ABSTRACT
Risk and protective factors for cognitive function in aging may affect how much individuals benefit from their environment or life experiences by preserving or improving cognitive abilities. We investigated the relations between such factors and outcome from episodic-memory training in 136 healthy young and older adults. Tested risk factors included carrying the ɛ4 variant of the apolipoprotein E allele (APOE), age, body mass index, blood pressure, and cholesterol. Protective factors included higher levels of education, intelligence quotient (IQ), physical activity, fatty acids, and vitamin D. Average increases in memory performance were seen after training, with ample variation between individuals. Being young, female, and having higher IQ were positive predictors of memory improvement. No other relationships were observed. Similar benefit was observed across APOE allelic variation. This indicates that beyond IQ, age, and sex, known risk -and protective factors of cognitive function in aging were not significantly related to memory plasticity.
1. Introduction
Cognitive decline, including lowered memory capacity, is a part of normal aging, in addition to being a hallmark symptom of dementia and Alzheimer´s disease (AD) (Buckner, Citation2004; Nyberg, Lövdén, Riklund, Lindenberger, & Bäckman, Citation2012; Salthouse, Citation2003). Several demographic, lifestyle -and genetic factors have been suggested to relate to both the risk and the course of age-related cognitive and neural changes, in addition to the onset of dementia and AD (Livingston et al., Citation2017). In the present study, we investigate the relationships between a series of such protective and risk factors and response to memory training in healthy young and older adults.
The factors of investigation were chosen based on their established relation to cognitive function in aging, as well as their availability in a relatively healthy sample (e.g., known risk and disease factors such as stroke and diabetes could not be studied). Of non-modifiable factors, we study age, APOE allelic variation, and sex. These were included as age is the primary predictor for sporadic AD, and carrying the ɛ4 variant of the APOE allele is the major known genetic risk factor (Farrer et al., Citation1997). As for sex, while females have a higher risk of AD, males have higher risk of, e.g., vascular dementia (Podcasy & Epperson, Citation2016). Being female has been associated with higher memory scores and lesser memory decline in healthy populations (Josefsson, de Luna, Pudas, Nilsson, & Nyberg, Citation2012). The relationship between sex and benefit from memory training should thus be studied. Education and IQ were included as they are often used as proxies for cognitive reserve, the adaptability of cognitive processes or day-to-day function to brain aging, pathology, or insult (Stern et al., 2018). Among lifestyle-associated factors, we included body mass index (BMI), blood pressure, physical activity, and blood levels of fatty acids, vitamin D, and cholesterol. On the one hand, vitamin D deficiency, elevated BMI, and hypertension are assumed to be risk factors (Balion et al., Citation2012; Josefsson et al., Citation2012; Kivipelto et al., Citation2005; Livingston et al., Citation2017; Nagai, Hoshide, Ishikawa, Shimada, & Kario, Citation2008; Profenno, Porsteinsson, & Faraone, Citation2010; Raz, Rodrigue, Kennedy, & Acker, Citation2007). On the other hand, education, IQ, physical activity, and high blood levels of fatty acids have been proposed as protective factors (Anstey & Christensen, Citation2000; Diaz-Asper, Schretlen, & Pearlson, Citation2004; Erickson, Gildengers, & Butters, Citation2013; Josefsson et al., Citation2012; Livingston et al., Citation2017; Lyketsos, Chen, & Anthony, Citation1999; Ratcliff, Thapar, & McKoon, Citation2011; Titova et al., Citation2013; Walhovd, Storsve, Westlye, Drevon, & Fjell, Citation2014). Interestingly, cholesterol has been related to both elevated and decreased risk of AD, age-related degenerative changes, and dementia (Mielke et al., Citation2005b; Solomon, Kivipelto, Wolozin, Zhou, & Whitmer, Citation2009). While the exact mechanisms by which these risk and protective factors may work are largely unknown, one possible pathway is by means of affecting cognitive plasticity, i.e., how much an individual can benefit from his or her environment and experience.
One approach to studying this is by inducing plastic responses through cognitive training interventions, and investigating whether risk and protective factors for cognition in aging are predictive of variation in outcome. Memory plasticity refers to the improvement in a specific memory ability after a memory training intervention. Of such memory abilities, episodic memory refers to the capacity to encode and retrieve previous events (Tulving & Thomson, Citation1973), and previous studies have shown that strategic memory training can induce episodic memory plasticity (de Lange et al., Citation2016; Engvig et al., Citation2010). Given that episodic memory is pivotal for everyday functioning in both healthy and clinical populations, it is of interest to study the premises of episodic memory plasticity through memory training. Here, we studied memory plasticity through measuring the change in episodic memory performance as a response to a memory training intervention, here measured in terms of performance differences at alternate versions of a criterion memory task pre- vs. post-training.
Effects of cognitive training on brain and cognition have been observed in both young and older adults, although benefits are commonly smaller in the latter group (Baltes & Kliegl, Citation1992; Burki, Ludwig, Chicherio, & de Ribaupierre, Citation2014; Dahlin, Nyberg, Backman, & Neely, Citation2008; de Lange et al., Citation2016; Engvig et al., Citation2010; Lövdén, Brehmer, Li, & Lindenberger, Citation2012; Lustig, Shah, Seidler, & Reuter-Lorenz, Citation2009; Nyberg et al., Citation2003). However, the degree to which lifestyle factors related to cognitive health can facilitate or limit cognitive plasticity is not well understood. In this regard, factors such as IQ, education, cardiovascular health, nutrition, and physical activity have been related to neurocognitive plasticity and changes (Anstey & Christensen, Citation2000; Erickson et al., Citation2013; Erickson, Weinstein, & Lopez, Citation2012; Lerche et al., Citation2018; Ngandu et al., Citation2015; Rapport et al., Citation1997). Additionally, APOE allelic variation has been assumed to relate to plastic responses to cognitive training interventions. Although results are inconclusive, it has been suggested that ε4 carriers may show less cognitive plasticity than non-carriers (Feng et al., Citation2015; Polito et al., Citation2015; Runge, Small, McFall, & Dixon, Citation2014; Zehnder et al., Citation2009). As the prevalence of people living with dementia is increasing (Livingston et al., Citation2017), it is of interest to study whether genetic variation, IQ, and modifiable lifestyle factors impact memory plasticity in a controlled experimental intervention. Investigating potentially modifiable factors (such as BMI and blood markers of nutrition) may help promote interventions adjusted to the characteristics of each particular individual. Further, to what extent individuals with risk factors can benefit from memory training is of relevance to clinical populations. This will also provide a broader understanding of factors of importance to cognitive plasticity.
