Abstract
Objective: Limb apraxia is a motor cognitive disorder that has been mainly studied in patients with dementia or left hemisphere stroke (LHS). However, limb apraxia has also been reported in patients with right hemisphere stroke (RHS), multiple sclerosis (MS) or traumatic brain injury (TBI). This study’s aim was to report detailed praxis performance profiles in samples suffering from these different neurological disorders by use of the Diagnostic Instrument for Limb Apraxia (DILA-S).
Method: 44 LHS patients, 36 RHS patients, 27 patients with dementia, 26 MS and 44 TBI patients participated. The diagnostics included the imitation of meaningless and meaningful hand gestures, pantomime of tool-use, single real tool-use as well as a multistep naturalistic action task (preparing breakfast).
Results: Apraxia occurred in all tested samples but to a varying degree and with dissimilar profiles. LHS patients demonstrated most severe deficits in pantomime, but they were also vulnerable to deficits in real tool-use. Dementia patients showed high incidence rates of apraxia in almost all subscales of the DILA-S. RHS patients demonstrated difficulties in imitation and pantomime of tool-use, but they did not show severe difficulties with real tool-use. TBI patients appeared challenged by multistep naturalistic actions. The tested MS sample did not show clinically relevant symptoms in the DILA-S.
Conclusion: Different types of patients display varying limb apraxic symptoms detectable by the DILA-S. In these limb apraxia susceptible populations, testing should be warranted as standard. Prospectively, individual error profiles may be helpful for shaping motor cognitive training.
Introduction
Limb apraxia is commonly defined as a “disorder of movement not caused by weakness, akinesia, deafferentation, abnormal tone or posture, movement disorders (such as tremors or chorea), intellectual deterioration, poor comprehension, or uncooperativeness” (Heilman & Rothi, Citation1993). It is characterized by impairments in motor cognitive tasks such as imitating meaningless gestures or meaningful emblems, impairments in pantomiming tool-use or deficits in real single-step or multi-step tool-use affecting both hands (e.g., Goldenberg, Citation2011). Limb apraxia can occur after several neurological disorders like stroke, neurodegenerative diseases, traumatic brain injury (TBI) or multiple sclerosis (MS; for references see below). Dependent on lesion localization and size, all of the above-named domains or just selective parts can be affected (Goldenberg, Citation2009). Despite the diversity in affected domains, most of the test instruments only evaluate performance in imitation and pantomime tasks when examining limb apraxia (e.g., the Dementia Apraxia Test: Johnen Frommeyer, et al., Citation2016; the Waterloo Apraxia Battery: Stamenova, Citation2010; the Short Screening Test for Ideomotor Apraxia: Tessari, Toraldo, Lunardelli, Zadini & Rumiati, Citation2015; the Apraxia Screen of TULIA: Vanbellingen et al., Citation2011b; the Test of Upper Limb Apraxia: Vanbellingen et al., Citation2010; or the Kölner Apraxie Screening: Weiss, Kalbe, Kessler & Fink, Citation2013).
Research on limb apraxia primarily focuses on stroke and dementia patients, whereby it is also known to occur in patients with neurologic disorders such as TBI (e.g., Acosta, Bennett & Heilman, Citation2014; McKenna, Thakur, Marcus & Barrett, Citation2013), MS (e.g., Kamm et al., Citation2012; Staff, Lucchinetti & Keegan, Citation2009), Parkinson’s disease (Foki et al., Citation2016; Kübel, Stegmayer, Vanbellingen, Walther & Bohlhalter, Citation2018), corticobasal syndrome (Acosta et al., Citation2014; Borroni et al., Citation2008; Peigneux et al., Citation2001; Stamenova et al., Citation2015), or Huntington´s disease (Hamilton, Haaland, Adair & Brandt, Citation2003) as well as in patients with psychiatric diseases like schizophrenia (Dutschke et al., Citation2017; Stegmayer et al., Citation2016).
One major challenge for diagnosing limb apraxia is the fact that measures of limb apraxia can be strongly affected by motor deficits (Goldenberg, Citation2011). This presents a diagnostic challenge in most neurologic disorders and in particular in those that go along with major symptoms of bilateral motor deficits (e.g., tremor and rigor), such as typically present in Parkinson’s disease, corticobasal syndrome or Huntington’s disease (Bader, Citation2014).
In the current paper we will focus on neurology patient groups suffering from unilateral stroke, dementia, MS or TBI. We included only patients with spared or minimally affected motor function in at least one upper limb. While we acknowledge that other neurology and even psychiatry patient groups may demonstrate apraxic symptoms, their inclusion would be beyond the scope of the here described study. In the following, we would like to briefly summarize results of limb apraxia prevalence rates described in the literature in patients with unilateral stroke, dementia, MS or TBI.
In studies of limb apraxia, stroke patients typically are divided into patient groups with first time right (RHS) or left (LHS) hemisphere stroke. The latter group usually is studied more extensively due to the frequently described association of limb apraxia with left hemisphere lesions. Accordingly, reported prevalence rates of limb apraxia range between 0 and 34% for patients with RHS (e.g., Donkervoort, Dekker, Van den Ende & Stehmann-Saris, Citation2000; Wirth et al., Citation2016) and 28–57% for patients with LHS (e.g., Donkervoort et al., Citation2000; Dovern, Fink & Weiss, Citation2012; Kaya, Unsal-Delialioglu, Kurt, Altinok & Ozel, Citation2006; Weiss et al., Citation2013).
Further, limb apraxia is a well-studied symptom in patients with dementia (e.g., Chandra, Isaac, & Abbas, 2015; Cotelli, Manenti, Brambilla & Balconi, Citation2014; Johnen, Frommeyer, et al., Citation2016). It is recognized as a diagnostic characteristic of Alzheimer’s disease and as such it is included in the criteria of the National Institute of Neurological and Communicative Diseases and Stroke-Alzheimer´s Disease and Related Disorders Association (NINCDS-ADRDA; McKhann et al., Citation1984; McKhann et al., Citation2011). Prevalence rates for difficulties in imitation and pantomime tasks range between 32 and 69% in Alzheimer’s disease patients (Ahmed, Baker, Thompson, Husain & Butler, Citation2016; Ozkan, Adapinar, Elmaci & Arslantas, Citation2013), while difficulties seem to increase with ongoing disease decline (Edwards, Deuel, Baum & Morris, Citation1991; Parakh, Roy, Koo & Black, Citation2004; Wu & Lin, Citation2015), and go along with worse Mini Mental State Examination (MMSE) scores and deficient language comprehension abilities (Cotelli et al., Citation2014; Stamenova, Roy & Black, Citation2014; Wu & Lin, Citation2015). Real tool-use impairment prevalence rates were reported with similar levels of 25–54% impaired patients (Del Ser, Hachinski, Merskey & Munoz, Citation2001; LeClerc, Wells, Sidani, Dawson & Fay, Citation2004; Wu & Lin, Citation2015). Moreover, studies indicated that Alzheimer’s disease and vascular dementia patients have higher apraxia incidence rates than patients with mild cognitive impairment (Ahmed et al., Citation2016; Ozkan et al., Citation2013), frontotemporal dementia (Ahmed et al., Citation2016; Chandra, Isaac, & Abbas, 2015; Johnen, Frommeyer, et al., Citation2016; Johnen et al., Citation2015) or semantic dementia (Baumard et al., Citation2016).
To date, we only found two articles that clinically diagnosed limb apraxia in a group of TBI patients: McKenna et al. (Citation2013) postulated that up to 35–60% of patients with TBI show symptoms of limb apraxia, while patients with co-occurring spinal cord injury were reported to be especially vulnerable to these symptoms. Falchook et al. (Citation2015) examined patients with severe TBI and resulting damage to the corpus callosum with the Apraxia Screen of TULIA (Vanbellingen et al., Citation2011b). They found this patient group on average to show apraxic symptoms for the left, but not the right hand. However, prevalence rates were not reported. Concluding, to date there is only limited diagnostic information available on limb apraxia in TBI patients.
Limb apraxia symptoms seem to occur in about 13–27% of MS patients, but mostly only mild deficits were reported (Harscher, Hirth-Walther, Buchmann, Dettmers & Randerath, Citation2017; Kamm et al., Citation2012; Rapaic, Medenica, Kozomara & Ivanovic, Citation2014; Staff et al., Citation2009). Low severity levels could be explained by the relatively small amount of 20% brain atrophy in the gray matter compared to 80% brain atrophy in the white matter (Gil Moreno et al., Citation2013). At the same time neuropsychological symptoms such as limb apraxia or aphasia are typically reported to involve gray matter lesions. In line with this, aphasia, which frequently co-occurs with limb apraxia, has been shown to occur in only 0.8% of MS patients (Larner & Lecky, Citation2007) and the frequency of patients affected with limb apraxia increases with a higher Expanded Disability Status Scale (EDSS: score measuring neurological deficits in MS patients; Kurtzke, Citation1983) and disease duration (Kamm et al., Citation2012; Rapaic et al., Citation2014). Whether patients with different subtypes of MS (PPMS: primarily chronic progressive MS, SPMS: secondarily chronic progressive MS, RRMS: relapsing remitting MS) vary in their prevalence rates of limb apraxia is a controversial issue (Kamm et al., Citation2012; Rapaic et al., Citation2014).
Because the described patient samples have clearly different neuropathological underpinnings, they may demonstrate differential apraxic profiles. Thus, it is of interest to see what praxic deficits occur predominantly per respective sample by use of the same test battery. This study may contribute to inform clinicians about aspects that deserve special attention for diagnosis and treatment in the respective patient population. To our knowledge, the described patient groups to date were examined with different apraxia tests which in most studies only included classical imitation or pantomime tasks. Here we would like to provide data on unilateral stroke, dementia, MS or TBI patients who were tested with the same limb apraxia test battery (DILA-S). The DILA-S includes both classical tasks assessing the imitation of hand postures and pantomime of tool-use, as well as different real tool-use tasks involving novel tools, familiar tools and a serial familiar object interaction task (making breakfast).
