Abstract
Objective: Mood- and stress-related disorders commonly cause attentional and memory impairments in middle-aged individuals. In memory testing, these impairments can be mistakenly interpreted as symptoms of dementia; thus, more reliable diagnostic approaches are needed. The present work defines the discriminant accuracy of the Dementia Apraxia Test (DATE) between psychiatric conditions and early-onset Alzheimer’s disease (AD) on its own and in combination with memory tests. Method: The consecutive sample included 50–70-year-old patients referred to dementia investigations for recent cognitive and/or affective symptoms. The DATE was administered and scored as a blinded measurement, and a receiver operating curve analysis was used to define the optimal diagnostic cut-off score. Results: A total of 24 patients were diagnosed with probable AD (mean age 61 ± 4) and 23 with a psychiatric condition (mean age 57 ± 4). The AD patients showed remarkable limb apraxia, but the psychiatric patients mainly performed at a healthy level on the DATE. The test showed a total discriminant accuracy of 87% for a total sum cut-off of 47 (sensitivity 79% and specificity 96%). The limb subscale alone reached an accuracy of 91% for a cut-off of 20 (sensitivity 83% and specificity 100%). All memory tests were diagnostically less accurate, while the combination of the limb praxis subscale and a verbal episodic memory test suggested a correct diagnosis in all but one patient. Conclusions: Apraxia testing may improve the accuracy of differentiation between AD and psychiatric aetiologies. Its potential in severe and chronic psychiatric conditions should be examined in the future.
Introduction
Alzheimer’s disease (AD) is the most common cause of dementia in individuals younger than 65 years of age (Hendriks et al., Citation2021). One-third to two-thirds of people with early-onset AD develop an atypical non-amnestic phenotype that is prone to misdiagnosis (Graff-Radford et al., Citation2021; Mendez et al., Citation2012; Vanhoutte et al., Citation2017). The atypical phenotypes debut with language, visuospatial or dysexecutive deficits instead of memory loss and disorientation (McKhann et al., Citation2011). Additionally, a parietal phenotype with prominent limb apraxia has been proposed (Mendez et al., Citation2018; Ross et al., Citation1996).
Affective disorders, depression most commonly, can be challenging to distinguish from mild AD (Brodaty & Connors, Citation2020; Olivieri et al., Citation2020; Woolley et al., Citation2011). This is because measurable impairments of memory, attention and executive functions appear in clinical anxiety (Bierman et al., Citation2005; Perin et al., Citation2022), depression (Bierman et al., Citation2005; Olaya et al., Citation2017; Rock et al., Citation2014) and stress-related exhaustion (Grossi et al., Citation2015; Nelson et al., Citation2021) in middle-aged and older people. Although the measured impairments are generally mild, late-life clinical anxiety or depression seems to affect overall cognition and memory more severely than at younger ages (Bora et al., Citation2013; DeLuca et al., Citation2005). Depression and other psychiatric conditions are therefore often misdiagnosed as young-onset dementia (Salem et al., Citation2012). On the other hand, affective symptoms are common in early-onset AD patients within the first years of the disease (Altomari et al., Citation2022; Ferreira et al., Citation2018), and depression is an independent risk factor of dementia (Cherbuin et al., Citation2015). Timely and accurate discrimination between a dementing and a reversible condition is crucial for the individual and for adequate treatment and prognosis (Brodaty & Connors, Citation2020; Merckelbach et al., Citation2012; Voros et al., Citation2020). Neuropsychological test batteries may not capture the atypical AD features and should more reliably discriminate between dementia and cognitive impairments caused by affective disorders (Graff-Radford et al., Citation2021).
‘Apraxia’ refers to the loss of voluntary skilled movement or action due to neural injury, which is not explained by primary sensory or motor deficits (Cubelli, Citation2017). Apraxias are common deficits in AD (Lesourd et al., Citation2013). The most intensively studied apraxia type in AD is upper limb apraxia (Lesourd et al., Citation2013), also called ideomotor apraxia; it originates from damage to the bilateral parietal and surrounding structures (Foster et al., Citation1986; Giannakopoulos et al., Citation1998; Johnen, Brandstetter, et al., Citation2016; Kas et al., Citation2011). Little is known about praxis in patients with affective disorders, but while they may show mild deficits in their gestural skills, frank apraxia is not expected (Pavlidou et al., Citation2021). Thus, apraxia could be used to distinguish between dementia and late-life psychiatric conditions.
The Dementia Apraxia Test (DATE) is a brief screen constructed to detect apraxia in the limbs and face and to facilitate the differentiation of healthy individuals from those with mild dementia (Johnen, Frommeyer, et al., Citation2016). The test has been validated for samples of AD, the four clinical variants of frontotemporal dementia (FTD) and Parkinson’s disease (Johnen, Frommeyer, et al., Citation2016; Johnen et al., Citation2018; Renftle et al., Citation2022). The DATE has shown good ability in detecting dementia in older individuals and even higher discriminative accuracy between young-onset AD patients and age-matched healthy control (HC) participants (Yliranta et al., Citation2023).
