ABSTRACT
Research studies exploring the association of cognitive complaints with objectively assessed cognitive decline report inconsistent results. However, many of these have methodological limitations. We investigated whether 1) more severe subjective cognitive decline (SCD) and subjective memory decline (SMD) predict change in objectively assessed global cognition, remote memory, recent memory, learning; 2) the predictive value of more severe SMD over change in objectively assessed remote memory, recent memory, and learning is stronger for individuals that report an SMD that started within the past five years than for those that report an SMD that started five or more years previously and/or stronger for those that experienced SMD within the past two years than for those who had not; and 3) greater depression and anxiety are associated with more severe SCD and SMD. We used two-year longitudinal data from the CFAS-Wales study (N = 1,531; mean (SD) age = 73.0 (6.0) years). We fitted linear regression models. More severe SCD and SMD did not predict change in objectively assessed global cognition, remote memory, and recent memory but predicted lower scores in learning. The prediction of SMD over change in learning was not stronger when individuals reported an SMD that started within the past five years compared to when they reported an SMD that started five or more years previously nor when individuals reported an SMD that started within the past two years than those who did not. Greater depression and anxiety were associated with more severe SCD and SMD. More severe SMD may be useful for predicting lower learning ability and for identifying individuals experiencing depression and anxiety.
It is estimated that 50 million people are living with dementia (World Health Organization, Citation2020). Given the negative impact that dementia has on the life of the individual, the social system, and the economy (World Health Organization, Citation2020), identifying strategies to prevent or delay cognitive decline and dementia is a global priority (Anstey, Citation2013). Early detection of cognitive decline provides an opportunity to implement interventions aiming to prevent or delay progression (Rakesh et al., Citation2017). Individuals who experience subtle cognitive decline may to some extent be aware of the changes experienced (Clare et al., Citation2010; Montejo et al., Citation2012). Assessing self-evaluations of cognitive decline may, therefore, be a way of identifying those individuals most likely to experience actual early cognitive decline (Reisberg et al., Citation2008; Slot et al., Citation2018). The term subjective cognitive decline (SCD) refers to the self-experienced worsening of cognitive functioning in the context of scores on neuropsychological tests that lie within normal limits (Jessen et al., Citation2014; Molinuevo et al., Citation2017; Tandetnik et al., Citation2015). It has been estimated that one-quarter of individuals aged over 60 report SCD (Roehr et al., Citation2020).
Research studies exploring the association of SCD with objectively assessed cognitive decline report inconsistent results. On the one hand, some studies found that individuals reporting SCD tend to experience greater objective cognitive decline and are more likely to develop pathological cognitive impairment such as mild cognitive decline (MCI) and dementia (Amariglio et al., Citation2012; Koppara et al., Citation2015; Peter et al., Citation2014; Reisberg et al., Citation2008; Rönnlund et al., Citation2015). Self-evaluations of cognitive decline can even be a stronger predictor of future levels of cognition than the assessment of current objective cognition (Amariglio et al., Citation2012; Rönnlund et al., Citation2015). This may be due to objective cognitive tests providing information about current cognitive ability only. In contrast, when individuals rate their cognitive ability, they may take into consideration their previous levels of cognitive ability and their own experience of trajectory of cognitive decline. In addition, those older individuals who are highly educated and/or whose occupation was cognitively demanding can perform within normal limits on many cognitive tests even though they may experience early cognitive decline (Grønkjær et al., Citation2019; Palmer et al., Citation1998; Van Oijen et al., Citation2007).
On the other hand, there are some studies that did not find an association between the presence of SCD and poorer objectively assessed cognition (Buckley et al., Citation2013; Hertzog et al., Citation2018). Discrepancy in the results of existing research may be due to SCD not having been measured in the same way across studies (Rabin et al., Citation2015; Tandetnik et al., Citation2015). The strength of the association of SCD and objective cognition varies depending on several characteristics of questionnaire items including format, phrasing, scaling, and reference points (Molinuevo et al., Citation2017). Regarding reference points, accumulating evidence suggests that SCD is most closely linked to objective cognitive performance and to the presence of dementia biomarkers when individuals are asked to compare their performance with that of their peers (Chapman et al., Citation2021; Perrotin, Mormino, Madison, Hayenga & Jagust, 2012; Tandetnik et al., Citation2015).
Most studies on this topic assessed SCD by adopting a categorical approach (presence versus absence of SCD) (Rabin et al., Citation2015), whereas few studies used a continuous approach (Amariglio et al., Citation2012). The use of categorical approaches may result in bias as there is no agreement regarding thresholds to determine when an individual has “significant” SCD. Moreover, continuous approaches make better use of the data than categorical approaches as they consider the whole range of scores. Continuous approaches are, therefore, preferred when the intent is to quantify the frequency and severity of SCD (Molinuevo et al., Citation2017). In sum, continuous approaches may make it possible to more accurately assess the association of more severe SCD with objectively assessed cognition.
Many studies investigated the association of SCD with objective cognition by focusing solely on the assessment of memory (Buckley et al., Citation2013; Hertzog et al., Citation2018); this approach may be too restrictive as some individuals may perceive a decline in multiple domains of their cognition. Indeed, compared to healthy controls without SCD, individuals with SCD show more decrements, not only in memory, but also in other cognitive domains including attention and decision-making (Smart & Krawitz, Citation2015; Smart et al., Citation2014). In addition, although some subtypes of MCI (e.g., amnestic MCI) and dementia (e.g., Alzheimer’s disease) are characterized by a memory impairment, other subtypes of MCI (e.g., non-amnestic MCI) and dementia (e.g., some cases of vascular dementia) are not (Kramer et al., Citation2003; Pusswald et al., Citation2013; Scheltens et al., Citation2016; Smits et al., Citation2015). Hence, it is sensible to assess perceived decline in global cognition and to explore it in association with scores on neuropsychological tests that assess global cognition. This would make it possible to investigate whether those who perceive a decline in multiple cognitive domains are more likely to experience objective cognitive decline or a more severe objective cognitive decline than those who perceive a decline only in one domain.
Finally, whereas some studies explored whether SCD predicts current cognitive functioning, others explored the predictive role of SCD for future cognitive functioning (Jessen et al., Citation2014; Jonker et al., Citation2000; Mendonça et al., Citation2016; Sabatini et al., Citation2021). Exploring whether SCD predicts future objective cognitive decline may be the preferred approach, especially with respect to testing the potential use of SCD to identify those individuals at higher risk of cognitive decline. With the intention to more accurately identify individuals with SCD who are at an increased risk of experiencing objective cognitive decline, the Subjective Cognitive Decline-Initiative (SCD-I) working group was created and produced the “SCD-Plus criteria” (See Jessen et al., Citation2014 for a complete list of the SCD-Plus criteria). One of the SCD-Plus criteria states that those individuals who perceive a decline in their memory are more likely to experience objective cognitive decline compared to those who report a perceived decline in other cognitive domains. The first aim of the current study is therefore to test whether among cognitively healthy individuals aged over 65 more severe subjective decline in global cognition (in this paper referred to as SCD) and subjective decline in memory (in this paper referred to as subjective memory decline; SMD) are, respectively, predictors of the occurrence of more severe decline in objectively assessed global cognition and memory.
Another SCD-Plus criterion states that those individuals who report an SMD that has started within the last five years experience a greater cognitive decline compared to those who report an SMD lasting for longer than five years. This is because when complaints persist without progressing to objective impairment, it may be less likely that they reflect underlying preclinical AD or other neurodegenerative dementias (Molinuevo et al., Citation2017). As further examination of the “SCD-Plus criteria” is warranted (Jessen et al., Citation2014), the second aim of this study is to test whether the predictive value of SMD over change in objectively assessed memory is stronger: 1) for those individuals reporting an SMD that has started within the past five years compared to those reporting an SMD that started five or more years ago; and 2) for those individuals experiencing SMD within the past two years compared to those who had not.
The small or non-significant associations of SCD and/or SMD with objectively assessed cognitive decline reported in existing studies may be due to SCD and SMD being influenced by psychosocial variables such as the presence of depression and anxiety (Chapman et al., Citation2019; Crane et al., Citation2007; Hill et al., Citation2016; Lubitz et al., Citation2018; Mendonça et al., Citation2016; Montejo et al., Citation2011; Schmidtke et al., Citation2008; Siebert et al., Citation2020; Yates et al., Citation2015; Zlatar et al., Citation2018). Indeed, some studies found that individuals experiencing symptoms of depression and/or anxiety often report SCD and SMD that is not objectively measurable (Hill et al., Citation2016; Siebert et al., Citation2020). Depression and anxiety are risk factors for cognitive decline (Anstey, Citation2013; Heser et al., Citation2013; Pietrzak et al., Citation2012; Rakesh et al., Citation2017; De Vito et al., Citation2019) and mood changes and the subsequent SCD may reflect early neurodegenerative changes that precede cognitive decline (Anstey, Citation2013). However, the associations of depression and anxiety with SCD and SMD may also be due to the experience and perception of cognitive failures leading to clinical features of depression and anxiety such as hopelessness and worry about the future (Abramson et al., Citation1989). A third objective of this study is, therefore, to explore whether the presence of baseline depression and anxiety are associated with baseline levels of SCD and SMD.
In summary, we hypothesized first that more severe baseline SCD and SMD predict two-year change in global cognition and memory, and that baseline SMD is a stronger predictor of two-year change in global cognition and memory compared to baseline SCD. Second, we hypothesized that the predictive role of more severe baseline SMD over objectively assessed two-year change in memory is stronger for individuals who have been experiencing an SMD that started within the past five years compared to those who had experienced an SMD that started five or more years ago and/or experienced an SMD within the past two years compared to those who had not. Finally, we expect more severe baseline depression and/or anxiety to be associated with more severe baseline SCD and SMD.
