?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Amnestic mild cognitive impairment (aMCI) and Alzheimer’s disease (AD) dementia are characterized by pathological changes to the medial temporal lobes, resulting in explicit learning and retention reductions. Studies demonstrate that implicit/procedural memory processes are relatively intact in these populations, supporting different anatomical substrates for differing memory systems. This study examined differences between explicit and procedural learning and retention in individuals with aMCI and AD dementia relative to matched healthy controls. We also examined anatomical substrates using volumetric MRI. Results revealed expected difficulties with explicit learning and retention in individuals with aMCI and AD with relatively preserved procedural memory. Explicit verbal retention was associated with medial temporal cortex volumes. However, procedural retention was not related to medial temporal or basal ganglia volumes. Overall, this study confirms the dissociation between explicit relative to procedural learning and retention in aMCI and AD dementia and supports differing anatomical substrates.
Introduction
Memory is a complex cognitive ability consisting of a variety of underlying processes and mechanisms. It has been hypothesized that memory can be broken down into various systems (Tulving, Citation1985). Broadly, memory abilities are often classified as declarative and nondeclarative (Squire, Citation2004). Declarative, or explicit memory, involves conscious recollection of facts and events, while nondeclarative, or implicit memory, involves abilities generally expressed through performance (Squire, Citation2004). This classification was prompted by early research indicating that certain implicit learning and memory abilities remain relatively intact in amnestic populations, including priming and motor learning abilities (Cohen & Squire, Citation1980; Graf et al., Citation1984; Milner et al., Citation1968). For instance, patient HM showed improved ability to complete a motor task across repeated trials without conscious memory of previous exposure to the task (Milner et al., Citation1968).
This literature on the distinction between explicit and implicit memory has been expanded to neurodegenerative processes. AD often involves a hallmark amnestic decline in explicit learning and memory ability. Medial temporal structures such as the hippocampus and entorhinal cortex show atrophy early in AD (Du et al., Citation2001; D. P. Devanand et al., Citation2012). Medial temporal atrophy in AD is associated with declines observed in explicit verbal learning and memory (Bonner-Jackson et al., Citation2015; Laakso et al., Citation1995; Di Paola et al., Citation2007). Yet, a growing body of research indicates that procedural learning and memory is relatively spared in AD (Beaunieux et al., Citation2012; Eslinger & Damasio, Citation1986; Deweer et al., Citation1993, Citation1994; Dick et al., Citation1995; see van Halteren-van Tilborg et al., Citation2007 for review). Citation2009Examination of procedural learning and memory could have diagnostic implications. Subcortical structures such as the basal ganglia are implicated in procedural memory (Doyon etal., Citation2009). The role of the basal ganglia in implicit memory has been explored, in part, through studies involving individuals with Parkinson’s disease, given its impact on striatal dopamine receptors. For instance, individuals with Parkinson’s disease have displayed impaired performances on incremental implicit learning tasks and relatively intact performances on explicit memory tasks, which is the opposite pattern observed in individuals with amnestic conditions (Bondi & Kaszniak, Citation1991; Knowlton et al., Citation1996). One study suggested that it may be possible to discriminate AD dementia and vascular dementia based on differential patterns of impairment on procedural versus explicit memory tests (Libon et al., Citation1998). Specifically, individuals with vascular dementia showed better recognition discriminability of a word list but worse performance on a pursuit rotor learning task relative to individuals with AD.
A recent systematic review and meta-analysis (De Wit et al., Citation2021) examined 17 studies comparing procedural learning in individuals with amnestic Mild Cognitive Impairment (aMCI, which is often, but not always, a precursor to AD) or AD dementia and healthy older adults. Included studies utilized a variety of pattern and motor learning tasks, including the serial reaction time task, mirror reading, mirror tracing, rotary pursuit, and prediction tasks. Overall, the mean difference in procedural learning in those with aMCI or AD dementia compared with healthy older adults was not statistically significant, suggesting that this ability remains preserved. However, strong conclusions could not be drawn regarding procedural learning comparisons between aMCI and AD dementia due to the low number of studies with an aMCI group. In addition, the effect sizes of studies comparing aMCI with healthy controls did appear smaller relative to those with AD.
Fewer studies have examined the retention of procedural learning. There is some evidence that patients with AD dementia have shown intact retention of performances on a mirror-drawing task after a 30-min delay (Rouleau et al., Citation2002), a coordinated tracing task after a range of 3–18 months (Mochizuki-Kawai et al., Citation2004), and a serial reaction task after 1–2 week delay (Knopman, Citation1991). However, these studies had notably small sample sizes. Additional research is warranted to further clarify the nature of procedural memory in AD and the potential role of disease severity (aMCI vs. AD dementia). Understanding and incorporating procedural memory assessment across the disease process is crucial and can potentially translate into rehabilitation practices. Capitalizing on preserved procedural memory may improve adaptive functioning and prolong independence through skill learning (Oudman et al., Citation2015) in patients with AD. Although there are several tasks designed to measure procedural learning and retention, these are rarely, if ever, used in routine clinical practice.
The current study is the first to our knowledge to directly investigate procedural and declarative learning and retention rates across individuals with AD dementia, aMCI, and healthy controls (HCs). This study is also novel in applying a new measure of procedural memory involving modification of a widely used neuropsychological test of graphomotor processing speed. Specifically, we compared the rates of learning and retention on the California Verbal Learning Test-II – Short Form (Delis et al., Citation2000) as a measure of declarative memory with the rate of learning and then retention of a modified version of the Trail Making Test – part A (Reitan & Wolfson, Citation1985) as a measure of procedural learning and retention. This revised measure was shown to be impaired in a case study involving a patient with bilateral hippocampal and basal ganglia lesions who presented with anterograde amnesia for both procedural and explicit/declarative memory (Haut et al., Citation2017). Given the results of this case study and our current understanding of the role of the medial temporal lobe and basal ganglia in explicit and procedural memory, respectively, we also sought to examine correlations between memory performances and volumes of these structures.
We hypothesized that individuals with AD dementia would show significantly worse declarative learning and retention with relatively preserved procedural learning and retention when compared to cognitively unimpaired older adults. We also hypothesized that individuals with aMCI would show a similar pattern though less severe impairment on declarative memory measures. More specifically, we hypothesized an interaction between group (HC > aMCI > AD) and type of measure (procedural < explicit) for both learning and retention. Finally, we hypothesized that basal ganglia volume would positively correlate with procedural retention and that entorhinal cortex and hippocampal volume would positively correlate with explicit retention.