However, comparing AD patients with asymptomatic cognitively intact individuals does not represent the true clinical environment in which differential diagnosis is made and may result in an overestimation of the discriminative accuracy (Chassé & Fergusson, Citation2019). The present diagnostic accuracy cohort study aims to define the DATE’s ability to distinguish between two groups of symptomatic patients: early-onset AD and late-life psychiatric conditions. We hypothesized that (1) the DATE enables diagnostic differentiation between early-onset AD patients and psychiatric patients and that (2) the DATE enhances diagnostic accuracy, specifically in cases of atypical non-amnestic AD presentations and cases with episodic memory impairment due to psychiatric causes.
Materials and methods
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethical Committee of Oulu University Hospital (19/2019). All participants gave their informed written consent.
Participants
The patient samples were drawn consecutively from individuals between 50 and 70 years of age who were referred to our hospital for neurological investigations due to insidious cognitive or affective symptoms. Our chief neurologist screened all of these referrals between August 2019 and January 2022. The symptom duration limit was set at a maximum of 36 months, and individuals with earlier severe psychiatric or neurologic diagnoses or a Mini-Mental State Examination (MMSE; Folstein et al., Citation1975) score below 19 were screened out. Individuals with excessive alcohol or drug abuse were also screened out. Previous mild and brief mood disorder episodes and benign neurologic conditions (such as migraine and transient ischemic attack) were allowed, and so were the following non-diagnostic imaging findings: minor chronic traumatic injury, single lacunar lesion, white matter changes Fazekas grade 1 and small cysts. Lacunar and minor traumatic lesions were considered acceptable as signs of past silent asymptomatic events clinically and temporally unrelated to the current insidious condition, as judged by our radiologists and neurologists. Fazekas grade 1 and single lacunae are common incidental findings in middle-aged populations (Schmidt et al., Citation2011; Smith et al., Citation2017) but they are as such insufficient to cause diagnosable vascular cognitive impairment (Sachdev et al., Citation2014).
A group of voluntary HC participants of comparable age range and education level was recruited from the same hospital district. They were normally functioning asymptomatic people without psychiatric or neurologic medical histories. The group consisted of hospital workers (unfamiliar with neurological investigations), their relatives, acquaintances and acquaintances of the relatives.
Procedure
Praxis assessment
The DATE consists of 20 items measuring limb, face, oral and verbal praxis. The test emphasizes manual and facial imitation tasks for which example stimuli are provided in . Separate sum scores are counted for limb items (limb subscore) and face items (face subscore), and these together constitute a total sum score. Each item is scored from 3 (fluent) to 0 (erroneous), thus, the limb and face subscores range from 0 to 30 points and the total sum ranges from 0 to 60 points. The test stimuli and scoring principles are described in detail by Johnen, Frommeyer, et al. (Citation2016). The DATE has shown good internal consistency and inter-rater agreement (Johnen, Frommeyer, et al., Citation2016; Renftle et al., Citation2022; Yliranta et al., Citation2023). Johnen, Frommeyer, et al. (Citation2016) proposed a cut-off score of 45 points to discriminate HC from older individuals with mild dementia, resulting in 0.91 sensitivity and 0.71 specificity. We previously reported the discriminative accuracy between the early-onset AD and HC groups described in this work (Yliranta et al., Citation2023). An optimal cut-off of 49 points showed 0.88 sensitivity and 1.0 specificity, and equal accuracy was reached with a limb subscore of 22 points (sensitivity 0.92, specificity 1.0).
Figure 1. Samples of the imitation items of the Dementia Apraxia Test (Johnen, Frommeyer, et al., 2016). Reprinted with permission from Dr Andreas Johnen and IOS Press.

Experienced neuropsychologists blinded to all referral information and medical records administered the DATE at the baseline visit. The DATE results were not revealed to the clinicians who ultimately established the diagnosis.
Neuropsychological assessment
After the DATE was presented and scored, a comprehensive clinical interview and a traditional test assessment were conducted. The assessment included three tests for learning and memory: logical memory (LM) and word lists (WL) from the Finnish standardised version of the Wechsler Memory Scale, 3rd version (WMS-III; Wechsler, Citation2007) and the Rey–Osterrieth Complex Figure Test (ROCFT; Osterrieth, Citation1944). In the LM subtest, the participants were asked to recall two short passages read aloud. The WL subtest required the participants to learn 12 words in four trials. In the ROCFT, the participants copied a complex figure and then drew it from their memory. Each test included an immediate and 30-min delayed recall condition; WL additionally included a delayed recognition condition.