Method
Study design and participants
This study is a longitudinal analysis of secondary data from the Cognitive Function & Aging Study (CFAS-Wales Study; http://www.cfas.ac.uk/cfas-wales/). The CFAS-Wales Study investigated the physical and cognitive health of people aged 65 and over and the environmental factors that may influence activity and participation in community life. The CFAS-Wales study obtained ethical approval from the NHS North Wales-West Research Ethics Committee (REC Ref No: 10/WNo01/37; IRAS Project No: 40,092). In the CFAS-Wales study, potential participants living in two areas of Wales and aged 65 and over were randomly selected from general practice registers, stratified by age group to ensure equal numbers in the 65 to 74 years and 75 years and over age groups. Selected participants provided written informed consent to take part in the CFAS-Wales study.
In the CFAS-Wales study, local interviewers were recruited from a range of backgrounds (those working with older people in the voluntary, health-care, and social-care sectors), and given identical training (overseen by one investigator) to deliver standardized computer-assisted in-depth interviews. This training consisted of an intense week-long course, with follow-up practice until each interviewer achieved the necessary quality standards, with ongoing quality control. Baseline data were collected between 2011 and 2013 and follow-up data were collected between 2013 and 2015.
To reduce the possibility of reverse causation, individuals identified as potentially having cognitive impairment at baseline were excluded from the current analyses. Hence, from the CFAS-Wales Study baseline sample (N = 3,593), 737 people were excluded from analyses of the current study as they met study criteria for a dementia diagnosis (Copeland et al., Citation1986) or had a Mini-Mental State Examination (Folstein et al., Citation1975) score below 25 indicating cognitive impairment. The CFAS-Wales study used an algorithmic approach to diagnosis of dementia to provide consistency across area and time, eliminating the variability that has been shown with clinical diagnosis. A diagnosis of dementia was indicated with a score of three or more on the Automated Geriatric Examination for Computer Assisted Taxonomy designed for use with the Geriatric Mental State Schedule (GMS-AGECAT). Change diagnoses of dementia made with the AGECAT have been shown to be as reliable as those made by a range of clinicians and the GMS-AGECAT has been validated against clinical diagnoses of dementia made according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders (DSM) III R (Matthews et al., Citation2013).
In addition, we excluded from the current analyses 580 participants as at baseline they scored 1 standard deviation (SD) below the mean sample score on three or more cognitive subtests of the Cambridge Cognitive Examination (CAMCOG; Huppert et al., Citation1995; Williams et al., Citation2003) or on two subtests assessing the same cognitive domain (e.g., remote and recent memory), indicating possible MCI (Bondi et al., Citation2014). Moreover, 90 participants were excluded as they had missing data at baseline, resulting in a baseline sample of 2,186 participants. Finally, 655 participants were excluded from the current analyses as they had missing data on the variables of interest at follow-up. This resulted in a sample of 1,531 participants for the current analyses.
Measures
Global cognition and memory – objective assessments
The CAMCOG (Huppert et al., Citation1995; Williams et al., Citation2003) was used to assess cognitive functioning. The CAMCOG is a useful indicator of objective cognition as it can differentiate between pathological cognitive impairment and age-related cognitive decline; even among individuals with high pre-morbid ability (Hobson & Meara, Citation1998; Huppert et al., Citation1995). It consists of 67 items organized in 10 subscales assessing orientation (with possible scoring range 0–10), language comprehension (0–9), language expression (0–21), remote memory (0–6), recent memory (0–4), learning (0–17), attention/calculation (0–9), praxis (0–12), abstract thinking (0–8), and perception (0–10). The total score (0–106) is calculated as the sum of subscale scores; higher scores indicate better cognitive functioning. In the current study, we used the total score on the CAMCOG as an indicator of global cognition and the scores on the subscales for remote memory, recent memory, and learning as indicators of memory.
In the remote memory task individuals are asked to recall people who were well-known in the past, but not much discussed in the present time (e.g., “What was Mae West famous for?”; “What was Edmund Hilary famous for?”). Hence, the term “remote memory” used in the current paper is reflective of the CAMCOG remote memory domain and not necessarily reflective of remote memory as it is commonly used in the literature (e.g., to describe remote autobiographical or episodic memory). In the recent memory task, participants are asked to remember the names of people who are often in the news (e.g., “What is the name of the prime minister?”). The learning task requires participants to recall and recognize verbal, non-verbal, and pictorial information learned both incidentally and intentionally during the administration of the CAMCOG. The total score on the CAMCOG has excellent internal consistency and test–retest reliability and the 10 subscales have acceptable reliability (Huppert et al., Citation1996).
Global cognition and memory – subjective evaluations
Some items of the Geriatric Mental State Examination (GMSE; Copeland et al., Citation1986; McWilliam et al., Citation1988), which is a standardized psychiatric interview for older people, were used to assess cognitive difficulties. Each item is dichotomous indicating whether the participant perceived or did not perceive a decline in the specific cognitive ability depicted in the item. Eight items were used to assess SCD (see Supplementary Table S1 for a full list of items). Seven items were used to assess SMD (see Supplementary Table S2 for the full list of items). To calculate the severity of SCD and SMD, we summed the single item scores to form a total score for SCD (0–8) and for SMD (0–7); higher scores indicate higher levels of perceived decline either in global cognition or memory. In the current sample Cronbach’s α was 0.57 for SCD and 0.61 for SMD. To assess the length of experience of SMD, we used two single-item questions. The first one (“When did you first notice this beginning?”) was asked after question “Do you have to make more effort to remember things than you used to?” and therefore it was asked only to those individuals who reported difficulty in remembering things. The item was analyzed as a dichotomous variable indicating whether the participant experienced an SMD starting within the past 5 years (score of 2) or more than 5 years ago (score of 1). The second question (“Would you say there has been a change in your memory over the last two years?”) was categorical and asked to everyone. Response options 0 = no change; 1 = better; 2 = worse; 3 = much worse.
Mood
Depression and anxiety were assessed with the Geriatric Mental State Automated Geriatric Examination for Computer Assisted Taxonomy (AGECAT; Copeland et al., Citation1986), which is a semi-structured standardized psychiatric interview validated against the DSM III R (American Psychiatric Association, Citation1980; Matthews et al., Citation2013). For both the depression and the anxiety subscales scores can range from 0 to 2. A score of zero indicates no clinical level of depression or anxiety; a score of one indicates mild depression or anxiety; and a score of two indicates a case-level of depression or anxiety.
Covariates
The demographic variables age, sex, and education (number of years spent in full-time education) were used as covariates, together with co-morbidity. To assess the presence and number of health conditions, participants were asked to indicate whether they had been diagnosed with any of the 20 listed health conditions (e.g., high/low blood pressure or cancer). Questions were mapped onto the Charlson Comorbidity Index (Charlson et al., Citation2008, Citation1987); in line with the Charlson Comorbidity Index, a summary score (0–22) was created by scoring each positive answer as one, except for cancer which was scored two or three depending on whether it was past or present. Higher scores reflect higher levels of illness.
Data-analytical design
To calculate participants’ two-year change in objective global cognition, we subtracted the CAMCOG two-year follow-up score from the CAMCOG score at baseline. To calculate participants’ two-year change in objective global cognition without memory, we subtracted the CAMCOG two-year follow-up score without the three memory tasks from the CAMCOG score at baseline without the three memory tasks. To calculate participants’ two-year change in remote memory, recent memory, and learning, we subtracted the two-year follow-up score from the score at baseline on the respective subscales. Hence, positive scores indicate objective cognitive decline.
To test our hypotheses that more severe SCD and SMD would have predicted greater two-year change in global cognition and memory, we fitted linear regressions with either baseline severity of SCD or SMD as the predictor and either two-year change in global cognition (both global cognition and global cognition without memory tasks) or memory (remote memory, recent memory, and learning) as the outcome. For each regression, we tested a crude model and a second model where we adjusted for demographic characteristics (age, sex, and education) and co-morbidity.
Moreover, as suggested by Clifton and Clifton (Citation2019), for each regression, we also tested a model with either baseline SMD or SCD as the predictor, two-year change in global cognition, global cognition without memory, remote memory, recent memory, or learning as the outcome, and baseline cognitive score on a specific task as a covariate. However, these follow-up analyses were only conducted for variables that were significant in the main analyses. Follow-up analyses were conducted using the linear regressions outlined above to investigate an alternative CAMCOG global cognition score that removed the memory items (remote memory, recent memory, and learning). We did this in order to explore whether SMD predicts cognitive abilities that do not rely on memory.
The hypothesis that the predictive role of more severe baseline SMD over greater objectively assessed two-year change in memory is stronger among individuals reporting an SMD that started within the past five years compared to those experiencing an SMD that has lasted for five years or longer was tested in a subsample of participants who reported SMD (N = 788). This is because this question was asked only to those participants who reported experiencing some level of memory decline but was skipped for those who did not report SMD. The hypothesis that the predictive role of more severe baseline SMD over greater objectively assessed two-year change in memory is stronger among individuals who have been experiencing an SMD within the past two years compared to those who had not was tested in the whole sample of participants. We fitted linear regression models containing a term for the interaction between the predictors length of time experiencing SMD (either within five years or within two years) and the severity of baseline SMD.