Materials and methods
Participants
The current study consisted of 50 patients from 225 consecutive patients seen for initial evaluation within an interdisciplinary memory clinic in an academic medical center between June 2020-September 2021 and 27 healthy controls. The clinical group was comprised of patients with probable AD dementia (N = 30) or aMCI (N = 20) based on National Institute on Aging-Alzheimer’s Association criteria (NIA-AA; Albert et al., Citation2013 McKhann et al., Citation2011). We further classified the patients with aMCI into single or multiple domain (aMCI-md) subtypes per the Petersen criteria (2004; aMCI = 11 aMCI-md = 9). There were no significant differences between these subtypes for demographic variables, MMSE total score, or any of the neuropsychological variables with the exception of CVLT learning (which was greater in the aMCI-md group; F(1,18) = 9.10, p < .05). Given our small sample size and observed similarities between subtypes, we left the groups collapsed into a single aMCI group.
Each patient completed a physical and neurological examination, standard laboratory values (B12, folate, TSH), a neuropsychological evaluation, and neuroimaging. Diagnosis for each affected subject was reached by consensus of an interdisciplinary team comprising Geriatrics, Neuropsychology, Neurology, Neuroradiology, and Psychiatry. MRI, or CT in two cases, was used to support a diagnosis of aMCI, aMCI-md, or AD dementia based on the presence of atrophy in the medial temporal lobes (Duara et al., Citation2008; Wahlund et al., Citation2000) and/or parietal lobes (Koedam et al., Citation2011; Lehmann et al., Citation2012). Additional biomarker data were available for select participants with aMCI and AD dementia. Specifically, fluorodeoxyglucose (FDG)–positron emission tomography (PET) with classic temporal/parietal hypometabolism was present in 7 participants with aMCI and 4 participants with AD dementia. Positive amyloid PET using florobetaben was present in 5 participants with aMCI and 2 participants with AD dementia. Finally, cerebrospinal fluid analysis with an abnormal Abeta42/ptau ratio was present in 2 participants with aMCI and 2 participants with AD dementia. Patients were excluded if they had a history of significant cerebrovascular disease, defined as cortical infarcts, subcortical lacunar infarcts, or at least moderate small vessel disease (i.e., Fazekas score > 1) as determined by a board-certified neuroradiologist. The Fazekas scale is a visual rating classification of white matter hyperintensities used to grade the severity of chronic small vessel ischemic disease on T-2 FLAIR images with a range of scores from 0 (no abnormal white matter hyperintensities) to 3 (large areas of confluent white matter hyperintensities) (Fazekas et al., Citation1987). Patients were also excluded if they were unable to complete both of the primary memory measures. A total of 52 patients were excluded due to significant vascular burden (e.g., lacunar infarctions, SAH, white matter disease severity), 14 were excluded due to being diagnosed with vascular MCI, and 11 were excluded due to being unable to complete the primary measures. All other excluded patients had a non-AD cognitive disorder diagnosis.
The HC group (N = 27) was ascertained between 2017 and 2019 using web announcements, community flyers, and patient caregivers. Inclusion criteria for the HC group included age older than 55, no subjective cognitive concerns, and MMSE > 26. Controls were excluded if untreated medical problems could impact cognition (e.g., diabetes, cardiac disease, depression, anxiety, traumatic brain injury, substance use).
Measures
As part of routine clinical care, the clinical groups completed a battery of neuropsychological tests from which several measures were selected to characterize the sample. Tests were administered and scored per their respective manuals.
Mini Mental Status Examination (MMSE). The MMSE (Folstein et al., Citation1975) was utilized to assess general mental status. A total score out of 30 was used as the dependent variable.
Wide Range Achievement Test, 4th Edition-Word Reading Subtest (WRAT-4-WR). The Word Reading subtest of the WRAT-4 (Wilkinson & Robertson, Citation2006) was used to estimate premorbid intellect. The standard score was used as the dependent variable.
Geriatric Depression Scale short form (GDS). Depressive symptoms were assessed using the 15-item GDS (Sheikh & Yesavage, Citation1986). A total score out of 15 was used as the dependent variable.
Functional Activities Questionnaire (FAQ). Functional status for the aMCI and AD dementia groups was quantified using informant ratings on the FAQ, which is a 10-item scale designed to assess patients’ abilities to complete activities common to older adults (Pfeffer et al., Citation1982). A total score out of 30 was used as the dependent variable.
Boston Naming Test (BNT). The BNT (Kaplan et al., Citation2001) is confrontation naming test consisting of 60 pictures. Total score out of 60 was used as the dependent variable.
Controlled Oral Word Association Test (COWAT) and Animal Naming. Semantic and phonemic fluency were examined using tests requiring patients to spontaneously generate as many words as possible in response either a semantic (animals) or phonemic (the letters F, A, and S) cue in 1 min. The number of words was used as the dependent variable.
Digit Span. This subtest from the Wechsler Adult Intelligence Scale (WAIS-IV; Wechsler, Citation2008) was utilized to measure basic auditory attention and working memory. Total raw score (i.e., correct trials) for the forwards, backwards, and sequencing conditions were utilized as dependent variables.
Similarities. This subtest from the Wechsler Adult Intelligence Scale (WAIS-IV; Wechsler, Citation2008) was utilized to measure abstract verbal reasoning and required examinees to describe how two words are similar. Total raw score was used as the dependent variable.
Trail Making Test. A modification of the trail making test (TMT-M) was used to measure procedural learning and retention. Specifically, Part A of the trail making test (TMT-A) from the Halstead-Reitan Neuropsychological Battery (Reitan & Wolfson, Citation1985) was repeatedly administered. This test of psychomotor processing speed requires the subject to connect dots in numerical order from 1 to 25 as quickly as possible. On the first trial, the task was administered according to standardized administration instructions from the manual. The next four trials were administered in immediate succession with abbreviated instructions. After a 30-min delay, the patient completed the same TMT-A task. The number of seconds to complete each trial was the primary variable of interest. A maximum time score was assigned if the subject discontinued or exceeded the time limit (i.e., 150 s). Similar to other tests of procedural learning with a motor component (e.g., rotor pursuit, mirror tracing), TMT-M involves skill learning with observable improvement in response to practice.
California Verbal Learning Test-II Short Form (CVLT-II SF). Episodic learning and retention were measured using the CVLT-II SF, which was administered using standard procedures (Delis et al., Citation2000). The number of words recalled across the four learning trials was the primary variable of interest. Standard learning and retention scores were obtained for both tasks. We chose not to use the learning slope calculation provided in the CVLT-II SF manual as our methods allow us to directly compare procedural and declarative learning taking into account total number of learning trials. The methods described below were used to compare both learning and retention for the CVLT-II SF and TMT-M.