Here, we aimed to investigate the relationships between response to memory training in healthy young and older adults to a series of assumed protective and risk factors of cognitive performance and dementia. We investigated memory training outcome in relation to age, sex, APOE status, IQ, education, cardiovascular health (systolic/diastolic blood pressure), physical activity level, BMI, and nutrition markers (blood levels of vitamin D, cholesterol, and the fatty acid docosahexaenoic acid [DHA]). Analyses were conducted to specifically test interactions between age and APOE status, as well as any factors shown to have a main effect on memory training outcomes.
2. Methods and materials
2.1. Sample
The sample was drawn from the project Neurocognitive Plasticity (NCP) at the Center for Lifespan Changes in Brain and Cognition (LCBC), Department of Psychology, University of Oslo. All procedures were approved by the regional ethical committee of Southern Norway, and written consent was obtained from all participants. The broader NCP study encompasses also brain MRIs and some participants not completing memory training (Brathen et al., Citation2018; de Lange et al., Citation2016). The present sample includes all NCP participants who underwent memory training. Participants were required to be either in or around their 20s or 70s, right-handed, fluent Norwegian speakers, and have normal or corrected to normal vision and hearing. For inclusion in the study, participants were required to score ≥26 on the Mini-Mental State Examination (MMSE) (Folstein, Folstein, & McHugh, Citation1975) and have scores within normal range (≥2 standard deviations below mean) for age and sex on the 5-min delayed recall subtest of the California Verbal Learning Test II (Delis, Kramer, Kaplan, & Ober, Citation2000). All participants further had to achieve an IQ above 85 on the Wechsler Abbreviated Scale of Intelligence (Wechsler, Citation1999). Three participants in the older group were excluded based on these criteria. Exclusion criteria were history of injury or disease known to affect central nervous system function, including neurological or psychiatric illness or serious head trauma, being under psychiatric treatment, and use of psychoactive drugs known to affect central nervous system functioning. The participants in the NCP study from which the sample is drawn were assigned to one of the three groups: memory training, active control (partaking in lectures, discussion groups, and tasks pertaining to popular science topics), or passive control (no intervention). The training group received 10 weeks of memory training including a single group session each week led by a research fellow (see below). The active controls partook in lectures, discussion groups, and tasks pertaining to popular science topics. The passive controls were merely tested with the same ten-week interval as the two other groups. In the NCP study, pools of around 20 participants were recruited at a time, and the participants were assigned to either the training group or the control groups at registration. The data collection was on-going and continuous for the conditions simultaneously, ensuring that participants in each group were tested interchangeably and thus reducing the possibility of group differences with regard to the assessment conditions. Although group assignments based on date do not comply with suggested criteria for randomization of participants (Schulz and Grimes, Citation2002), practical considerations forced a compromise due to the extensive data collection with strict time intervals and assessments locked to specific dates to ensure that all participants were tested with the same interval. We have previously reported that memory improvements were found exclusively in the memory-training group (de Lange et al., Citation2016). That is, only the training group improved their memory performance, indicating that the change in memory performance was not due to retest effects. Here, we proceeded to investigate factors moderating the memory training outcome. Thus, only the participants who completed the memory-training program (N = 136) were included in the analyses. The sample included all the participants from the memory-training group of the NCP study, 51 participants in their 20s and 85 participants in their 70s from the memory-training group. Some of the passive control-participants in NCP (15 young adults and 41 older adults) completed the memory training after 10 initial weeks as passive controls. 22.79 percent of the sample was carriers of the ɛ4 allele. The percentage of ɛ4 carriers differed slightly between the young and the older participants (23.5% and 22.45%, respectively). However, an independent sample t-test showed that this difference was not significant (t = .157, p = .875). For the demographics of the whole sample, see . For demographics of the ɛ4 carriers and non-carriers separately, see . For demographics of the active and passive control groups, in addition to a detailed description of the design and the control-group conditions, see (de Lange et al., Citation2016).
Table 1. Demographics of the participants. MMSE (Mini-Mental State Examination), CVLT (California Verbal Learning Test), MET (metabolic rate), IQ (intelligence quotient), BP Systolic (Systolic blood pressure), BP Diastolic (diastolic blood pressure), DHA (docosahexaenoic acid), BMI (body mass index)
Table 2. Demographics of the non-carriers and carriers of the APOE ɛ4 allele. MMSE (Mini-Mental State Examination), CVLT (California Verbal Learning Test), MET (metabolic rate), IQ (intelligence quotient), BP Systolic (Systolic blood pressure), BP Diastolic (diastolic blood pressure), DHA (docosahexaenoic acid), BMI (body mass index)
2.2. Assessments
The participants in all the groups underwent a neuropsychological assessment immediately before and after the training or control conditions. Thus, there was a 10-week interval between the test sessions in the training group (interval mean ± SD weeks across ages; 10.95 ± .0.52, in the young; 11.05 ± 0.57, in the older: = 10.86 ± 0.42), as well as in the passive control group (mean ± SD weeks across ages; 10.85 ± 0.79, in the young; 10.74 ± 1.25, older; 10.91 ± 0.31) and before and after the active control intervention (10.95 ± 0.2 weeks, young; 11.1 ± 0.26, older; 10.95 ± .0.16). Data regarding the risk/protective factors were collected at baseline, within the week preceding the commencement of the memory training. Blood samples were extracted for analyses of cholesterol, vitamin D, and fatty acids, namely docosahexaenoic acid (DHA). The blood samples were monitored in dried blood spots as developed by Vitas (www.vitas.no). DNA was extracted from saliva samples collected using Oragene DNA saliva tubes. APOE genotyping was determined by polymerase chain reaction using TaqMan genotyping assays (5ʹ-nuclease assay, Applied Biosystems) for amino acid positions 112 and 158 and an allelic discrimination method on the ABI-7500 platform (Hui, DelMonte, & Ranade, Citation2008; Koch et al., Citation2002; Livak, Citation1999). The samples were analyzed at Akershus University Hospital. Blood pressure was measured with an AND UA-767 + 30 digital upper arm blood pressure monitor. Height and weight were measured manually. Height was measured with a wall-mounted stadiometer with a headpiece to ensure accurate height measures across participants. BMI was calculated as weight (kg)/height × height (m). Level of physical activity was mapped using multiples of the resting metabolic rate (METs) as calculated by administered a short version of the International Physical Activity Questionnaire (IPAQ) in Norwegian (Hagstromer, Oja, & Sjostrom, Citation2006). IQ was calculated according to the Wechsler Abbreviated Scale of Intelligence (Wechsler, Citation1999), while years of education were self-reported and used as a continuous variable in the analyses. Given that the structure of the Norwegian educational system differed between the two age groups due to education reforms, academic level, and degrees reported were verified according to the Norwegian Standard Classification as provided by Statistics Norway, https://www.ssb.no/en/.
