ABSTRACT
Purpose
To determine the validity of the validate the adult patient-reported outcome measure tools, the Michigan Retinal Degeneration Questionnaire (MRDQ) and Michigan Vision-Related Anxiety Questionnaire (MVAQ), in adolescent patients with inherited retinal diseases (IRDs).
Methods
Ninety-one adolescent patients diagnosed with IRDs were recruited at the Hospital for Sick Children (University of Toronto) and the Kellogg Eye Center (University of Michigan). The patients were administered the MRDQ, MVAQ, and Patient Health Questionnaire-4 (PHQ-4). Test-retest variability was assessed in eighteen patients within 14 days of the initial administration. Adolescent responses were analyzed for validity and reliability. As a further validation step, comparisons were made to adult data from the original MRDQ and MVAQ studies to ensure consistency in response ranges.
Results
The existing MRDQ and MVAQ content and format could accurately detect the impact of IRD on activities of daily living in adolescents with IRDs. No floor/ceiling effects were identified, test-retest reliability was established (r = 0.73–0.86), and no items were excluded after differential item functioning analysis. Domain and trait associations with visual acuity and IRD phenotypes were similar between adolescents and adults.
Conclusions
The MRDQ and MVAQ are psychometrically validated questionnaires for which we have shown validity for use in adolescent patients with IRDs.
Introduction
Inherited retinal diseases (IRDs) refer to a diverse group of rare hereditary diseases with photoreceptor malfunction that can lead to blindness (Citation1). IRDs are genetically heterogeneous and show variability in the severity of signs and symptoms, such as photophobia and nyctalopia. The visual impairment of patients with IRDs is most often silent and invisible, even though these changes impact patients’ daily lives and their ability to perform activities of daily living (Citation2–4). These challenges and health outcomes meaningful to patients with IRDs are not captured by routine ophthalmic clinical tests, which are primarily retinal functional and structural tests (Citation5–7). This requires the development of patient-reported outcome measures (PROMs); questionnaires that assess the preferred health needs and outcomes of patients (Citation7–9). PROMs provide an important insight into the impact of a condition on patient experience, translating to a meaningful assessment that optimizes clinician decision-making (Citation2,Citation7,Citation10). This could involve measuring the impact of IRDs on a patient’s activities of daily living (Citation11–13), which could then be used to best interpret outcomes (Citation2).
Over the years, several PROMs were developed to assess for meaningful changes in vision; however, until recently, the tools available were not developed or validated for patients with IRDs (Citation2,Citation5). Only recently was a set of IRD-specific tools designed for adult patients: the Michigan Retinal Degeneration Questionnaire (MRDQ) (Citation3) and Michigan Vision-Related Anxiety Questionnaire (MVAQ) (Citation4). PROMs like the MRDQ and MVAQ are becoming critical for patients with IRDs, given challenges to the recent U.S. Food and Drug Administration (FDA), European Medical Agency, and Health Canada approvals of novel therapies such as gene replacement therapy for the RPE65-related IRD and the many other upcoming therapeutic trials for IRDs (Citation1). MRDQ and MVAQ assess the impact of an IRD on adult patients’ daily activities. However, many IRDs manifest in childhood and, therefore, most amenable to gene therapy treatment also in childhood. There remains a great unmet need to develop PROMs tailored to pediatric patients with IRDs (Citation1,Citation9).
Considering the original development of MRDQ and MVAQ included 18-year-old patients with IRD, the present study aims to determine if these tools are valid PROMs for adolescent patients. While children and adults have different experiences with their conditions (Citation14), we hypothesize that the MRDQ and MVAQ can be used as patient-reported outcome measures (PROMs) in adolescents with inherited retinal disorders (IRDs) because of the similarities in the functional vision-related difficulties and limitations between adolescents and adults with IRDs (Citation15–17). Novel PROMs for children are being created in a separate study. As with the development of the MRDQ and MVAQ, these tools were validated in accordance with FDA guidelines (Citation10,Citation18) and addressed a critical unmet need in clinical practice and research.