To test whether baseline depression and anxiety are associated with baseline levels of SCD and SMD, we fit linear regression models with either baseline depression or anxiety as the predictor variable and either baseline SCD or SMD as the outcome variable.
Standardized regression coefficients are reported to indicate the effect size. Coefficients ≤0.09 were considered negligible, 0.10–0.29 small, 0.30–0.49 moderate, and ≥0.50 large (Cohen, Citation1988). We conducted analyses using the STATA version 16 (StataCorp, Citation2017).
Results
Descriptive data
The sample comprised 1,531 participants, having a mean age of 73.0 years (SD = 6.0; range: 65 to 97) and mean 12.3 years of education; 50.2% were women. At baseline, 690 (45.1%) participants perceived cognitive difficulties in at least one of the eight items assessing SCD; 788 (51.5%) participants perceived cognitive difficulties in at least one of the seven items assessing SMD; 500 (32.7%) participants perceived both SCD and SMD. Of those who reported experiencing SMD (N = 788), 145 (18.4%) experienced an SMD that started within five years. Out of the study sample, 1,129 (74.0%) perceived no change in their memory over the last two years, 23 (1.5%) participants perceived an increase in their memory ability over the last two years, 370 (24.3%) and 4 (0.3%) participants, respectively, perceived that their memory got worse and much worse over the past two years. At baseline participants’ mean (SD) score on the CAMCOG and on the CAMCOG without the three subtasks assessing memory were 95.0 (4.1) and 72.2 (3.6), respectively, indicating intact global cognition (De Koning et al., Citation1998). At baseline participants’ mean (SD) scores on the remote memory, recent memory, and learning subscales of the CAMCOG were 4.9 (0.9), 3.9 (0.3), and 13.9 (1.5), respectively.
Participants’ two-year mean (SD; range) change in global cognition was 0.7 (4.6; range: −14 to 24), in global cognition without the three tests assessing memory was 0.6 (3.9; range: −20 to 19), in remote memory was 0.05 (0.83; −3 to 4), in recent memory was 0.02 (0.4; range: −2 to 3), and in learning was −0.1 (1.7; range: −8 to 8); hence, over two years participants’ scores on global cognition, remote and recent memory slightly lowered whereas scores on learning slightly increased. Most participants did not experience depression at baseline (77.8% of participants) nor at follow-up (78.5% of participants). Similarly, most participants did not experience anxiety at baseline (95.2% of participants) nor at follow-up (96.6% of participants). Hence, over the study period the percentage of depression and anxiety experienced by participants remained stable. More information about descriptive statistics for the study sample can be found in (). We report descriptive statistics for the 65 to 74 years and 75 years and over age groups. The two age groups have similar demographic characteristics and scores in health-related measures and in cognitive change.
Table 1. Descriptive statistics of demographic variables and main study variables for the study sample.
The predictive role of severity of baseline SCD and SMD for two-year change in cognition
Results from simple and multiple regressions with either severity of baseline SCD or SMD as predictor of two-year change in cognition are reported in (). More severe baseline SCD was not a significant predictor of two-year change in global cognition, global cognition without memory, remote memory, and recent memory. More severe baseline SCD predicted two-year change in learning both before and after adjusting for covariates. However, the amount of variance explained by the severity of the baseline SCD over two-year change in learning was small.
Table 2. Linear regression analyses with baseline subjective cognitive decline as the predictor and two-year change in global cognition, global cognition without memory, remote memory, recent memory, or learning as the outcome: Unstandardized (B) and standardized (β) regression coefficients.
Table 3. Linear regression analyses with subjective memory decline as the predictor and two-year change in global cognition, global cognition without memory, remote memory, recent memory, or learning as the outcome: Unstandardized (B) and standardized (β) regression coefficients.
More severe baseline SMD was not a significant predictor of two-year change in global cognition, global cognition without memory, remote memory, and recent memory. More severe baseline SMD predicted two-year change in learning both before and after adjusting for covariates. Again, the amount of variance explained by the severity of the baseline SMD for two-year change in learning was small. () shows the predictive value of baseline SCD and SMD, respectively, over a two-year change in learning.
Figure 1. a) Severity of baseline subjective cognitive decline (SCD) as predictor of two-year change in learning. Positive values are indicative of worse performance over time. R2 = 0.04%. p = .01. b) Severity of baseline subjective memory decline (SMD) as predictor of two-year change in learning. Positive values are indicative of worse performance over time. R2 = 1%. p < .001.
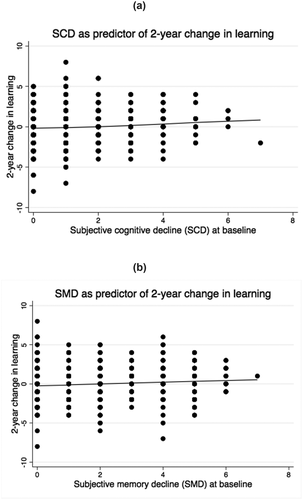
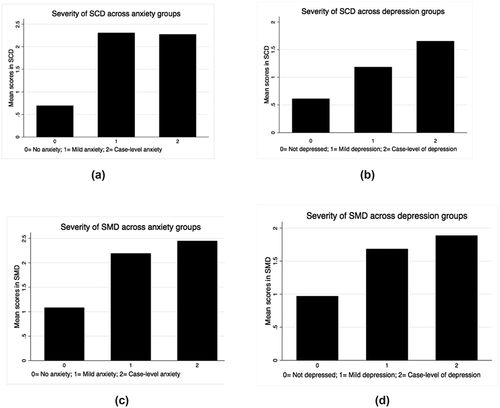
Figure 2. a) Baseline mean level of subjective cognitive decline (SCD) across individuals with no anxiety, mild anxiety, and case-level anxiety. Levels of SCD were significantly different between individuals without anxiety and individuals with mild anxiety (p < .001) and between individuals without anxiety and individuals with case-level of anxiety (p < .001). b) Baseline mean level of SCD across individuals with no depression, mild depression, and case-level depression. Levels of SCD were significantly different between individuals without depression and individuals with mild depression (p < .001), between individuals with mild depression and individuals with case-level of depression (p < .001), and between individuals without depression and individuals with case-level of depression (p < .001). c) Baseline mean level of subjective memory decline (SMD) across individuals with no anxiety, mild anxiety, and case-level anxiety. Levels of SMD were significantly different between individuals without anxiety and individuals with mild anxiety (p < .001) and between individuals without anxiety and individuals with case-level of anxiety (p < .001). d) Baseline mean level of SMD across individuals with no depression, mild depression, and case-level depression. Levels of SMD were significantly different between individuals without depression and individuals with mild depression (p < .001) and between individuals without depression and individuals with case-level of depression (p < .001).
We also conducted linear regression analyses with either severity of baseline SCD or SMD as the predictor, two-year change in learning as the outcome, and baseline learning as a covariate (see Supplementary Table S3). The severity of the baseline SCD explained a small, statistically significant, amount of the variance in two-year change in learning. Similarly, the baseline SMD explained a small, statistically significant, amount of variance in two-year change in learning.
Tests of interaction showed that the predictive role of baseline SMD over two-year change in learning was not stronger when individuals reported an SMD that started within the past five years compared to when they reported an SMD that started five or more years previously (interaction p = .69) nor when individuals experienced an SMD over the past two years compared to those who had not (interaction p = .97). In sum, the length of time over which individuals have been experiencing SMD does not moderate the association between the severity of the baseline SMD and two-year change in learning.
Associations of baseline depression and anxiety with severity of baseline SCD and SMD
At baseline, individuals with case-level depression had more severe SCD than those with mild level of depression (ß = 0.24; 95% CI: 0.19 to 0.29; p < .001; R2 = 6%) and those with mild level of depression had more severe SCD compared to those without depression (ß = 0.19; 95% CI: 0.14 to 0.23; p < .001; R2 = 3%). At baseline, individuals with case-level anxiety had more severe SCD than those with mild level of anxiety (ß = 0.25; 95% CI: 0.57 to 0.68; p < .001; R2 = 6%) and those with mild level of anxiety had more severe SCD compared to those without anxiety (ß = 0.19; 95% CI: 0.14 to 0.23; p < .001; R2 = 4%).
At baseline, individuals with case-level depression had more severe SMD than those with mild level of depression (ß = 0.16; 95% CI: 0.11 to 0.20; p < .001; R2 = 2%) and those with mild level of depression had more severe SMD compared to those without depression (ß = 0.17; 95% CI: 0.12 to 0.22; p < .001; R2 = 3%). Individuals with case-level anxiety had more severe SMD than those with mild level of anxiety (ß = 0.16; 95% CI: 0.11 to 0.20; p < .001; R2 = 2%) and those with mild level of anxiety had more severe SMD compared to those without anxiety (ß = 0.10; 95% CI: 0.05 to 0.15; p < .001; R2 = 1%).
Discussion
This study tested, in a group of cognitively healthy individuals aged 65 and over, whether (1) more severe baseline SCD and SMD predict greater two-year change in global cognition and three aspects of memory (remote memory, recent memory, and learning) and whether the predictive role of more severe baseline SMD over greater two-year change in global cognition and/or memory is stronger than the predictive role of more severe baseline SCD over greater two-year change in global cognition and/or memory; (2) the predictive role of severity of baseline SMD over two-year change in learning is weaker for those individuals who have been experiencing SMD for longer time compared to those who have been experiencing SMD for less time; (3) more severe baseline depression and anxiety are associated with more severe baseline SCD and SMD. Overall, we found that more severe SCD and SMD predicted greater objective change only in learning (one aspect of memory). The length of time over which individuals have been experiencing SMD did not moderate the association between SMD and objective change in learning. More severe baseline depression and anxiety are associated with more severe baseline SCD and SMD.