The rest of the baseline battery consisted of globally used tests of attention, working memory, executive function, processing speed, visual and verbal intelligence, academic skills and specific tasks for visual and language abilities (detailed in Supplemental Table 1). The HC participants completed a limited set of tests. We avoided cognitively demanding psychiatric symptom questionnaires and recorded these symptoms from interviews, referral information, the 15-item Geriatric Depression Scale (GDS-15; Sheikh & Yesavage, Citation1986) and the modified Frontal Behaviour Inventory (FBI-mod; Heidler-Gary & Hillis, Citation2007; Suhonen et al., Citation2017) completed by an informant.
Table 1. Demographic characteristics and selected descriptive scores at baseline.
Diagnoses
The clinical AD diagnoses were made by neurologists according to the National Institute on Ageing and Alzheimer’s Association guidelines (McKhann et al., 2011) based on observed performance decline and supportive evidence from neuropsychological assessment, imaging and/or biomarker analysis. The diagnostic investigations included a 1.5 T brain magnetic resonance imaging with neurodegenerative sequences, a comprehensive neuropsychological assessment, a neurological status examination and an informant interview on daily life functioning. For some patients, cerebrospinal fluid (CSF) analysis was needed to confirm or exclude the diagnosis of AD, and almost all patients returned for a follow-up visit after 1 year.
The symptom aetiology was defined as psychiatric (PSY) for patients (a) fulfilling the diagnostic requirements for a mood disorder, a stress-related or somatoform disorder (including burnout) or a first-ever psychotic outbreak; (b) showing no diagnostic neurologic imaging or status findings and (c) showing a stable or relieved symptom status at follow-up. Mild unspecific non-focal cerebral atrophy was allowed in this group. The psychiatric diagnoses were defined according to the International Classification of Diseases 10th Revision (World Health Organization, Citation2016). A minority of the patients had received an adequate psychiatric diagnosis at the referring unit, and for missing diagnoses, a general hospital psychiatrist was consulted.
Statistics
Group differences in demographic and test data were inspected with the chi-squared test for sex, one-way analysis of variance or Welch’s t test for normally distributed continuous variables and Kruskal–Wallis or Mann–Whitney U tests for non-normally distributed variables. Diagnostic status was set as an independent variable, and demographic and test variables were set as dependent variables in these comparisons. Group differences in the DATE scores were analysed with a general linear model adjusted for age and years of education. For this analysis, group status, age and education were set as independent variables and the DATE scores as dependent variables.
A receiver operating characteristic (ROC) analysis was conducted using the DATE total sum, limb subscore and face subscore as test variables to classify the patients in the AD (positive state category) or PSY groups (negative state category). DeLong’s test was used to investigate which ROC curve (independent variable) resulted in a larger area under the curve (AUC; dependent variable). ROC was repeated as an additional analysis between AD and the others (PSY and HC). The results of the ROC analysis between AD patients and HC participants have been reported elsewhere (Yliranta et al., Citation2023).
We inspected the post-hoc diagnostic ability of the seven memory test conditions (LM immediate, LM delayed, WL immediate, WL delayed, WL recognition, ROCFT immediate and ROCFT delayed) by counting how many AD patients scored pathologically and how many PSY patients scored normally in each test condition. For LM and WL, the age-corrected scaled score of 8 and above is regarded as normal, 6–7 as mildly impaired and 5 and below as pathological. As there are no local norm values for ROCFT, it was included in the HC test battery, and the results are reported here. Scores between −1 and −2 SD below the healthy average were defined as mildly impaired, and those below −2 SD were pathological.
ROC analysis was not applied to the memory tests because they were part of the reference standard procedure and they are not meant for dichotomous discrimination. All analyses were run on R v. 4.0.0 using the following packages: tableone version 0.13.0 (Yoshida & Bartel, Citation2021), pROC (Robin et al., Citation2011), cutpointr (Thiele & Hirschfeld, Citation2021), ggplot2 (Wickham, Citation2016) and rstatix version 0.7.0 (Kassambar, Citation2021).
Results
Samples
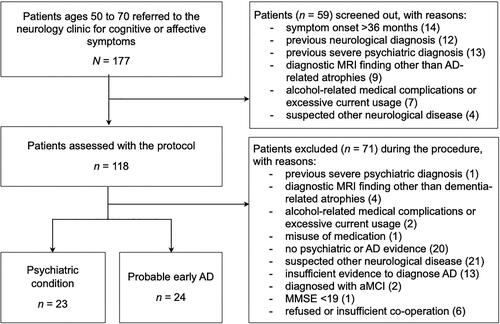
Our chief neurologist screened out 59 of 177 referrals based on medical records for reasons detailed in . During the investigations, eight more patients were found to meet the exclusion criteria. In 21 patients, a neurological diagnosis other than AD was suspected or diagnosed, and 20 patients did not show evidence of psychiatric or neurologic conditions. Thirteen AD suspects did not show enough evidence for a reliable diagnosis, one AD patient was at a moderate disease stage and two were diagnosed with amnestic mild cognitive impairment. Two participants who presented with a paranoid episode refused follow-up visits; as their conditions remained unconfirmed, they were excluded. The symptom aetiology could be defined reliably as AD in 24 patients and as PSY in 23 patients. All individuals were white native Finnish speakers, one with a bilingual background.
