often lead to diagnosis being delayed by up to 20 years (Citation3). The potentially fatal consequences underline the importance of correct early diagnosis and possible early treatment with enzyme replacement therapy (ERT) or the pharmacologic chaperone migalastat (Citation4).
The accumulation of Gb3 leads to corneal manifestations occurring early (Citation5) and easily detected by slit-lamp examination. The curled epithelium opacities named cornea verticillata are reported in 80–100% of the patients (Citation6). In the early stages, fine horizontal lines are seen, developing into featherlike brownish-cream-colored changes, progressing into curving lines emanating from a single vortex often in the inferonasal cornea or sometimes a homogeneous epithelial haze instead (Citation7). Differential diagnosis includes the use of medication like amiodarone or chloroquine (Citation8,Citation9). Two specific subtypes of cataract, an anterior subcapsular cataract and a radial posterior subcapsular cataract, are described (Citation10). The anterior cataract is generally bilateral and wedge-shaped with radial distribution. The posterior subcapsular cataract with a whiteish spoke-like appearance is very specific and is therefore termed “Fabry cataract” (Citation11).
Irregularities of the conjunctival vessels occur due to deposition within the endothelial cells, pericytes, and smooth muscle cells of vessel walls. This results in tortuosity and aneurysmal dilatation of the conjunctival-vessels (Citation12) most commonly in the inferior bulbar conjunctiva. Retinal vessels may also show an increased tortuosity, arteriolar narrowing, segmental venous dilatation, and arteriovenous nicking (Citation13) including retinal artery- and vein-occlusions (Citation14–16).
Other ophthalmological findings include lid edema (Citation17), chronic chemosis (Citation18), dry eye syndrome (Citation19), papilledema or optic atrophy (Citation20), and anterior ischemic optic neuropathy (AION) (Citation21).
The ocular abnormalities in FD are rarely of any visual significance and do not usually increase patient referral to an ophthalmologist. Nevertheless, ophthalmologists have responsibility to identify patients with FD during routine eye examinations, so increased knowledge about the ocular abnormalities of FD is important to avoid unnecessary delay of diagnosis.
The purpose of the study was to evaluate visual outcome and ocular pathology in patients with FD, to correlate these to somatic associated morbidities, and to evaluate the patients’ visual health-related quality of life.
Material and methods
The patients were recruited from the Department of Endocrinology or Pediatric Neurology, Karolinska University Hospital. In total, 26 patients eligible for inclusion agreed to participate in the cross-sectional cohort study with prospective longitudinal follow-up and 25/26 were assessed at our department between 2017 and 2021. One patient only committed to studies of medical records.
Diagnosis of FD in male patients was based on low alfa-galactosidase A activity measurements as well as the presence of a hemizygous pathogenic variant in the GLA gene. Some of the female patients were probands, who were diagnosed based on their organ manifestations and having a reduced alfa-galactosidase A activity and a heterozygous pathogenic variant in the GLA gene, whereas other females had been diagnosed through family screening as being heterozygous for the pathogenic GLA variant but had not developed any or only mild symptoms and organ manifestations. Pathogenic GLA variants in our cohort were p.(His46Gln;Met51Ile;Cys56Phe), p.(Cys52Gly), p.(Gln157*), p.(Met187Val), p.(Leu191Pro), p.(His225Gln;Trp226Arg), p.(Arg227*), p.(Gly260Arg), p.(Pro409Ala), and p.(Lys426fs).
Clinical information was obtained from the patients’ medical records and included age, sex, presence of acroparestesias, angiokeratomas, CNS, heart and renal manifestations, and the patients’ current treatment. The FAbry STabilization indEX (FASTEX) (Citation22,Citation23) was used to score disease severity in the cardiac, renal, and nervous system domains. FASTEX has been shown to have a good correlation with the more comprehensive scoring systems MSSI and DS3 but is easier to use and is not dependent on ocular parameters (Citation22).
The clinical ophthalmological examinations performed by the same orthoptist and ophthalmologist included a history of subjective visual- or ocular problems, best corrected visual acuity (BCVA) (Konstantin Motakis Ortho KM, Lund, Sweden), ocular alignment/strabismus evaluation including Lang I test for stereopsis, and microscopy/slit-lamp evaluation of the anterior and posterior segment. The autorefractor was used for the measurement of refractive errors. Keratometry was also assessed by topography performed with Schwind Sirius (SCHWIND eye-tech-solutions, GmbH & Co. KG, Kleinostheim, Germany) and evaluated by a corneal specialist.