2.3. Design and memory-training program
The participants underwent 10 weeks of memory training using the mnemonic technique Method of Loci (MoL) (Bower, Citation1970), which has previously been reported to improve serial recall substantially in both young and older adults (de Lange et al., Citation2016; Engvig et al., Citation2010; Kliegl, Smith, & Baltes, Citation1990; Nyberg et al., Citation2003). The method involves creating a mental travel route of a familiar place, such as one´s home. When memorizing a list of items, one visualizes placing the items along this travel route. For retrieval of the items, one imagines walking through the travel route, recollecting the items previously placed along the route.
The memory training involved learning and practicing the MoL specifically aiming to improve episodic memory performance. The first group session included a presentation of the project, an introduction to the MoL with instructions, and an initial word list task consisting of 15 words. The research fellow leading the group session was available for questions, and provided further explanations and repetition of instructions to ensure that all participants were able to use the technique. The following weekly group sessions included updating of the strategy, clarification of instructions, and a new word list task, which was increased by five words each week to ensure a continuous challenge. However, the participants were additionally encouraged to individually adjust the difficulty level of the tasks both in class and of the home assignments. This was done with the aim of achieving a challenging but manageable training level across all the participants. The home assignments were completed online and all responses in addition to time spent on the tasks were registered to a database. Both age groups underwent the same program. The number of total tasks completed was on average 48.1% in the young training group and 71.8% in the older training group. Memory improvement was measured in a training-specific task by change in correct written recall of a word list consisting of 100 nouns administrated in the laboratory on the neuropsychological test sessions at baseline and after the training intervention. The task measured change in correct written recall of a word list consisting of 100 nouns. The participants were given 5 min to memorize the word list, followed by 10 min to recall as many words as possible. The lists differed between the two time points, and the extensive length of the lists was chosen to avoid ceiling effects. For more details regarding the memory-training program, course sessions, and the individual adjustments (see de Lange et al., Citation2017).
3. Statistical analyses
Differences between participants who dropped out of the study versus those who completed the training were assessed by independent samples t-tests for all studied factors. Correlation analyses were performed to check for any relations between the factors investigated and memory at baseline. Change in memory performance (based on scores on different versions of the 100-word test as described above) after the training intervention was used as the measure of memory improvement. To account for baseline differences in memory in the analyses, two approaches were taken 1) Standardized residuals were used as the measure of memory improvement. The residuals were calculated from a linear regression analysis, using memory performance at time point 2 as the dependent variable and memory performance at baseline as the independent variable. This approach takes into account differences in relative improvement across persons by removing baseline variance. 2) The change in memory performance was modeled as a time factor (memory pre- vs post-training) in repeated measures GLMs. In order to test for an effect of age group and sex as well as their possible interactions on memory improvement, a repeated measures GLM was performed with time (memory score at time point 1, memory score at time point 2) x age group (young, older) x sex (male, female). Greenhouse-Geisser corrections for violation of sphericity were used where applicable. As it has been suggested that APOE can affect the age groups differentially through antagonistic pleiotropy (Tuminello & Han, Citation2011), we tested for possible effects of APOE and age interaction, by a repeated measures GLM with time (memory pre- vs post-training) x APOE (presence, absence of e4) x age group (young, older) as between-subject factors, using sex as a covariate. Correlations analyses for age and sex and partial correlation analyses using age and sex as covariates, were conducted for all factors (IQ, education, BMI, DHA, cholesterol, vitamin D, blood pressure, physical activity, APOE status), and memory improvement with training (residuals). For any factor shown related to memory outcome, analyses were conducted to check for possible interactions (see below). Finally, a multiple regression analysis was carried out introducing all the above factors, as well as age and sex, to investigate whether variance in memory change could be explained by any of the factors when taking into account all the other variables.
4. Results
4.1. Drop out analysis
A total of 42 participants (21 young, 21 older) dropped out before the follow up session, and were thus excluded from the analyses. The group of participants who dropped out after the first test session performed lower than the rest of the sample in terms of IQ (mean ± SD drop outs = 110.3 ± 11.7; included = 118.1 ± 10.3; t(176) = 4.126, p = .000), MMSE score (mean ± SD drop outs = 28.5 ± 1.4; included = 29.0 ± 1.1; t(176) = 2.2, p = .024) and DHA levels (mean ± SD drop outs = 1.89 ± .78; included = 2.23 ± .78; t(133) = 2.3, p = .024). Cholesterol showed a tendency toward lower levels in the drop outs (mean ± SD drop outs = 5.61 ± .94; included = 5.92 ± .83; t(133) = 1.9, p = .060). None of the other factors differed between the drop outs and the participants who completed the training. See (de Lange et al., Citation2017) for further analysis addressing the selection bias in the current sample.
4.2. Correlations at baseline
Age correlated −.54 (p < .001) with memory performance at baseline, sex did not correlate (r = −.037 (females coded as 0, males coded as 1), p = .627). These relationships did not change when controlling for sex and age, respectively. Partial correlation analysis controlling for age and sex showed no significant relationships between any of the risk/protective factors and memory performance at baseline (see Supplementary Table 1).
4.3. Memory improvement and effects of age, sex, and APOE status
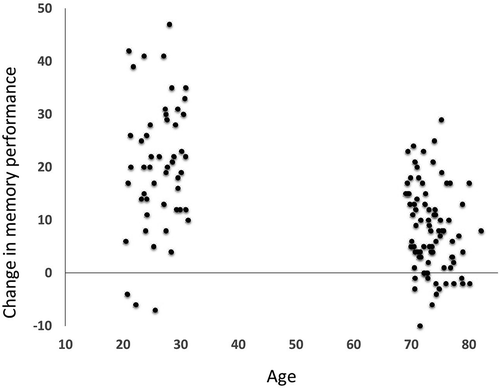
Repeated measures analyses with time (pre-, post-training score) x age group (young, older) x sex (male, female) showed a significant main effect of time in terms of improvement in memory performance from baseline to time point 2, (F(1,132) = 253.606, p < .001), a main effect of age group (F(1,132) = 161.301, p < .001), and a marginal effect of sex (F(1,132) = 3.203, p = 071). The trend for sex was due to a marginally greater increase in memory performance for women (means (SDs) for memory pre- vs. post-training were 13.8 (7.6) and 27.2 (13.2) for females and 13.3 (7.0) and 24.9 (14.0) for males, respectively). The only significant interaction or trend toward such (p < .10) was observed for age group, interacting with time (F(1,132) = 49.091, p < .001). As seen in , both age groups improved, but the group of young adults improved more than the older adults (mean difference = 12.37, p < .001). Ample variation was found in memory change both across the sample (mean change = 12.7, SD = 11.44), and when exploring the age groups separately (young; mean change = 20.43, SD = 12.11, old; mean change = 8.06, SD = 8.06).