Our goal was to provide an overview of sample-related limb apraxia profiles including information on different domains in order to contribute to a better understanding of the disorder’s impact (Deutsch-Lezak, Howieson, Bigler & Tranel, Citation2012).
Methods
Participants
Participants were recruited from four different clinics in Germany and Switzerland. The four different inpatient groups are described below. For the distinct patient groups, several tests assessing aphasia, working memory, semantic processing, motor difficulties and neglect were used. For demographic data and data on the conducted neuropsychological tests please see .
Table 1. Demographic and clinic data.
Since patients with limb apraxia frequently also demonstrate impairments in language production and comprehension (Goldenberg & Randerath, Citation2015), it is not surprising that the current study includes patients for whom comprehension difficulties were detected by aphasia assessment (measured with the “Token” subtest of the Aachener Aphasia Test (AAT; Huber, Poeck, Weniger & Willmes, Citation1983). Distributions of patients without, with mild, moderate or severe aphasia are shown in . Please note, only patients who understood the instructions of the Token Test remained included in the study. Further, it was important to ensure that praxic but not language difficulties were measured by the limb apraxia assessment. The DILA-S provides up to three practice trials for each apraxia task (see section Assessment of Limb Apraxia) to verify comprehension of simple task instructions and to familiarize the patient with the upcoming task. In these practice trials, patients either have to demonstrate correct solutions or at least attempts to produce a skillful movement. Practice trials were pre-tested with 33 LHS patients with comprehension problems and were solvable for more than 95% of patients (Buchmann & Randerath, Citation2017). All of the here included patients were able to follow the instructions.
In addition, visuospatial processing performance may influence test results and was assessed by the Star Cancellation and Line Bisection Tests (Plummer, Morris & Dunai, Citation2003). Care was taken to minimize an influence of visuo-spatial deficits on the assessment of limb apraxia. In case such difficulties were apparent, the photos and items of the tasks were shifted towards the unaffected hemi-space. Before beginning the tasks, participants normally are instructed only once to pay attention to each of the three tools (see section Assessment of Limb Apraxia for task structure) and material on the table. However, patients with visuospatial deficits received this prompt before each trial and in case they could not find certain objects on the table (e.g., “Please make sure to look to your left/right.”).
Of course it would also be interesting to report representative data on participants who preferred to use their left hand before onset of illness. Left hand dominance typically amounts to 10% of the population (Hardyck & Petrinovich, Citation1977). However, for our relatively small samples it was verified that all patients were initially right hand dominant (diagnosed with lateralization quotient >60; Salmaso & Longoni, Citation1983) in order to avoid the potential heterogeneity introduced by left handedness (please see Goldenberg, Citation2013 for a discussion on apraxia in stroke-patients who were left hand dominant before the incident).
All patients were able to withstand testing sessions of at least 30 minutes.
Further, to determine the patient’s neurologic disease, we consulted the medical record and in case of stroke and TBI patients also their MRI or CT scans. A participant was excluded if additional to the neurological disease for which the patient was recruited a co-morbid neurological or psychiatric diagnosis was mentioned.
The study design was approved by the ethical committee of the University of Konstanz and the Cantonal Ethics Committee in Thurgovia on research involving humans. All patients took part in the study voluntarily. Informed consent was obtained, and privacy rights were observed. The study was conducted in accordance with the Declaration of Helsinki.
Stroke patients
Participants were recruited from the neurorehabilitation clinic “Kliniken Schmieder” in Allensbach, Germany. All patients suffered from first time stroke at least 19 days ago (see ) and were classified by ICD-10 code (International Statistical Classification of Diseases and Related Health Problems) as I60 (subarachnoid haemorrhage), I61 (intracerebral haemorrhage), I62 (other nontraumatic intracranial haemorrhage), I63 (cerebral infarction), or I64 (stroke, not specified as haemorrhage). A total of 80 patients with left (LHS, N = 44) or right hemisphere lesions (RHS, N = 36), verified by MRI or CT scan, participated. None of the patients suffered from any other neurological or psychiatric disease. 56.8% of LHS patients suffered from comprehension difficulties, diagnosed with the Aachener Aphasia Test (Huber et al., Citation1983), whereas in the RHS patient group only 8.3% showed mild aphasia symptoms. All of the patients were able to understand task instructions (for further information see above). Patients were tested with their neurologically unaffected ipsilesional hand (LHS: left, RHS: right).
TBI patients
Participants were recruited from the neurorehabilitation clinic “Kliniken Schmieder” in Allensbach, Germany (N = 28) and from the neurorehabilitation clinic “Rehaklinik Zihlschlacht Neurologisches Rehabilitationszentrum” in Zihlschlacht, Switzerland (N = 16). All patients suffered from first time TBI at least 27 days ago (see ) and did not suffer from any other neurological or psychiatric disease. TBI was classified via ICD-10 as S06 intracranial injury, mostly accompanied by traumatic subarachnoid hemorrhage (S06.6). 20.5% of the patients were female, which is in line with previous studies showing that men are more often affected by TBI than women, have longer hospitalization times and show higher degrees of TBI severity (Röhrer, Citation2013; Seebauer, Citation2015), which accounts for the higher number of men in rehabilitation clinics. Patients were tested with the hand that showed better motor functions according to their performance in a short version of the Wolf Motor Function Test (N = 6 with left hand, N = 29 with right hand; Wolf et al., Citation2001). Patients who did not demonstrate motor impairments in either hand were tested with their preferred dominant hand (N = 26) or were tested with both hands (N = 9). For patients being tested with both hands, performance did not differ between hands (Z ≤ −.520, p ≥ .156), therefore mean performance of both hands was used for further analysis.
MS patients
Participants were recruited from the neurorehabilitation clinic “Kliniken Schmieder” in Konstanz, Germany. The patient population was first described in Harscher et al. (Citation2017). All patients (N = 26) suffered from MS, diagnosed with the revised McDonald criteria (Polman et al., Citation2011) as ICD-10 code G35 (MS). 15% (N = 4) of patients were diagnosed with PPMS, 42% (N = 11) with SPMS and 39% (N = 10) with RRMS. One patient (4%) had a secured MS diagnosis, but the diagnosis was not further specified. With the exception of fatigue (a frequent symptom of MS; Sehle, Neumann, Spiteri & Dettmers, Citation2014) or adjustment disorder, the patients did not suffer from any other neurological or psychiatric diseases. To minimize the influence of motor deficits patients were tested with their stronger hand (N = 7 left; N = 19 right). To be included in the study, the tested hand needed to have a minimal muscle power of 4 kg on an electronic hand dynamometer (Trailite TL-LSC100 from LiteXpress GmbH; 2012) and a minimum of 15 mm discrimination sensitivity (Two-Point Discriminator Touch Test from North Coast Medical, Inc. 2013). 77% of patients were female, which is in line with reported prevalence rates of MS in Germany (see https://www.dmsg.de/multiple-sklerose-infos/was-ist-ms/).
Dementia patients
Participants were recruited from the clinic for geriatric psychiatry at the “Zentrum für Psychiatrie” Reichenau in Konstanz, Germany. Inclusion criteria were Alzheimer’s disease (N = 20) or vascular dementia (N = 7), diagnosed by an expert (G.R., head physician of the clinic for geriatric psychiatry). Patients with Alzheimer´s disease and vascular dementia are both defined by ICD-10 code as belonging to the dementia subgroup. Patients with Alzheimer’s disease were classified by ICD-10 code F00.0 (dementia in Alzheimer’s disease with early onset), F00.1 (dementia in Alzheimer’s disease with late onset) or F00.2 (dementia in Alzheimer´s disease, atypical or mixed type). Patients with vascular dementia were classified by ICD-10 code F01.1 (multi-infarct dementia), F01.2 (subcortical vascular dementia) or F01.3 (mixed cortical and subcortical vascular dementia). In this clinic patients with vascular dementia typically had suffered from multiple minor strokes, and their clinical progression including functional impairments usually occurred step by step. There is some evidence that suggests that Alzheimer’s disease and vascular dementia groups do not differ in their praxis performance (Ahmed et al., Citation2016; Ozkan et al., Citation2013). Stroke onset in vascular dementia patients was at least two years ago (as opposed to a considerably shorter time since stroke onset in the stroke patient groups, LHS: M = 89.5 days ago, RHS: M = 60.31 days ago). A total of 27 patients were included. None of the patients showed any accompanying psychiatric disease. The patients with Alzheimer’s disease did not suffer from any neurological disorder, all patients with vascular dementia suffered from stroke. 43.5% of all dementia patients had comprehension difficulties ranging from mild (N = 7) to moderate (N = 3) symptoms. All patients showed no symptoms of hemiparesis and were tested with their right hand. Patients with Alzheimer’s disease or vascular dementia did not differ in most neuropsychological tests (U ≥ 35.00, p ≥ .283). Consequently, these test results are reported for the entire sample in . The BOSU (Bogenhausener Semantikuntersuchung) subtest sorting for major features results are reported for vascular dementia and Alzheimer’s disease separately, since group comparisons showed that vascular dementia patients scored worse in this test (U = 33.00, p = .028).
Description of patient groups
For group comparisons across all demographic variables please see Table S.3 (supplementary material). Patients differed in gender proportion (H (4) = 24.26, p = .000). There were significantly more men in the TBI group than in all other patient groups (U ≥ 249.00, p ≤ .023). In the patient group with MS significantly more females were represented compared to the LHS (U = 392.00, p = .013) and TBI (U = 249.00, p = .000) patient groups. There were no differences between gender distribution found between MS and RHS (U = 368.00, p = .109) or dementia (U = 289.00, p = .241) patient groups. All other group comparisons regarding gender did not reach significance (U ≥ 289.00, p ≥ .241).