Optical coherence tomography (OCT) images and color fundus photography were obtained using the Topcon SS-OCT (Triton; Topcon Medical Systems, Tokyo, Japan). Optical coherence tomography angiography (OCT-A) was obtained with AngioVue XR Avanti (Optovue, Fremont, CA, US). The vascular density and the avascular area in the superior capillary plexus, FAZ area, were recorded using the automatic measurement tool in the associated software. The OCT images and color fundus photographs were evaluated by a retinal specialist.
To evaluate vision- and health-related quality of life in adult patients with FD (>18 years of age), a 25-item Visual Function Questionnaire, from the National Eye Institute (NEI) termed VFQ-25, was used (Citation24). The VFQ 25 contains 25 questions divided into the following subscales: general health, general vision, ocular pain, near activities, distance activities, social functioning, mental health, role difficulties, dependency, driving, colour vision, and peripheral vision. In addition to scoring each sub-scale, an overall composite score for the VFQ-25 was calculated by averaging the subscale scores, excluding the general health rating question.
For statistical analyses, only adult patients >18 years of age (10 men and 10 women) were used. The influence of age, gender, and eye health measurements on the FASTEX total severity score (TSS) was investigated using linear regression. Linear regression was chosen as the method of analysis primarily because we wanted to have a simple and well-established evaluation metric (the adjusted R-squared) that showed how much of the variance in TTS that sex, age, and measurements of eye health could explain. Although it is possible to get approximations of R-squared also using more complex models, such as mixed-effects models, it is less clear how to interpret R-squared in these contexts, as different versions of R-squared exist and interpretations vary and are not always agreed upon. A linear model was first fitted with age and sex as the only covariates. Eye health measurement variables which showed a significant correlation to TSS were subsequently entered as additional covariates in the regression model to evaluate whether eye health measurements contributed additional predictive information, over and above the variance explained by age and sex in the model.
The study was performed according to the Helsinki Declaration, with ethical approval from the local committee. Informed consent was obtained from all patients older than 15 years of age and in addition from parents/guardians for patients <18 years of age.
Results
Demographics, FASTEX score, and treatment
In total 26 patients (16 males and 10 females) were included. The mean age of the male patients was 29.6 years (range 5.0–57.5 years), and the mean age of the female patients was 47.2 years (range 30.2–63.5 years) at the time of inclusion. The female patients were significantly older than the male patients (p = 0.008). Six patients, all boys, were below 18 years of age (median 15, range 8–17 years). Twelve of the male patients and five of the female patients were on treatment with enzyme replacement therapy (ERT). One male and one female patient were on migalastat treatment. Thus, overall, 13 out of 16 (81%) male patients and 6 out of 10 (60%) female patients were on Fabry-specific treatment.
Among the adult patients (>18 years), there were 10 men, mean age 40.2 years (range 25.2–60 years), and 10 women, mean age 47.2 years (range 30.2–63.5 years). The average TSS was 59.5 (range 3.5–100) in the adult group. The mean score was 65.9 (range 25.8–100) among men and 53.2 (range 3.5–93) among women. TSS correlated significantly with age (Pearson’s r = 0.53, p < 0.05) (), but there was no significant difference in TSS between men and women.
Figure 1. Scatterplot showing the correlation between age and total severity score (FASTEX) with data points grouped by sex.
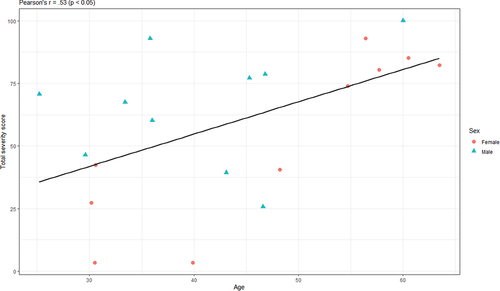
A linear regression model including both age and sex as covariates could explain about 38% of the variance in TSS among adults. A univariate model including only age as predictor explained 24.4% of the variance in TSS. By adding sex, the model explained an additional 13.4% points. However, sex was not a significant predictor by itself. Only when combined with age did sex show a significant relationship with TTS. The coefficient for age indicated that TSS increased by an average of 1.6% points with every year increased in age (p < 0.01), and the coefficient for sex indicated that the men had 24% points higher total severity score than women, on average (p < 0.05).