In the current study, we found marginal changes in global cognition, remote memory, recent memory, but scores on learning ability increased (reflected by the negative change scores) (Beigneux et al., Citation2007; Small, Citation2001). In line with our first hypothesis, more severe baseline SMD predicted a larger two-year change in learning but not a two-year change in either remote memory or recent memory. Hence, SMD may better predict a certain type of memory change, such as learning new information, as opposed to others, as such recalling semantic information from remote or recent memory. Indeed, whereas the remote memory and recent memory tasks used in the current study rely on semantic memory, the learning task assesses the ability to encode and temporarily store information. More severe baseline SCD also predicted a broader two-year change in learning even though the amount of variance explained by the severity of SCD over a two-year change in learning was very small. Linear regression analyses with either severity of baseline SCD or SMD as the predictor, two-year change in learning as the outcome, and baseline learning as covariate showed that even after controlling for baseline learning, severity of baseline SCD or SMD remain significant predictors of variance in two-year change in learning. Given the higher risk of more extensive cognitive decline among those experiencing more severe SCD and SMD, individuals reporting more extensive SCD and/or SMD may benefit from interventions such as cognitive training programs (Buschkuehl et al., Citation2008; Clare & Woods, Citation2004; Corbett et al., Citation2015), aiming to prevent or delay the progression of cognitive decline.
Contrary to our first hypothesis, those individuals who reported more severe baseline SCD and/or SMD did not experience a greater change in global cognition, remote memory, and recent memory. Lack of support for an association between severity of SCD and SMD and objectively assessed cognition has been reported in a previous literature on the topic (Hertzog et al., Citation2018), suggesting that severity of SCD and SMD may not be informative of future decline in global cognition. Alternatively, the lack of an association for the severity of SCD and SMD with objectively assessed global cognition may be due to scores on global cognition having been obtained from the sum of participants’ performance in several cognitive subdomains. However, in older age, some cognitive abilities (e.g., attention) tend to decline more than others (e.g., knowledge) (Christensen, Citation2001; Deary et al., Citation2009; Lövdén et al., Citation2004; Park & Festini, Citation2017). Future studies could explore the individual associations of severity of SCD and SMD with objective scores on several cognitive subdomains. Some may argue that when calculating a score for objective global cognition, memory tasks should be excluded as their inclusion may lead to significant associations with severity of SCD and SMD that are not due to the presence of complaints in other cognitive domains. However, in our study, the predictive values of severity of SCD and SMD over global cognition remained the same regardless of whether or not the score for objective global cognition included the memory tasks.
Contrary to our second hypothesis, we found that the predictive role of severity of SMD over change in memory did not depend on the length of the perceived memory decline. As some empirical studies showed that SMD can have a duration of up to 15 years prior to progression to MCI (Prichep et al., Citation2006; Reisberg, Citation1986; Reisberg & Gauthier, Citation2008), the length of experience of SMD may not be useful to identify those individuals at higher risk of objective cognitive decline.
In line with our third hypothesis and with existing literature, we found that greater depression and anxiety have cross-sectional associations with more severe SCD and SMD (Heser et al., Citation2013). In older age SCD and SMD often coexist with the experience of poor mood (Cargin et al., Citation2008). Indeed, perceived cognitive dysfunction (e.g., diminished ability to think or concentrate) is a clinical symptom of, and a diagnostic criteria for, both depression and anxiety (American Psychiatric Association, Citation2013; Nordhus, Citation2008; Pietrzak et al., Citation2012). The associations of greater depression and anxiety with more severe SCD and SMD are particularly relevant given the high proportion of older individuals reporting SCD and SMD (Roehr et al., Citation2020) and the higher risk of objective physical and cognitive decline that characterizes individuals with depression, anxiety, SCD, and/or SMD (Bhalla et al., Citation2009; Bunce et al., Citation2014; Gallo et al., Citation2005; Ho et al., Citation2014; Jonker et al., Citation2000; Mendonça et al., Citation2016; Szanto et al., Citation2012).
Even though our results support the cross-sectional association of depression and anxiety with SCD and SMD, future longitudinal studies need to understand the direction of the association of SCD or SMD with poor mood. This is an essential step in order to deliver interventions that optimize the psychological health of individuals with SCD and/or SMD. On the one hand, poor mood may precede SCD and SMD; indeed, both depression and anxiety are risk factors for cognitive decline (Anstey, Citation2013; Heser et al., Citation2013; Pietrzak et al., Citation2012; Rakesh et al., Citation2017; Sachs-Ericsson et al., Citation2005; De Vito et al., Citation2019) and can reflect early neurodegenerative changes that precede cognitive decline (Anstey, Citation2013). As a consequence, targeting depressive and anxiety symptoms may help to decrease SCD and SMD. However, so far only a few studies have explored the association of mood with SCD over time (e.g., Hill et al., Citation2020). In our study, the percentage of participants that were depressed and anxious did not change from baseline to follow-up. Hence, it was not possible to explore whether and how levels of depression and anxiety are related to perceived and objective cognitive change.
On the other hand, poorer cognition and the subsequent perception of cognitive decline and fear of loss of function may predict symptoms of depression and anxiety (Hill et al., Citation2016; Kessler et al., Citation2012; Mol et al., Citation2008). If this is the case, promoting the acceptance of age-related cognitive changes (Hahn & Lachman, Citation2015; Huntley et al., Citation2017; Sabatini et al., Citation2020, Citation2020a, Citation2021), boosting positive effects, decreasing negative effects (Crane et al., Citation2007), and consultation with health professionals (Hill et al., Citation2016) may all be potential strategies to reduce SCD and SMD. Alternatively, SCD, SMD, depression and anxiety may all be caused by other factors; for instance, they may both be the consequence of cognitive decline and of neurodegenerative processes.
The association of depression and anxiety with more severe SCD and SMD that we identified may also be due to all these variables being related to levels of introspection (Roberts et al., Citation2009) and negative thoughts such as hopelessness, worry (Abramson et al., Citation1989), and anxiety about developing dementia (Kessler et al., Citation2012). Finally, the more severe SCD and SMD experienced by those with higher levels of depression and anxiety may be due to some of the items assessing severity of SCD overlapping with symptoms of depression and anxiety (Hill et al., Citation2016; Jessen et al., Citation2007). Nonetheless, future efforts could be directed toward understanding factors underlying the co-occurrence of depressive and anxiety symptoms with SCD and SMD.
As mentioned in the introduction of this paper, evidence in support of the association of SCD and/or SMD with objective cognitive decline is mixed (Buckley et al., Citation2013; Hertzog et al., Citation2018). Numerous studies have investigated the characteristics of those individuals showing a mismatch between subjective perceptions and objectively assessed cognitive decline (Blackburn et al., Citation2014). Evidence suggests that those individuals experiencing symptoms of depression and anxiety, especially those whose symptoms are severe, frequently perceive a cognitive decline that does not correspond to an objective assessment of their cognitive performance (Hill et al., Citation2016; Jessen et al., Citation2007; Siebert et al., Citation2020). Concepts such as functional memory decline (FMD) have specifically been developed to refer to perceived cognitive decline that is unlikely to be due to objective cognitive decline (Schmidtke & Metternich, Citation2009; Schmidtke et al., Citation2008) but is more likely due to a variety of psychosocial factors including poor mental health, distress, and negative attitudes toward own aging (Sabatini et al., Citation2020b; Siebert et al., Citation2020).
Nonetheless, among individuals with SCD and/or SMD, it is difficult to tease apart those who are at high and low risk of experiencing future cognitive decline, as low mood is both a risk factor and a prodromal symptom of cognitive decline and dementia (Anstey, Citation2013; Heser et al., Citation2013; Pietrzak et al., Citation2012; Rakesh et al., Citation2017; De Vito et al., Citation2019). The SCD-I working group was created in order to define guidelines, the SCD-Plus criteria (Jessen et al., Citation2014), that help to more accurately identify, among individuals with SCD, those at increased risk of experiencing objective cognitive decline. The SCD-Plus criteria state that those individuals who report a perceived decline in their memory (rather than other cognitive domains), that has started within the last five years, are aged 60 years or over, experience concerns (such as worry) associated with SCD, feel that their performance is worse compared to their peers, have the APOE ε4 genotype or biomarkers of AD, and/or their SCD is confirmed by an informant are more likely to experience objective cognitive decline compared to those who do not report these characteristics.
Our study made it possible to test some of the SCD-Plus criteria. Overall, our findings support the criterion stating that severity of SMD is a more accurate predictor of future objective cognitive change than subjective complaints about a decline in any other cognitive domain, but do not support the criterion stating that the predictive role of severity of SCD over objectively assessed cognition is stronger when the cognitive complaints have been present for less than five years. The lack of support for this criterion may, however, be related to the limitations of our study.
Strengths and limitations
First, on average, participants’ scores on global cognition and the subscales of CAMCOG assessing remote memory and recent memory were only slightly lower at follow-up compared to the baseline score. The small cognitive change experienced by participants may be due to a two-year follow-up period being insufficient to observe substantial change in cognitive performance in a sample of cognitively healthy participants. In addition, the administration of the same neuropsychological assessment both at baseline and at follow-up may have given rise to a learning effect (Goldberg et al., Citation2015) and obscured any cognitive decline experienced by participants. However, there is research suggesting that a practice effect may be normal and expected in cognitively healthy older individuals, whereas it is not present among individuals experiencing some sort of pathological cognitive decline (Duff et al., Citation2010, Citation2007).