Further, patients also differed in age (F (4) = 35.92, p = .000). Patients with dementia were significantly older than patients in other groups (p = .000). Additionally, LHS and RHS patients were older than TBI and MS patients (p ≤ .002). The other groups did not differ from each other with respect to age (p = 1.000).
Gender and age variables influence cut-off values of apraxia tasks (see Table S.1).
For group comparison we refrained from using absolute performance scores. Instead, distributions of apraxia severity per subscale were compared (see Statistical Analyses section).
As described in , the distributions of tested hand differed in the patient groups (LHS: 100% left, RHS: 100% right; TBI: 66% right, 14% left, 20% both; MS: 73% right, 27% left; dementia: 100% right). Please note that in healthy right handed participants a between subjects analysis revealed that testing the right versus the left hand did not differ in performance in any of the DILA-S subtests (U ≥ 646.50, p ≥ .114; see Randerath, Buchmann, Liepert & Büsching, Citation2017).
Assessment of limb apraxia – the diagnostic instrument for limb apraxia
The applied Diagnostic Instrument for Limb Apraxia – Short Version (DILA-S) is described in detail in Randerath et al. (Citation2017).1 Existing versions of subtests were integrated with permission from the author (imitation of meaningless gestures: Goldenberg, Citation1996). Other subtests were revised (novel tools test: Goldenberg & Hagmann, Citation1998; pantomime of tool-use: Goldenberg, Hartmann & Schlott, Citation2003; naturalistic action test - breakfast task: Schwartz, Segal, Veramonti, Ferraro & Buxbaum, Citation2002) or newly developed (imitation of meaningful gestures, familiar tools test). To calculate cut-off values the 5th percentile was computed for each task based on the performance of a normative healthy right handed control group (N = 82). A distinction between mild, moderate and severe apraxia was drawn for each subset in reference to the performance of 20 LHS and 5 RHS patients who were diagnosed apraxic (Buchmann & Randerath, Citation2017). Based on their performance distribution, a diagnosis of mild apraxia was attributed between the 75th and 100th percentile, moderate apraxia between the 50th and 74th percentile and severe apraxia below the 50th percentile. All cut-off values are listed in Table S.1 (see supplementary material).
In some cases it was not possible to perform all tasks of the DILA-S test battery, particularly in the dementia group. In the dementia group three patients did not perform the novel tools test and seven patients did not perform the naturalistic action test - breakfast task. For all other groups, data of maximally one patient per subtest is missing. For a distinct group description, including information on missing data, please see Table S.2 (supplementary material).
Testing and rating was completed by trained students. Interrater reliability has been shown to be sufficiently high (τ ≥ .577, p ≤ .038; Randerath et al., Citation2017).
Classical tests: imitation of hand gestures and pantomime of tool-use
Patients were requested to imitate meaningless (IML) or meaningful (IMF) hand gestures shown by the experimenter. For evaluation, patients achieved 0–2 points per item depending on the correct performance at the first (2 points) or second (1 point) attempt, or if both attempts were incorrect then 0 points were given.
Further, patients were instructed to pantomime the use of an object (PTU) by both verbal and visual cues (e.g., verbal: “Show me how to hit a nail with a hammer”, visual: picture of a hammer). Two evaluation scales were used. The production scale included the qualitative rating of different movement aspects such as grip-formation, movement-content, and movement-orientation. Further, the execution scale evaluated the amount of needed attempts with the three-tier evaluation system similar to the imitation tasks (0–2 points).
Real tool-use tests: novel and familiar tools test
Patients were asked to select the one tool that is best suitable to safely lift a cylinder out of a socket (novel tools test: NTT) or to manipulate a familiar object (familiar tools test: FTT). An item consisted of three tools and one recipient cylinder (NTT) or one recipient familiar object to be manipulated (FTT), respectively. For the NTT and FTT three evaluation scales were used. First, selection of the correct tool was awarded with 0–2 points per item. Second, for the evaluation of an appropriate usage the following movement aspects were assessed: grip-formation, grip-orientation, movement-content, and movement-orientation. Similar to the above described pantomime task two scales were applied that either focus on the quality of the finally produced movement (production scale) or the amount of needed attempts based on a three-tier evaluation system (execution scale).
Naturalistic action test- breakfast task
Patients were asked to prepare a breakfast consisting of a toasted slice of bread with butter and jam as well as a cup of tea with sugar (NAT; adapted from Schwartz et al., Citation2002). For evaluation, the naturalistic action test score (NAT score) was derived by combining the accomplishment score and error score. For the accomplishment score the amount of completed steps is counted. The error score indicated the amount of errors made while completing the task. When patients were not able to solve one step of the task then participants would receive support by the person who administered the test. For example if it was clear that they did not perceive the needed objects in one visuospatial hemifield, the experimenter asked the patients verbally and with gestures to explore the affected hemifield.
Statistical analyses
All behavioral analyses were conducted using IBM SPSS Statistics 25.
Parametric tests were used to determine age effects, since this variable was normally distributed (tested with Chi2, p = 1.000). Group differences in age were analyzed with one-way ANOVA and post-hoc t-tests with Bonferroni correction.
Chi2 indicated that all other variables were not normally distributed (p = .000). Accordingly, for calculating differences between patient groups non parametric Mann-Whitney-U-Tests were used. Alpha level was set to 5%. p values lower than ≤ .050 were interpreted as significant.
For apraxia profile descriptions we used average percentage scores across subscales of each subtest. The following values were extracted: execution scores in IML, IMF, PTU, and NAT and the mean of execution and selection scores in NTT and FTT, respectively.
Group comparisons were not run for total scores, since for some tasks age- and gender-specific cut-off values have to be used (see Table S.1 in the supplementary material) and patient groups differed in these two variables (see above). Instead, for group comparisons patient-data was classified relative to the normative data. They were classified due to their performance as being not impaired or as being mildly, moderately or severely impaired in the respective task. The distributions per group regarding impairment severity were then compared between all patient groups.
Furthermore, the production scale parameters were used to describe the distribution of committed error types in pantomime of tool-use and real tool-use tasks (for a distinct description of production and execution scale please see Buchmann & Randerath, Citation2017). Average percentage scores per patient group were used.
Sometimes, patients were not able to solve all tasks (for a distinct list of missing data please see supplementary material Table S.2). In this case, patients were excluded from analysis for this respective task. Except for two tests in the dementia group (NTT and NAT) not more than data of one person per test was missing.
Results
Limb apraxia prevalence in the different patient groups
For each of the eleven subscales of the DILA-S, the number and percentage of patients per sample who showed no, mild, moderate or severe apraxic symptoms can be found in Table S.2 (supplementary material). Further, the specific profile of each patient group is shown descriptively in . See “Comparison of performance between patient groups” section for detailed group comparison reports. For group comparisons in all DILA-S subscales please see Table S.3 (supplementary material).
Figure 1. The figure displays apraxia profiles. It includes the profile description for the respective patient groups (LHS = stroke in the left hemisphere, n = 44; RHS = stroke in the right hemisphere, n = 36; TBI = traumatic brain injury, n = 44; MS = multiple sclerosis, n = 26; Dem = dementia, n = 27) for the average performance across subscales per subtest of the DILA-S (IML = imitation of meaningless gestures; IMF = imitation of meaningful gestures; PTU = pantomime; NTT = novel tools test; FTT = familiar tools test; NAT = naturalistic action test - breakfast task). For pantomime of tool-use the execution scale is shown. For novel and familiar tools test, the mean performance of selection and execution subscales is shown.
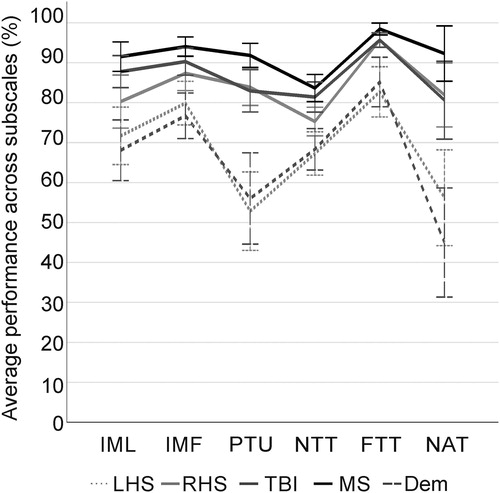
Next, we will describe the specific profiles of each patient group in the distinct apraxia subtests.
Stroke patients
In line with the known left lateralization of limb apraxia, patients with left (LHS, N = 44) versus right (RHS, N = 36) brain damage demonstrated worse task performance in several subscales of the DILA-S (five out of eight subscales: U ≥ 385.00, p ≤ .018). Consequently, we analyzed data of LHS and RHS patients separately. 84.1% of LHS patients (N = 37) and 55.6% of RHS patients (N = 20) showed apraxic symptoms in at least one subscale. RHS patients most frequently were classified apraxic in only few subscales of the DILA-S. The two most affected RHS patients showed impairments in four of the eight DILA-S subscales, whereas 27.2% LHS patients (N = 12) showed apraxic symptoms in five or more subscales including 9.1% of patients (N = 4) being impaired in all eight subscales. The highest incidence of apraxia in LHS patients occurred in executing proper tool-use pantomimes (PTU execution scale: 61% (N = 23) impaired patients), and in RHS patients the imitation tasks were most frequently affected (in IML and IMF both 19% (N = 7) impaired patients).
TBI patients
45.5% (N = 20) of TBI patients showed apraxic symptoms in at least one subscale of the DILA-S. Most of the TBI patients (19%, N = 8) were unable to complete the NAT Breakfast Task correctly. While half of the patients who were apraxic only showed symptoms in one or two subscales (50.0% (N = 10) of apraxic patients), the other half of patients (N = 10) was impaired in up to seven out of eight subscales of the DILA-S.