Figure 2. The flow chart of the patient selection. AD: Alzheimer’s disease; aMCI: amnestic mild cognitive impairment; MMSE: Mini-Mental State Examination; MRI; magnetic resonance imaging.
All AD patients were in the mild disease stage in terms of daily functioning; their cognitive performance was compromised, but they still managed daily routines independently. The clinical AD presentation was defined as predominantly amnestic for eleven patients, visuospatial/posterior for five patients, dysexecutive for two patients and linguistic for two patients. Four patients showed remarkable deficits in multiple domains without a clear predominance. Two AD patients exhibited isolated moderate memory impairment across the three test types (scaled score 4–5 or approximately SD −2). All others had severe test impairment (scaled score 1–3 or below SD −2.5) in at least one cognitive domain accompanied by milder defects in other domains. Select deficits, such as amnesia, disorientation, visuoperceptual disorder, acalculia, alexia or agraphia, were evident in 19 patients.
The CSF analysis was completed for 18 AD patients. Sixteen patients had abnormal beta-amyloid1–42 values, and 13 of these had abnormal tau values. Two patients showed merely abnormal tau but were diagnosed with clinical AD (one with amnestic and one with posterior variants) after follow-up. Hippocampal or mediotemporal atrophy (Scheltens score 1–2) was present in all AD patients and was isolated or prominent in 11 patients. Of these 11 patients, eight showed a predominantly amnestic syndrome. Fifteen patients presented with atrophies in the central, peri-Sylvian, parietal or fronto-parietal areas.
In the PSY group, two patients presented with mild and seven patients with moderate depressive episodes, two with non-psychotic severe depression, six with a persistent mood disorder, four with an adjustment disorder, one with a paranoid episode and one with a psychogenic aphasic attack. Half of the patients complained of severe work-related burnout, and an equal number reported chronic behavioural sleep disorders. Five reported primary learning disorders. The CSF analysis was ordered for two PSY patients at our clinic and had previously been completed for one patient in the referring unit. None of the patients showed pathological values.
The AD, PSY and HC groups did not significantly differ in terms of education or sex distribution, but the PSY patients were an average of four years younger than the AD and HC participants (). The mean symptom duration approached 1.5 years in both patient groups. The MMSE scores ranged from 19 to 28 among the AD patients and from 26 to 30 among the PSY patients, except for one anxious patient in the latter group, who scored 21. The same patient performed poorly across a selection of verbal tests, but his cognitive symptoms diminished at follow-up after his pain medication was changed. The PSY patients obtained better scores than the AD patients on the Frontal Assessment Battery (FAB; Dubois et al., Citation2000) and reported more depressive symptoms on the GDS, but the groups did not differ in behavioural symptoms, as assessed by informants on the FBI-mod. Descriptive results of the standard test battery can be found in Supplemental Table 1.
A total of 13 AD patients and 12 PSY patients lost points during the 30-min delay in the verbal memory tests (a loss of 1–3 scaled scores in the AD group and 1–5 scores in the PSY group). In the ROCFT, five AD patients were not able to draw anything at immediate recall, and 13 AD patients, 7 PSY patients and 10 HC participants lost material during the delay (retention rate <100%).
The discriminant accuracy of the DATE
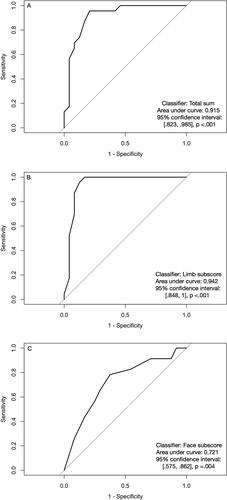
The HC and PSY groups obtained significantly better scores than the AD group on the DATE limb subscore, face subscore and total sum (). The PSY group reached the healthy level on both subscores and all item types, but they scored lower than HC for the total sum. The smallest differences between the groups were found for oral and verbal items. The generalised linear model showed that the group explained most of the variance in total sum (R2 = 0.62, F(2, 66) = 53.98, p <.001) and limb subscore (R2 = 0.66, F(2, 66) = 63.40, p< .001) but less in face subscore (R2 = 0.25, F(2, 66) = 11.25, p <.001). Adding age and education did not improve the model (total sum adjusted R2 = 0.60; limb subscore adjusted R2 = 0.65; face subscore adjusted R2 = 0.22).