Learning indices
The following standardized learning value formulas were used to compare procedural and episodic memory from the TMT-M and CVLT-II SF:
Standard Learning Value = Learning Value * (max possible gain)−1
For CVLT-II SF, Learning Value was computed as:
Because higher time scores on TMT are associated with worse performance, the minuend and subtrahend values in the above formula were reversed when computing the TMT-M Learning Value:
Next, to account for the impact of baseline performance (e.g., improvement is more difficult to achieve when performance is relatively good at baseline), the learning value was multiplied by the inverse of the maximum possible gain. For CVLT-II SF, the maximum possible gain was calculated as the difference between maximum possible recall (i.e., 9) and words recalled on CVLT-II SF Trial 1. For TMT-M, the maximum possible gain was calculated as the difference between the fastest TMT Trial 5 completion time in the current sample (10 seconds) and TMT Trial 1 completion time for every subject. Thus, the final standardized learning values for each task were computed as follows:
Procedural Learning Value:
Episodic Learning Value:
Retention indices
The percent retained was calculated for TMT-M and CVLT-II SF to compare procedural and episodic retention between groups. Because longer completion times on TMT are associated with worse performance, the inverse of TMT scores was used for the following formula.
Standardized Procedural Retention:
Standardized Episodic Retention:
MRI data acquisition
A subset of clinical participants completed an MRI scan using a Siemens Prisma 3 T scanner with 20 channel head coil to obtain a 3D T1 MPRAGE with either sagittal or axial acquisition and a minimum resolution of 1x1x1 mm (TR/TE = 2300 ms/2.26 msFlip angle: 8 degrees). Three participants received their imaging on a GE Architect 3 T scanner with a 48 channel head coil using a comparable T1 Bravo SPGR sequence with 1x1x1 mm resolution (TR/TE = 8.5 ms/3.3 ms). Comparing quality metrics between the Siemens and GE T1 images using CAT12 (see below) did not reveal any significant differences for resolution (F < 1), noise (F(1,42) = 1.28), bias (F(1,42) = 2.99), or the composite measure IQR (F(1,42) = 1.11; all ps > .05). In addition, removing the three participants who received their imaging on a GE Architect scanner did not alter the correlations reported here. MRI data were not available for seven clinical participants (AD = 5; aMCI = 2) for the reason of not being able to safely obtain an MRI (N = 2) or MRI was not of comparable quality or resolution (N = 5). The T1 images were processed using Spatially Localized Atlas Network Tiles (SLANT; Y. Huo et al., Citation2019; Yuankai Huo et al., Citation2018), which was utilized for rapid imaging processing and produced 132 brain segmentations for volumetric analysis. We selected the volumes of the hippocampus, entorhinal cortex, and basal ganglia (caudate + putamen + globus pallidus) as our measures of interest and calculated each as a percentage of total intracranial volume to correct for head size. For the hippocampus and entorhinal cortex, we averaged the right and left volumes as has been done in prior AD research (e.g., Hays et al., Citation2020; Killiany et al., Citation2002). Total intracranial volume was calculated using CAT12 (http://www.neuro.uni-jena.de/cat/) segmentation routine implemented within SPM12 (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/) and operated with in the MATLAB environment (R2021b; https://www.mathworks.com).
Data analysis
One-way ANOVAs and a Chi-Square test were used to assess group differences on demographic characteristics (i.e., age, education, estimated premorbid intellect, gender). Post-hoc contrasts were investigated with Tukey tests. The primary analysis consisted of a one-way MANOVA comparing the four dependent variables (procedural learning, procedural retention, episodic learning, and episodic retention) between three diagnostic groups (AD dementia, aMCI, and HC). Significant findings from the MANOVA were followed up with mixed-model repeated measure ANOVAs to examine for the hypothesized group by task interaction. One assessed the two learning measures between the three groups, while the other assessed the two retention measures between the three groups. Finally, Pearson correlations were calculated between the percent retained on CVLT-II SF and percent retained on TMT-M with each of the MRI volumetric measures (hippocampus, entorhinal cortex, and basal ganglia). We corrected the p-value for the number of correlations hypothesized to be significant (0.05/12 = .004). All analyses were conducted using IBM SPSS Statistics, Version 27.0.
Results
Participant characteristics
Participant characteristics are displayed in . Overall, the sample was well educated (M = 14.84 years of education, SD = 3.08) and almost exclusively white. There were no significant differences between groups for age, gender, education, or WRAT-4-WR. As expected, MMSE significantly differed as a function of group (HC > aMCI > AD), and functional status differed between the two clinical groups (aMCI < AD). Using the MMSE as a marker of level of impairment, the patients with AD dementia were generally mildly affected with only four patients obtaining an MMSE < 19. Participant groups differed in their performances on learning trials and delayed recall for procedural and verbal memory tests (HC > aMCI > AD). There was no significant difference between the two clinical groups on reported depression, and mean GDS scores were below the level of clinical significance. As expected, the participants with aMCI performed better than the participants with AD dementia on all cognitive measures. The participants with AD dementia performed worse than both aMCI and HC on all components of the CVLT-II SF and TMT. The aMCI performed worse than HCs on all components of the CVLT-II SF except trial 1. Of note, the aMCI group displayed a high degree of variability in retention scores as indicated by the standard deviation. This is not unexpected given the heterogenous nature of aMCI (Ezzati et al., Citation2020; Nettiksimmons et al., Citation2014) and the inclusion of several subtypes (e.g., amnestic and multiple domain) in the present study. There were no significant differences in performance between the HC and aMCI groups on the TMT.
Table 1. Participant characteristic data.
Primary analyses
A one-way MANOVA revealed a statistically significant difference between groups (i.e., AD dementia, aMCI, and HC) on the combined dependent variables (CVLT-II SF learning, CVLT-II SF retention, TMT-M learning and TMT-M retention), F(8, 142) = 11.70, p < .001, Wilks’ Λ = .363, ηp2 = .397. Follow-up ANOVAs were conducted to understand the specific differences between groups and tasks.