MS patients
Limb apraxia was shown in 26.9% (N = 7) of MS patients. Each of these patients showed difficulties in only one subscale with mild (19%, N = 5) to moderate (8%, N = 2) symptom manifestations. None of the patients showed apraxic symptoms in the FTT or NTT execution scales. Patient groups with PPMS, SPMS or RRMS demonstrated a similar level of performance in all of the DILA-S tasks (H (2) ≤ 6.675, p ≥ .217). Due to the small number of patients per subgroup and the absence of differences between these subgroups, the MS patient group was not subdivided for later group comparisons.
Dementia patients
Patient groups with Alzheimer´s disease (N = 20) or vascular dementia (N = 7) did not show performance differences in any of the applied DILA-S subscales (U ≤ 35.50, p ≥ .427). Therefore, the dementia group was not subdivided for subsequent group comparisons. In total 88.9% of dementia patients (N = 24) showed apraxic symptoms in at least one subscale. The severity of deficits varied immensely within the group of apraxic patients. Patients’ impairments ranged from mild difficulties in one subscale up to severe difficulties in six out of eight subscales. Deficits occurred most frequently in pantomiming tool-use (69% (N = 18/26) impaired patients) and preparing breakfast (70% (N = 14/20) impaired patients).
Comparison of performance between patient groups
The performance of patient groups differed in all subtests but the NTT execution subscales of the DILA-S (H (4) ≥ 12.85, p ≤ .010). Differences between the tested clinical samples will be detailed below.
Classical tests: imitation and pantomime of tool-use
As shown in , LHS patients scored worse than TBI and MS patients in IML and IMF (U ≥ 374.50, p ≤ .036). Patients with dementia also scored worse in both IML and IMF compared to patients with MS or TBI (U ≥ 174.00, p ≤ .002). Further, dementia patients scored worse than RHS patients in IML (U = 305.50, p = .006). All other group comparisons did not reach significance (U ≤ 764.00, p ≥ .051).
Figure 2. The figure displays performance in the imitation of meaningless (left graph) and meaningful (middle graph) gestures and pantomime of tool-use (right graph). It includes the distribution of apraxia severity (no, mild, moderate and severe apraxia) in percent per group for the distinct patient groups (patients with stroke in the left hemisphere [LHS, n = 44], stroke in the right hemisphere [RHS, n = 36], traumatic brain injury [TBI, n = 44], multiple sclerosis [MS, n = 26], dementia [Dem, n = 27]) in the subscales imitation of meaningless gestures (left), imitation of meaningful gestures (middle) and pantomime of tool-use (right). Significant group differences are marked with stars: **p ≤ .010, *p ≤ .050.
![Figure 2. The figure displays performance in the imitation of meaningless (left graph) and meaningful (middle graph) gestures and pantomime of tool-use (right graph). It includes the distribution of apraxia severity (no, mild, moderate and severe apraxia) in percent per group for the distinct patient groups (patients with stroke in the left hemisphere [LHS, n = 44], stroke in the right hemisphere [RHS, n = 36], traumatic brain injury [TBI, n = 44], multiple sclerosis [MS, n = 26], dementia [Dem, n = 27]) in the subscales imitation of meaningless gestures (left), imitation of meaningful gestures (middle) and pantomime of tool-use (right). Significant group differences are marked with stars: **p ≤ .010, *p ≤ .050.](/cms/asset/bdbe7a95-820d-4b87-9190-6afd02b82632/ntcn_a_1585575_f0002_b.jpg)
In pantomime, patients with LHS or dementia scored worse than patients with MS, TBI or RHS (U ≥ 110.50, p = .000). All other group comparisons did not reveal significant differences (U ≤ 774.00, p ≥ .108).
Real tool-use tests: novel and familiar tools test
As shown in , in NTT selection scale, the frequency of patients showing apraxic behavior was higher in the LHS group than in the RHS, TBI, and MS patient groups (U ≥ 408.00, p ≤ .018). Similarly, in the NTT selection scale the frequency of apraxic patients with dementia was higher than the frequency of apraxic MS and TBI patients (U ≥ 244.50, p ≤ .043). The NTT execution scale did not differentiate between the here tested patient samples (H (4) = 6.28, p =.143).
Figure 3. The figure displays the distribution of apraxia severity (no, mild, moderate, and severe apraxia) in the novel (left) and familiar tools (right) tests. The subscales selection (above) and execution (below) are shown for both tests. Apraxia severity is shown in percent per group for the distinct patient groups (patients with stroke in the left hemisphere [LHS, n = 44], stroke in the right hemisphere [RHS, n = 36], traumatic brain injury [TBI, n = 44], multiple sclerosis [MS, n = 26], dementia [Dem, n = 27]). Significant group differences are marked with stars: **p ≤ .010, *p ≤ .050.
![Figure 3. The figure displays the distribution of apraxia severity (no, mild, moderate, and severe apraxia) in the novel (left) and familiar tools (right) tests. The subscales selection (above) and execution (below) are shown for both tests. Apraxia severity is shown in percent per group for the distinct patient groups (patients with stroke in the left hemisphere [LHS, n = 44], stroke in the right hemisphere [RHS, n = 36], traumatic brain injury [TBI, n = 44], multiple sclerosis [MS, n = 26], dementia [Dem, n = 27]). Significant group differences are marked with stars: **p ≤ .010, *p ≤ .050.](/cms/asset/8158bbc9-94c4-4f09-bad5-9ba6ed71c030/ntcn_a_1585575_f0003_b.jpg)
In FTT selection, the percentage of apraxic LHS patients was significantly higher than RHS, TBI, and MS patients (U ≥ 460.00, p ≤ .023). In the FTT execution scale, LHS and dementia patients were more frequently affected compared to RHS, TBI, and MS patients (U ≥ 234.00, p ≤ .036). All other group comparisons did not reveal significant results (U ≤ 775.00, p ≥ .132).
Naturalistic action test - breakfast task
As shown in , in the breakfast task the percentage of impaired patients was significantly higher in the LHS and dementia groups compared to the RHS, TBI, and MS groups (U ≥ 86.50, p ≤ .012). All other group comparisons did not reach significance (U ≤ 729.50, p ≥ .095).
Figure 4. The distribution of apraxia severity (no, mild, moderate, and severe apraxia) in the naturalistic action test - breakfast task is presented. Apraxia severity is shown in percent per group for the distinct patient groups (patients with stroke in the left hemisphere [LHS, n = 44], stroke in the right hemisphere [RHS, n = 36], traumatic brain injury [TBI, n = 44], multiple sclerosis [MS, n = 26], dementia [Dem, n = 27]). Significant group differences are marked with stars: **p ≤ .010, *p ≤ .050.
![Figure 4. The distribution of apraxia severity (no, mild, moderate, and severe apraxia) in the naturalistic action test - breakfast task is presented. Apraxia severity is shown in percent per group for the distinct patient groups (patients with stroke in the left hemisphere [LHS, n = 44], stroke in the right hemisphere [RHS, n = 36], traumatic brain injury [TBI, n = 44], multiple sclerosis [MS, n = 26], dementia [Dem, n = 27]). Significant group differences are marked with stars: **p ≤ .010, *p ≤ .050.](/cms/asset/f05c10b0-9e1d-4691-87d3-b79ead8a7830/ntcn_a_1585575_f0004_b.jpg)
Committed error types
Descriptive profiles for committed error types in pantomime of tool-use, novel, and familiar tools test are shown in . These are based on production parameters described in Section Assessment of Limb Apraxia. For pantomime of tool-use, LHS patients produced most errors when forming their grip, whereas dementia patients performed most errors in orienting the movement. RHS, TBI, and MS patients did not demonstrate any particular problem with certain production parameters. For the novel tools test, all groups showed the highest error rates for movement-content but produced nearly perfect grip-formation and thumb-orientation. For the familiar tools test, LHS and dementia patients produced most errors again in the movement-content parameter, while RHS, TBI, and MS patients did not show any erroneous behavior. Across tests, LHS patients demonstrated consistently errors for movement-content, while dementia patients show differential profiles depending on the subtest.
Figure 5. The average performance on production parameters in pantomime of tool-use (left), novel (middle) and familiar tools test (right) is presented. Mean percentage scores of performance in grip-formation, orientation of thumb, movement-content, orientation of movement and body-part-as-object-errors are shown for the distinct patient groups (patients with stroke in the left hemisphere [LHS, n = 44], stroke in the right hemisphere [RHS, n = 36], traumatic brain injury [TBI, n = 44], multiple sclerosis [MS, n = 26], dementia [Dem, n = 27]).
![Figure 5. The average performance on production parameters in pantomime of tool-use (left), novel (middle) and familiar tools test (right) is presented. Mean percentage scores of performance in grip-formation, orientation of thumb, movement-content, orientation of movement and body-part-as-object-errors are shown for the distinct patient groups (patients with stroke in the left hemisphere [LHS, n = 44], stroke in the right hemisphere [RHS, n = 36], traumatic brain injury [TBI, n = 44], multiple sclerosis [MS, n = 26], dementia [Dem, n = 27]).](/cms/asset/21130249-2195-4eab-b0da-7112e87d7ea7/ntcn_a_1585575_f0005_b.jpg)
Discussion
To our knowledge the present study is the first one to examine four in-clinic patient samples with different neurological disorders by use of the same limb apraxia test battery (DILA-S). In this manuscript we describe and display the apraxic profiles of each group for the imitation of meaningless and meaningful hand gestures, pantomime of tool-use, the selection and use of novel, and familiar tools as well as a multistep naturalistic action task that comprised the preparation of breakfast.