Figure 1. Change in memory performance in the two age groups. Individual change is illustrated as change in correct number of words recalled on the 100 words test from baseline to time point
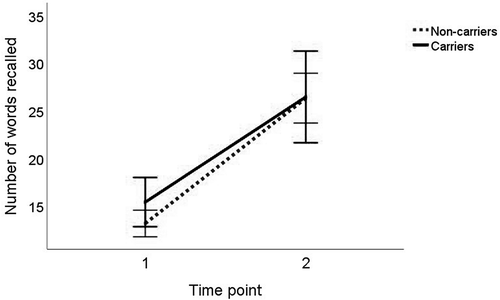
The repeated measures GLM carried out with time (memory pre- vs post-training) x APOE (presence, absence of e4) x age group (young, older), using sex as a covariate, showed no main (F(1,131) = .750, p = .388) or interaction effects of APOE, or trends toward such (all p s > .10)
Increase in memory performance from baseline to time point 2 was observed both in the carriers and the non-carriers (see ).
4.4. Effects of other factors on memory improvement
Partial correlation analyses of memory improvement (residuals) and the factors studied (IQ, education, BMI, DHA, cholesterol, vitamin D, blood pressure, physical activity, APOE status) controlling for age and sex, showed a positive correlation between memory-training improvement and IQ (r = .24, p = .012). No other significant relationships or trends toward such (p < .10) were observed. Correlation analyses with all risk/protective factors were undertaken to assess whether possible collinearity could affect results when variables were entered in a multiple regression (see Supplementary Table 2). In general, extremely high correlations between variables were not observed, all r < .60). Multiple regression analysis including all the factors as predictors showed that sex, age, and IQ contributed as predictors to explained variance in memory improvement (sex; β = −.185, p = .043, age; β = −.737, p < .001, IQ; β = .258, p = .007), while physical activity showed a trend (β = .157 p = .069). None of the other variables showed any significant relationship or trend toward such. Given the observed significance of IQ and age as predictors of memory improvement, we proceeded to split the sample in two based on the IQ distribution, the lower and upper half (IQ ≤ 118, n = 69, and IQ ≥ 119, n = 67), and check for possible interactions of time (pre- vs post-training) x IQ group (lower, upper) x age group (young, older), using sex as a covariate in a repeated measures GLM. This analysis showed significant main effects of time (F(1,131) = 168.128, p < .001), age (F(1,131) = 160.017, p < .001), IQ group (F(1,131) = 6.580, p = .011) and sex (F(1,131) = 6.197, p = .014) and an interaction of time x age (F(1,131) = 52.462, p < .001) and trends toward interactions of time x IQ (F(1,131) = 3.416, p = .067) and time x sex (F(1,131) = 3.086, p = .081).
5. Discussion
The current results only partly support relationships between response to memory training in healthy young and older adults to a series of assumed protective and risk factors of cognitive performance and dementia: Being younger and having higher IQ were factors positively related to memory benefit, being female was marginally related, whereas variation in years of education, APOE status, BMI, blood pressure, blood cholesterol, fatty acids, and vitamin D were not.
Ample training effects were observed across ages, with considerable variation. The younger adults showed a greater increase in memory performance relative to the older adults. This was expected given that young adults tend to improve more than older adults from cognitive training interventions. Although older participants have at some tasks been shown to improve proportionately as much as younger (Brehmer, Shing, Heekeren, Lindenberger, & Backman, Citation2016; Carretti, Borella, & De Beni, Citation2007; Lovden et al., Citation2012), training appears to increase individual differences (Baltes & Kliegl, Citation1992; de Lange et al., Citation2017; Kliegl et al., Citation1990), with less improvement in older age (Baltes & Kliegl, Citation1992; Burki et al., Citation2014; Dahlin et al., Citation2008; de Lange et al., Citation2017; Nyberg et al., Citation2003). Relatedly, the young participants completed a lower amount of tasks relative to the older participants. Although merely speculative, this suggests that the improvement in the young is largely dependent on properly learning and applying the technique. Very high recall scores have been observed in “memory athletes” applying mnemonic training techniques (e.g., Dresler et al., Citation2017). It may be that with more time invested in the training, the young group could have improved even more, potentially broadening the performance gap between young and older participants further. The marginal advantage for females with regard to memory training benefit, is in accordance with observed sex differences in cognitive performance in previous studies, in that females have been observed to perform better than males on episodic memory tasks, and to more often be classified as cognitive maintainers relative to males (Herlitz, Nilsson, & Bäckman, Citation1997; Josefsson et al., Citation2012).
The suggested protective factor IQ was positively related to memory-training outcome. IQ is assumed to be relatively stable throughout the lifespan (Deary, Whalley, Lemmon, Crawford, & Starr, Citation2000; Deary et al., Citation2012; Gow et al., Citation2011), and although performance may change with age, an individual’s relative ability level, as compared to the ability level of same-age peers, tends to remain highly stable (Deary et al., Citation2000). IQ has been related to years of schooling (Johnson, Deary, & Iacono, Citation2009), and learning abilities in adults (Cowan, Fristoe, Elliott, Brunner, & Saults, Citation2006; Deary, Strand, Smith, & Fernandes, Citation2007; Diaz-Asper et al., Citation2004; Markant & Amso, Citation2014). Hence, it could be that a higher IQ is advantageous in the usage of the MoL, both in terms of managing the cognitive challenges to promote a successful use if the strategy, and by facilitating a rapid learning curve (Cowan et al., Citation2006; Deary et al., Citation2007; Diaz-Asper et al., Citation2004; Markant & Amso, Citation2014). Moreover, cortical integrity is related to cognitive abilities (Fjell & Walhovd, Citation2010; Nyberg et al., Citation2012; Raz & Rodrigue, Citation2006), and such brain–cognition relationships have been suggested to remain stable throughout the lifespan (Walhovd et al., Citation2016). Childhood IQ has further been related to cognitive health and pathology in aging, observed through decreased risk for, or delayed onset of dementia (McGurn, Deary, & Starr, Citation2008). Additionally, a subject´s motivation to perform well can affect the outcome of IQ tests (Duckworth, Quinn, Lynam, Loeber, & Stouthamer-Loeber, Citation2011). While we do not believe that the IQ scores were mere products of performance motivation, it is possible that each participant´s determination to perform well was also reflected in the dedication to the memory training. Thus, the possible pathways through which IQ relates to memory-training outcomes are many. Overall, the factors that contribute to high IQ, and stability of IQ relative to same-age peers through the lifespan, may be partially the same as those facilitating learning, whether in terms of genetic propensity to seek out stimulating environments and challenges, brain characteristics, or health throughout the lifespan. Both IQ and education are commonly treated as proxies of cognitive reserve (Stern et al., 2018), and we hypothesized that education would also relate to the memory improvement. However, no relationship was observed, suggesting that the implication of IQ might not be a reflection of the propensity to seek out stimulating challenges in terms of amount of schooling. Thus, the pathway through which IQ affects plasticity might be more related to an inherent capacity of successful comprehension and application of the MoL, rather than the amount of educational stimulation per se.