Table 2. Group comparisons of Dementia Apraxia Test scores at baseline.
ROC analysis produced an optimal total sum cut-off of 47 points to discriminate between AD and PSY. This cut-off correctly classified 19 of 24 AD patients and 22 of 23 PSY patients (sensitivity 0.79, specificity 0.96, total accuracy 0.87, Youden’s J = 0.75). The limb subscore showed higher accuracy values with a cut-off of 20 points (sensitivity 0.83, specificity 1.00, total accuracy 0.91, J = 0.83), and it correctly classified 20 of 24 AD patients and all PSY patients. The AUC values for the total sum and limb subscore () did not significantly differ (Z = 1.20, p = .23, 95% confidence interval [−0.02, 0.07]). Using the face subscore as a classifier resulted in lower values (optimal cut-off of 26 points: sensitivity 0.78, specificity 0.63, total accuracy 0.70, J = 0.41). All HC participants scored above these cut-offs, and adding them to the ROC analysis (AD vs. others) improved the diagnostic accuracy (Supplemental Table 2).
Memory tests and the DATE
The five verbal memory tasks labelled 66–74% of cases correctly as AD or PSY, and the LM delayed condition achieved the highest precision. For ROCFT, immediate and delayed recall were equally precise (78% vs. 77%). shows each patient’s score for LM delayed and ROCFT immediate recall on the y-axis and DATE limb subscores on the x-axis. (Note that the ROCFT score is missing for two AD patients, as they were not able to copy the figure.) Patients with pathological memory and praxis are located towards the bottom left, and those with normal memory and praxis are towards the top right. The grey zone denotes mild memory impairment that is diagnostically unspecific.
Figure 4. (A) Verbal delayed and (b) visual immediate memory performances in relation to limb praxis. The grey zone denotes mild, diagnostically unspecific memory impairment. The vertical line denotes the optimal limb subscore cut-off (set at 20.5 for clarity). Both memory tests resulted in six participants in the undiagnostic grey zone and misdiagnosis of six participants. The praxis cut-off classified most of these cases correctly. False negative cases are circled; neither of the tests distinguished these cases as AD. For Logical Memory, nAD = 24, nPSY = 23. For ROCFT, nAD = 22, nPSY = 23, nHC = 22. AD: Alzheimer’s disease; DATE: Dementia Apraxia Test; HC: healthy control; PSY: psychiatric aetiology; ROCFT: Rey–Osterrieth Complex Figure Test.
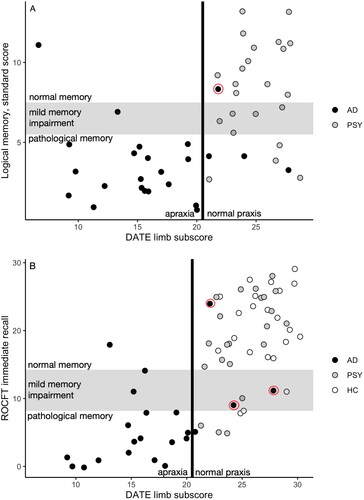
If we had relied on memory scores alone, the diagnostically problematic cases would be all those with a mild unspecific impairment or a result that would suggest a false diagnostic label (AD patients with normal memory and PSY patients with pathological memory). LM alone would have resulted in six patients being diagnostically unspecified and another six given a false label (). Adding the limb cut-off line classified all unspecified patients and four false-label patients correctly according to their diagnoses, leaving most AD patients on the affected left side and all PSY patients on the healthy right side. The combination of these two tests resulted in one case of false-negative AD.
ROCFT alone left five patients and one HC in the unspecific range between −1 and −2 SD, and it falsely labelled five patients and one HC (). The limb cut-off correctly resolved nine of these cases. This test combination resulted in three cases of false-negative AD.
Discussion
Our diagnostic accuracy cohort study aimed to assess the diagnostic value of praxis testing as part of dementia investigations among late middle-aged participants with cognitive and affective symptoms. The DATE total score or limb scale differentiated mild AD from psychiatric aetiologies with a total accuracy of 87–91%. A small portion of the AD patients showed normal or just mildly impaired memory performances, and most of them were correctly diagnosed using the limb scale. Importantly, the limb scale detected the PSY patients whose memory performances were mildly impaired or pathological. Together with a verbal episodic memory test, the limb scale correctly classified almost all patients (46/47).