Overall, our results confirm previously shown prevalence rates of limb apraxia in the different patient groups for the classical imitation and pantomime tests. By adding the assessment of novel and familiar tool-use tasks and a multistep naturalistic action test, prevalence rates slightly increased. LHS and dementia patients showed the highest frequencies of affected patients in all six tasks of the DILA-S. They scored significantly worse compared to RHS, TBI and MS patient groups on almost all subscales except for the novel tools test execution scale.
Limb apraxia profiles across subtests at first sight demonstrated similar patterns for LHS and dementia patients. However, observation of error-types across pantomime and real novel and familiar tool-use tests revealed some particularities for both patient groups. While LHS patients consistently produced movement-content errors across tool-use tasks, dementia patients showed differential profiles depending on the subtest. In the pantomime task for which there was no tool in hand and no recipient object to manipulate the dementia group mainly produced errors for movement-orientation.
Prevalence rates of limb apraxia
At first, the overall high prevalence rates of more than 80% patients in the LHS and the dementia patient groups demonstrating apraxic behavior in at least one of the subtests of the DILA-S may be surprising but can be explained by the wide-ranging limb apraxia test instrument used here which not only included the classic subtests on pantomime or imitation, but also targeted other domains in real tool-use. Previous work already demonstrated that patients may exhibit impaired behavior in selective domains (Buchmann & Randerath, Citation2017). For the classic subtests our prior psychometric evaluation of the DILA-S in stroke patients (Buchmann & Randerath, Citation2017; Randerath et al., Citation2017) revealed satisfying concurrent validity. Significant correlations with the Kölner Apraxie Screening (Weiss et al., Citation2013) and the Apraxia Screen of TULIA (Vanbellingen et al., Citation2011a) support the idea that similar concepts are measured. Further, the incidence rates of apraxia in classic subtests of pantomime and imitation appeared to be on a similar level compared to other test instruments. Other research groups reported prevalence rates of around 28–57% in LHS patients (e.g., Donkervoort et al., Citation2000; Dovern et al., Citation2012; Kaya et al., Citation2006; Weiss et al., Citation2013) and of 32–69% in Alzheimer´s disease patients (Ahmed et al., Citation2016; Ozkan et al., Citation2013), but these considered performance in imitation and pantomime tasks only. Had we similarly considered these tasks only (IML, IMF, and PTU), prevalence rates of apraxia for LHS patients would be at 52% for imitation and at 61% for pantomime tasks, which is comparable to previously reported results.
For the here tested dementia patients, prevalence rates reach 63% for imitation and 67% for pantomime tasks. This number appears to be in line with the aforementioned reports, while at the higher end. Two factors may have contributed to that. First, it needs to be pointed out that the age range of the normative sample of the DILA-S was limited to a maximum of 80 years whereas in the dementia patient group, 15 patients (56%) were older than 80 years. For tasks with age specific cut-off values such as the imitation tasks and FTT selection, this fact could have skewed the results towards an overestimation of apraxia-diagnoses. However, the possibility of overestimation of apraxia in the dementia group seems less likely in view of the fact that the vast majority of apraxic dementia patients also were impaired on the non-age sensitive subscales. Therefore, the lack of people older than 80 in the normative group may have played only a minor role in explaining the relatively high prevalence of apraxia in the dementia group. Instead a better explanation may be provided by the patient recruitment in a psychiatric clinic. Dementia severity in the recruited sample appears to be higher than in previously reported studies. The patient group tested in the presented study was predominantly moderately impaired in Mini Mental State Exam (MMSE, N = 17 (65%); Crum, Anthony, Bassett & Folstein, Citation1993). With a mean of 16.7 points in MMSE, this patient group showed lower MMSE scores than patient groups of previous dementia patient studies (average scores: Cotelli et al., Citation2014: 20.1 points; Johnen, Frommeyer, et al., Citation2016: 23.2 points; Johnen et al., Citation2015: 22.4 points; Ozkan et al., Citation2013: 17.2 points in Alzheimer´s disease and 19.4 points in vascular dementia patients). As shown before, prevalence rates of apraxia increase with ongoing disease decline (Edwards et al., Citation1991; Parakh et al., Citation2004; Wu & Lin, Citation2015) and lower MMSE scores (Cotelli et al., Citation2014; Stamenova et al., Citation2014; Wu & Lin, Citation2015). In the current sample patients with Alzheimer´s disease and vascular dementia did not differ in their praxis performance, which is in line with previous results (Ahmed et al., Citation2016; Ozkan et al., Citation2013).
Similarly, the prevalence rates of RHS patients (56%) also seem to be rather high in this patient group compared to previous studies, which showed apraxia prevalence rates of 0–34% in RHS patients (e.g., Donkervoort et al., Citation2000; Wirth et al., Citation2016). Again, when only considering classical imitation and pantomime tasks, prevalence rates decrease to 31% in imitation and 17% in pantomime tasks. It should also be emphasized that symptoms occur in less subscales and in a milder manifestation than in LHS patients, which is in line with previous study results (Buchmann & Randerath, Citation2017; Wirth et al., Citation2016).
As expected, MS patients showed the lowest apraxia prevalence rates with 27% of affected patients, which is comparable to previous study results (Kamm et al., Citation2012; Rapaic et al., Citation2014). However, compared to prior reports of other research groups, our MS patient sample seemed not as strongly affected by limb apraxia. Only a few MS patients demonstrated difficulties on even a single subscale of the DILA-S. This could be due to the method of merely testing the stronger hand (which controlled for motor deficits), which has been applied here, compared to testing both hands in studies of other research groups (Kamm et al., Citation2012; Rapaic et al., Citation2014). Further, in the current MS sample, limb apraxia symptoms did not reach clinical relevance, which was shown by absent complaints by the patients as well as absent notes on apraxia in the discharge report of the rehabilitation clinic (Harscher et al., Citation2017).
A different profile was found for TBI patients, who scored better than LHS and dementia patients on almost all subscales. When interpreted in reference to the healthy normative sample, TBI patients are most strongly impaired in the NAT Breakfast Task. Here, even patients who did not show any apraxic behavior in the other tasks, showed striking behavior such as infusing the tea in the sugar bowl or sticking the knife in the toaster to check whether the bread is toasted. These difficulties may potentially be explained by the typically high number of TBI patients suffering from frontal brain damage (Gentry, Godersky & Thompson, Citation1988; Levin et al., Citation1987) affecting executive functions. Frequently, this leads to action planning and inhibition disabilities (e.g., Wallesch & Bartels, Citation2009). Thus, a lack of impulse control may explain behavior such as the before described actions.
Task specifics
Except for the MS patient group, overall performance of each clinical group showed task specific deficits on the DILA-S. In LHS and dementia patients, the pantomime task was most prone to errors, whereas for RHS patients, imitation of gestures and for TBI patients the NAT Breakfast Task were mostly affected by apraxic behavior.
It has been shown before that imitation and pantomime tasks are more sensitive to erroneous behavior compared to real tool-use tasks in LHS and RHS patient groups (De Renzi, Faglioni & Sorgato, Citation1982; Randerath, Goldenberg, Spijkers, Li & Hermsdörfer, Citation2011). This similarly has been shown for dementia patients. Lesourd et al. (Citation2013) for example demonstrated that pantomime production deficits seem to increase with more severe forms of dementia, while real tool-use including familiar tool-use and mechanical problem solving seems to remain stable at least from mild to moderate stages of the disease. The greater extent of contextual information curtails action opportunities (affordances) provided in real tool-use tasks and might minimize demands on working memory, and thus leads to fewer errors. However, despite the relative improvement of behavior in real tool-use settings, patients can still be significantly affected (Randerath et al., Citation2011). Moreover, while the familiar tools test seems to be easier for healthy adults (verified by higher cut-off values than in the novel tools test), it seems to be more difficult for LHS and dementia patients than mechanical problem solving. Two reasons could account for this result: First, since cut-off values in the familiar tools test are near to ceiling, patients only need to make few errors to score below cut-off. In contrast, the cut-off-values for the novel tools test are lower, so patients can make some errors without scoring below cut-off. Second, stroke- or atrophy-induced lesions in LHS or dementia patients, are frequently located in temporo-parietal regions, which are responsible for semantic processing and thus involved in familiar tool-use (Goldenberg & Hagmann, Citation1998; Hodges, Bozeat, Lambon Ralph, Patterson & Spatt, Citation2000).
Profile specificity
General praxic performance profiles across subtests demonstrated similar patterns for LHS and dementia patients. However, more specific observations show that preferences for error types appear to differ, especially in the pantomime task. One feasible approach to explain these results are findings from brain imaging studies in these patient groups.