Including the eye health variables which were significantly correlated with TSS did not significantly improve the model fit. Therefore, no evidence was found that eye health variables explain any additional variability in TSS in a model that already accounts for the variability due to age and sex. The FASTEX TSS and the cardiac, renal, and nervous system domains for all patients are presented in .
Table 1. Age distribution, total severity score (FASTEX), and treatment in patients with Fabry disease.
A majority of patients fulfilled the criteria for Fabry-specific treatment. Among males of all ages, 11 were treated with agalsidase beta, one with agalsidase alpha, and one with the pharmacologic chaperone migalastat. Among the females, four patients were treated with agalsidase beta, one with agalsidase alpha, and one migalastat ().
Subjective symptoms, best corrected visual acuity, intraocular pressure, and refraction
Subjective symptoms of poor visual acuity, sensitiveness to light or dry eyes, were reported by 8/16 male patients and 6/8 (information missing in two) female patients. Glasses to refractive errors were worn by 10/16 males and 9/10 females. Five patients had A REDUCED BCVA of 0.5 BCVA in one eye. Among the male patients, one had amblyopia due to esotropia, another patient had severe astigmatism (−6D), but the last one was young and improved to have full vision during follow-up. Among the women, one with esotropia had amblyopia and another cataract.
Details on BCVA, intraocular pressure (IOP), autorefractor spherical equivalent, and astigmatism are presented in .
Table 2. Visual and ocular outcome including subjective symptoms, strabismus, best corrected visual acuity, spherical equivalent, astigmatism, corneal findings, cataract, and vessel characteristics in patients with Fabry disease.
Strabismus, ocular alignment, spherical equivalent
Manifest strabismus and negative stereopsis, that is lack of binocularity and difficulties in perceiving visual objects in depth, were registered in four adult patients.
One patient had abnormal motility with difficulties to initiate saccades and saccadic smooth pursuit eye movements. He also showed horizontal nystagmus of low frequency. No patient had ptosis.
Anterior segment
In total, conjunctival vessel tortuosity and/or aneurysmal dilatation were observed in 9/16 of male patients and 5/10 female patients. Chemosis occurred in 2/16 men (). Cornea verticillate was detected in 14/16 of the male patients and 9/10 of the female patients (). Lens opacities or cataract (anterior, nuclear, or posterior) occurred in 12/16 of the males and 7/10 of the females ().
Figure 2. a–c. Photos showing conjunctival chemosis and cataract in a male patient 60 years of age with Fabry disease (FD). Twenty years earlier he had experienced problems with a swollen eye. An ophthalmologist noticed eye lid oedema, chemosis and corneal horizontal pigmentation but suspected allergy. FD was diagnosed a few years later due to other organ manifestations.
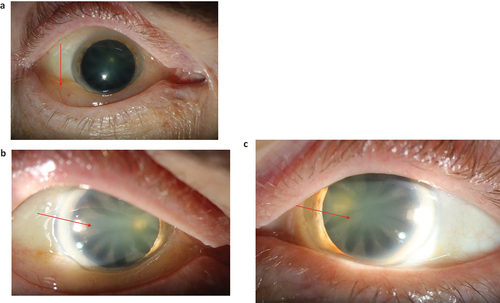
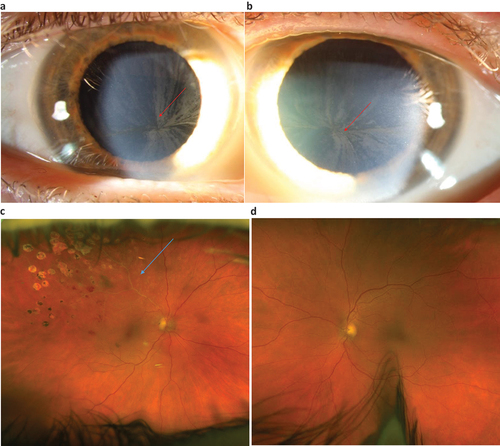
Figure 3. a–d. Photos showing cornea verticillate right and left eye () and branch vein occlusion (BVO) in the right eye () and anormal ocular fundus left eye () in a woman with FD. She was diagnosed with FD at 60 years of age when an ophthalmologist detected cornea verticillate. Cataract surgery has been performed as well as laser treatment for the BVO (). Best corrected visual acuity was 0.7/1.0 right eye/left eye at latest follow up. (Photos published with permission from the patient).