The mean differences in limb praxis between AD participants and other participants were substantial within 1.5 years from symptom onset. The result is logical, considering the shared areas of neural damage in apraxia and early-stage AD. Current evidence suggests that the root of praxic functions locates in the inferior and superior aspects of the parietal lobes and the intraparietal sulci (Caspers et al., Citation2010; Ishibashi et al., Citation2016; Lesourd et al., Citation2018; Niessen et al., Citation2014; Reynaud et al., Citation2016). Additional contributions come from inferior frontal, premotor and supplemental motor areas, insula and the posterior temporal structures. Temporo-parietal hypometabolism is an established early biomarker of AD, even in the prodromal phase, irrespective of onset age (Jacobs et al., Citation2012; Jagust, Citation2018). Atrophy patterns and tau burden in the occipital, temporoparietal and frontal cortices that underlie the atypical non-memory phenotypes (Graff-Radford et al., Citation2021; Jagust, Citation2018; Vanhoutte et al., Citation2017) overlap widely with the limb praxis network (Lesourd et al., Citation2018; Reynaud et al., Citation2016). Preliminary evidence locates imaging correlates of apraxia in AD in bilateral parietal structures Johnen, Brandstetter, et al., Citation2016; Kas et al., Citation2011).
The participants who eventually were diagnosed with a psychiatric condition did not exhibit apraxia. This could be expected in the absence of focal neural damage. Affective disorders, specifically late-life conditions, may be associated with cortical thinning and functional changes, but the best-known neurobiological alterations are centred in the prefrontal and orbitofrontal sites, as well as in the limbic system (Colloby & O’Brien, Citation2013; Kaltenboeck & Harmer, Citation2018; Luan Phan, Citation2015).
The PSY patients mostly reached the healthy performance level on the DATE but, as a group, gained a few total sum points less than the healthy participants. This result parallels what Pavlidou et al. (Citation2021) found for depressed young adults, namely that a majority of them show mild gesturing deficits but not at the level of limb apraxia in the Test of Upper Limb Apraxia (TULIA; Vanbellingen et al., Citation2010). The authors assigned the cause of the mild deficits to working memory dysfunction, as they found a moderate correlation of TULIA with digit span backwards task but not with executive functioning, as measured with FAB. We agree that the mild difficulties shown by PSY patients are secondary to other cognitive factors, but it could be that it is executive ability more importantly than working memory that affects performance in DATE. Obervations of apraxia in AD led to a hypothesis that bimanual imitation essentially depends on visual analysis and executive control to produce complex hand and finger configurations according to a model (Lesourd et al., Citation2013). The DATE emphasizes bimanual limb postures and complex facial imitation, but the stimulus photograph of each item is kept in sight during the attempt. This diminishes working memory load as compared to TULIA but increases the visuoperceptual and visuoconstructive demands. The DATE and bimanual imitations in general correlate with tests that require visual attention, planning, constructive deduction and action monitoring (such as location matching, complex figure copy and trail making) (Johnen et al., Citation2018; Sanin & Benke, Citation2017; Tian et al., Citation2019; Yliranta et al., Citation2023). We suggest that mild dysfunction in these aspects is the cause of the loss of a few points in the PSY group.
Another possible cause is suboptimal test effort (McMillan et al., Citation2009). Effort was not formally assessed in this work, which is clearly a limitation. Among psychiatric samples, inadequate effort is most often associated with psychotic and personality disorders and is not as systematic among depressed individuals (McWhirter et al., Citation2020), although the impact of motivation, momentary well-being and experienced difficulty on test performance in depression has been documented (Gass & Patten, Citation2020; Moritz et al., Citation2022; for a contrasting view, see Hammers & Weisenbach, Citation2020). An essential effort-reducing factor is assessment-related anxiety, which tends to compromise verbal memory and executive control in healthy older adults (Dorenkamp et al., Citation2021; Williams et al., Citation2017). Observations of test behaviour across the present samples suggest better effort and lower test anxiety in the DATE than in tests of attention and memory. The latter tests seemed to provoke anticipation of failure in dementia-worried patients, while none of them expected difficulties in moving their hands or mimicking faces.
Each episodic memory test suggested a correct diagnosis for 66–78% of the patients across the groups. The accuracy is low, considering that all of the AD patients and none of the PSY patients showed hippocampal or mediotemporal atrophy. Notably, the same tests were part of the standard diagnostic procedure, and this likely inflates the accuracy. Higher discriminant accuracies have been reported between late-onset mild AD and depression (Coen et al., Citation1997; Dierckx et al., Citation2007, Citation2011), but we could not find previous data for middle-aged patients. Several factors could explain the low accuracy: First, the AD sample included non-amnestic phenotypes with relatively spared episodic memory and hippocampi, reflecting the heterogeneity of early-onset AD (Graff-Radford et al., Citation2021; Pollet et al., Citation2021; Vanhoutte et al., Citation2017). A more important reason is that several PSY patients failed to perform normally in the episodic memory tests. This has been observed for young middle-aged and older depressed people (Douglas et al., Citation2018; Lanza et al., Citation2020a; Masse et al., Citation2021; Moritz et al., Citation2022; Sexton et al., Citation2012). Last, loss of points in delayed memory retrieval is not specific to AD but appeared often in the PSY and HC groups, too (Butters et al., Citation2011; Lanza et al., Citation2020b).