In stroke and dementia patients, brain damage frequently affects regions along the perisylvian area. Functional imaging studies in healthy subjects provide ample evidence for bilateral activity in these regions when being occupied by motor cognitive tasks. However, in line with lesion studies in stroke patients (Buxbaum, Kyle, Grossman & Coslett, Citation2007; Buxbaum, Sirigu, Schwartz & Klatzky, Citation2003; Finkel, Hogrefe, Frey, Goldenberg & Randerath, Citation2018; Goldenberg & Randerath, Citation2015; Niessen, Fink & Weiss, Citation2014; Rumiati et al., Citation2004; Weiss et al., Citation2016), functional imaging studies have emphasized a strongly left lateralized fronto-temporo-parietal network being involved in praxic skills including tool-use, gesturing, hand posture-imitation or grip-selection (e.g., Binkofski & Buxbaum, Citation2013; Frey, Citation2007; Króliczak & Frey, Citation2009; Lewis, Citation2006; Przybylski & Króliczak, Citation2017; Randerath, Valyear, Philip & Frey, Citation2017; Valyear & Frey, Citation2015). A recent lesion study further disentangled the limb apraxic error types for pantomime of tool-use in left brain damaged stroke patients. The study results demonstrated communicative gesturing errors (BPO) to be associated with anterior inferior frontal and temporal regions and motor cognitive error types (movement-content) being associated with posterior inferior parietal regions (Finkel et al., Citation2018). Early studies investigating dementia patients (Hodges et al., Citation2000; Spatt, Bak, Bozeat, Patterson & Hodges, Citation2002) have discussed particularly the temporal regions to be a major player in difficulties with semantic aspects of tool-use tasks. However, more recently Johnen, Brandstetter et al. (Citation2016) analyzed reduced grey matter volume in dementia patients and found bilateral volume loss in superior parietal regions to be associated with deficits in imitating and more strikingly they found reduced grey matter volume at the right temporo-occipito-parietal junction being associated with deficits in pantomiming. The authors proposed a greater influence of visuospatial impairments and spatial body representation deficits on praxis performance coming to the fore in patients with dementia. While these findings at first seem surprising, they are in line with the profiles portrayed in the current study demonstrating specificity in error types in pantomime tasks for LHS versus dementia patients. While movement-content errors were consistently found across tasks in LHS patients, dementia patients showed differential profiles depending on the subtest. In the pantomime task the dementia group mainly produced errors for movement-orientation. In contrast to the real tool- use tasks, for pantomime movements affordances leading to correct movements are reduced (for a similar discussion see Randerath et al., Citation2011). There is no tool in hand and no recipient object to manipulate. Patients with deficiencies in visuospatial aspects of motor cognition, e.g., the visuospatial movement parameter (movement orientation), should be particularly susceptible to erroneous behavior, which is reflected by the current results.
Thus, in line with discussions in the literature, our current results support the presence of differential limb apraxia error profiles in dementia versus LHS patients.
Limitations and future directions
To our knowledge, this was the first study that examined four groups of in-clinic patients with different neurological disorders with the same limb apraxia test instrument. However, the comparison of different neurological samples naturally provokes several challenges. Of course, one major biasing factor may be the recruitment. Here we only included patients admitted to local clinics and thereby concentrated on the most vulnerable samples. Other patient cohorts (e.g., recruited in nursing homes, support groups etc.) may be of interest as well. Our study cohorts were representative for inpatients in the respective clinics. The samples were very heterogeneous. Heterogeneity was present within groups (e.g., based on aphasia distribution, Barthel Index or Expanded Disability Status Scale) and between patient groups (e.g., shown by different amounts of aphasic patients and distribution of cognitive restrictions). Levels of overall cognitive impairment between these patient groups could not be compared.
In the current study, the clinical groups demonstrated different degrees of impairment on limb praxis measures in some cases (e.g., MS versus TBI versus dementia versus stroke) as well as in error profiles (e.g., dementia versus stroke). On the other hand, we also observed similar degrees of impairment in some groups (e.g., dementia, stroke, and TBI). These findings are intriguing, but clarification must await further study given the heterogeneity within our groups and the overlap of neuropathology between some groups.
Ideally, age-matched normative data would be available for dementia patients older than 80 years. However, in this study normative data did not include healthy participants beyond 80 years of age. Moreover, DILA-S classifications of level of severity are based on a group of stroke patients from 30 to 80 years of age (Randerath et al., Citation2017).
Another limitation of this study is its exploratory nature. Comparisons based on five patient groups and eight subscales of the DILA-S clearly introduce the drawbacks of multiple comparisons. Although we used nonparametric measures the results need to be interpreted with caution.
Future studies should aim at including larger samples with same sized patient groups for statistically ideal group comparisons. The current exploratory study may support generating predefined hypotheses. Further, additional neuroimaging data will help to better explain sample-specific profiles and deliver a more holistic view on the underlying mechanisms of limb apraxia. In rare cases, patients demonstrate unilateral limb apraxia symptoms, for example when suffering from corpus callosum lesions. Thus, future studies should consider examining larger samples of TBI or MS patients and test both hands, if motor abilities permit and are controlled for.
Based on the profile specific results it is suggested to analyze error types, and to consistently include the evaluation of visuo-spatial abilities. Prospectively, the evaluation of individual error profiles may be helpful for shaping motor cognitive training tailored to the individual’s needs.
Last, extending the study to other patient groups for which limb apraxia symptoms were shown before (e.g., patients with Parkinson’s disease, Huntington’s disease, corticobasal syndrome or schizophrenia) may help to better understand the underlying neuronal mechanisms in motor cognitive networks.
Conclusion
To our knowledge the current study is the first study to compare praxis performance of groups with distinct neurological diseases by use of the same limb apraxia test battery. Overall, except for the MS patient group, patients tested here had wide ranging difficulties in the presented tasks. LHS as well as dementia patients scored worst in all tasks, while RHS and TBI patients only showed difficulties in some tasks and MS patients did not show any clinically relevant limb apraxia symptoms. In patient groups with apraxic deficits, error profiles may differ depending on the neurologic condition. Concluding, testing for apraxia should be warranted for all these populations as standard, rather than concentrating on patients suffering from LHS. Prospectively, individual error profiles may be helpful for shaping motor cognitive training.
| Abbreviations | ||
| DILA-S | = | Diagnostic Instrument for Limb Apraxia – Short Version |
| FTT | = | Familiar tools test |
| IMF | = | Imitation of meaningful hand gestures |
| IML | = | Imitation of meaningless hand gestures |
| LHS | = | Left hemisphere stroke |
| MMSE | = | Mini Mental State Examination |
| MS | = | Multiple sclerosis |
| NAT | = | Naturalistic action test |
| NTT | = | Novel tools test |
| PPMS | = | Primarily chronic progressive multiple sclerosis |
| PTU | = | Pantomime of tool-use |
| RHS | = | Right hemisphere stroke |
| RPMS | = | Relapsing remitting multiple sclerosis |
| SPMS | = | Secondarily chronic-progressive multiple sclerosis |
| TBI | = | Traumatic brain injury |
Acknowledgments
We thank our colleagues Kathi Harscher, Celina Hirth-Walter, Patrick Lipfert, Cedric Rosati, Sebastian Schöne, Tobias Schmidt and Farida Vildanova for their help with data collection. We thank Dr. Thomas Hassa, Dr. Stefan Spiteri and Lisa Friedrich-Schmieder for their support with administrative questions in the rehabilitation center of the Kliniken Schmieder, as well as all participants.
Disclosure Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Funding
Notes
1 For materials and examples please see https://www.moco.uni-konstanz.de/publikationen/assessments/
References
- Acosta, L. M., Bennett, J. A., & Heilman, K. M. (2014). Callosal disconnection and limb-kinetic apraxia. Neurocase, 20(6), 599–605. doi: 10.1080/13554794.2013.826683
- Ahmed, S., Baker, I., Thompson, S., Husain, M., & Butler, C. R. (2016). Utility of testing for apraxia and associated features in dementia. Journal of Neurology, Neurosurgery & Psychiatry, 87(11), 1158–1162. doi: 10.1136/jnnp-2015-312945
- Bader, P. (2014). Spezifische Störungsbilder in der Neurorehabilitation. In F. Müller, E. Walther & J. Herzog (Eds.), Praktische Neurorehabilitation. Behandlungskonzepte nach Schädigung des Nervensystems (vol. 1, pp. 35–79). Stuttgart: W. Kohlhammer GmbH.
- Baumard, J., Lesourd, M., Jarry, C., Merck, C., Etcharry-Bouyx, F., Chauviré, V., … Le Gall, D. (2016). Tool use disorders in neurodegenerative diseases: Roles of semantic memory and technical reasoning. Cortex, 82, 119–132. doi: 10.1016/j.cortex.2016.06.007
- Binkofski, F., & Buxbaum, L. J. (2013). Two action systems in the human brain. Brain and Language, 127(2), 222–229. doi: 10.1016/j.bandl.2012.07.007
- Borroni, B., Garibotto, V., Agosti, C., Brambati, S. M., Bellelli, G., Gasparotti, R., … Perani, D. (2008). White matter changes in corticobasal degeneration syndrome and correlation with limb apraxia. Archives of Neurology, 65(6), 796–801.
- Buchmann, I., & Randerath, J. (2017). Selection and application of familiar and novel tools in patients with left and right hemispheric stroke: Psychometrics and normative data. Cortex, 94, 49–62. doi: 10.1016/j.cortex.2017.06.001
- Buxbaum, L. J., Kyle, K., Grossman, M., & Coslett, H. B. (2007). Left inferior parietal representations for skilled hand-object interactions: Evidence from stroke and corticobasal degeneration. Cortex, 43(3), 411–423. doi: 10.1016/S0010-9452(08)70466-0
- Buxbaum, L. J., Sirigu, A., Schwartz, M. F., & Klatzky, R. (2003). Cognitive representations of hand posture in ideomotor apraxia. Neuropsychologia, 41(8), 1091–1113. doi: 10.1016/S0028-3932(02)00314-7
- Chandra, S. R., Issac, T. G., & Abbas, M. M. (2015). Apraxias in Neurodegenerative dementias. Indian Journal of Psychological Medicine, 37(1), 42–47. doi: 10.4103/0253-7176.150817
- Cotelli, M., Manenti, R., Brambilla, M., & Balconi, M. (2014). Limb apraxia and verb processing in Alzheimer's disease. Journal of Clinical and Experimental Neuropsychology, 36(8), 843–853. doi: 10.1080/13803395.2014.948389
- Crum, R. M., Anthony, J. C., Bassett, S. S., & Folstein, M. (1993). Population based norms for the Mini-Mental-Status Examination by age and education level. JAMA: The Journal of the American Medical Association, 269(18), 2386–2391. doi: 10.1001/jama.269.18.2386
- De Renzi, E., Faglioni, P., & Sorgato, P. (1982). Modality-specific and supramodal mechanisms of apraxia. Brain, 105(Pt 2), 301–312.