Methods
This study was jointly approved by the Institutional Review Boards of the Hospital for Sick Children (HSC), Toronto, Ontario, and the University of Michigan (UM), Ann Arbor, Michigan. Informed consent and informed assent were obtained as required by the local institutions. All research procedures respected the tenets of the Declaration of Helsinki.
Participants
Adolescent patients (aged 13 to <18 years) with IRD were recruited between March and December 2021 at two collaborative sites: the Department of Ophthalmology and Vision Sciences (HSC, Toronto) and Kellogg Eye Center (UM, Michigan). Participants at HSC were recruited during their routine clinic visits or from the Fighting Blindness Canada (FBC) patient registry (Citation19), which provides information for patients with IRDs, either consented by the patient or their caregivers. Patients at Kellogg Eye Center were identified by searching the electronic medical records for patients with IRDs. Potential participants were then sent invitation letters, including the consent form, and were later contacted by telephone.
Inclusion criteria were adolescents aged 13 to <18 years with an IRD diagnosis per clinical evaluation by a fellowship-trained IRD specialist. Information collected included age, sex, full-field electroretinogram (ERG), retinal imaging (optical coherence tomography and fundus autofluorescence), widefield perimetry, and genetic testing results from CLIA-approved laboratories when available. According to established guidelines, participants’ phenotypes (hereafter “IRD phenotype”) were classified as rod-cone dystrophy, cone/cone-rod dystrophy, or macular dystrophy (Citation20). Of note, transsynaptic-retinal dystrophies (X-linked retinoschisis and X-linked congenital stationary night blindness) were included in the above three phenotypes based on ERG amplitude evaluation and categorization. Patients were excluded if they had insufficient command of the English language, were unable to complete the PROM according to the judgment of their caregivers, or had an impairment that affected their ability to understand and provide informed assent.
Interview protocols
The Michigan Retinal Degeneration Questionnaire (MRDQ) measures the impact of visual disability on activities of daily living in patients with IRDs (Citation3). The questionnaire consists of 59 Likert-scaled items spanning seven scored domains: Central Vision (11 questions), Color Vision (Citation4 questions), Contrast Sensitivity (Citation7 questions), Scotopic Function (Citation12 questions), Photopic Peripheral Vision (Citation9 questions), Mesopic Peripheral Vision (Citation9 questions), and Photosensitivity (Citation7 questions). The Michigan Vision-Related Anxiety Questionnaire (MVAQ) assesses the patient’s psychosocial well-being associated with their IRD condition (Citation4). This tool consists of 14 Likert-scaled items spanning two separately scored domains: Rod Function Anxiety (Citation6 questions) and Cone Function Anxiety (Citation8 questions). The MRDQ and MVAQ contain maintained appropriate language for the adolescent population, as evidenced by a Flesch-Kincaid reading score of 8 for participants 13 and up (Citation21,Citation22). This was reaffirmed by administering both tools to an independent set of unaffected adolescents (n = 5) to verify the relevance and comprehensibility of items to their age group prior to the larger study. Items from the MRDQ and MVAQ are detailed in Lacy et al. (Citation3) and Lacy et al. (Citation4), respectively.
In addition to the MRDQ and MVAQ, the participants completed the shortened version of the Patient Health Questionnaire (PHQ-4), a validated tool for detecting clinical depression and anxiety that is widely used and designed to minimize respondent burden. The PHQ-4 questionnaire provides a total score of 0–12 and two subscales: depression (0–6) and anxiety (0–6) (Citation23,Citation24).
This study occurred during the COVID-19 pandemic, and adaptations were made to the methodology to minimize infection risk. The MRDQ and MVAQ were administered verbally by research assistants in-person, over the phone, or on the institution-provided ZOOM (Zoom Video Communications, Inc., San Jose, CA, USA) video call. Subjects had the option to complete a second administration 14 days following their initial interview to assess the test-retest variability of the two patient-reported outcome measures. If the visual acuity had not been assessed within six months before the first interview, it was measured using one of three teleophthalmic visual acuity tests based on patient preference and access to devices (laptop/tablet, android phone, or iOS phone): Freiburg Vision Test (“FrACT,” http://michaelbach.de/fract/) (Citation25), Verana Vision Test (Verana Health, NYC, NY, USA) (Citation26), or Peek Acuity (Peek Vision Ltd, Berkhamsted, England, UK) (Citation27).