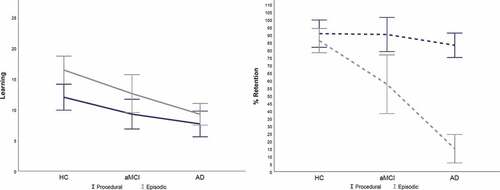
Learning. There was a main effect of learning, F(1,74) = 14.82, p < .001, ηp2 = .167, observed power = .967, with higher mean learning scores on the CVLT-II SF (M = 12.74, SD = 6.39) than TMT-M (M = 9.68, SD = 5.64). There was also a main effect of diagnosis, F(2,74) = 14.95, p < .001, ηp2 = .288, observed power = .999, with HC demonstrating higher learning (M = 14.489, SE = .83) > aMCI (M = 10.92, SE = .93) > AD dementia (M = 8.45, SE = .762). There was no significant interaction between diagnostic group and type of learning measure, F(2,74) = 1.26, p > .05, ηp2 = .033, observed power = .266 (see ).
Retention. There was a main effect of retention, F(1,74) = 62.68, p < .001, ηp2 = .459, observed power = 1.0, with higher mean retention scores on the TMT-M (M = 87.34, SD = 22.42) than CVLT-II SF (M = 50.90, SD = 41.84). There was also a main effect of diagnosis, F(2,74) = 36.91, p < .001, ηp2 = .499, observed power = 1.0, with HC displaying higher retention scores (M = 87.89, SE = .3.32) > aMCI (M = 73.84, SE = 3.86) > AD dementia (M = 49.09, SE = .3.15). There was a significant interaction between diagnostic group and type of retention measure, F(2,74) = 20.15, p < .05, ηp2 = .353, observed power = 1.0. Follow-up Tukey tests were conducted to isolate the source of the interaction (as displayed in ). HC retained a greater amount of information on the CVLT-II SF than aMCI who retained more information than patients with AD dementia (HC: M = 85.99, SD = 19.48 > aMCI: M = 57.47, SD = 41.35 > AD: M = 15.05, SD = 25). However, there was no difference in retention scores on TMT-M between the three groups (HC: M = 89.89, SD = 22.29, = aMCI: M = 90.20, SD = 24.09, = AD: M = 83.12, SD = 21.50).
Another way of examing this interaction is displayed in . Independent t tests were utilized to compare the HC group to aMCI and AD dementia separately for both explicit and procedural learning and retention. Cohen’s d effect sizes are also displayed in and demonstrate the largest effect between the HC and AD dementia groups for CVLT retention. The smallest effect was observed between the HC and aMCI group for TMT-M retention.
Table 2. Cohen’s d effect sizes for aMCI vs. HC and AD vs. HC.
MRI analyses
As hypothesized, the entorhinal cortex positively correlated with percent retained on the CVLT-II SF (r = 0.445 p < 0.001) but not percent retained on the TMT-M. However, percent retained on the TMT-M did not correlate with total basal ganglia volume, and additional exploratory analyses did not reveal any significant associations with the basal ganglia component parts, including the caudate, putamen, or globus pallidus. While not surviving correction, the following correlations were also noted: the hippocampus correlated positively with TMT-M learning and percent retained on the CVLT-II SF, and the entorhinal cortex correlated with learning on both the CVLT-II SF and TMT-M. The total volume of the basal ganglia did positively correlate with retention on the CVLT-II-SF. All correlations are displayed in .
Table 3. Correlations Between Memory Measures and Neuroimaging (MCI N = 18, AD N = 25).
Table 4. Correlations between procedural learning/retention and demographic/cognitive variables.
Exploratory correlations
To better understand the measure, we tested whether learning and retention on TMT-M was correlated with demographic and neuropsychological tasks (see ). We had no specific hypotheses. After correcting for multiple comparisons (p < .0023), the only correlation surviving correction was TMT-B, with greater procedural learning associated with faster time to complete TMT-B. While not surviving correction, there were tentative relationships with age (older age was associated with lower TMT-M learning), and TMT-M learning was positively associated with MMSE, and CVLT learning and retention. TMT-M retention was only associated with the BNT, in that better naming was associated with better retention of procedural learning.
Discussion
The current study directly compared episodic learning and retention with procedural learning and retention across individuals with aMCI, AD dementia, and HCs. Relationships between these measures and volumes of select brain regions were also examined. This study showed that while patients showed lower procedural learning relative to each other and controls, there was no difference in procedural retention for the groups. This confirmed our primary hypothesis that procedural memory would be relatively preserved in a population of patients with aMCI and AD dementia relative to healthy controls. As expected, patients with AD dementia displayed worse explicit retention than patients with aMCI who displayed worse explicit retention than HCs. Thus, patients with aMCI and AD dementia have relative preservation of procedural learning retention compared to explicit information retention. In addition, as hypothesized, retention of explicit verbal information was positively associated with the medial temporal cortex, while retention of procedural learning was not associated with these structures. However, procedural retention was not associated with basal ganglia volume as hypothesized. We will discuss each of these findings in turn.
With respect to learning, we predicted that patients with aMCI and AD dementia would show lower explicit learning with relatively preserved procedural learning. In contrast, patients with AD dementia showed lower learning relative to those with aMCI, who showed lower learning relative to HCs for both procedural and explicit memory tasks. While a preponderance of literature has shown intact procedural learning in aMCI and AD dementia (see De Wit et al., Citation2021 for meta-analysis; van Halteren-van Tilborg et al., Citation2007 for review), there have also been several isolated studies that failed to show this (Grober et al., Citation1992; Merbah et al., Citation2011). Multiple factors likely account for this discrepancy. The procedural memory test utilized in this study may not be directly comparable to previously studied tasks. Methodology, as it pertains to type of task and measurement of learning, has varied significantly across prior studies; for instance, outcome measures of interest have included reaction time, accuracy, the difference in learning across trials, speed, and accuracy. While patients with AD dementia have historically shown some level of learning on procedural tasks, there is more variability regarding whether their overall performance on these tasks differs from healthy controls. Our outcome measure considers initial performance, total learning, number of trials, and maximum possible gain. While patients with aMCI and AD dementia did show procedural learning with repeated exposure to this task, their overall learning performance was reduced relative to healthy controls.
As expected, patients with aMCI and AD dementia showed no significant differences in procedural retention (i.e., retention of improved completion time) compared with HCs. This is consistent with previous studies suggesting that patients with AD dementia have relatively preserved retention of procedural learning after delays (Knopman, Citation1991; Mochizuki-Kawai et al., Citation2004; Rouleau et al., Citation2002). Further, our study expands on existing literature in applying an adapted clinical measure that closely resembles the structure of commonly administered explicit memory tasks (e.g., repeatedly administered learning trials with a retention trial following a delay). This structure allows for more direct comparison to explicit memory tests, examination of both learning and retention components separately, and greater potential clinical utility.