- Del Ser, T., Hachinski, V., Merskey, H., & Munoz, D. G. (2001). Clinical and pathologic features of two groups of patients with dementia with Lewy bodies: Effect of coexisting Alzheimer-type lesion load. Alzheimer Disease and Associated Disorders, 15(1), 31–44. doi: 10.1097/00002093-200101000-00005
- Deutsch-Lezak, M., Howieson, D. B., Bigler, E. D., & Tranel, D. (2012). Construction and motor performance: Examining for apraxia. In M. Deutsch-Lezak, D. B. Howieson, E. D. Bigler & D. Tranel (Eds.), Neuropsychological assessment (5th ed., pp. 607–609). New York: Oxford University Press.
- Donkervoort, M., Dekker, J., Van den Ende, E., & Stehmann-Saris, J. (2000). Prevalence of apraxia among patients with a first left hemisphere stroke in rehabilitation centres and nursing homes. Clinical Rehabilitation, 14(2), 130–136. doi: 10.1191/026921500668935800
- Dovern, A., Fink, G. R., & Weiss, P. H. (2012). Diagnosis and treatment of upper limb apraxia. Journal of Neurology, 259(7), 1269–1283. doi: 10.1007/s00415-011-6336-y
- Dutschke, L. L., Stegmayer, K., Ramseyer, F., Bohlhalter, S., Vanbellingen, T., Strik, W., & Walther, S. (2017). Gesture impairments in schizophrenia are linked to increased movement and prolonged motor planning and execution. Schizophrenia Research, 200, 42–49.
- Edwards, D. F., Deuel, R. K., Baum, C. M., & Morris, J. C. (1991). A quantitative analysis of apraxia in senile dementia of the Alzheimer type: Stage-related differences in prevalence and type. Dementia and Geriatric Cognitive Disorders, 2(3), 142–149. doi: 10.1159/000107189
- Falchook, A. D., Porges, E. C., Nadeau, S. E., Leon, S. A., Williamson, J. B., & Heilman, K. M. (2015). Cognitive-motor dysfunction after severe traumatic brain injury: A cerebral interhemispheric disconnection syndrome. Journal of Clinical and Experimental Neuropsychology, 37(10), 1062–1073. doi: 10.1080/13803395.2015.1077930
- Finkel, L., Hogrefe, K., Frey, S. H., Goldenberg, G., & Randerath, J. (2018). It takes two to pantomime: Communication meets motor cognition. NeuroImage: Clinical, 19, 1008–1017. doi: 10.1016/j.nicl.2018.06.019
- Foki, T., Vanbellingen, T., Lungu, C., Pirker, W., Bohlhalter, S., Nyffeler, T., … Beisteiner, R. (2016). Limb‐kinetic apraxia affects activities of daily living in Parkinson's disease: A multi‐center study. European Journal of Neurology, 23(8), 1301–1307. doi: 10.1111/ene.13021
- Frey, S. H. (2007). What puts the how in where? Tool use and the divided visual streams hypothesis. Cortex, 43(3), 368–375.
- Gentry, L. R., Godersky, J. C., & Thompson, B. (1988). MR imaging of head trauma: Review of the distribution and radiopathologic features of traumatic lesions. American Journal of Roentgenology, 150(3), 663–672. doi: 10.2214/ajr.150.3.663
- Gil Moreno, M. J., Cerezo Garcia, M., Marasescu, R., Pinel Gonzalez, A., Lopez Alvarez, L., & Aladro Benito, Y. (2013). Neuropsychological syndromes in multiple sclerosis. Psicothema, 25(4), 452–460.
- Goldenberg, G. (1996). Defective imitation of gestures in patients with damage in the left or right hemispheres. Journal of Neurology, 61, 176–180. doi: 10.1136/jnnp.61.2.176
- Goldenberg, G. (2009). Apraxia and the parietal lobes. Neuropsychologia, 47(6), 1449–1459. doi: 10.1016/j.neuropsychologia.2008.07.014
- Goldenberg, G. (2011). Apraxien. Göttingen: Hogrefe Verlag GmbH & Co. KG.
- Goldenberg, G. (2013). Apraxia in left-handers. In G. Goldenberg (Ed.), Apraxia. The Cognitive Side of Motor Control (pp. 184–190). Oxford: Oxford University Press.
- Goldenberg, G., & Hagmann, S. (1998). Tool use and mechanical problem solving in apraxia. Neuropsychologia, 36(7), 581–589. doi: 10.1016/S0028-3932(97)00165-6
- Goldenberg, G., Hartmann, K., & Schlott, I. (2003). Defective pantomime of object use in left brain damage: Apraxia or asymbolia?. Neuropsychologia, 41(12), 1565–1573.
- Goldenberg, G., & Randerath, J. (2015). Shared neural substrates of apraxia and aphasia. Neuropsychologia, 75, 40–49. doi: 10.1016/j.neuropsychologia.2015.05.017
- Hamilton, J. M., Haaland, K. Y., Adair, J. C., & Brandt, J. (2003). Ideomotor limb apraxia in Huntington's disease: Implications for corticostriate involvement. Neuropsychologia, 41(5), 614–621. doi: 10.1016/S0028-3932(02)00218-X
- Hardyck, C., & Petrinovich, L. F. (1977). Left-handedness. Psychological Bulletin, 84(3), 385–404.
- Harscher, K. M., Hirth-Walther, C., Buchmann, I., Dettmers, C., & Randerath, J. (2017). Gliedmaßenapraxie bei Patienten mit Multipler Sklerose. Zeitschrift Für Neuropsychologie, 38(3-4), 1–11.
- Heilman, K. M., & Rothi, L. J. (1993). Apraxia. In K. M. Heilman & E. Valenstein (Eds.), Clinical neuropsychology (pp. 141–164). New York, Oxford: Oxford University Press.
- Hodges, J. R., Bozeat, S., Lambon Ralph, M. A., Patterson, K., & Spatt, J. (2000). The role of conceptual knowledge in object use evidence from semantic dementia. Brain, 123(9), 1913–1925. doi: 10.1093/brain/123.9.1913
- Huber, W., Poeck, K., Weniger, D., & Willmes, K. (1983). Aachener Aphasie Test. Göttingen: Hogrefe.
- Johnen, A., Brandstetter, L., Kärgel, C., Wiendl, H., Lohmann, H., & Duning, T. (2016). Shared neural correlates of limb apraxia in early stages of Alzheimer´s dementia and behavioural variant frontotemporal dementia. Cortex, 84, 1–14. doi: 10.1016/j.cortex.2016.08.009
- Johnen, A., Frommeyer, J., Modes, F., Wiendl, H., Duning, T., & Lohmann, H. (2016). Dementia Apraxia Test (DATE): A brief tool to differentiate behavioral variant frontotemporal dementia from Alzheimer's dementia based on apraxia profiles. Journal of Alzheimer's Disease, 49(3), 593–605.
- Johnen, A., Tokaj, A., Kirschner, A., Wiendl, H., Lueg, G., Duning, T., & Lohmann, H. (2015). Apraxia profile differentiates behavioural variant frontotemporal from Alzheimer's dementia in mild disease stages. Journal of Neurology, Neurosurgery & Psychiatry, 86(7), 809–815. doi: 10.1136/jnnp-2014-308773
- Kamm, C. P., Heldner, M. R., Vanbellingen, T., Mattle, H. P., Muri, R., & Bohlhalter, S. (2012). Limb apraxia in multiple sclerosis: Prevalence and impact on manual dexterity and activities of daily living. Archives of Physical Medicine and Rehabilitation, 93(6), 1081–1085. doi: 10.1016/j.apmr.2012.01.008
- Kaya, K., Unsal-Delialioglu, S., Kurt, M., Altinok, N., & Ozel, S. (2006). Evaluation of ideomotor apraxia in patients with stroke: A study of reliability and validity. Journal of Rehabilitation Medicine, 38(2), 108–112. doi: 10.1080/16501970500312255
- Króliczak, G., & Frey, S. H. (2009). A common network in the left cerebral hemisphere represents planning of tool use pantomimes and familiar intransitive gestures at the hand-independent level. Cerebral Cortex, 19(10), 2396–2410. doi: 10.1093/cercor/bhn261
- Kübel, S., Stegmayer, K., Vanbellingen, T., Walther, S., & Bohlhalter, S. (2018). Deficient supplementary motor area at rest: Neural basis of limb kinetic deficits in Parkinson's disease. Human Brain Mapping, 39(9), 3691–3700. doi: 10.1002/hbm.24204
- Kurtzke, J. F. (1983). Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology, 33(11), 1444. doi: 10.1212/WNL.33.11.1444
- Larner, A. J., & Lecky, B. R. (2007). Acute aphasia in MS revisited. International MS Journal, 14(3), 76–77.
- LeClerc, C. M., Wells, D. L., Sidani, S., Dawson, P., & Fay, J. (2004). A feeding abilities assessment for persons with dementia. Alzheimer's Care Today, 5(2), 123–133.
- Lesourd, M., Le Gall, D., Baumard, J., Croisile, B., Jarry, C., & Osiurak, F. (2013). Apraxia and Alzheimer's disease: Review and perspectives. Neuropsychology Review, 23(3), 234–256. doi: 10.1007/s11065-013-9235-4
- Levin, H. S., Amparo, E., Eisenberg, H. M., Williams, D. H., High, W. M., McArdle, C. B., & Weiner, R. L. (1987). Magnetic resonance imaging and computerized tomography in relation to the neurobehavioral sequelae of mild and moderate head injuries. Journal of Neurosurgery, 66(5), 706–713. doi: 10.3171/jns.1987.66.5.0706
- Lewis, J. W. (2006). Cortical networks related to human use of tools. The Neuroscientist, 12(3), 211–231. doi: 10.1177/1073858406288327
- McKenna, C., Thakur, U., Marcus, B., & Barrett, A. M. (2013). Assessing limb apraxia in traumatic brain injury and spinal cord injury. Frontiers in Bioscience (Scholar Edition), 5, 732.