Data analysis
Analysis was conducted in R (4.1.2, R Foundation for Statistical Computing, Vienna, Austria). Patient responses were input to the existing MRDQ and MVAQ graded response models to calculate nine domain scores. Results from this study were then compared to the findings in the original adult patient with IRDs cohort used for the creation and psychometric validation of MRDQ and MVAQ (Citation3,Citation4).
Graded response model
Building the MRDQ and MVAQ models is detailed previously (Citation3,Citation4). Briefly, graded response models were built using Cai’s Metropolis-Hastings Robbins-Monro algorithm (Citation28), implemented in the R (version 3.6.3) package mirt (Citation29). A graded response model gives each person one parameter score (θ) that quantifies their visual disability (for MRDQ) or vision-related anxiety (for MVAQ). The scales were created with a mean of 0 and a variance of 1 from the original adult population. Higher person scores indicate greater disability or anxiety in relation to IRD. These models were used to score the adolescent responses (Citation3,Citation4).
Performance by measure domain
The presence of floor or ceiling effects was investigated for each domain of the MRDQ and MVAQ to establish whether the questionnaires were properly centered, ensuring the domain’s ability to capture different levels of visual disability and vision-related anxiety within the IRD population.
Test-retest variability
Test-retest reliability was quantified for each domain using the Pearson correlation between participants’ scores on the first and second tests, the mean change between administrations, and the estimated standard deviation (SD) of measurement error (Citation30). The measurement error SD estimate is based on the bias-corrected Bland-Altman difference scores method, and confidence intervals (CI) were computed by jackknife resampling (Citation31).
Differential item functioning
Differential item functioning (DIF) was investigated to determine whether responses to an item depended on a covariate after controlling for overall disability or anxiety. DIF was independently investigated for each of the 73 items (59 MRDQ and 14 MVAQ) and each of 7 covariates: logMAR corrected visual acuity in the better eye (logMAR BE) and worse eye (logMAR WE), age, sex, administration site (HSC and UM), IRD phenotype (rod-cone, cone/cone-rod, and macular dystrophy), and total PHQ-4 scores (0–12 scale). Multiple linear regression was used for each item and covariate combination to quantify the correlation between the mean response to the item and the covariate, controlling for the domain score. The p value of the coefficient of the covariate was used to judge DIF. Only item/covariate combinations with a p < .001 were reported due to the many comparisons that were conducted.
Domain and trait associations
Patient covariates were summarized by counts and percentages or medians and ranges. The proportion of missing data was summarized for each item. Coefficients of determination quantified correlation between domains and covariates. Domain correlations with covariates were assessed by simple linear models: regression for continuous covariates and ANOVA for categorical covariates. The following traits were investigated for known-group validity: logMAR BE, logMAR WE, age (13 to <18 years), sex, administration site (HSC and UM), and IRD phenotype (rod-cone, cone/cone-rod, and macular dystrophy). Comparing domain scores between different phenotypes was done as a test of instrument validity.
Patient health Questionnaire-4 and Michigan Vision-Related Anxiety Questionnaire association
After completing the MVAQ, participants were asked items from the validated PHQ-4 to assess symptoms of depression and anxiety (Citation23,Citation24). Responses were compared with MVAQ using linear regression to establish convergent validity.
Comparison to adults with inherited retinal diseases
Results from the univariate analyses of domain and trait associations in adolescents were compared to those from adults who had participated in the original validation studies (Supplementary Material 1) (Citation3,Citation4). Additionally, nine (one for each scale) multivariable regression models were used to assess the equality of associations between the MRDQ/MVAQ domains and visual acuity (logMAR BE), age, sex, and IRD phenotype in adults and adolescents using interaction terms. Differences in coefficients (adolescent minus adult) are reported with 95% confidence intervals.