This is the first study, to our knowledge, to investigate procedural memory in both aMCI and AD dementia. We demonstrated that patients with aMCI were no different in procedural retention from patients with AD dementia despite differences in explicit verbal retention. Only a few studies have examined implicit and procedural memory in aMCI (Baker et al., Citation2015; Gobel et al., Citation2013; Hong et al., Citation2020; Luft et al., Citation2015). Consistent with our results, these prior studies have shown relatively intact implicit memory in aMCI (through possible recruitment of alternate neural circuits as observed with functional neuroimaging, Luft et al., Citation2015). While it may be intuitive that if patients with AD dementia tend to show preserved procedural memory, then those with aMCI would also show this pattern given their milder level of impairment, this has not been thoroughly studied. Examining both aMCI and AD dementia groups is beneficial as it increases understanding of these abilities at multiple stages of disease severity.
Regarding structural neuroimaging findings, we partially demonstrated a dissociation between explicit and procedural retention relationships with the underlying neuroanatomy. While the expected positive relationship between the hippocampal and entorhinal cortex volumes with explicit retention was demonstrated (i.e., decreased volume was associated with decreased explicit retention), procedural retention was not correlated with hippocampal or entorhinal volumes. This makes intuitive sense, as we would not expect intact retention of procedural learning to correlate with a structure known to be affected by the AD process. These findings are consistent with previous research showing that reduced declarative memory on this particular word learning task is associated with smaller left hippocampal volume (Ystad et al., Citation2009). Similarly, a prior study examining neuroimaging correlates of declarative and procedural memory in AD also found verbal memory to be associated with hippocampal formation volume. However, procedural memory correlations with white matter alterations were examined rather than basal ganglia to differentiate vascular dementia from AD dementia. While the basal ganglia were not directly examined in that study, relationships between white matter disease and procedural memory were thought to indicate disrupted projections from the neostriatum (Libon et al., Citation1998).
In terms of neuroanatomical correlates of learning, the entorhinal cortex was associated with both explicit and procedural learning. In both cases, increased volume was associated with increased learning across trials. The entorhinal cortex has been associated with memory for many years with some studies reporting relationships with learning in addition to retention. Thus, our results support that the entorhinal cortex is associated with learning for both explicit and procedural learning, but not for retention. Procedural learning occurs, in part, in association with the entorhinal cortex, but retention is not associated with the entorhinal cortex or the hippocampus and may occur elsewhere. It is also notable that the hippocampus was associated with procedural learning but not explicit learning. Further work is necessary to better understand the neuroanatomical correlates of learning in patients with MCI and AD dementia.
Contrary to our predictions, procedural retention as measured by our task was not associated with basal ganglia volume despite previous evidence that populations with impairment to the basal ganglia (e.g., Parkinson’s dementia or focal lesions as seen in Haut et al., Citation2017) are impaired on many procedural tasks (Allain et al., Citation1995; Sarazin et al., Citation2002; Vakil et al., Citation2000). The absence of this hypothesized relationship may be a function of several factors. First, any potential changes in the volume of the basal ganglia may be subtle within our groups. Therefore, a relationship may not be detectable within this range of relatively intact procedural memory performances. Indeed, the range of performance was smaller for TMT-M retention than CVLT-II SF retention. For the TMT-M retention, the standard deviation was consistently about 20%, but for the CVLT-II SF the standard deviation was as much as 40%. Second, it has been demonstrated that measuring the relationship between basal ganglia structure and impaired cognitive abilities requires more subtle/sophisticated measurements than standard MRI volumetrics. For example, it has been demonstrated that there is a relationship between working memory and basal ganglia shape, but not volume, in schizophrenia spectrum disorders (Cobia et al., Citation2021) and with cannabis use (Smith et al., Citation2014). Future work using surface-based representations of changes in the shape of the basal ganglia may reveal a relationship between the basal ganglia and procedural learning and retention.
There are several strengths to this study. First, we have a relatively well-characterized sample diagnosed by consensus using established criteria. We also compared procedural and declarative memory across individuals with both aMCI and AD dementia, which has not been previously examined. Further, the inclusion of MRI volumes with memory performance allowed us to explore the underlying anatomical substrates of these tasks. Finally, we demonstrated that a commonly used clinical measure could be adapted for procedural learning and retention with limited extra time (approximately 5–10 minutes total additional time). There may also be rehabilitation implications; for instance, one study (Zanetti et al., Citation2001) found that patients with mild AD showed significant reduction in time to complete basic and instrumental activities of daily living after participation in a training program utilizing procedural learning. A practical example from our clinic involves teaching a patient to remember to use their cane. We instruct family members to place the cane against their leg while they are sitting in a chair and have the patient grab the cane as they arise from the chair repeatedly until it is “automatic.”
There are also several limitations to the study that must be considered. While well characterized, patients were clinically diagnosed, and many participants did not have biomarker confirmation of amyloid by either PET or CSF. In addition, the healthy controls did not receive a structural MRI for comparison with the clinical groups. Furthermore, our sample is relatively well educated and almost exclusively white, and thus generalization of our findings has limitations. In addition, there were differences between the number of learning trials for the explicit (the CVLT-II SF has been standardized with four learning trials) vs. the implicit (five learning trials were administered during TMT-M) task, but it is doubtful that led to the large effect of group by task interaction. The calculation of the learning slope and the retention variables accounted for learning ability regardless of the total number of trials. It is important to note that TMT-M has not yet been validated as a measure of procedural learning and retention through direct comparisons with other established measures, and this should be a focus of future studies. However, groups showed improved completion time of the task across repeated trials with some retention of this time improvement after a delay, and this pattern has been previously demonstrated with other procedural learning tasks such as mirror tracing (Gabrieli et al., Citation1993). Finally, we have a relatively modest sample of healthy control participants that will need to be expanded to provide normative information for clinical use of TMT-M. Despite these limitations, we demonstrated medium to large effect sizes in the hypothesized directions.