- McKhann, G. M., Drachman, D., Folstein, M., Katzman, R., Price, D., & Stadlan, E. M. (1984). Clinical diagnosis of Alzheimer's disease. Report of the NINCDS‐ADRDA Work Group* under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology, 34(7), 939–939. doi: 10.1212/WNL.34.7.939
- McKhann, G. M., Knopman, D. S., Chertkow, H., Hyman, B. T., Jack, C. R., Jr., Kawas, C. H., … Phelps, C. H. (2011). The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & Dementia, 7(3), 263–269. doi: 10.1016/j.jalz.2011.03.005
- Niessen, E., Fink, G. R., & Weiss, P. H. (2014). Apraxia, pantomime and the parietal cortex. Neuroimage: Clinical, 5, 42–52. doi: 10.1016/j.nicl.2014.05.017
- Ozkan, S., Adapinar, D. O., Elmaci, N. T., & Arslantas, D. (2013). Apraxia for differentiating Alzheimer's disease from subcortical vascular dementia and mild cognitive impairment. Neuropsychiatric Disease and Treatment, 9, 947–951.
- Parakh, R., Roy, E., Koo, E., & Black, S. (2004). Pantomime and imitation of limb gestures in relation to the severity of Alzheimer's disease. Brain and Cognition, 55(2), 272–274. doi: 10.1016/j.bandc.2004.02.049
- Peigneux, P., Salmon, E., Garraux, G., Laureys, S., Willems, S., Dujardin, K., … Van der Linden, M. (2001). Neural and cognitive bases of upper limb apraxia in corticobasal degeneration. Neurology, 57(7), 1259–1268. doi: 10.1212/WNL.57.7.1259
- Plummer, P., Morris, M. E., & Dunai, J. (2003). Assessment of unilateral neglect. Physical Therapy, 83(8), 732–740.
- Polman, C. H., Reingold, S. C., Banwell, B., Clanet, M., Cohen, J. A., Filippi, M., … Wolinsky, J. S. (2011). Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Annals of Neurology, 69(2), 292–302. doi: 10.1002/ana.22366
- Przybylski, L., & Króliczak, G. (2017). Planning functional grasps of simple tools invokes the hand-independent praxis representation network: An fMRI study. Journal of the International Neuropsychological Society, 23(02), 108–120. doi: 10.1017/S1355617716001120
- Randerath, J., Buchmann, I., Liepert, J., & Büsching, I. (2017). Diagnostic Instrument for Limb Apraxia - Short Version (DILA-S). Konstanz.
- Randerath, J., Goldenberg, G., Spijkers, W., Li, Y., & Hermsdörfer, J. (2011). From pantomime to actual use: How affordances can facilitate actual tool-use. Neuropsychologia, 49(9), 2410–2416. doi: 10.1016/j.neuropsychologia.2011.04.017
- Randerath, J., Valyear, K. F., Philip, B. A., & Frey, S. H. (2017). Contributions of the parietal cortex to increased efficiency of planning-based action selection. Neuropsychologia, 105, 135–143. doi: 10.1016/j.neuropsychologia.2017.04.024
- Rapaic, D., Medenica, V., Kozomara, R., & Ivanovic, L. (2014). Limb apraxia in multiple sclerosis. Vojnosanitetski Pregled, 71(9), 821–827.
- Röhrer, S. (2013). Das Schädel-Hirn-Trauma in Europa. (Dissertation), Universität Ulm, Ulm.
- Rumiati, R. I., Weiss, P. H., Shallice, T., Ottoboni, G., Noth, J., Zilles, K., & Fink, G. R. (2004). Neural basis of pantomiming the use of visually presented objects. NeuroImage, 21(4), 1224–1231. doi: 10.1016/j.neuroimage.2003.11.017
- Salmaso, D., & Longoni, A. M. (1983). Hand preference in an Italian sample. Perceptual and Motor Skills, 57, 1029–1042.
- Schwartz, M. F., Segal, M., Veramonti, T., Ferraro, M., & Buxbaum, L. J. (2002). The Naturalistic Action Test: A standardised assessment for everyday action impairment. Neuropsychological Rehabilitation, 12(4), 311–339. doi: 10.1080/09602010244000084
- Seebauer, B. (2015). Das schwere Schädel-Hirn-Trauma - gibt es geschlechtsspezifische Unterschiede? (Dissertation), Medizinische Universität Graz, Graz.
- Sehle, A., Neumann, M., Spiteri, S., & Dettmers, C. (2014). Fatigue und kognitive Beeinträchtigungen bei der MS. neuroreha, 06(01), 22–28. doi: 10.1055/s-0034-1372453
- Spatt, J., Bak, T., Bozeat, S., Patterson, K., & Hodges, J. R. (2002). Apraxia, mechanical problem solving and semantic knowledge: Contributions to object usage in corticobasal degeneration. Journal of Neurology, 249(5), 601–608. doi: 10.1007/s004150200070
- Staff, N. P., Lucchinetti, C. F., & Keegan, B. M. (2009). Multiple sclerosis with predominant, severe cognitive impairment. Archives of Neurology, 66(9), 1139–1143. doi: 10.1001/archneurol.2009.190
- Stamenova, V. (2010). A Model-based Approach to Limb Apraxia: Evidence from Stroke and Corticobasal Syndrome (PhD thesis), University of Toronto, Toronto.
- Stamenova, V., Roy, E. A., & Black, S. E. (2014). A model-based approach to limb apraxia in Alzheimer's disease. Journal of Neuropsychology, 8(2), 246–268. doi: 10.1111/jnp.12023
- Stamenova, V., Roy, E. A., Szilagyi, G., Honjo, K., Black, S. E., & Masellis, M. (2015). Progression of limb apraxia in corticobasal syndrome: Neuropychological and functional neuroimaging report of a case series. Neurocase, 21(5), 642–659. doi: 10.1080/13554794.2014.964730
- Stegmayer, K., Bohlhalter, S., Vanbellingen, T., Federspiel, A., Moor, J., Wiest, R., … Walther, S. (2016). Structural brain correlates of defective gesture performance in schizophrenia. Cortex, 78, 125–137. doi: 10.1016/j.cortex.2016.02.014
- Tessari, A., Toraldo, A., Lunardelli, A., Zadini, A., & Rumiati, R. I. (2015). STIMA: a short screening test for ideomotor apraxia, selective for action meaning and bodily district. Neurological Sciences, 36(6), 977–984. doi: 10.1007/s10072-015-2203-4
- Valyear, K. F., & Frey, S. H. (2015). Human posterior parietal cortex mediates hand-specific planning. NeuroImage, 114, 226–238. doi: 10.1016/j.neuroimage.2015.03.058
- Vanbellingen, T., Kersten, B., Van de Winckel, A., Bellion, M., Baronti, F., Muri, R., & Bohlhalter, S. (2011a). A new bedside test of gestures in stroke: The apraxia screen of TULIA (AST). Journal of Neurology, Neurosurgery & Psychiatry, 82(4), 389–392. doi: 10.1136/jnnp.2010.213371
- Vanbellingen, T., Kersten, B., Van De Winckel, A., Bellion, M., Baronti, F., Müri, R., & Bohlhalter, S. (2011b). A new bedside test of gestures in stroke: the apraxia screen of TULIA (AST). Journal of Neurology, Neurosurgery, and Psychiatry, 82(4), 389–392. doi: 10.1136/jnnp.2010.213371
- Vanbellingen, T., Kersten, B., Van Hemelrijk, B., Van de Winckel, A., Bertschi, M., Müri, R., … Bohlhalter, S. (2010). Comprehensive assessment of gesture production: A new test of upper limb apraxia (TULIA). European Journal of Neurology, 17(1), 59–66. doi: 10.1111/j.1468-1331.2009.02741.x
- Wallesch, C. W., & Bartels, C. (2009). Neuropsychologische Defizite nach Schädel-Hirn-Trauma. In W. Sturm, M. Herrmann & T. F. Münte (Eds.), Lehrbuch der Klinischen Neuropsychologie. Grundlagen, Methoden, Diagnostik, Therapie (Vol. 2, pp. 719–725). Heidelberg: Springer.
- Weiss, P. H., Kalbe, E., Kessler, J., & Fink, G. R. (2013). Das Kölner Apraxie Screening. Göttingen: Hogrefe Verlag.
- Weiss, P. H., Ubben, S. D., Kaesberg, S., Kalbe, E., Kessler, J., Liebig, T., & Fink, G. R. (2016). Where language meets meaningful action: A combined behavior and lesion analysis of aphasia and apraxia. Brain Structure and Function, 221(1), 563–576. doi: 10.1007/s00429-014-0925-3
- Wirth, K., Held, A., Kalbe, E., Kessler, J., Saliger, J., Karbe, H., … Weiss, P. H. (2016). A new diagnostic tool for apraxia in patients with right hemisphere stroke: The Revised Cologne Apraxia Screen (CAS-R). Fortschritte Der Neurologie Und Psychiatrie, 84, 633–639.
- Wolf, S. L., Catlin, P. A., Ellis, M., Archer, A. L., Morgan, B., & Piacentino, A. (2001). Assessing Wolf motor function test as outcome measure for research in patients after stroke. Stroke, 32(7), 1635–1639. doi: 10.1161/01.STR.32.7.1635
- Wu, H. S., & Lin, L. C. (2015). Comparing cognition, mealtime performance, and nutritional status in people with dementia with or without ideational apraxia. Biological Research for Nursing, 17(2), 199–206. doi: 10.1177/1099800414536773