Results
Participants
Ninety-one adolescent patients with clinically diagnosed IRDs were administered the MRDQ and MVAQ (Supplementary Material 2). Participants took, on average, approximately 13 minutes to complete the MRDQ and 4 minutes to complete the MVAQ. The sample consisted of 51 males (56%), and the ages ranged from 13 to 17.9 years (mean 16.0) years old. Forty-seven (51.6%) presented with a rod-cone dystrophy phenotype, while 33 (36.3%) and 11 (12.1%) presented with cone/cone-rod and macular dystrophy phenotypes, respectively. Seventy-nine patients had conclusive genetic testing available (Supplementary Material 3).
Data analysis
All items considered for analysis were answered by 97% of participants on average. Questions deemed not applicable by the participants were considered unanswered. No items were removed, and no domains were adapted in the analysis.
Performance by measure domain
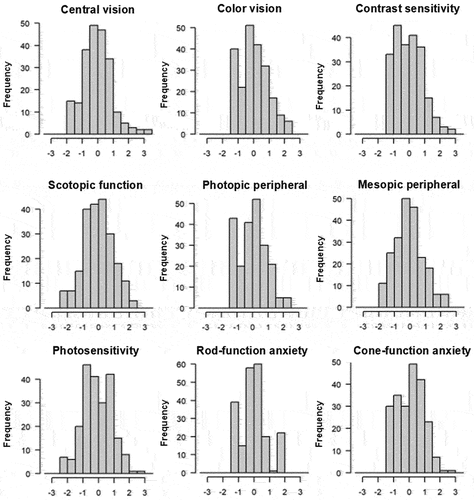
The presence of floor or ceiling effects was not identified from any domain score (θ) distributions for the MRDQ and MVAQ ().
Test-retest variability
Eighteen subjects from the initial sample participated in a second administration to measure test-retest reliability. There was low variability observed in the mean change in patient responses across repeat administrations of both PROMs obtained in 12–15 days across all domains. The MRDQ’s and MVAQ’s Pearson correlations between the first and second administrations exceed 0.7, the average changes in θ between administrations are less than 0.2, and the SDs of measurement error are less than<0.34 (). All values are comparable to the initial adult studies (Citation3,Citation4).
Table 1. Test-retest variability of domain scores (θ).
Differential item functioning
DIF was not found in any item for covariates, such as age, sex, and site. Five items were significantly correlated with visual acuity (MRDQ items 26 and 29 from the Scotopic Function domain, item 30 from the Mesopic Peripheral Vision domain, and items 2 and 3 from the MVAQ Cone Function Anxiety domain), and two items (MRDQ items 29 and 30) were associated with IRD phenotype (rod-cone, cone/cone-rod, or macular dystrophy). No items were removed or domains adapted.
Domain and trait associations
The correlations between domain scores (θ) and patients’ clinical characteristics (logMAR BE, logMAR WE, age, sex, IRD phenotype) are summarized in . The Central Vision domain had a statistically significant positive correlation with logMAR BE and logMAR WE but not with the IRD phenotype. The Color Vision, Contrast Sensitivity, Scotopic Function, Photopic Peripheral Vision, and Mesopic Peripheral Vision domains had statistically significant positive correlations with logMAR BE, logMAR WE, and IRD phenotype.
Table 2. Associations between domain scores (θ) and participant characteristics measured by adjusted R2 with 95% confidence interval of the linear model and by the p value of the F test of no association.
Patient Health Questionnaire-4 and Michigan Vision-Related Anxiety Questionnaire association
A statistically significant relationship was observed between each of the two domains of anxiety (Rod Function Anxiety and Cone Function Anxiety) and PHQ-4 screening scores in adolescents (). A positive linear correlation was observed between PHQ-4 total scores and both Rod Function Anxiety (R2 = 11.8%, p = .001) and Cone Function Anxiety (R2 = 9.7%, p = .003).