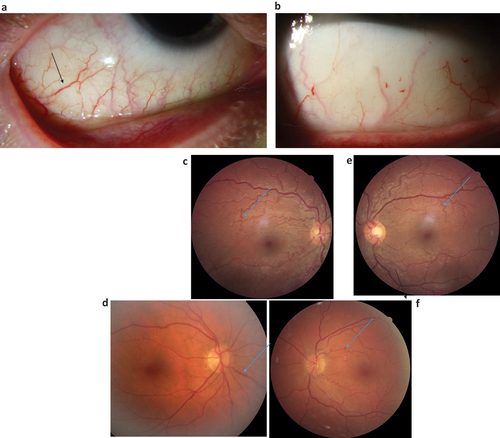
Among children or teenagers <18 years of age, the most common abnormal finding was conjunctival tortuosity and retinal tortuosity (), each present in 4/6 boys, followed by cornea verticillate in 3/6, and cataract in 3/6.
Figure 4. a–f. Photos showing torturous and dilated vessels in the conjunctivae () and in the ocular fundii () in different patients with Fabry.
Intraocular pressure (IOP) measured in 20 patients with ICare (and pneumotonometry in one patient) had a mean of 16.2 mm Hg RE (range 8.0–25 mmHg) and 15.9 mm Hg LE (range 8.0–23 mm Hg).
Visual and ocular details are presented in .
Corneal topography and tomography
Twelve out of 26 patients were examined with corneal topography and tomography: five women, five men, and two boys. One of the patients had steep symmetric corneas and high with the rule (WTR) astigmatism but none of the patients presented with ectasia.
Astigmatism was regular in all cases: WTR astigmatism in seven patients (two boys, three men, and two women) and oblique in two patients, both men. In three patients, all women, the astigmatism was mixed: against the rule (ATR), in one eye and WTR in the other eye, oblique in one eye and WTR or ATR in the other eye, with one patient for each of these combinations.
Among women, the median Sim K-max was 43.77D (42.92D–46.50D), among men: 44.50 (43.30D–50.75D), and mean sim K-Max for the boys was 43.35D. The median astigmatism among women was 0.85 D (0.19D–1.8D), among men: 1.3D (0.12 D − 6.46 D), and the mean astigmatism for the two boys was 2.14D.
One of the patients, a woman, was also diagnosed with early-stage Fuchs dystrophy. The median for the thinnest point of the cornea was 566 µm (540−604 µm) in the female group, with 586 µm (497−599 µm) in men and 507 µm for the two boys.
Posterior segment
OCT was performed in 15/16 men and in 9/10 women. The OCT was abnormal in 0/15 males and 2/9 women (p = 0.13 between genders). Both women with abnormal OCT showed small non-central cysts.
Color fundus photography was performed in 25 patients. Evidence of tortuous vessels, either slightly or moderately, was observed in 14/16 males and in 6/9 females. There were no gender-specific differences in vessel tortuosity in the study population (p = 0.21)
OCT angiography
OCT angiography was obtained in 9/25 patients and showed normal vessel density of the superficial capillary plexus in all evaluated subjects. All patients had a normal sized FAZ.
Health related quality of life—VFQ 25
In total, 15/20 adult patients >18 years of age, eight men and seven women, completed the VFQ 25 questionnaire. The median composite score, computed across all VFQ-subscales and patients, was 93.6 (range: 78.1–100). Grouped by sex, the median composite score in women was 90.6 (range: 78.1–100) and 94.5 (range: 87.6–100) in men (). There was no statistically significant difference between male and female patients in any of the VFQ-subscales or the composite score. The largest difference between men and women was observed in the VFQ-score for distance activities, where the median score was 95.8 for men and 83.3 for women (p = 0.15, Mann-Whitney). In the group as a whole, a significant relationship was found between the VFQ-25 subscale general health and FASTEX TSS, having a moderate correlation of −0.64 (p = 0.01, Pearson’s r).