The measures that were used in the CFAS-Wales study to assess the severity of SCD and SMD are fairly crude and may not have accurately captured perceived changes in global cognition and memory. For instance, items used in the CFAS-Wales study to assess SCD and SMD did not ask participants to consider themselves in relation to their peers; however, accumulating evidence suggests that SCD is most closely linked to objective cognitive performance when SCD is assessed through comparison with others of the same age (Tandetnik et al., Citation2015). The measures used in the current study may have captured participants’ general disposition to complain. In addition, some of the items used to assess the severity of SCD may have captured symptoms of depression rather than perceived cognitive decline (Jessen et al., Citation2007). Moreover, recent evidence suggests that there is a variability in the reports of SCD and SMD (Pearman, Citation2020; Vanderhill et al., Citation2010). Assessing SCD and SMD is, however, difficult and there is little agreement regarding the most reliable way of assessing SCD and SMD (Rabin et al., Citation2015; Tandetnik et al., Citation2015).
A further limitation of the present study is that the AGECAT algorithm used to categorize the level of depression and anxiety may not be the most effective at classifying people with less severe but more frequent depressive and anxiety symptoms, which are quite common in older age (Buchtemann et al., Citation2012; Meeks et al., Citation2011; Nordhus, Citation2008). As a consequence, the AGECAT algorithm may miss individuals with subclinical levels of depression and/or anxiety. In addition, as symptoms of depression and anxiety assume peculiar features in older people (Lenze & Wetherell, Citation2009; Morrison, Citation2008; Therrien & Hunsley, Citation2012), the questions regarding depression and anxiety in the AGECAT may not be sensitive enough to capture depression and anxiety in older people. Older people are generally reluctant to report depression and/or anxiety, as they tend to trivialize the symptoms or to regard them as a normal part of the aging process (Morrison, Citation2008).
Despite the above limitations, the study has several strengths. First, the overall sample size is large, and includes both community dwellers and people living in residential care. Moreover, the study sample included people living in both urban and rural areas. We consider these characteristics of study participants as a strength as they make the study sample more representative of the older population in Wales.
Second, the use of the CAMCOG (Huppert et al., Citation1995) to assess cognition is a strength of the current study as it is a well-established tool for use with older people, and covers a broad range of cognitive domains. This made it possible to obtain separate scores for the memory domains as well as a score for global cognitive performance, and, therefore, to examine whether both memory and global cognition, respectively, are related to subjective memory and cognitive change. Moreover, the CAMCOG was designed to detect mild degrees of cognitive impairment, so it can differentiate between pathological cognitive impairment and age-related cognitive decline (Hobson & Meara, Citation1998; Huppert et al., Citation1995). This is important as those older adults with higher education or who have had a cognitively stimulating job for most of their lives can perform well on objective cognitive tests even when they are experiencing a subtle cognitive decline (Grønkjær et al., Citation2019; Palmer et al., Citation1998; Van Oijen et al., Citation2007).
Third, another strength of this study is the assessment of both severity of SCD and SMD, whereas the majority of existing studies have assessed either SCD or SMD but not both. Assessing only SCD or SMD may leave a proportion of individuals with some sort of cognitive complaints undetected. Indeed, in the current study, some participants reported either SCD or SMD but not both. Fourth, unlike most previous evidence on the topic (Hill et al., Citation2016), to assess SCD and SMD we used a continuous approach that allowed us capture the severity of cognitive complaints (Molinuevo et al., Citation2017). Fifth, also unlike many existing studies on the topic (Hill et al., Citation2016), the current study is based on longitudinal data, which makes it possible to investigate the predictive value of SCD and SMD over objective change in global cognition and memory. However, as only two time points were used in the current study, it was not possible to determine whether the fluctuations that we found in SMD and SCD scores are normal variation or indicative of a pattern. Thus, additional research using more than two time points is needed to better clarify these longitudinal trajectories.
Conclusions
This study aimed to increase knowledge about the associations of SCD and SMD with depression, anxiety, and objectively assessed two-year change in cognition and memory by overcoming some of the limitations of existing studies on the topic. Results showed that information about SCD and SMD does not help to predict future levels of global cognition but helps to predict change in learning (one aspect of memory). Therefore, future research could focus on SCD and SMD to identify individuals at higher risk of objective decline in learning ability. However, the length of experience of SMD does not help to predict future levels of learning ability. Finally, study results suggest that those individuals with more severe SCD and SMD may be experiencing concurrent depression and anxiety. Future studies should explore variables that underlie the associations of severity of SCD and SMD with depression and anxiety.
Supplemental Material
Download MS Word (15.2 KB)Acknowledgments
We are grateful to the University of Exeter for funding a PhD scholarship for Serena Sabatini to carry out this work.
This paper represents independent research funded by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. Obi Ukoumunne was supported by the National Institute for Health Research (NIHR) Applied Research Collaboration (ARC) South West Peninsula. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Availability of data and materials
This study was conducted using secondary data from CFAS-Wales. The data is publicly available and can be accessed through the UK data archive http://doi.org/10.5255/UKDA-SN-8281-1
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Abramson, L. Y., Metalsky, G. I., & Alloy, L. B. (1989). Hopelessness depression: A theory-based subtype of depression. Psychological Review, 96(2), 358–372. https://doi.org/10.1037/0033-295X.96.2.358
- Amariglio, R. E., Becker, J. A., Carmasin, J., Wadsworth, L. P., Lorius, N., Sullivan, C., Maye, J. E., Gidicsin, C., Pepin, L. C., Sperling, R. A., Johnson, K. A., & Rentz, D. M. (2012). Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia, 50(12), 2880–2886. https://doi.org/10.1016/j.neuropsychologia.2012.08.011
- American Psychiatric Association. (1980). Diagnostic and statistical manual of mental disorders (3rd ed.).
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (DSM-5®).
- Anstey, K. J. (2013). Optimizing cognitive development over the life course and preventing cognitive decline: Introducing the Cognitive Health Environment Life Course Model (CHELM). International Journal of Behavioral Development, 38(1), 1–10. https://doi.org/10.1177/0165025413512255
- Beigneux, K., Plaie, T., & Isingrini, M. (2007). Aging effect on visual and spatial components of working memory. The International Journal of Aging and Human Development, 65(4), 301–314. https://doi.org/10.2190/AG.65.4.b
- Bhalla, R. K., Butters, M. A., Becker, J. T., Houck, P. R., Snitz, B. E., Lopez, O. L., Aizenstein, H. J., Raina, K. D., DeKosky, S. T., & Reynolds, C. F. (2009). Patterns of mild cognitive impairment after treatment of depression in the elderly. The American Journal of Geriatric Psychiatry, 17(4), 308–316. https://doi.org/10.1097/JGP.0b013e318190b8d8
- Blackburn, D. J., Harkness, K., Reuber, M., Venneri, A., Shanks, M. F., & Wakefield, S. (2014). Memory difficulties are not always a sign of incipient dementia: A review of the possible causes of loss of memory efficiency. British Medical Bulletin, 112(1), 71–81. https://doi.org/10.1093/bmb/ldu029
- Bondi, M. W., Edmonds, E. C., Jak, A. J., Clark, L. R., Delano-Wood, L., McDonald, C. R., Galasko, D. J., Au, R., Galasko, D., Salmon, D. P., & Nation, D. (2014). Neuropsychological criteria for mild cognitive impairment improves diagnostic precision, biomarker associations, and progression rates. Journal of Alzheimer’s Disease, 42(1), 275–289. https://doi.org/10.3233/JAD-140276
- Buchtemann, D., Luppa, M., Bramesfeld, A., & Riedel-Heller, S. (2012). Incidence of late-life depression: A systematic review. Journal of Affective Disorders, 142(1–3), 172–179. https://doi.org/10.1016/j.jad.2012.05.010
- Buckley, R., Saling, M. M., Ames, D., Rowe, C. C., Lautenschlager, N. T., Macaulay, S. L., Savage, G., O’Meara, T., Savage, G., Szoeke, C., Villemagne, V. L., Ellis, K. A., & Martins, R. N. (2013). Factors affecting subjective memory complaints in the AIBL aging study: Biomarkers, memory, affect, and age. International Psychogeriatrics, 25(8), 1307–1315. https://doi.org/10.1017/S1041610213000665
- Bunce, D., Batterham, P. J., Christensen, H., & Mackinnon, A. J. (2014). Causal associations between depression symptoms and cognition in a community-based cohort of older adults. The American Journal of Geriatric Psychiatry, 22(12), 1583–1591. https://doi.org/10.1016/j.jagp.2014.01.004
- Buschkuehl, M., Jaeggi, S. M., Hutchison, S., Perrig-Chiello, P., Däpp, C., Müller, M., Breil, F., Hoppeler, H., & Perrig, W. J. (2008). Impact of working memory training on memory performance in old-old adults. Psychology and Aging, 23(4), 743–753. https://doi.org/10.1037/a0014342
- Cargin, J. W., Collie, A., Masters, C., & Maruff, P. (2008). The nature of cognitive complaints in healthy older adults with and without objective memory decline. Journal of Clinical and Experimental Neuropsychology, 30(2), 245–257. https://doi.org/10.1080/138003390701377829
- Chapman, S., Sunderaraman, P., Joyce, J. L., Azar, M., Colvin, L. E., Barker, M. S., McKeague, I., Kreisl, W. C., & Cosentino, S. (2021). Optimizing subjective cognitive decline to detect early cognitive dysfunction. Journal of Alzheimer’s Disease, 80(3), 1185–1196. https://doi.org/10.3233/JAD-201322
- Chapman, S., Weiss, D., Broulíková, H. M., Joyce, J. L., Sunderaraman, P., McKeague, I. W., & Cosentino, S. (2019). Age stereotypes influence subjective cognitive decline via depression. Alzheimer’s & Dementia, 15(7), 1366. https://doi.org/10.1016/j.jalz.2019.06.3896
- Charlson, M. E., Charlson, R. E., Peterson, J. C., Marinopoulos, S. S., Briggs, W. M., & Hollenberg, J. P. (2008). The Charlson comorbidity index is adapted to predict costs of chronic disease in primary care patients. Journal of Clinical Epidemiology, 61(12), 1234–1240. https://doi.org/10.1016/j.jclinepi.2008.01.006
- Charlson, M. E., Pompei, P., Ales, K. L., & MacKenzie, C. R. (1987). A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. Journal of Chronic Diseases, 40(5), 373–383. https://doi.org/10.1016/0021-9681(87)90171-8
- Christensen, H. (2001). What cognitive changes can be expected with normal ageing? Australian and New Zealand Journal of Psychiatry, 35(6), 768–775. https://doi.org/10.1046/j.1440-1614.2001.00966.x
- Clare, L., Whitaker, C. J., & Nelis, S. M. (2010). Appraisal of memory functioning and memory performance in healthy ageing and early-stage Alzheimer’s disease. Aging, Neuropsychology, and Cognition, 17(4), 462–491. https://doi.org/10.1080/13825580903581558
- Clare, L., & Woods, R. T. (2004). Cognitive training and cognitive rehabilitation for people with early-stage Alzheimer’s disease: A review. Neuropsychological Rehabilitation, 14(4), 385–401. https://doi.org/10.1080/09602010443000074
- Clifton, L., & Clifton, D. A. (2019). The correlation between baseline score and post-intervention score, and its implications for statistical analysis. Trials, 20(1), 1–6. https://doi.org/10.1186/s13063-018-3108-3
- Cohen, J. (1988). Statistical power analysis for the behavioral sciences. Lawrence Earlbaum Associates.