Table 3. MVAQ domain score correlations with PHQ-4.
Comparison to adults with inherited retinal diseases
Univariate analyses were performed to identify the presence of an association between the PROM’s domains and each covariate (). The association between the Central Vision domain and the logMAR BE differed statistically between adults and adolescents (p = .049), as did the association between age and the Cone Function Anxiety domain (P = 0.029), the association between the Color Vision domain and sex (p = .009), and the association between the Mesopic Peripheral domain and IRD phenotype (p = .024). However, when controlling for variables altogether in the multivariate analysis, there were no differences (p > .05) found between adults and adolescents when comparing domain and trait associations (). Supplementary Material 4 shows the relationship of domain scores (θ) of each MRDQ/MVAQ domain with logMAR BE, sex, and IRD phenotype (rod-cone, cone/cone-rod, and macular dystrophy) between adolescents and adults upon multivariate analysis (Citation3,Citation4).
Table 4. Multivariate analysis for differences of beta coefficients with 95% confidence intervals between adolescent and adults.
Discussion
Considering the growing number of novel therapeutic opportunities being assessed, there is an urgent need for tailored PROMs to assess the impact of IRD on the daily living of affected adolescents. As such, this study validated the MRDQ and MVAQ as useful PROMs for adolescent patients with IRD. This new knowledge addresses a pressing need as IRDs are most often of early onset, and there are no other tailored tools currently available for this group.
The strength of the MRDQ and MVAQ is that the validation in adolescents was performed using a graded response model (GRM)—this was built in the original studies (Citation3,Citation4). Unlike a Rasch model, which requires uniform item discrimination, a GRM accounts for variance and distribution of patient responses when fitting the model. Further, the MRDQ and MVAQ provided response scales anchored in a contextual meaning rather than a simple numerical Likert scale, which provided to establish consistency across the population and over time.
The GRM analysis gives an overall person score (θ), the numerical equivalent of an individual’s ability or disability in a domain/trait. With scores captured from each domain of MRDQ and MVAQ, participants’ responses underwent rigorous testing against different validity and reliability guidelines to establish their suitability in adolescent patients with IRDs. Importantly, the tools’ performance in adolescents did not show any floor or ceiling effects, therefore showing a sufficient ability to capture the range of vision-related disabilities (MRDQ) and vision-related anxieties (MVAQ) in adolescent patients with IRDs. Additionally, both tools (domain correlations with covariates) performed similarly in the adolescent participants and the adults who participated in the original validation of MRDQ and MVAQ (Citation3,Citation4).
The MRDQ and MVAQ domain test-retest reliability measurements were very similar in adolescent patients compared to the previously published results in adult patients (Citation3,Citation4). Verifying test-retest reliability in adolescents was an essential metric for validating functional outcome measures in the IRD populations, as it allows interpretation of change in a given functional ability over time in the setting of a clinical trial or natural history study (Citation2). The test-retest variability of adolescents is comparable to that of adults with IRDs (Citation3,Citation4).
Correlations between domain scores and clinical characteristics were found in adolescents with IRDs. Of note, visual acuity only modestly correlated with different domains (), highlighting that each domain measures a form of functional vision beyond visual acuity and foveal function per se (Citation32). Furthermore, differential item functioning measures confirms that individual items in each domain of these PROMs are not specifically affected by covariates such as age and sex. Upon multivariate analysis, no differences were found for any domain regarding their trait associations between adolescents and the previously studied adult cohort (Citation3,Citation4), further supporting that MRDQ and MVAQ are suitable for use in adolescents with IRDs.
The traits measured by MVAQ agreed with symptoms of clinical depression and anxiety in adolescents. This was verified through correlations identified between MVAQ and PHQ-4 scores, confirming convergent validity. Furthermore, this correlation was also found in the adult population utilized for the original validation of MVAQ (Citation4).