Table 3. Visual-related quality of life in eight adult males (median age: 39.6 years, min: 29.6, max: 60.0) and seven adult females (median age: 56.4, min: 30.2, max: 63.5).
Discussion
The purpose of the study was to evaluate and compare visual outcome and ocular pathology with somatic associated morbidities in patients with FD. Further aims were to evaluate their visually related QoL.
There was a slight predominance for males in our cohort, as expected for an X-linked disorder in which males have more pronounced organ manifestations and are subjected to ophthalmological evaluations to a greater extent.
The FASTEX TSS was best explained by the variables of age and sex. Ocular variables did not explain any additional variability in TSS in the current study, once age and sex had been accounted for. This contrasts with a study by Pitz et al., in which patients with cornea verticillate had a more severe disease than those without ocular signs, even after adjusting for age (Citation25). This could be due to a smaller cohort in the current study or the fact that we also included less severe versions of cornea verticillate.
BCVA was good, not below 0.5 in any patient in the present study. The most common ophthalmic feature was cornea verticillate found in 14/16 of the men and 9/10 of the women. This is a slightly higher number than presented in the study by Sodi et al. (n = 173) where cornea verticillate was reported in 73% of the males and 76.9% of the females (Citation26). Even higher prevalence, with cornea verticillate in all males above 4 years of age and all women above 10 years of age, was reported by Sher et al. (n = 62) (Citation10).
Moiseev et al. found cornea verticillate in 65% of the patients (n = 69) with a similar frequency in males and females (56% vs 77%) (Citation27). Similarly to our study, the corneal manifestations were not associated with the severity of the disease. In a large study by Pitz et al., which included 1203 adult patients, cornea verticillate was found in adults in an equal percentage in men and women (50.8% vs 51.1%) (Citation25).
When the children in the present study were analyzed separately, the most common abnormal finding was conjunctival tortuosity and retinal tortuosity, each present in 4/6 boys, followed by cornea verticillate and cataract, each in 3/6. In a study by Ries et al. of 23 boys with a median age of 15 years (range 6–18 years), mild cornea verticillate was found in 88%, whereas vascular tortuosity was less common than in our study and found in 36% (Citation28).
In the whole group, conjunctival vessel tortuosity and/or aneurysmal dilatation were observed in 9/16 of the male patients and 5/10 female patients, while retinal tortuosity was even more common. It occurred in 14/16 of the males and in 6/9 females in the present study. These are higher frequencies than the 49% in males and 22% in females in a study by Sodi (Citation26). However, in their study, unlike ours, vessel tortuosity correlated to disease severity.
Retinal vascular changes were also frequent among the 62 patients (37 male and 25 female patients) especially among males 70% vs 25% in females reported by Sher et al. (Citation10).
Cataract of any type (anterior, nuclear, or posterior) occurred in 12/16 of the men and 7/10 of the women in our study. These figures are higher than reported by Sodi et al., but they reported only FD cataract which occurred in 10% of the females and 23% of the males (Citation26). Two of our patients underwent cataract surgery during follow-up.
In a study of one family with 23 members: eight male patients (mean age 32.3 years) and 15 female patients (mean age 26.9 years), cornea verticillate was present in all patients. Anterior capsule opacity occurred in 2/8 and FD-cataract in 1/8 of the males. Conjunctival and/or retinal vessel tortuosity was present in the majority of male patients 5/8 and 6/8 but was more uncommon in females (6/15 and 2/15) (Citation29).
Retinal vascular involvement has been reported in FD, with retinal arteriolar narrowing, dilation, and irregularity of the retinal veins, and increased tortuosity of the retinal vessels (Citation10,Citation26,Citation30). In the current study, fundus photography showed tortuous or dilated vessels in a majority of the patients although there was no gender-specific difference. OCT showed small non-central cysts in two female patients. OCT angiography obtained in 9/26 patients demonstrated normal vessel density (VD), superficial capillary plexus, and a normal sized FAZ. This contrasts with the lower VD values and enlarged FAZ that suggest possible changes in retinal microvasculature in patients with FD (Citation31).
To the best of our knowledge, VFQ-25 has not previously been used in studies of FD. Although this is a small group of individuals, the study may provide valuable information about there being no statistically significant difference between male and female patients in any of the VFQ-subscales or the composite score. In the group as a whole, a significant relationship was found between the VFQ-25 subscale for general health and FASTEX TSS, having a moderate correlation of 0.64 (p = 0.01, Pearson’s r).