- Copeland, J. R. M., Dewey, M. E., & Griffiths-Jones, H. M. (1986). A computerized psychiatric diagnostic system and case nomenclature for elderly subjects: GMS and AGECAT. Psychological Medicine, 16(1), 89–99. https://doi.org/10.1017/S0033291700057779
- Corbett, A., Owen, A., Hampshire, A., Grahn, J., Stenton, R., Dajani, S., Burns, A., Howard, R., Williams, N., Williams, G., & Ballard, C. (2015). The effect of an online cognitive training package in healthy older adults: An online randomized controlled trial. Journal of the American Medical Directors Association, 16(11), 990–997. https://doi.org/10.1016/j.jamda.2015.06.014
- Crane, M. K., Bogner, H. R., Brown, G. K., & Gallo, J. J. (2007). The link between depressive symptoms, negative cognitive bias and memory complaints in older adults. Aging & Mental Health, 11(6), 708–715. https://doi.org/10.1080/13607860701368497
- De Koning, I., Van Kooten, F., Dippel, D. W. J., Van Harskamp, F., Grobbee, D. E., Kluft, C., & Koudstaal, P. J. (1998). The CAMCOG: A useful screening instrument for dementia in stroke patients. Stroke, 29(10), 2080–2086. https://doi.org/10.1161/01.STR.29.10.2080
- De Vito, A., Calamia, M., Greening, S., & Roye, S. (2019). The association of anxiety, depression, and worry symptoms on cognitive performance in older adults. Aging, Neuropsychology, and Cognition, 26(2), 161–173. https://doi.org/10.1080/13825585.2017.1416057
- Deary, I. J., Corley, J., Gow, A. J., Harris, S. E., Houlihan, L. M., Marioni, R. E., Penke, L., Rafnsson, S. B., & Starr, J. M. (2009). Age-associated cognitive decline. British Medical Bulletin, 92(1), 135–152. https://doi.org/10.1093/bmb/ldp033
- Duff, K., Beglinger, L. J., Moser, D. J., Paulsen, J. S., Schultz, S. K., & Arndt, S. (2010). Predicting cognitive change in older adults: The relative contribution of practice effects. Archives of Clinical Neuropsychology, 25(2), 81–88. https://doi.org/10.1093/arclin/acp105
- Duff, K., Beglinger, L. J., Schultz, S. K., Moser, D. J., McCaffrey, R. J., Haase, R. F., Westervelt, H., Langbehn, D., & Paulsen, J., & Huntington’s Study Group. (2007). Practice effects in the prediction of long-term cognitive outcome in three patient samples: A novel prognostic index. Archives of Clinical Neuropsychology, 22(1), 15–24.
- Folstein, M. F., Folstein, S. E., & McHugh, P. R. (1975). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–198. https://doi.org/10.1016/0022-3956(75)90026-6
- Gallo, J. J., Bogner, H. R., Morales, K. H., Post, E. P., Ten Have, T., & Bruce, M. L. (2005). Depression, cardiovascular disease, diabetes, and two-year mortality among older, primary-care patients. The American Journal of Geriatric Psychiatry, 13(9), 748–755. https://doi.org/10.1176/appi.ajgp.13.9.748
- Goldberg, T. E., Harvey, P. D., Wesnes, K. A., Snyder, P. J., & Schneider, L. S. (2015). Practice effects due to serial cognitive assessment: Implications for preclinical Alzheimer’s disease randomized controlled trials. Alzheimer’s & Dementia, 1(1), 103–111. https://doi.org/10.1016/j.dadm.2014.11.003
- Grønkjær, M., Osler, M., Flensborg-Madsen, T., Sørensen, H. J., & Mortensen, E. L. (2019). Associations between education and age-related cognitive changes from early adulthood to late midlife. Psychology and Aging, 34(2), 177–186. https://doi.org/10.1037/pag0000332
- Hahn, E. A., & Lachman, M. E. (2015). Everyday experiences of memory problems and control: The adaptive role of selective optimization with compensation in the context of memory decline. Neuropsychology, Development, and Cognition. Section B, Aging, Neuropsychology and Cognition, 22(1), 25–41. https://doi.org/10.1080/13825585.2014.888391
- Hertzog, C., Hülür, G., Gerstorf, D., & Pearman, A. (2018). Is subjective memory change in old age based on accurate monitoring of age-related memory change? Evidence from two longitudinal studies. Psychology and Aging, 33(2), 273–287. https://doi.org/10.1037/pag0000232
- Heser, K., Tebarth, F., Wiese, B., Eisele, M., Bickel, H., Köhler, M., Mösch, E., Weyerer, S., Werle, J., König, -H.-H., Leicht, H., Pentzek, M., Fuchs, A., Riedel-Heller, S. G., Luppa, M., Prokein, J., Scherer, M., Maier, W., & Wagner, M. (2013). Age of major depression onset, depressive symptoms, and risk for subsequent dementia: Results of the German study on ageing, cognition, and dementia in primary care patients (AgeCoDe). Psychological Medicine, 43(8), 1597–1610. https://doi.org/10.1017/s0033291712002449
- Hill, N. L., Mogle, J., Bhargava, S., Bell, T. R., Bhang, I., Katz, M. J., & Sliwinski, M. J. (2020). Longitudinal relationships among depressive symptoms and three types of memory self-report in cognitively intact older adults. International Psychogeriatrics, 32(6), 719–732. https://doi.org/10.1017/S104161021900084X
- Hill, N. L., Mogle, J., Wion, R., Munoz, E., DePasquale, N., Yevchak, A. M., & Parisi, J. M. (2016). Subjective cognitive impairment and affective symptoms: A systematic review. The Gerontologist, 56(6), e109–e127. https://doi.org/10.1093/geront/gn-w091
- Ho, C., Feng, L., Fam, J., Mahendran, R., Kua, E. H., & Ng, T. P. (2014). Coexisting medical comorbidity and depression: Multiplicative effects on health outcomes in older adults. International Psychogeriatrics, 26(7), 1221–1229. https://doi.org/10.1017/s1041610214000611
- Hobson, P., & Meara, J. (1998). Screening for “cognitive impairment, no dementia” in older adults. Journal of the American Geriatrics Society, 46(5), 659-659. https://doi.org/10.1111/j.1532-5415.1998.tb01091.x
- Huntley, J. D., Hampshire, A., Bor, D., Owen, A., & Howard, R. J. (2017). Adaptive working memory strategy training in early Alzheimer’s disease: Randomised controlled trial. The British Journal of Psychiatry, 210(1), 61–66. https://doi.org/10.1192/bjp.bp.116.182048
- Huppert, F. A., Brayne, C., Gill, C., Paykel, E. S., & Beardsall, L. (1995). CAMCOG–A concise neuropsychological test to assist dementia diagnosis: Socio-demographic determinants in an elderly population sample. British Journal of Clinical Psychology, 34(4), 529–541. https://doi.org/10.1111/j.2044-8260.1995.tb01487.x
- Huppert, F. A., Jorm, A. F., Brayne, C., Girling, D. M., Barkley, C., Beardsall, L., & Paykel, E. S. (1996). Psychometric properties of the CAMCOG and its efficacy in the diagnosis of dementia. Aging, Neuropsychology, and Cognition, 3(3), 201–214. https://doi.org/10.1080/13825589608256624
- Jessen, F., Amariglio, R. E., Van Boxtel, M., Breteler, M., Ceccaldi, M., Chetelat, G., Dubois, B., Dufouil, C., Ellis, K. A., Van Der Flier, W. M., Glodzik, L., Van Harten, A. C., De Leon, M. J., McHugh, P., Mielke, M. M., Molinuevo, J. L., Mosconi, L., Osorio, R. S., Perrotin, A., Rabin, L. A., … Wagner, M. (2014). A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimer’s & Dementia, 10(6), 844–852. https://doi.org/10.1016/j.jalz.2014.01.001
- Jessen, F., Wiese, B., Cvetanovska, G., Fuchs, A., Kaduszkiewicz, H., Kölsch, H., Luck, T., Mösch, E., Pentzek, M., Riedel-heller, S. G., Werle, J., Weyerer, S., Zimmermann, T., Maier, W., & Bickel, H. (2007). Patterns of subjective memory impairment in the elderly: Association with memory performance. Psychological Medicine, 1753–1762. https://doi.org/10.1017/S0033291707001122
- Jonker, C., Geerlings, M. I., & Schmand, B. A. (2000). Are memory complaints predictive for dementia? A review of clinical and population-based studies. International Journal of Geriatric Psychiatry, 15(11), 983–991. https://doi.org/10.1002/1099-1166(200011)15:11