Limb apraxia and episodic memory disturbance are unrelated impairments although they commonly co-occur in AD, and that is why they are useful to test in combination. Meaningless imitation is a task type that does not rely on prior knowledge or experience, and many praxic actions recruit semantic or working memory systems but not episodic memory (Bardakan et al., Citation2022; Baumard et al., Citation2018; Buxbaum, Citation2017; Kleineberg et al., Citation2018; Osiurak et al., Citation2021). Furthermore, the praxis network is essentially distinct from the prefrontal-mediotemporal circuitry responsible for episodic memory and its loss in AD (Eichenbaum, Citation2017; Jagust, Citation2018).
The face scale barely discriminated between the patient groups, and the PSY patients performed as well as the healthy participants. Renftle et al. (Citation2022) also noted the low discriminatory power of the face scale. The original purpose of testing face praxis with the DATE was to detect FTD (Johnen, Frommeyer, et al., Citation2016; Johnen et al., Citation2018). Late-age psychiatric syndromes considerably resemble the behavioural variant of FTD in terms of emotional and behavioural changes, and distinguishing between the two aetiologies is a challenge (Pressman et al., Citation2021). Our results put forward a hypothesis for future studies that the intactness of facial imitation in psychiatric conditions might support resolving this challenge.
Age was not associated with praxis. This may be explained by the narrow age range in this sample and by the fact that the effect of age may manifest only after the age of 70 (Baumard et al., Citation2020). The finding contrasts with observations that the DATE scores tend to be higher in younger groups (Johnen, Frommeyer, et al., Citation2016; Yliranta et al., Citation2023) and that in Parkinson’s disease, increasing age was associated with weaker limb praxis (Renftle et al., Citation2022).
Limitations
Our inferences are limited to outpatient populations and to individuals who receive their first psychiatric diagnosis in late middle-age. We do not know how the most severely depressed patients with hypomimia (Mergl et al., Citation2005) would perform for the face items. The picture of limb apraxia also becomes more complicated towards the severe end of the psychiatric disease spectrum; gesturing deficits have been documented in schizophrenia as milder at first episode and more remarkable in chronic conditions (Stegmayer et al., Citation2016; Walther et al., Citation2013). How chronic or recurring affective and psychotic disorders might alter DATE results remains a clinically relevant question for future research.
The heterogeneity of psychiatric aetiologies prevents us from generalizing the results to any single condition. For each patient, only one main diagnosis was selected and reported here, while several patients experienced comorbid disorders. In addition, we did not measure or control for symptom levels or insomnia, although they affect cognitive performance (Bierman et al., Citation2005; Fortier-Brochu et al., Citation2012). The sample included miscellaneous disorders of diverse severity levels, and some were easily discriminated against from neurologic diseases.
Due to limited diagnostic procedures, there were no imaging data available for the HC participants, and most PSY patients were not referred for CSF puncture. We cannot exclude latent disease pathology for their part, as incidental cerebral atrophies and AD-type CSF abnormalities are not uncommon at this age (Brugulat-Serrat et al., Citation2017; Randall et al., Citation2013). In addition, the HC group is a convenience sample that may not accurately represent the general population.
Conclusions
The DATE reliably identifies people with AD among middle-aged patients exhibiting various cognitive and affective symptoms. High accuracy can be reached if it is combined with episodic memory tests, but it seems useful as a stand-alone test.
| Abbreviations | ||
| AD | = | Alzheimer’s disease |
| aMCI | = | amnestic mild cognitive impairment |
| AUC | = | area under curve |
| CSF | = | cerebrospinal fluid |
| DATE | = | Dementia Apraxia Test |
| FAB | = | Frontal Assessment Battery |
| FTD | = | fronto-temporal dementia |
| GDS | = | Geriatric Depression Scale |
| HC | = | healthy comparison |
| MMSE | = | Mini-Mental State Examination |
| LM | = | Logical Memory subtest |
| MRI | = | magnetic resonance imaging |
| PSY | = | psychiatric aetiology |
| ROC | = | receiver operating curve |
| ROCFT | = | Rey–Osterrieth Complex Figure Test |
| TULIA | = | Test of Upper Limb Apraxia |
| WL | = | Word Lists subtest |
| WMS | = | Wechsler Memory Scale |
Supplemental Material
Download MS Word (21.1 KB)Acknowledgments
We wish to thank Teemu Karjalainen and Antti Liikkanen for their valuable help in the process.
Disclosure statement
The authors report no competing interests to declare.
Data availability statement
The reported data and code are available from the corresponding author upon request.