Conclusion
In conclusion, a majority of patients with FD had ocular changes. No gender-specific differences were seen in any ocular parameters that were examined. Conjunctival blood vessel and retinal blood vessel tortuosity as well as cornea verticillate were common already from childhood, so early ophthalmological signs could be a clue to help early FD diagnosis. Despite ocular changes, BCVA was usually good and more severe manifestations, such as branch vein occlusion and cataract needing surgery, were rare.
Ophthalmologic assessment can not only be of diagnostic help but is also useful to evaluate the need for glasses and risk for amblyopia in children through continuous follow-up. In adults, the ophthalmologist can monitor the cataract, initiate surgery when needed and recognize typical FD manifestations like severe chronic chemosis, which can be problematic for the patient and lead to unnecessary investigations.
Acknowledgements
The authors thank all the patients who participated in the study.
The VFQ 25 questionnaire was developed at RAND under the sponsorship of the National Eye Institute.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Meikle PJ, Hopwood JJ, Clague AE, Carey WF. Prevalence of lysosomal storage disorders. Jama. 1999 Jan 20;281 (3): 249–54. doi:10.1001/jama.281.3.249.
- Germain DP. Fabry disease. Orphanet J Rare Dis. 2010;5(1):30. doi:10.1186/1750-1172-5-30.
- Mehta A, Ricci R, Widmer U, Dehout F, Garcia de Lorenzo A, Kampmann C, Linhart A, Sunder-Plassmann G, Ries M, Beck M. Fabry disease defined: baseline clinical manifestations of 366 patients in the Fabry Outcome Survey. Eur J Clin Invest. 2004;34(3):236–42. doi:10.1111/j.1365-2362.2004.01309.x.
- Schiffmann R, Kopp JB, Austin III HA, Sabnis S, Moore DF, Weibel T, Balow JE, Brady RO. Enzyme replacement therapy in Fabry disease: a randomized controlled trial. Jama. 2001;285(21):2743–49. doi:10.1001/jama.285.21.2743.
- Franceschetti AT. Fabry disease: ocular manifestations. Birth Defects Orig Artic Ser. 1976;12(3):195–208.
- Samiy N. Ocular features of Fabry disease: diagnosis of a treatable life-threatening disorder. Surv Ophthalmol. 2008;53(4):416–23.
- Sivley MD, Benjamin WJ. Fabry keratopathy: manifestations and changes over time. Br J Ophthalmol. 2020;104(8):1148–55.
- Slowik C, Somodi S, von Gruben C, Richter A, Guthoff R. Erfassung morphologischer Hornhautveränderungen infolge Chloroquintherapie mit Hilfe der konfokalen In-vivo-Mikroskopie. Ophthalmologe. 1997;94(2):147–51. doi:10.1007/s003470050096.
- Wasielica-Poslednik J, Pfeiffer N, Reinke J, Pitz S. Confocal laser-scanning microscopy allows differentiation between Fabry disease and amiodarone-induced keratopathy. Graefes Arch Clin Exp Ophthalmol. 2011;249(11):1689–96. doi:10.1007/s00417-011-1726-5.
- Sher NA, Letson RD, Desnick RJ. The ocular manifestations in Fabry’s disease. Arch Ophthalmol. 1979;97(4):671–76.
- Spaeth GL, Frost P. Fabry’s disease. Its ocular manifestations. Arch Ophthalmol. 1965;74(6):760–69.
- Libert J, Toussaint D. Tortuosities of retinal and conjunctival vessels in lysosomal storage diseases. Birth Defects Orig Artic Ser. 1982;18(6):347–58.
- Font RL, Fine BS. Ocular pathology in Fabry’s disease. Histochemical and electron microscopic observations. Am J Ophthalmol. 1972;73(3):419–30.
- Sher NA, Reiff W, Letson RD, Desnick RJ. Central retinal artery occlusion complicating Fabry’s disease. Arch Ophthalmol. 1978;96(5):815–17. doi:10.1001/archopht.1978.03910050421003.