It remains unknown why the other factors did not predict the memory-training outcome, but it could be related to the characteristics of the specific sample of the study. Interestingly, APOE allelic distribution did not affect the memory-training outcome. Presence of the APOE ɛ4 allele is the main known genetic risk factor of AD (Farrer et al., Citation1997), and previous studies suggest a genetic influence of APOE allelic variation on neurocognitive health and plasticity. Consequently, we expected the ε4 carriers to show less memory plasticity relative to the non-carriers. Few cognitive training studies have investigated APOE as a moderator of cognitive plasticity. In accordance with our results, plastic responses to cognitive training were found in ε4 carriers in a multi-domain training study (Feng et al., Citation2015). The participants underwent training sessions including different memory tasks such as word recall training, and story recall training, in addition to physical exercise and painting. Similarly, no difference in cognitive benefit between ε4 carriers and non-carriers was found in another multi-domain lifestyle intervention study (Solomon et al., Citation2018). The intervention included participation in social activities, and an individual nutrition plan, in addition to physical and cognitive training. However, and more commonly, it has also been suggested that cognitive plasticity is limited amongst ɛ4 carriers. In one training study, ε4 carriers showed less improvement on visuospatial tasks relative to the non-carriers after the intervention (Polito et al., Citation2015). Another study reported that the effects on language comprehension after a cognitive training program were restricted to the non-carriers of the sample (Lopez-Higes et al., Citation2017). Furthermore, it was shown that cognitively stimulating activities more likely moderated cognitive performance in non-carriers (Runge et al., Citation2014). Thus, the scarce literature points mainly toward limited plastic potential in the ε4 carriers. While the reasons for the inconclusive literature are not understood, it could be that the differences in training paradigms, cognitive domains trained and intensity of the training interventions can affect the degree to which genetic variation relates to the effects of the interventions. Additionally, as the prevalence of APOE ε4 is geographically dependent (Lucotte, Loirat, & Hazout, Citation1997), the variety of regional localizations of the studies is a factor that could potentially underlie the discrepancies in training outcome. Either way, memory improvement was not dependent on APOE ε4 status in our sample, and showed no trend toward being so. In our study, the significant memory increases also among ε4 carriers show that individuals with this risk factor for AD can benefit from memory training.
In terms of BMI, previous literature mainly point toward overweight or obesity as risk factors for cognitive ability (Kivipelto et al., Citation2005; Livingston et al., Citation2017). Although mean BMI of the sample (mean BMI = 24.5) was close to the limit of what is considered to be overweight (normal BMI = 18.5–25), the majority of the participants was largely within what is considered normal BMI. This could explain why no relationship between BMI and memory plasticity was observed. Previous literature has shown both negative and positive effects of cholesterol on cognitive abilities (Anstey, Ashby-Mitchell, & Peters, Citation2016; Mielke et al., Citation2005a; Solomon et al., Citation2009). Thus, the expected effect of this factor was also largely uncertain. Similarly, although levels of fatty acids have been related to neurocognitive health in previous studies, findings are mixed, and it has been suggested that the inconsistent results are due methodological differences or effects of variables not accounted for here, such as environmental pollutions or interactions with other nutrients (Huang, Citation2010).
We recently found that after participating in two 10-week training periods separated by a 10-week passive period, age-related decline in white matter microstructure was mitigated by the training periods specifically (de Lange et al., Citation2018). However, memory improvement was to a great degree maintained throughout both the passive and active periods. Thus, cognitive improvements from the training might not rely on consistent training during short time intervals to the same degree as some brain characteristics. These results are promising in terms of a possibly more moderate need for continuous training to maintain results, possibly also in clinical samples.
6. Limitations
This study has several limitations. While no group differences in memory improvement were detected between the ɛ4 carriers and the non-carriers, this could be due to the sample size. However, both the carriers and the non-carriers significantly improved their memory performance after the training intervention. Prevalence of ɛ4 carriers varies across geographical regions, and ɛ4 has in Nordic countries been observed in 13–23% of the population (Lucotte et al., Citation1997). In our sample, 22.79% of the participants were ɛ4 allele carriers, with no age difference. Consequently, the results are based on a relatively representative sample in terms of allelic variation in the general population. The presence of the ɛ4 allele has been shown to negatively affect longevity in older adults (Brooks-Wilson, Citation2013). There is a possibility that some ɛ4 carriers in this sample are “super-carriers,” not equally affected by other risk factors of age-related disease as the general population of ɛ4 carriers. Regardless, the results indicate that ɛ4 status alone does not severely restrict memory plasticity. Accordingly, our results convey that healthy ɛ4 carriers can experience memory plasticity comparable to the plasticity experienced by non-carriers. Participants were required to score 26 or above on the MMSE, which is considered to be a general lower threshold for normal functioning. Studies have shown that scores of 23 or 24 are valid cut-offs for dementia diagnosis (Fountoulakis et.al, Citation2000), while scores of 27–28 could be appropriate for the diagnosis of mild cognitive impairment in adults aged over 60 (Ciesielska et al., Citation2016). Thus, the threshold of MMSE scores is considered sufficiently liberal to capture the variance of the healthy population, regardless of APOE status.