There are a few limitations to the study. This study was conducted during the COVID-19 pandemic, which challenged recruitment and forced remote assessment. As most participants preferred to be assessed remotely, different visual acuity assessment tools had to be used based on the accessibility of devices by the patients. Additionally, participants were provided a choice from different modes of test administration (i.e., in-person, over the phone, or on the institution-provided Zoom). A minor limitation is our cohort did not include all genetic subtypes of IRDs; however, it reflects the general distribution of clinical phenotypes for a group of diseases classified as orphan diseases (Citation20,Citation33). It is worth noting that the macular dystrophy group had a small sample size (n = 11) compared to the other IRD phenotypes, which is consistent with the typical distribution of IRD phenotypes.
The domain/trait correlations for MRDQ and MVAQ in adolescents were similar to those previously published in adults (Citation3,Citation4). Although adolescents and the previously studied adults evaluated were at different stages of their disease process, the domains of MRDQ and MVAQ capture the degree of disability and distress in both groups. Hence, these two tools can be used to longitudinally track the self-reported impact of visual disability and vision-related anxiety into adulthood without the difficulty of having different scoring systems.
In summary, there is an urgent need to integrate adolescent IRD patients’ perspectives and preferences into their care alongside measuring their vision function and structure (Citation7,Citation11–13). Adolescence is a stressful physical, social, and emotional transition period, which an IRD diagnosis and progressive vision loss can significantly impact. As most IRDs are of early onset, adolescence seems to be a strategic period to initiate a potential therapy. Understanding the impact of an IRD on the patient’s activities of daily living and distress using tools such as the MRDQ and MVAQ can assist eye care providers in optimizing the management of their patient’s needs and better understanding of response to any intervention. The MRDQ and MVAQ are now validated to reliably assess for vision-related disability and IRD-related distress in adolescents with IRDs.
Author contributions
EH contributed to the conceptualization of the study. KS, RA, MFA, AV, CAA, KTJ, and EH were responsible for the investigation and methodology. CAA and RA conducted the analysis, while CAA and GD ensured validation techniques. KS led the write-up of the study. All authors contributed to the manuscript write-up and review.
Supplemental Material
Download PDF (108.9 KB)Supplemental Material
Download PDF (151.6 KB)Supplemental Material
Download PDF (151.9 KB)Acknowledgments
The authors would like to thank Abigail Fahim, Kari Branham, Naheed Khan, and Dana Schlegel for their contributions to the work.
Disclosure statement
EH is consultant for Novartis, Janssen, and on a DSMB (for Sanofi Atsena). AV is a consultant for Novartis and Adverum Biotechnologies, Inc. DCM is a consultant for Editas Medicine, Inc. KTJ, GDL, and MFA are the co-inventors of the Michigan Retinal Degeneration Questionnaire and Michigan Vision-Related Anxiety Questionnaire, which are licensed and copyrighted by the University of Michigan. The remaining authors indicate no financial support or conflicts of interest.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/13816810.2023.2179074
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Prado DA, Acosta-Acero M, Maldonado RS. Gene therapy beyond luxturna: a new horizon of the treatment for inherited retinal disease. Curr Opin Ophthalmol. 2020 May;31(3):147–54. doi:10.1097/ICU.0000000000000660.
- Lacy GD, Abalem MF, Musch DC, Jayasundera KT. Patient-reported outcome measures in inherited retinal degeneration gene therapy trials. Ophthalmic Genet. 2020 Jan 2;41(1):1–6. doi:10.1080/13816810.2020.1731836.
- Lacy GD, Abalem MF, Andrews CA, Popova LT, Santos EP, Yu G, Rakine HY, Baig N, Ehrlich JR, Fahim AT, et al. The Michigan Retinal Degeneration Questionnaire: a patient-reported outcome instrument for inherited retinal degenerations. Am J Ophthalmol. 2021 Feb 1;222:60–68. doi:10.1016/j.ajo.2020.08.032
- Lacy GD, Abalem MF, Andrews CA, Abuzaitoun R, Popova LT, Santos EP, Yu G, Rakine HY, Baig N, Ehrlich JR, Fahim AT, et al. The Michigan Vision-related Anxiety Questionnaire: a psychosocial outcomes measure for inherited retinal degenerations. Am J Ophthalmol. 2021 May 1;225:137–46. doi:10.1016/j.ajo.2020.12.001.