- Andersen MV, Dahl H, Fledelius H, Nielsen NV. Central retinal artery occlusion in a patient with Fabry’s disease documented by scanning laser ophthalmoscopy. Acta Ophthalmol (Copenh). 1994;72(5):635–38. doi:10.1111/j.1755-3768.1994.tb07193.x.
- Oto S, Kart H, Kadayifçilar S, Özdemir N, Aydin P. Retinal vein occlusion in a woman with heterozygous Fabry’s disease. Eur J Ophthalmol. 1998;8(4):265–67. doi:10.1177/112067219800800412.
- Rahman AN. The ocular manifestations of hereditary dystopic lipidosis (angiokeratoma corporis diffusum universale). Arch Ophthalmol. 1963;69(6):708–16. doi:10.1001/archopht.1963.00960040714005.
- Edwards JD, Bower KS, Brooks DB. Fabry disease and chemosis. Cornea. 2009;28(2):224–27.
- Klein P. Ocular manifestations of Fabry’s disease. J Am Optom Assoc. 1986;57(9):672–74.
- Velzeboer CM, de Groot WP. Ocular manifestations in angiokeratoma corporis diffusum (Fabry). Br J Ophthalmol. 1971;55(10):683–92.
- Abe H, Sakai T, Sawaguchi S, Hasegawa S, Takagi M, Yoshizawa T, Usui T, Horikawa Y. Ischemic optic neuropathy in a female carrier with Fabry’s disease. Ophthalmologica. 1992;205(2):83–88. doi:10.1159/000310318.
- Mignani R, Pieruzzi F, Berri F, Burlina A, Chinea B, Gallieni M, Pieroni M, Salviati A, Spada M. FAbry STabilization indEX (FASTEX): an innovative tool for the assessment of clinical stabilization in Fabry disease. Clin Kidney J. 2016;9(5):739–47. doi:10.1093/ckj/sfw082.
- Mignani R, Pieroni M, Pisani A, Spada M, Battaglia Y, Verrecchia E, Mangeri M, Feriozzi S, Tanini I, De Danieli G, et al. New insights from the application of the FAbry STabilization indEX in a large population of Fabry cases. Clin Kidney J. 2019;12(1):65–70. doi:10.1093/ckj/sfy108.
- Mangione CM, Lee PP, Gutierrez PR, Spritzer K, Berry S, Hays RD. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol. 2001;119(7):1050–58.
- Pitz S, Kalkum G, Arash L, Karabul N, Sodi A, Larroque S, Beck M, Gal A. Ocular signs correlate well with disease severity and genotype in Fabry disease. PLoS One. 2015;10(3):e0120814. doi:10.1371/journal.pone.0120814.
- Sodi A, Ioannidis AS, Mehta A, Davey C, Beck M, Pitz S. Ocular manifestations of Fabry’s disease: data from the Fabry outcome survey. Br J Ophthalmol. 2007;91(2):210–14. doi:10.1136/bjo.2006.100602.
- Moiseev SV, Ismailova DS, Moiseev AS, Bulanov NM, Karovaikina EA, Nosova NR, Fomin VV. Cornea verticillata in Fabry disease. Ter Arkh. 2018;90(12):17–22. doi:10.26442/00403660.2018.12.000003.
- Ries M, Gupta S, Moore DF, Sachdev V, Quirk JM, Murray GJ, Rosing DR, Robinson C, Schaefer E, Gal A, et al. Pediatric Fabry disease. Pediatrics. 2005;115(3):e344–55. doi:10.1542/peds.2004-1678.
- Morier AM, Minteer J, Tyszko R, McCann R, Clarke MV, Browning MF. Ocular manifestations of Fabry disease within in a single kindred. Optometry. 2010;81(9):437–49. doi:10.1016/j.optm.2010.02.011.
- Eng CM, Germain DP, Banikazemi M, Warnock DG, Wanner C, Hopkin RJ, Bultas J, Lee P, Sims K, Brodie SE, et al. Fabry disease: guidelines for the evaluation and management of multi-organ system involvement. Genet Med. 2006;8(9):539–48. doi:10.1097/01.gim.0000237866.70357.c6.
- Cakmak AI, Atalay E, Cankurtaran V, Yaşar E, Turgut FH. Optical coherence tomography angiography analysis of Fabry disease. Int Ophthalmol. 2020;40(11):3023–32. doi:10.1007/s10792-020-01486-2.