IQ was the only suggested factor that moderated the memory-training response. It should be noted that the sample showed a mean IQ superior to what is normal in the general population. Consequently, the sample suffers a selection bias skewed toward a higher-than-normal functioning group of adults in respect to IQ scores. Selection bias of the project participants is addressed in (de Lange et al., Citation2017). Physical activity was the only self-reported factor investigated. To ensure that the data were as accurate as possible, participants were thoroughly instructed (item by item) by the research fellow upon filling out the questionnaire. The research fellow was further available for questions related to the questionnaire during the test sessions.
7. Conclusions
Being younger and having higher IQ were predictive of greater memory improvement in the current training paradigm. None of the other suggested risk and protective factors of cognitive performance and development of dementia were related to the benefit of the memory training, with the exception of a marginal advantage for females over males. Thus, the present results do not indicate that these factors affect the cognitive decline in healthy adults through direct modulation of memory plasticity. Although a larger sample could potentially yield a relationship between the factors and the plastic response to cognitive training, we show that cognitive plasticity is not strictly related to the absence or presence of any of these factors. Thus, our results indicate that even adults with risk factors of cognitive decline and dementia may benefit from cognitive training interventions.
Supplemental Material
Download Zip (22.6 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Anstey, K., & Christensen, H. (2000). Education, activity, health, blood pressure and apolipoprotein E as predictors of cognitive change in old age: A review. Gerontology, 46(3), 163–177.
- Anstey, K. J., Ashby-Mitchell, K., & Peters, R. (2016). Updating the evidence on the association between serum cholesterol and risk of late-life dementia: Review and meta-analysis. Journal of Alzheimer’s Disease, 56(1), 215–228.
- Balion, C., Griffith, L. E., Strifler, L., Henderson, M., Patterson, C., Heckman, G., … Raina, P. (2012). Vitamin D, cognition, and dementia; A systematic review and meta-analysis. Neurology, 79(13), 1397–1405.
- Baltes, P. B., & Kliegl, R. (1992). Further testing of limits of cognitive plasticity: Negative age differences in a mnemonic skill are robust. Developmental Psychology, 28(1), 121–125.
- Bower, G. H. (1970). Analysis of a mnemonic device: Modern psychology uncovers the powerful components of an ancient system for improving memory. American Scientist, 58(5), 496–510.
- Brathen, A. C. S., de Lange, A. G., Rohani, D. A., Sneve, M. H., Fjell, A. M., & Walhovd, K. B. (2018). Multimodal cortical and hippocampal prediction of episodic-memory plasticity in young and older adults. Human Brain Mapping, 39(11), 4480–4492.
- Brehmer, Y., Shing, Y. L., Heekeren, H. R., Lindenberger, U., & Backman, L. (2016). Training-induced changes in subsequent-memory effects: No major differences among children, younger adults, and older adults. NeuroImage, 131, 214–225.
- Brooks-Wilson, A. R. (2013). Genetics of healthy aging and longevity. Human Genetics, 132(12), 1323–1338.
- Buckner, R. L. (2004). Memory and executive function in aging and AD: Multiple factors that cause decline and reserve factors that compensate. Neuron, 44(1), 195–208.
- Burki, C. N., Ludwig, C., Chicherio, C., & de Ribaupierre, A. (2014). Individual differences in cognitive plasticity: An investigation of training curves in younger and older adults. Psychological Research, 78(6), 821–835.
- Carretti, B., Borella, E., & De Beni, R. (2007). Does strategic memory training improve the working memory performance of younger and older adults? Experimental Psychology, 54(4), 311–320.
- Ciesielska, N., Sokołowski, R., Mazur, E., Podhorecka, M., Polak-Szabela, A., & Kędziora-Kornatowska, K. (2016). Is the montreal cognitive assessment (moca) test better suited than the mini-mental state examination (mmse) in mild cognitive impairment (mci) detection among people aged over 60? meta-analysis. Psychiatr Pol, 50(5), 1039–1052. doi: 10.12740/pp/45368
- Cowan, N., Fristoe, N. M., Elliott, E. M., Brunner, R. P., & Saults, J. S. (2006). Scope of attention, control of attention, and intelligence in children and adults. Memory & Cognition, 34(8), 1754–1768.
- Dahlin, E., Nyberg, L., Backman, L., & Neely, A. S. (2008). Plasticity of executive functioning in young and older adults: Immediate training gains, transfer, and long-term maintenance. Psychology and Aging, 23(4), 720–730.
- de Lange, A. G., Brathen, A. C. S., Rohani, D. A., Grydeland, H., Fjell, A. M., & Walhovd, K. B. (2017). The effects of memory training on behavioral and microstructural plasticity in young and older adults. Human Brain Mapping, 38, 5666–5680.
- de Lange, A.-M. G., Bråthen, A. C. S., Grydeland, H., Sexton, C., Johansen-Berg, H., Andersson, J. L. R., … Walhovd, K. B. (2016). White matter integrity as a marker for cognitive plasticity in aging. Neurobiology of Aging, 47, 74–82.
- de Lange, A.-M. G., Bråthen, A. C. S., Rohani, D. A., Fjell, A. M., & Walhovd, K. B. (2018). The temporal dynamics of brain plasticity in aging. Cerebral Cortex, 28(5), 1857–1865. doi: 10.1093/cercor/bhy003
- Deary, I. J., Strand, S., Smith, P., & Fernandes, C. (2007). Intelligence and educational achievement. Intelligence, 35(1), 13–21.
- Deary, I. J., Whalley, L. J., Lemmon, H., Crawford, J. R., & Starr, J. M. (2000). The stability of individual differences in mental ability from childhood to old age: Follow-up of the 1932 Scottish mental survey. Intelligence, 28(1), 49–55.
- Deary, I. J., Yang, J., Davies, G., Harris, S. E., Tenesa, A., Liewald, D., … Visscher, P. M. (2012). Genetic contributions to stability and change in intelligence from childhood to old age. Nature, 482(7384), 212–215.
- Delis, D. C., Kramer, J. H., Kaplan, E., & Ober, B. A. (2000). California verbal learning test - Second Edition (CVLT - II). San Antonio, TX: The Psychological Corporation.
- Diaz-Asper, C. M., Schretlen, D. J., & Pearlson, G. D. (2004). How well does IQ predict neuropsychological test performance in normal adults? Journal of the International Neuropsychological Society: JINS, 10(1), 82–90.
- Dresler, M., Shirer, W. R., Konrad, B. N., Muller, N. C. J., Wagner, I. C., Fernandez, G., … Greicius, M. D. (2017). Mnemonic Training Reshapes Brain Networks to Support Superior Memory. Neuron, 93(5), 1227–1235.e1226.