- Selvan K, Abalem MF, Lacy GD, Vincent A, Héon E. The state of patient-reported outcome measures for pediatric patients with inherited retinal disease. Ophthalmol Ther. 2022 Jun;11(3):1031–46. doi:10.1007/s40123-022-00514-x.
- El-Alti L, Sandman L, Munthe C. Person centered care and personalized medicine: irreconcilable opposites or potential companions? Health Care Anal. 2019 Mar 1;27(1):45–59. doi:10.1007/s10728-017-0347-5.
- Dean S, Mathers JM, Calvert M, Kyte DG, Conroy D, Folkard A, Southworth S, Murray PI, Denniston AK. “The patient is speaking”: discovering the patient voice in ophthalmology. Br J Ophthalmol. 2017 Jun 1;101(6):700–08. doi:10.1136/bjophthalmol-2016-309955.
- Poochikian-Sarkissian S, Sidani S, Ferguson-Pare M, Doran D. Examining the relationship between patient-centred care and outcomes. Can J Neurosci Nurs. 2010 Dec;32(4):14–21.
- Napier MP, Selvan K, Hayeems RZ, Shuman C, Chitayat D, Sutherland JE, Day MA, Heon E. Gene therapy: perspectives from young adults with Leber’s congenital amaurosis. Eye. 2022 Nov;36(11):2088–93. doi:10.1038/s41433-021-01763-z.
- Patrick DL, Burke LB, Powers JH, Scott JA, Rock EP, Dawisha S, O’Neill R, Kennedy DL. Patient-reported outcomes to support medical product labeling claims: FDA perspective. Value Health. 2007 Nov;10:S125–37. doi:10.1111/j.1524-4733.2007.00275.x.
- Aarts JWM, Huppelschoten AG, van Empel IWH, Boivin J, Verhaak CM, Kremer JAM, Nelen WL. How patient-centred care relates to patients’ quality of life and distress: a study in 427 women experiencing infertility. Hum Reprod. 2012 Feb 1;27(2):488–95. doi:10.1093/humrep/der386.
- Apers S, Dancet EAF, Aarts JWM, Kluivers KB, D’hooghe TM, Nelen WLDM. The association between experiences with patient-centred care and health-related quality of life in women with endometriosis. Reprod Biomed Online. 2018 Feb 1;36(2):197–205. doi:10.1016/j.rbmo.2017.10.106.
- Spertus JA. Quality of life in EMPEROR-reduced: emphasizing what is important to patients while identifying strategies to support more patient-centred care. Eur Heart J. 2021 Apr 1;42(13):1213–15. doi:10.1093/eurheartj/ehab057.
- Rokach A, Parvini M. Experience of adults and children in hospitals. Early Child Dev Care. 2011 Jun;181(5):707–15. doi:10.1080/03004430.2010.483115.
- Solans M, Pane S, Estrada M-D, Serra-sutton V, Berra S, Herdman M, Alonso J, Rajmil L. Health-related quality of life measurement in children and adolescents: a systematic review of generic and disease-specific instruments. Value Health. 2008;11(4):742–64. doi:10.1111/j.1524-4733.2007.00293.x.
- Varni JW, Limbers CA, Burwinkle TM. How young can children reliably and validly self-report their health-related quality of life?: an analysis of 8,591 children across age subgroups with the PedsQLTM 4.0 generic core scales. Health Qual Life Outcomes. 2007;5(1):1–13. doi:10.1186/1477-7525-5-1.
- Juniper EF, Guyatt GH, Feeny DH, Griffith LE, Ferrie PJ. Minimum skills required by children to complete health-related quality of life instruments for asthma: comparison of measurement properties. Eur Respir J. 1997;10(10):2285–94. doi:10.1183/09031936.97.10102285.