Future work should include replicating the findings using TMT-M with a more diverse sample and investigating the task with other clinical groups such as patients with motor-based diseases (e.g., Parkinson’s disease dementia and Lewy body disease). Exploring potential differences in procedural learning and retention in aMCI versus aMCI-md may also be worthwhile. Examination of changes in basal ganglia shape or complexity may be required to demonstrate a relationship between procedural learning and retention in populations with relatively intact basal ganglia structures. In addition, examination of individual data suggests that not all participants showed relatively intact procedural learning and retention. Larger samples would allow exploration of which patients fail to show procedural learning and retention relative to those who do. Validation of TMT-M with an established measure of procedural motor learning will be important. Finally, further normative work is needed to assist in the clinical interpretation of the Trail Making Test modification to measure procedural learning and retention.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Albert, M. S., DeKosky, S. T., Dickson, D., Dubois, B., Feldman, H. H., Fox, N. C., Phelps, C. H., Holtzman, D. M., Jagust, W. J., Petersen, R. C., Snyder, P. J., Carrillo, M. C., Thies, B., & Phelps, C. H. (2013). The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the national institute on aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Focus, 11(1), 96–106. doi:https://doi.org/10.1176/appi.focus.11.1.96
- Allain, H., Lieury, A., Quemener, V., Thomas, V., Reymann, J. M., & Gandon, J. M. (1995). Procedural memory and Parkinson’s disease. Dementia and Geriatric Cognitive Disorders, 6(3), 174–178. doi:https://doi.org/10.1159/000106942
- Baker, R., Bentham, P., & Kourtzi, Z. (2015). Learning to predict is spared in mild cognitive impairment due to Alzheimer’s disease. Experimental Brain Research, 233(10), 2859–2867. doi:https://doi.org/10.1007/s00221-015-4356-z
- Beaunieux, H., Eustache, F., Busson, P., De La Sayette, V., Viader, F., & Desgranges, B. (2012). Cognitive procedural learning in early Alzheimer’s disease: Impaired processes and compensatory mechanisms. Journal of Neuropsychology, 6(1), 31–42. doi:https://doi.org/10.1111/j.1748-6653.2011.02002.x
- Bondi, M. W., & Kaszniak, A. W. (1991). Implicit and explicit memory in Alzheimer’s disease and Parkinson’s disease. Journal of Clinical and Experimental Neuropsychology, 13(2), 339–358. doi:https://doi.org/10.1080/01688639108401048
- Bonner-Jackson, A., Mahmoud, S., Miller, J., & Banks, S. J. (2015). Verbal and non-verbal memory and hippocampal volumes in a memory clinic population. Alzheimer’s Research & Therapy, 7(1), 1–10 doi:10.1186/s13195-015-0147-9.
- Cobia, D., Rich, C., Smith, M. J., Mamah, D., Csernansky, J. G., & Wang, L. (2021). Basal ganglia shape features differentiate schizoaffective disorder from schizophrenia. Psychiatry Research: Neuroimaging, 317, 111352. doi:https://doi.org/10.1016/j.pscychresns.2021.111352
- Cohen, N. J., & Squire, L. R. (1980). Preserved learning and retention of pattern-analyzing skill in amnesia: dissociation of knowing how and knowing that. Science, 210(4466), 207–210. doi:https://doi.org/10.1126/science.7414331
- De Wit, L., Marsiske, M., O’Shea, D., Kessels, R. P., Kurasz, A. M., DeFeis, B., Smith, G. E., & Smith, G. E. (2021). Procedural learning in individuals with amnestic mild cognitive Impairment and Alzheimer’s dementia: A systematic review and meta-analysis. Neuropsychology Review, 31(1), 103–114. doi:https://doi.org/10.1007/s11065-020-09449-1
- Delis, D. C., Kramer, J. H., Kaplan, E. (2000). California verbal learning test - second edition. Adult Version. Manual.
- Devanand, D. P., Bansal, R., Liu, J., Hao, X., Pradhaban, G., & Peterson, B. S. (2012). MRI hippocampal and entorhinal cortex mapping in predicting conversion to Alzheimer’s disease. Neuroimage, 60(3), 1622–1629. doi:https://doi.org/10.1016/j.neuroimage.2012.01.075
- Deweer, B., Pillon, B., Michon, A., & Dubois, B. (1993). Mirror reading in AlzheimerAlzheimer’s disease: normal skill learning and acquisition of item-specific information. Journal of Clinical and Experimental Neuropsychology, 15(5), 789–804. doi:https://doi.org/10.1080/01688639308402596
- Deweer, B., Ergis, A. M., Fossati, P., Pillon, B., Boller, F., Agid, Y., & Dubois, B. (1994). Explicit memory, procedural learning and lexical priming in Alzheimer’s disease. Cortex, 30(1), 113–126. doi:https://doi.org/10.1016/S0010-9452(13)80327-9
- Di Paola, M., Macaluso, E., Carlesimo, G. A., Tomaiuolo, F., Worsley, K. J., Fadda, L., & Caltagirone, C. (2007). Episodic memory impairment in patients with Alzheimer’s disease is correlated with entorhinal cortex atrophy. Journal of Neurology, 254(6), 774–781. doi:https://doi.org/10.1007/s00415-006-0435-1
- Dick, M. B., Nielson, K. A., Beth, R. E., Shankle, W. R., & Cotman, C. W. (1995). Acquisition and long-term retention of a fine motor skill in Alzheimer's-disease. Brain and Cognition, 29(3), 294–306. doi:https://doi.org/10.1006/brcg.1995.1283
- Doyon, J., Bellec, P., Amsel, R., Penhune, V., Monchi, O., Carrier, J., Benali, H., & Benali, H. (2009). Contributions of the basal ganglia and functionally related brain structures to motor learning. Behavioural Brain Research, 199(1), 61–75. doi:https://doi.org/10.1016/j.bbr.2008.11.012
- Du, A. T., Schuff, N., Amend, D., Laakso, M. P., Hsu, Y. Y., Jagust, W. J., … Weiner, M. W. (2001). Magnetic resonance imaging of the entorhinal cortex and hippocampus in mild cognitive impairment and Alzheimer’s disease. Journal of Neurology, Neurosurgery, and Psychiatry, 71(4), 441–447. doi:https://doi.org/10.1136/jnnp.71.4.441