- Duckworth, A. L., Quinn, P. D., Lynam, D. R., Loeber, R., & Stouthamer-Loeber, M. (2011). Role of test motivation in intelligence testing. Proceedings of the National Academy of Sciences, 108(19), 7716–7720.
- Engvig, A., Fjell, A. M., Westlye, L. T., Moberget, T., Sundseth, O., Larsen, V. A., & Walhovd, K. B. (2010). Effects of memory training on cortical thickness in the elderly. NeuroImage, 52(4), 1667–1676.
- Erickson, K. I., Gildengers, A. G., & Butters, M. A. (2013). Physical activity and brain plasticity in late adulthood. Dialogues in Clinical Neuroscience, 15(1), 99–108.
- Erickson, K. I., Weinstein, A. M., & Lopez, O. L. (2012). Physical activity, brain plasticity, and Alzheimer’s disease. Archives of Medical Research, 43(8), 615–621.
- Farrer, L. A., Cupples, L. A., Haines, J. L., Hyman, B., Kukull, W. A., Mayeux, R., … van Duijn, C. M. (1997). Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer disease meta analysis consortium. Jama, 278(16), 1349–1356.
- Feng, W., Yokoyama, J. S., Yu, S., Chen, Y., Cheng, Y., Bonham, L. W., … Li, C. (2015). APOE Genotype affects cognitive training response in healthy Shanghai community-dwelling elderly individuals. Journal of Alzheimer’s Disease: JAD, 47(4), 1035–1046.
- Fjell, A. M., & Walhovd, K. B. (2010). Structural brain changes in aging: Courses, causes and cognitive consequences. Reviews in the Neurosciences, 21(3), 187–221.
- Folstein, M. F., Folstein, S. E., & McHugh, P. R. (1975). Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–198.
- Fountoulakis, K. N, Tsolaki, M, Chantzi, H, & Kazis, A. (2000). Mini mental state examination (mmse): A validation study in greece. American Journal of Alzheimer's Disease, 15(6), 342–345. doi: 10.1177/153331750001500604
- Gow, A. J., Johnson, W., Pattie, A., Brett, C. E., Roberts, B., Starr, J. M., & Deary, I. J. (2011). Stability and change in intelligence from age 11 to ages 70, 79, and 87: The Lothian Birth Cohorts of 1921 and 1936. Psychology and Aging, 26(1), 232–240.
- Hagstromer, M., Oja, P., & Sjostrom, M. (2006). The International Physical Activity Questionnaire (IPAQ): A study of concurrent and construct validity. Public Health Nutrition, 9(6), 755–762.
- Herlitz, A., Nilsson, L.-G., & Bäckman, L. (1997). Gender differences in episodic memory. Memory & Cognition, 25(6), 801–811.
- Huang, T. L. (2010). Omega-3 fatty acids, cognitive decline, and alzheimer's disease: A critical review and evaluation of the literature. Journal of Alzheimer's Disease, 21(3), 673–690. doi: 10.3233/jad-2010-090934
- Hui, L., DelMonte, T., & Ranade, K. (2008). Genotyping using the TaqMan assay. Current Protocols in Human Genetics Chapter 56(1): 2.10.11-12.10.18.
- Johnson, W., Deary, I. J., & Iacono, W. G. (2009). Genetic and environmental transactions underlying educational attainment. Intelligence, 37(5), 466–478.
- Josefsson, M., de Luna, X., Pudas, S., Nilsson, L. G., & Nyberg, L. (2012). Genetic and lifestyle predictors of 15-year longitudinal change in episodic memory. Journal of the American Geriatrics Society, 60(12), 2308–2312.
- Kivipelto, M., Ngandu, T., Fratiglioni, L., Viitanen, M., Kareholt, I., Winblad, B., … Nissinen, A. (2005). Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Archives of Neurology, 62(10), 1556–1560.
- Kliegl, R., Smith, J., & Baltes, P. B. (1990). On the locus and process of magnification of age differences during mnemonic training. Developmental Psychology, 26(6), 894–904.
- Koch, W., Ehrenhaft, A., Griesser, K., Pfeufer, A., Müller, J., Schömig, A., & Kastrati, A. (2002). TaqMan systems for genotyping of disease-related polymorphisms present in the gene encoding apolipoprotein E. Clinical Chemistry And Laboratory Medicine : Cclm / FESCC, 40(11), 1123–1131.
- Lerche, S., Gutfreund, A., Brockmann, K., Hobert, M. A., Wurster, I., Sünkel, U., … Berg, D. (2018). Effect of physical activity on cognitive flexibility, depression and RBD in healthy elderly. Clinical Neurology and Neurosurgery, 165, 88–93.
- Livak, K. J. (1999). Allelic discrimination using fluorogenic probes and the 5’ nuclease assay. Genetic Analysis: Biomolecular Engineering, 14(5–6), 143–149.
- Livingston, G., Sommerlad, A., Orgeta, V., Costafreda, S. G., Huntley, J., Ames, D., … Mukadam, N. (2017). Dementia prevention, intervention, and care. The Lancet, 390(10113), 2673–2734.
- Lopez-Higes, R., Rodriguez-Rojo, I. C., Prados, J. M., Montejo, P., Del-Rio, D., Delgado-Losada, M. L., … Barabash, A. (2017). APOE epsilon4 modulation of training outcomes in several cognitive domains in a sample of cognitively intact older adults. Journal of Alzheimer’s Disease: JAD, 58(4), 1201–1215.
- Lövdén, M., Brehmer, Y., Li, S. C., & Lindenberger, U. (2012). Training-induced compensation versus magnification of individual differences in memory performance. Frontiers in Human Neuroscience, 6, 141.
- Lovden, M., Schaefer, S., Noack, H., Bodammer, N. C., Kuhn, S., Heinze, H. J., … Lindenberger, U. (2012). Spatial navigation training protects the hippocampus against age-related changes during early and late adulthood. Neurobiology of Aging, 33(3), 620.e629-620.e622.
- Lucotte, G., Loirat, F., & Hazout, S. (1997). Pattern of gradient of apolipoprotein E allele *4 frequencies in western Europe. Human Biology, 69(2), 253–262.
- Lustig, C., Shah, P., Seidler, R., & Reuter-Lorenz, P. A. (2009). Aging, training, and the brain: A review and future directions. Neuropsychology Review, 19(4), 504–522.
- Lyketsos, C. G., Chen, L.-S., & Anthony, J. C. (1999). Cognitive decline in adulthood: An 11.5-year follow-up of the Baltimore epidemiologic catchment area study. American Journal of Psychiatry, 156(1), 58–65.