- Kessler, E.-M., Bowen, C. E., Baer, M., Froelich, L., & Wahl, H.-W. (2012). Dementia worry: A psychological examination of an unexplored phenomenon. European Journal of Ageing, 9(4), 275–284. https://doi.org/10.1007/s10433-012-0242-8
- Koppara, A., Wagner, M., Lange, C., Ernst, A., Wiese, B., König, -H.-H., … Weyerer, S. (2015). Cognitive performance before and after the onset of subjective cognitive decline in old age. Alzheimer’s & Dementia, 1(2), 194–205. https://doi.org/10.1016/j.dadm.2015.02.005
- Kramer, J. H., Jurik, J., Sha, S. J., Rankin, K. P., Rosen, H. J., Johnson, J. K., & Miller, B. L. (2003). Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer Disease. Cognitive and Behavioral Neurology, 16(4), 211–218. https://doi.org/10.1097/00146965-200312000-00002
- Lenze, E. J., & Wetherell, J. L. (2009). Bringing the bedside to the bench, and then to the community: A prospectus for intervention research in late-life anxiety disorders. International Journal of Geriatric Psychiatry, 24(1), 1–14. https://doi.org/10.1002/gps.2074
- Lövdén, M., Rönnlund, M., Wahlin, Å., Bäckman, L., Nyberg, L., & Nilsson, L.-G. (2004). The extent of stability and change in episodic and semantic memory in old age: Demographic predictors of level and change. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 59(3), 130–134. https://doi.org/10.1093/geronb/59.3.P130
- Lubitz, A. F., Eid, M., & Niedeggen, M. (2018). Complainer Profile Identification (CPI): Properties of a new questionnaire on subjective cognitive complaints. Aging, Neuropsychology, and Cognition, 25(1), 99–121. https://doi.org/10.1080/13825585.2016.1267325
- Matthews, F. E., Arthur, A., Barnes, L. E., Bond, J., Jagger, C., Robinson, L., & Collaboration, A. (2013). A two-decade comparison of prevalence of dementia in individuals aged 65 years and older from three geographical areas of England: Results of the Cognitive Function and Ageing Study I and II. The Lancet, 382(9902), 1405–1412. https://doi.org/10.1016/S0140-6736(13)61570-6
- McWilliam, C., Copeland, J. R. M., Dewey, M. E., & Wood, N. (1988). The geriatric mental state examination as a case-finding instrument in the community. The British Journal of Psychiatry, 152(2), 205–208. https://doi.org/10.1192/bjp.152.2.205
- Meeks, T. W., Vahia, I. V., Lavretsky, H., Kulkarni, G., & Jeste, D. V. (2011). A tune in “a minor” can “b major”: A review of epidemiology, illness course, and public health implications of subthreshold depression in older adults. Journal of Affective Disorders, 129(1–3), 126–142. https://doi.org/10.1016/j.jad.2010.09.015
- Mendonça, M. D., Alves, L., & Bugalho, P. (2016). From subjective cognitive complaints to dementia: Who is at risk?: A systematic review. American Journal of Alzheimer’s Disease & Other Dementias, 31(2), 105–114. https://doi.org/10.1177/1533317515592331
- Mol, M. E., Ruiter, R. A., Verhey, F. R., Dijkstra, J., & Jolles, J. (2008). A study into the psychosocial determinants of perceived forgetfulness: Implications for future interventions. Aging & Mental Health, 12(2), 167–176. https://doi.org/10.1080/13607860801972503
- Molinuevo, J. L., Rabin, L. A., Amariglio, R. E., Buckley, R. F., Dubois, B., Ellis, K. A., Rami, L., Klöppel, S., Rami, L., Reisberg, B., Saykin, A. J., Sikkes, S., Smart, C. M., Snitz, B. E., Sperling, R., Van Der Flier, W. M., Wagner, M., Jessen, F., & Ewers, M. (2017). Implementation of subjective cognitive decline criteria in research studies. Alzheimer’s & Dementia, 13(3), 296–311. https://doi.org/10.1016/j.jalz.2016.09.012
- Montejo, P., Montenegro, M., Fernandez, M. A., & Maestu, F. (2011). Subjective memory complaints in the elderly: Prevalence and influence of temporal orientation, depression and quality of life in a population-based study in the city of Madrid. Aging & Mental Health, 15(1), 85–96. https://doi.org/10.1080/13607863.2010.501062
- Montejo, P., Montenegro, M., Fernández, M. A., & Maestú, F. (2012). Memory complaints in the elderly: Quality of life and daily living activities. A population based study. Archives of Gerontology and Geriatrics, 54(2), 298–304. https://doi.org/10.1016/j.archger.2011.05.021
- Morrison, V. (2008). Ageing and physical health. In R. T. Woods & L. Clare (Eds.), Handbook of the clinical psychology of ageing. Wiley. J. & Sons.
- Nordhus, I. H. (2008). Manifestations of depression and anxiety in older adults. In R. T. Woods & L. Clare (Eds.), Handbook of the clinical psychology of ageing (2nd ed., pp. 99–110). Wiley. J. & Sons.
- Palmer, B. W., Boone, K. B., Lesser, I. M., & Wohl, M. A. (1998). Base rates of “impaired” neuropsychological test performance among healthy older adults. Archives of Clinical Neuropsychology, 13(6), 503–511. https://doi.org/10.1093/arclin/13.6.503
- Park, D. C., & Festini, S. B. (2017). Theories of memory and aging: A look at the past and a glimpse of the future. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 72(1), 82–90. https://doi.org/10.1093/geronb/gbw066
- Pearman, A. (2020). Short-term variability in subjective memory decline: The influence of age and neuroticism. Aging, Neuropsychology, and Cognition, 1–14. https://doi.org/10.1080/13825585.2020.1809631
- Perrotin, A. (2012). Subjective cognition and amyloid deposition imaging: A Pittsburgh Compound B positron emission tomography study in normal elderly individuals. Archives of Neurology, 69(2), 223–229. https://doi.org/10.1001/archneurol.2011.666
- Peter, J., Scheef, L., Abdulkadir, A., Boecker, H., Heneka, M., Wagner, M., Koppara, A., Klöppel, S., & Jessen, F. (2014). Gray matter atrophy pattern in elderly with subjective memory impairment. Alzheimer’s & Dementia, 10(1), 99–108. https://doi.org/10.1016/j.jalz.2013.05.1764
- Pietrzak, R. H., Maruff, P., Woodward, M., Fredrickson, J., Fredrickson, A., Krystal, J. H., Southwick, S. M., & Darby, D. (2012). Mild worry symptoms predict decline in learning and memory in healthy older adults: A 2-year prospective cohort study. The American Journal of Geriatric Psychiatry, 20(3), 266–275. https://doi.org/10.1097/JGP.0b013e3182107e24
- Prichep, L. S., John, E. R., Ferris, S. H., Rausch, L., Fang, Z., Cancro, R., Torossian, C., & Reisberg, B. (2006). Prediction of longitudinal cognitive decline in normal elderly with subjective complaints using electrophysiological imaging. Neurobiology of Aging, 27(3), 471–481. https://doi.org/10.1016/j.neurobiolaging.2005.07.021
- Pusswald, G., Moser, D., Gleiß, A., Janzek-Hawlat, S., Auff, E., Dal-Bianco, P., & Lehrner, J. (2013). Prevalence of mild cognitive impairment subtypes in patients attending a memory outpatient clinic—Comparison of two modes of mild cognitive impairment classification. Results of the Vienna Conversion to Dementia Study. Alzheimer’s & Dementia, 9(4), 366–376. https://doi.org/10.1016/j.jalz.2011.12.009
- Rabin, L. A., Smart, C. M., Crane, P. K., Amariglio, R. E., Berman, L. M., Boada, M., Ellis, K. A., Dubois, B., Ellis, K. A., Gifford, K. A., Jefferson, A. L., Jessen, F., Katz, M. J., Lipton, R. B., Luck, T., Maruff, P., Mielke, M. M., Molinuevo, J. L., Naeem, F., Bayer, T., & Buckley, R. F. (2015). Subjective cognitive decline in older adults: An overview of self-report measures used across 19 international research studies. Journal of Alzheimer’s Disease, 48(s1), S63–S86. https://doi.org/10.3233/JAD-150154
- Rakesh, G., Szabo, S. T., Alexopoulos, G. S., & Zannas, A. S. (2017). Strategies for dementia prevention: Latest evidence and implications. Therapeutic Advances in Chronic Disease, 8(8–9), 121–136. https://doi.org/10.1177/2040622317712442
- Reisberg, B. (1986). Dementia: A systematic approach to identifying reversible causes. Geriatrics, 41(4), 30–46.