- Duara, R., Loewenstein, D. A., et-al, P. E., Appel, J., Greig, M. T., Urs, R., Shen, Q., Raj, A., Small, B., Barker, W., Schofield, E., Wu, Y., & Potter, H. (2008). Medial temporal lobe atrophy on MRI scans and the diagnosis of Alzheimer disease. Neurology, 71(24), 1986–1992. doi:https://doi.org/10.1212/01.wnl.0000336925.79704.9f
- Eslinger, P. J., & Damasio, A. R. (1986). Preserved motor learning in Alzheimer’s disease: implications for anatomy and behavior. Journal of Neuroscience, 6(10), 3006–3009. doi:https://doi.org/10.1523/JNEUROSCI.06-10-03006.1986
- Ezzati, A., Zammit, A. R., Habeck, C., Hall, C. B., & Lipton, R. B. (2020). Detecting biological heterogeneity patterns in ADNI amnestic mild cognitive impairment based on volumetric MRI. Brain Imaging and Behavior, 14(5), 1792–1804. doi:https://doi.org/10.1007/s11682-019-00115-6
- Fazekas, F., Chawluk, J. B., Alavi, A., Hurtig, H. I., & Zimmerman, R. A. (1987). MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. American Journal of Roentgenology, 149(2), 351–356. doi:https://doi.org/10.2214/ajr.149.2.351
- Folstein, M., McHugh, P., & McHugh, P. R. (1975). Mini mental state a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–198. doi:https://doi.org/10.1016/0022-3956(75)90026-6
- Gabrieli, J. D. E., Corkin, S., Mickel, S. F., & Growdon, J. H. (1993). Intact acquisition and long-term retention of mirror-tracing in Alzheimer’s disease and global amnesia. Behavioral Neuroscience, 107(6), 899–910. doi:https://doi.org/10.1037/0735-7044.107.6.899
- Gobel, E. W., Blomeke, K., Zadikoff, C., Simuni, T., Weintraub, S., & Reber, P. J. (2013). Implicit perceptual-motor skill learning in mild cognitive impairment and Parkinson’s disease. Neuropsychology, 27(3), 314. doi:https://doi.org/10.1037/a0032305
- Graf, P., Squire, L. R., & Mandler, G. (1984). The information that amnesic patients do not forget. Journal of Experimental Psychology. Learning, Memory, and Cognition, 10(1), 164. doi:https://doi.org/10.1037//0278-7393.https://doi.org/10.1.164
- Grober, E., Ausubel, R., Sliwinski, M., & Gordon, B. (1992). Skill learning and repetition priming in Alzheimer’s disease. Neuropsychologia, 30(10), 849–858. doi:https://doi.org/10.1016/0028-3932(92)90030-P
- Haut, M. W., Hogg, J. P., Marshalek, P. J., Suter, B. C., & Miller, L. E. (2017). Amnesia associated with bilateral hippocampal and bilateral basal ganglia lesions in anoxia with stimulant use. Frontiers in Neurology, 8, 27. doi:https://doi.org/10.3389/fneur.2017.00027
- Hays, C. C., Zlatar, Z. Z., Meloy, M. J., Bondi, M. B., Gilbert, P. E., Lui, T. H., L, J., & Wierenga, C. W. (2020). Interaction of APOE, cerebral blood flow, and cortical thickness in the entorhinal cortex predicts memory decline. Brain Imaging and Behavior, 14(2), 369–382. doi:https://doi.org/10.1007/s11682-019-00245-x
- Hong, Y., Alvarado, R. L., Jog, A., Greve, D. N., & Salat, D. H. (2020). Serial reaction time task performance in older adults with neuropsychologically defined mild cognitive impairment. Journal of Alzheimer’s Disease, 74(2), 491–500. doi:https://doi.org/10.3233/JAD-191323
- Huo, Y., Zhoubing, X., Aboud, K., Parvathaneni, P., Bao, S., Bermudez, C., Resnick, S. M., Cutting, L. E., & Landman, B. A. 2018“Spatially localized atlas network tiles enables 3D whole brain segmentation” In International Conference on Medical Image Computing and Computer-Assisted Intervention, MICCAI (Cham: Springer) .
- Huo, Y., Xu, Z., Xiong, Y., Aboud, K., Parvathaneni, P., Bao, S., Landman, B. A., Resnick, S. M., Cutting, L. E., & Landman, B. A. (2019). 3D whole brain segmentation using spatially localized atlas network tiles. NeuroImage, 194, 105–119. doi:https://doi.org/10.1016/j.neuroimage.2019.03.041
- Kaplan, E., Goodglass, H., & Weintraub, S. (2001). Boston naming test. PRO-ED Inc.
- Killiany, R. J., Hyman, B. T., t, G.-I., Moss, M. B., Kikinis, R., Jolesz, F., Tanzi, R., Jones, K., & Albert, M. S. (2002). MRI measures of entorhinal cortex vs. hippocampus in preclinical AD. Neurology, 58(8), 1188–1196. doi:https://doi.org/10.1212/WNL.58.8.1188
- Knopman, D. (1991). Long-term retention of implicitly acquired learning in patients with Alzheimer’s disease. Journal of Clinical and Experimental Neuropsychology, 13(6), 880–894 doi:10.1080/01688639108405105.
- Knowlton, B. J., Mangels, J. A., & Squire, L. R. (1996). A neostriatal habit learning system in humans. Science, 273(5280), 1399–1402. doi:https://doi.org/10.1126/science.273.5280.1399
- Koedam, E. L., Lehmann, M., van der Flier, W. M., Scheltens, P., Pijnenburg, Y. A. L., Fox, N., Barkhof, F., & Wattjes, M. P. (2011). Visual assessment of posterior atrophy development of a MRI rating scale. European Radiology, 21(12), 2618–2625. doi:https://doi.org/10.1007/s00330-011-2205-4
- Laakso, M. P., Soininen, H., Partanen, K., Helkala, E. L., Hartikainen, P., Vainio, P., Riekkinen, P. J., Sr, Hänninen, T., & Riekkinen Sr, P. J. (1995). Volumes of hippocampus, amygdala and frontal lobes in the MRI-based diagnosis of early Alzheimer’s disease: Correlation with memory functions. Journal of Neural transmission-Parkinson’s Disease and Dementia Section, 9(1), 73–86. doi:https://doi.org/10.1007/BF02252964
- Lehmann, M., Koedam, E. L., et-al, B. J., Bartlett, J. W., Ryan, N. S., Pijnenburg, Y. A. L., Barkhof, F., Wattjes, M. P., Scheltens, P., & Fox, N. C. (2012). Posterior cerebral atrophy in the absence of medial temporal lobe atrophy in pathologically-confirmed Alzheimer’s disease. Neurobiology of Aging, 33(3), 627.e1–627.e12. doi:https://doi.org/10.1016/j.neurobiolaging.2011.04.003