- U.S. Department of Health and Human Services FDA Center for Drug Evaluation and Research, U.S. Department of Health and Human Services FDA Center for Biologics Evaluation and Research, U.S. Department of Health and Human Services FDA Center for Devices and Radiological Health. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes. 2006 Oct 11;4(1):79.
- Patient registry | fighting blindness Canada [Internet]. Fighting Blindness Canada (FBC). [ accessed 2022 Apr 16]. https://www.fightingblindness.ca/patient-registry/.
- Thompson DA, Ali RR, Banin E, Branham KE, Flannery JG, Gamm DM, Hauswirth WW, Heckenlively JR, Iannaccone A, Jayasundera KT, et al. Advancing therapeutic strategies for inherited retinal degeneration: recommendations from the Monaciano symposium. Investig Ophthalmol Vis Sci. 2015 Feb;56(2):918–31. doi:10.1167/iovs.14-16049.
- Williamson JM, Martin AG. Analysis of patient information leaflets provided by a district general hospital by the Flesch and Flesch–Kincaid method. Int J Clin Pract. 2010;64(13):1824–31. doi:10.1111/j.1742-1241.2010.02408.x.
- Nickles MA, Ramani SL, Tegtmeyer K, Zhao J, Lio PA. Readability of online patient education materials for juvenile dermatomyositis. Pediatr Dermatol. 2021;38(2):544–46. doi:10.1111/pde.14513.
- Kroenke K, Spitzer RL, Williams JBW, Löwe B. An ultra-brief screening scale for anxiety and depression: the PHQ–4. Psychosomatics. 2009 Nov 1;50(6):613–21. doi:10.1016/S0033-3182(09)70864-3.
- Watson SE, Spurling SE, Fieldhouse AM, Montgomery VL, Wintergerst KA. Depression and anxiety screening in adolescents with diabetes. Clin Pediatr (Phila). 2020 May;59(4–5):445–49. doi:10.1177/0009922820905861.
- Kollbaum PS, Jansen ME, Kollbaum EJ, Bullimore MA. Validation of an iPad test of letter contrast sensitivity. Optom Vis Sci. 2014;91(3):291–96. doi:10.1097/OPX.0000000000000158.
- Khurana RN, Hoang C, Khanani AM, Steklov N, Singerman LJ. A smart mobile application to monitor visual function in diabetic retinopathy and age-related macular degeneration: the CLEAR study. Am J Ophthalmol. 2021 Jul 1;227:222–30. doi:10.1016/j.ajo.2021.03.033.
- Satgunam P, Thakur M, Sachdeva V, Reddy S, Rani PK. Validation of visual acuity applications for teleophthalmology during COVID-19. Indian J Ophthalmol. 2021 Feb;69(2):385–90. doi:10.4103/ijo.IJO_2333_20.
- Cai L. High-dimensional exploratory item factor analysis by a Metropolis–Hastings Robbins–Monro algorithm. Psychometrika. 2010;75(1):33–57. doi:10.1007/s11336-009-9136-x.
- Chalmers RP. Mirt: a multidimensional item response theory package for the R environment. J Stat Softw. 2012;48(1):1–29. doi:10.18637/jss.v048.i06.
- Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8(2):135–60. doi:10.1177/096228029900800204.
- Abou-Hanna JJ, Andrews CA, Khan NW, Musch DC, Jayasundera KT. Calculation of test-retest variability in phase I/IIa clinical trials for inherited retinal degenerations. Ophthalmic Genet. 2021 Jun;42(3):283–90. doi:10.1080/13816810.2021.1897848.
- Wojciechowski R. Nature and nurture: the complex genetics of myopia and refractive error. Clin Genet. 2011;79(4):301–20. doi:10.1111/j.1399-0004.2010.01592.x.
- Hardcastle AJ, Sieving PA, Sahel JA, Jacobson SG, Cideciyan AV, Flannery JG, Beltran WA, Aguirre GD. Translational retinal research and therapies. Transl Vis Sci Technol. 2018;7(5):8. doi:10.1167/tvst.7.5.8.