- Reisberg, B., & Gauthier, S. (2008). Current evidence for subjective cognitive impairment (SCI) as the pre-mild cognitive impairment (MCI) stage of subsequently manifest Alzheimer’s disease. International Psychogeriatrics, 20(1), 1–16. https://doi.org/10.1017/S1041610207006412
- Reisberg, B., Prichep, L., Mosconi, L., John, E. R., Glodzik-Sobanska, L., Boksay, I., Ashraf, N., Vedvyas, A., Ashraf, N., Jamil, I. A., De Leon, M. J., & Monteiro, I. (2008). The pre-mild cognitive impairment, subjective cognitive impairment stage of Alzheimer’s disease. Alzheimer’s & Dementia, 4(1), S98–S108. https://doi.org/10.1016/j.jalz.2007.11.017
- Roberts, J. L., Clare, L., & Woods, R. T. (2009). Subjective memory complaints and awareness of memory functioning in mild cognitive impairment: A systematic review. Dementia and Geriatric Cognitive Disorders, 28(2), 95–109. https://doi.org/10.1159/000234911
- Roehr, S., Pabst, A., Riedel-Heller, S. G., Jessen, F., Turana, Y., Handajani, Y. S., … Sachdev, P. S. (2020). Estimating prevalence of subjective cognitive decline in and across international cohort studies of aging: A COSMIC study. MedRxiv. https://doi.org/10.1101/2020.05.20.20106526
- Rönnlund, M., Sundström, A., Adolfsson, R., & Nilsson, L.-G. (2015). Subjective memory impairment in older adults predicts future dementia independent of baseline memory performance: Evidence from the Betula prospective cohort study. Alzheimer’s & Dementia, 11(11), 1385–1392. https://doi.org/10.1016/j.jalz.2014.11.006
- Sabatini, S., Silarova, B., Martyr, A., Collins, R., Ballard, C., Anstey, K. J., Clare, L., & Kim, S. (2020). Associations of awareness of age-related change with emotional and physical well-being: A systematic review and meta-analysis. The Gerontologist, 60(6), e477–e490. https://doi.org/10.1093/geront/gnz101
- Sabatini, S., Ukoumunne, O. C., Ballard, C., Brothers, A. F., Kaspar, R., Collins, R., … Clare, L. (2020b). International relevance of two measures of awareness of age-related change (AARC). Review. https://doi.org/10.21203/rs.3.rs-18586/v1
- Sabatini, S., Ukoumunne, O. C., Ballard, C., Brothers, A. F., Kaspar, R., Collins, R., Kim, S., Corbett, A., Aarsland, D., Hampshire, A., Brooker, H., & Clare, L. (2020a). International relevance of two measures of awareness of age-related change (AARC). BMC Geriatrics, 20(1), 359. https://doi.org/10.1186/s12877-020-01767-6
- Sabatini, S., Ukoumunne, O. C., Ballard, C., Collins, R., Anstey, K. J., Diehl, M. K., Brothers, A., Wahl, H.-W., Corbett, A., Hampshire, A., Brooker, H., & Clare, L. (2021). Cross-sectional association between objective cognitive performance and perceived age-related gains and losses in cognition. International Psychogeriatrics, 1–15. https://doi.org/10.1017/S1041610221000375
- Sachs-Ericsson, N., Joiner, T. E., Plant, E. A., & Blazer, D. G. (2005). The influence of depression on cognitive decline in community-dwelling elderly persons. The American Journal of Geriatric Psychiatry, 13(5), 402–408. https://doi.org/10.1097/00019442-200505000-00009
- Scheltens, N. M. E., Galindo-Garre, F., Pijnenburg, Y. A. L., Van Der Vlies, A. E., Smits, L. L., Koene, T., Scheltens, P., Wattjes, M. P., Scheltens, P., Van Der Flier, W. M., & Teunissen, C. E. (2016). The identification of cognitive subtypes in Alzheimer’s disease dementia using latent class analysis. Journal of Neurology, Neurosurgery, and Psychiatry, 87(3), 235–243. https://doi.org/10.1136/jnnp-2014-309582
- Schmidtke, K., & Metternich, B. (2009). Validation of two inventories for the diagnosis and monitoring of functional memory disorder. Journal of Psychosomatic Research, 67(3), 245–251. https://doi.org/10.1016/j.jpsychores.2009.04.005
- Schmidtke, K., Pohlmann, S., & Metternich, B. (2008). The syndrome of functional memory disorder: Definition, etiology, and natural course. The American Journal of Geriatric Psychiatry, 16(12), 981–988. https://doi.org/10.1097/JGP.0b013e318187ddf9
- Siebert, J. S., Braun, T., & Wahl, H. W. (2020). Change in attitudes toward aging: Cognitive complaints matter more than objective performance. Psychology and Aging, 35(3), 357–368. https://doi.org/10.1037/pag0000451
- Slot, R. E. R., Verfaillie, S. C. J., Overbeek, J. M., Timmers, T., Wesselman, L. M. P., Teunissen, C. E., … Barkhof, F. (2018). Subjective Cognitive Impairment Cohort (SCIENCe): Study design and first results. Alzheimer’s Research & Therapy, 10(1), 1–13. https://doi.org/10.1186/s13195-018-0390-y
- Small, S. A. (2001). Age-related memory decline: Current concepts and future directions. Archives of Neurology, 58(3), 360–364. https://doi.org/10.1001/archneur.58.3.360
- Smart, C. M., & Krawitz, A. (2015). The impact of subjective cognitive decline on Iowa Gambling Task performance. Neuropsychology, 29(6), 971. https://doi.org/10.1037/neu0000204
- Smart, C. M., Segalowitz, S. J., Mulligan, B. P., & MacDonald, S. W. S. (2014). Attention capacity and self-report of subjective cognitive decline: A P3 ERP study. Biological Psychology, 103, 144–151. https://doi.org/10.1016/j.biopsycho.2014.08.016
- Smits, L. L., Van Harten, A. C., Pijnenburg, Y. A. L., Koedam, E. L. G. E., Bouwman, F. H., Sistermans, N., Scheltens, P., Lemstra, A. W., Scheltens, P., Van Der Flier, W. M., & Reuling, I. E. W. (2015). Trajectories of cognitive decline in different types of dementia. Psychological Medicine, 45(5), 1051–1059. https://doi.org/10.1017/S0033291714002153
- StataCorp. (2017). Stata statistical software: Release 16.
- Szanto, K., Dombrovski, A. Y., Sahakian, B. J., Mulsant, B. H., Houck, P. R., Reynolds, C. F., & Clark, L. A. (2012). Social emotion recognition, social functioning, and attempted suicide in late-life depression. The American Journal of Geriatric Psychiatry, 20(3), 257–265. https://doi.org/10.1097/JGP.0b013e31820eea0c
- Tandetnik, C., Farrell, M. T., Cary, M. S., Cines, S., Emrani, S., Karlawish, J., Cosentino, S., Tales, A., Jessen, F., Butler, C., Wilcock, G., Phillips, J., & Bayer, T. (2015). Ascertaining subjective cognitive decline: A comparison of approaches and evidence for using an age-anchored reference group. Journal of Alzheimer’s Disease, 48(s1), S43–S55. https://doi.org/10.3233/JAD-150251
- Therrien, Z., & Hunsley, J. (2012). Assessment of anxiety in older adults: A systematic review of commonly used measures. Aging & Mental Health, 16(1), 1–16. https://doi.org/10.1080/13607863.2011.602960
- Van Oijen, M., De Jong, F. J., Hofman, A., Koudstaal, P. J., & Breteler, M. M. B. (2007). Subjective memory complaints, education, and risk of Alzheimer’s disease. Alzheimer’s & Dementia, 3(2), 92–97. https://doi.org/10.1016/j.jalz.2007.01.011
- Vanderhill, S., Hultsch, D. F., Hunter, M. A., & Strauss, E. (2010). Self-reported cognitive inconsistency in older adults. Aging, Neuropsychology, and Cognition, 17(4), 385–405. https://doi.org/10.1080/13825580903265699
- Williams, J. G., Huppert, F. A., Matthews, F. E., & Nickson, J. (2003). Performance and normative values of a concise neuropsychological test (CAMCOG) in an elderly population sample. International Journal of Geriatric Psychiatry, 18(7), 631–644. https://doi.org/10.1002/gps.886
- World Health Organization. (2020). Retrieved https://www.who.int/news-room/fact-sheets/detail/dementia
- Yates, J. A., Clare, L., Woods, R. T., Matthews, F. E., Tales, A., Jessen, F., Butler, C., Wilcock, G., Phillips, J., & Bayer, T. (2015). Subjective memory complaints are involved in the relationship between mood and mild cognitive impairment. Journal of Alzheimer’s Disease, 48(s1), S115–S123. https://doi.org/10.3233/JAD-150371
- Zlatar, Z. Z., Muniz, M., Galasko, D., & Salmon, D. P. (2018). Subjective cognitive decline correlates with depression symptoms and not with concurrent objective cognition in a clinic-based sample of older adults. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 73(7), 1198–1202. https://doi.org/10.1093/geronb/bgw207