- Markant, J., & Amso, D. (2014). Leveling the playing field: Attention mitigates the effects of intelligence on memory. Cognition, 131(2), 195–204.
- McGurn, B., Deary, I. J., & Starr, J. M. (2008). Childhood cognitive ability and risk of late-onset Alzheimer and vascular dementia. Neurology, 71(14), 1051–1056.
- Mielke, M. M., Zandi, P. P., Sjogren, M., Gustafson, D., Ostling, S., Steen, B., & Skoog, I. (2005a). High total cholesterol levels in late life associated with a reduced risk of dementia. Neurology, 64(10), 1689–1695.
- Mielke, M. M., Zandi, P. P., Sjögren, M., Gustafson, D., Östling, S., Steen, B., & Skoog, I. (2005b). High total cholesterol levels in late life associated with a reduced risk of dementia. Neurology, 64(10), 1689–1695.
- Nagai, M., Hoshide, S., Ishikawa, J., Shimada, K., & Kario, K. (2008). Ambulatory blood pressure as an independent determinant of brain atrophy and cognitive function in elderly hypertension. Journal of Hypertension, 26(8), 1636–1641.
- Ngandu, T., Lehtisalo, J., Solomon, A., Levälahti, E., Ahtiluoto, S., Antikainen, R., … Kivipelto, M. (2015). A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomised controlled trial. The Lancet, 385(9984), 2255–2263.
- Nyberg, L., Lövdén, M., Riklund, K., Lindenberger, U., & Bäckman, L. (2012). Memory aging and brain maintenance. Trends in Cognitive Sciences, 16(5), 292–305.
- Nyberg, L., Sandblom, J., Jones, S., Neely, A. S., Petersson, K. M., Ingvar, M., & Backman, L. (2003). Neural correlates of training-related memory improvement in adulthood and aging. Proceedings of the National Academy of Sciences of the United States of America, 100(23), 13728–13733.
- Podcasy, J. L., & Epperson, C. N. (2016). Considering sex and gender in Alzheimer disease and other dementias. Dialogues in Clinical Neuroscience, 18(4), 437–446.
- Polito, L., Abbondanza, S., Vaccaro, R., Valle, E., Davin, A., Degrate, A., … Guaita, A. (2015). Cognitive stimulation in cognitively impaired individuals and cognitively healthy individuals with a family history of dementia: Short-term results from the “Allena-Mente” randomized controlled trial. International Journal of Geriatric Psychiatry, 30(6), 631–638.
- Profenno, L. A., Porsteinsson, A. P., & Faraone, S. V. (2010). Meta-analysis of Alzheimer’s disease risk with obesity diabetes, and related disorders. Biological Psychiatry, 67(6), 505–512.
- Rapport, L. J., Axelrod, B. N., Theisen, M. E., Brines, D. B., Kalechstein, A. D., & Ricker, J. H. (1997). Relationship of IQ to verbal learning and memory: Test and retest. Journal of Clinical and Experimental Neuropsychology, 19(5), 655–666.
- Ratcliff, R., Thapar, A., & McKoon, G. (2011). Effects of aging and IQ on item and associative memory. Journal of Experimental Psychology. General, 140(3), 464–487.
- Raz, N., & Rodrigue, K. M. (2006). Differential aging of the brain: Patterns, cognitive correlates and modifiers. Neuroscience and Biobehavioral Reviews, 30(6), 730–748.
- Raz, N., Rodrigue, K. M., Kennedy, K. M., & Acker, J. D. (2007). Vascular health and longitudinal changes in brain and cognition in middle-aged and older adults. Neuropsychology, 21(2), 149–157.
- Runge, S. K., Small, B. J., McFall, G. P., & Dixon, R. A. (2014). APOE moderates the association between lifestyle activities and cognitive performance: Evidence of genetic plasticity in aging. Journal of the International Neuropsychological Society: JINS, 20(5), 478–486.
- Salthouse, T. A. (2003). Memory aging from 18 to 80. Alzheimer Disease and Associated Disorders, 17(3), 162–167.
- Schulz, K, & Grimes, D. (2002). Blinding in randomised trials: Hiding who got what. The Lancet, 359, 696–700. doi: 10.1016/S0140-6736(02)07816-9
- Solomon, A., Kivipelto, M., Wolozin, B., Zhou, J., & Whitmer, R. A. (2009). Midlife serum cholesterol and increased risk of Alzheimer’s and vascular dementia three decades later. Dementia and Geriatric Cognitive Disorders, 28(1), 75–80.
- Solomon, A., Turunen, H., Ngandu, T., Peltonen, M., Levalahti, E., Helisalmi, S., … Kivipelto, M. (2018). Effect of the apolipoprotein E genotype on cognitive change during a multidomain lifestyle intervention: A subgroup analysis of a randomized clinical trial. JAMA Neurology, 75, 462.
- Titova, O. E., Sjogren, P., Brooks, S. J., Kullberg, J., Ax, E., Kilander, L., … Benedict, C. (2013). Dietary intake of eicosapentaenoic and docosahexaenoic acids is linked to gray matter volume and cognitive function in elderly. Age (Dordrecht Netherlands), 35(4), 1495–1505.
- Tulving, E., & Thomson, D. M. (1973). Encoding specificity and retrieval processes in episodic memory. Psychological Review, 80(5), 352–373.
- Tuminello, E. R., & Han, S. D. (2011). The apolipoprotein e antagonistic pleiotropy hypothesis: review and recommendations. Int J Alzheimers Dis, 2011, 726197. doi: 10.4061/2011/726197.
- Walhovd, K. B., Krogsrud, S. K., Amlien, I. K., Bartsch, H., Bjørnerud, A., Due-Tønnessen, P., … Fjell, A. M. (2016). Neurodevelopmental origins of lifespan changes in brain and cognition. Proceedings of the National Academy of Sciences, 113(33), 9357–9362.
- Walhovd, K. B., Storsve, A. B., Westlye, L. T., Drevon, C. A., & Fjell, A. M. (2014). Blood markers of fatty acids and vitamin D, cardiovascular measures, body mass index, and physical activity relate to longitudinal cortical thinning in normal aging. Neurobiology of Aging, 35(5), 1055–1064.
- Wechsler, D. (1999). Wechsler abbreviated scale of intelligence. San Antonio, TX: The Psychological Corporation.
- Zehnder, A. E., Blasi, S., Berres, M., Monsch, A. U., Stahelin, H. B., & Spiegel, R. (2009). Impact of APOE status on cognitive maintenance in healthy elderly persons. International Journal of Geriatric Psychiatry, 24(2), 132–141.