- Libon, D. J., Bogdanoff, B., Cloud, B. S., Skalina, S., Giovannetti, T., Gitlin, H. L., & Bonavita, J. (1998). Declarative and procedural learning, quantitative measures of the hippocampus, and subcortical white alterations in Alzheimer’s disease and ischaemic vascular dementia. Journal of Clinical and Experimental Neuropsychology, 20(1), 30–41. doi:https://doi.org/10.1076/jcen.20.1.30.1490
- Luft, C. D. B., Baker, R., Bentham, P., & Kourtzi, Z. (2015). Learning temporal statistics for sensory predictions in mild cognitive impairment. Neuropsychologia, 75, 368–380. doi:https://doi.org/10.1016/j.neuropsychologia.2015.06.002
- McKhann, G. M., Knopman, D. S., Chertkow, H., Hyman, B. T., Jack, C. R., Jr, Kawas, C. H., Klunk, W. E., Koroshetz, W. J., Manly, J. J., Mayeux, R., Mohs, R. C., Morris, J. C., Rossor, M. N., Scheltens, P., Carrillo, M. C., Thies, B., Weintraub, S., & Phelps, C. H. (2011). The diagnosis of dementia due to Alzheimer’s disease: recommendations from the national institute on aging‐Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia, 7(3), 263–269. doi:https://doi.org/10.1016/j.jalz.2011.03.005
- Merbah, S., Salmon, E., & Meulemans, T. (2011). Impaired acquisition of a mirror-reading skill in Alzheimer’s disease. Cortex, 47(2), 157–165. doi:https://doi.org/10.1016/j.cortex.2009.11.006
- Milner, B., Corkin, S., & Teuber, H. L. (1968). Further analysis of the hippocampal amnesic syndrome: 14-year follow-up study of HM. Neuropsychologia, 6(3), 215–234. doi:https://doi.org/10.1016/0028-3932(68)90021-3
- Mochizuki-Kawai, H., Kawamura, M., Hasegawa, Y., Mochizuki, S., Oeda, R., Yamanaka, K., & Tagaya, H. (2004). Deficits in long-term retention of learned motor skills in patients with cortical or subcortical degeneration. Neuropsychologia, 42(13), 1858–1863. doi:https://doi.org/10.1016/j.neuropsychologia.2004.03.012
- Nettiksimmons, J., DeCarli, C., Landau, S., & Beckett, L., & Alzheimer’s Disease Neuroimaging Initiative. (2014). Biological heterogeneity in ADNI amnestic mild cognitive impairment. Alzheimer’s & Dementia, 10(5), 511–521. doi:https://doi.org/10.1016/j.jalz.2013.09.003
- Oudman, E., Nijboer, T. C., Postma, A., Wijnia, J. W., & Van der Stigchel, S. (2015). Procedural learning and memory rehabilitation in Korsakoff’s syndrome-a review of the literature. Neuropsychology Review, 25(2), 134–148. doi:https://doi.org/10.1007/s11065-015-9288-7
- Pfeffer, R. I., Kurosaki, T. T., Harrah, C. H., Chance, J. M., & Filos, S. (1982). Measurement of functional activities in older adults in the community. Journal of Gerontology, 37(3), 323–329. doi: https://doi.org/10.1093/geronj/37.3.323
- Reitan, R. M., & Wolfson, D. (1985). The Halstead–Reitan Neuropsycholgical Test Battery: Therapy and clinical interpretation. Tucson, AZ: Neuropsychological Press.
- Rouleau, I., Salmon, D. P., & Vrbancic, M. (2002). Learning, retention and generalization of a mirror tracing skill in Alzheimer’s disease. Journal of Clinical and Experimental Neuropsychology, 24(2), 239–250. doi:https://doi.org/10.1076/jcen.24.2.239.997
- Sarazin, M., Deweer, B., Merkl, A., Von Poser, N., Pillon, B., & Dubois, B. (2002). Procedural learning and striatofrontal dysfunction in Parkinson’s disease. Movement Disorders: Official Journal of the Movement Disorder Society, 17(2), 265–273. doi:https://doi.org/10.1002/mds.10018
- Sheikh, J. I., & Yesavage, J. A. (1986). Geriatric depression scale (GDS): recent evidence and development of a shorter version. Clinical Gerontologist: The Journal of Aging and Mental Health, 5(1–2), 165–173. doi: https://doi.org/10.1300/J018v05n01_09
- Smith, M. J., Cobia, D. J., Wang, L., Alpert, K. I., Cronenwett, W. J., Goldman, M. B., and Csernansky, J. G. (2014). Cannabis-related working memory deficits and associated subcortical morphological differences in healthy individuals and schizophrenia subjects. Schizophrenia Bulletin, 40(2), 287–299.
- Squire, L. R. (2004). Memory systems of the brain: A brief history and current perspective. Neurobiology of Learning and Memory, 82(3), 171–177. doi:https://doi.org/10.1016/j.nlm.2004.06.005
- Tulving, E. (1985). How many memory systems are there? American Psychologist, 40(4), 385. doi:https://doi.org/10.1037/0003-066X.40.4.385
- Vakil, E., Kahan, S., Huberman, M., & Osimani, A. (2000). Motor and non-motor sequence learning in patients with basal ganglia lesions: The case of serial reaction time (SRT). Neuropsychologia, 38(1), 1–10. doi:https://doi.org/10.1016/S0028-3932(99)00058-5
- van Halteren-van Tilborg, I. A., Scherder, E. J., & Hulstijn, W. (2007). Motor-skill learning in Alzheimer’s disease: A review with an eye to the clinical practice. Neuropsychology Review, 17(3), 203–212. doi:https://doi.org/10.1007/s11065-007-9030-1
- Wahlund, L. O., Julin, P., Johansson, S. E., & Scheltens, P. (2000). Visual rating and volumetry of the medial temporal lobe on magnetic resonance imaging in dementia: A comparative study. Journal of Neurology Neurosurgery Psychiatry, 69(5), 630–635. doi:https://doi.org/10.1136/jnnp.69.5.630
- Wechsler, D. (2008). Wechsler adult intelligence scale - fourth edition. Psychological Corporation: San Antonio, TX, USA.
- Wilkinson, G. S., & Robertson, G. J. (2006). WRAT 4: wide range achievement test. Lutz, FL: Psychological Assessment Resources.
- Ystad, M. A., Lundervold, A. J., Wehling, E., Espeseth, T., Rootwelt, H., Westlye, L. T., & Lundervold, A. (2009). Hippocampal volumes are important predictors for memory function in elderly women. BMC Medical Imaging, 9(1), 1–15. doi:https://doi.org/10.1186/1471–2342-9-17
- Zanetti, O., Zanieri, G., Giovanni, G. D., De Vreese, L. P., Pezzini, A., Metitieri, T., & Trabucchi, M. (2001). Effectiveness of procedural memory stimulation in mild Alzheimer’s disease patients: A controlled study. Neuropsychological Rehabilitation, 11(3–4), 263–272. doi:https://doi.org/10.1080/09602010042000088