Additional information
Funding
References
- Altomari, N., Bruno, F., Laganà, V., Smirne, N., Colao, R., Curcio, S., di Lorenzo, R., Frangipane, F., Maletta, R., Puccio, G., & Bruni, A. C. (2022). A comparison of behavioral and psychological symptoms of dementia (BPSD) and BPSD sub-syndromes in early-onset and late-onset Alzheimer’s disease. Journal of Alzheimer’s Disease, 85(2), 691–699. https://doi.org/10.3233/JAD-215061
- Baumard, J., Lesourd, M., Remigereau, C., Jarry, C., Etcharry-Bouyx, F., Chauviré, V., Osiurak, F., & Le Gall, D. (2018). Tool use in neurodegenerative diseases: Planning or technical reasoning? Journal of Neuropsychology, 12(3), 409–426. https://doi.org/10.1111/jnp.12121
- Baumard, J., Lesourd, M., Remigereau, C., Lucas, C., Jarry, C., Osiurak, F., & Le Gall, D. (2020). Imitation of meaningless gestures in normal aging. Neuropsychology, Development, and Cognition. Section B, Aging, Neuropsychology and Cognition, 27(5), 729–747. https://doi.org/10.1080/13825585.2019.1674773
- Bardakan, M. M., Schmidt, C. C., Hesse, M. D., Fink, G. R., & Weiss, P. H. (2022). Neuropsychological evidence for motor working memory subsystem related to apraxia. Journal of Cognitive Neuroscience, 34(11), 2016–2027. https://doi.org/10.1162/jocn_a_01893
- Bierman, E., Comijs, H., Jonker, C., & Beekman, A. (2005). Effects of anxiety versus depression on cognition in later life. The American Journal of Geriatric Psychiatry, 13(8), 686–693. https://doi.org/10.1176/appi.ajgp.13.8.686
- Bora, E., Harrison, B. J., Yücel, M., & Pantelis, C. (2013). Cognitive impairment in euthymic major depressive disorder: A meta-analysis. Psychological Medicine, 43(10), 2017–2026. https://doi.org/10.1017/S0033291712002085
- Brodaty, H., & Connors, M. H. (2020). Pseudodementia, pseudo-pseudodementia, and pseudodepression. Alzheimer’s and Dementia: Diagnosis, Assessment and Disease Monitoring, 12(1), e12027. https://doi.org/10.1002/dad2.12027
- Brugulat-Serrat, A., Rojas, S., Bargalló, N., Conesa, G., Minguillón, C., Fauria, K., Gramunt, N., Molinuevo, J. L., & Gispert, J. D. (2017). Incidental findings on brain MRI of cognitively normal first-degree descendants of patients with Alzheimer’s disease: A cross-sectional analysis from the ALFA (Alzheimer and Families) project. BMJ Open, 7(3), e013215. https://doi.org/10.1136/bmjopen-2016-013215
- Butters, M. A., Bhalla, R. K., Andreescu, C., Wetherell, J. L., Mantella, R., Begley, A. E., & Lenze, E. J. (2011). Changes in neuropsychological functioning following treatment for late-life generalised anxiety disorder. The British Journal of Psychiatry: The Journal of Mental Science, 199(3), 211–218. https://doi.org/10.1192/bjp.bp.110.090217
- Buxbaum, L. J. (2017). Learning, remembering, and predicting how to use tools: Distributed neurocognitive mechanisms: Comment on Osiurak and Badets (2016). Psychological Review, 124(3), 346–360. https://doi.org/10.1037/rev0000051
- Caspers, S., Zilles, K., Laird, A. R., & Eickhoff, S. B. (2010). ALE meta-analysis of action observation and imitation in the human brain. NeuroImage, 50(3), 1148–1167. https://doi.org/10.1016/j.neuroimage.2009.12.112
- Chassé, M., & Fergusson, D. A. (2019). Diagnostic accuracy studies. Seminars in Nuclear Medicine, 49(2), 87–93. https://doi.org/10.1053/j.semnuclmed.2018.11.005
- Cherbuin, N., Kim, S., & Anstey, K. J. (2015). Dementia risk estimates associated with measures of depression: A systematic review and meta-analysis. BMJ Open, 5(12), e008853. https://doi.org/10.1136/bmjopen-2015-008853
- Coen, R. F., Kirby, M., Swanwick, G. R., Maguire, C. P., Walsh, J. B., Coakley, D., O’Neill, D., & Lawlor, B. A. (1997). Distinguishing between patients with depression or very mild Alzheimer’s disease using the Delayed-Word-Recall test. Dementia and Geriatric Cognitive Disorders, 8(4), 244–247. https://doi.org/10.1159/000106638
- Colloby, S. J., & O’Brien, J. T. (2013). Structural neuroimaging in late-life mood disorders. In H. Lavretsky, M. Sajatovic, & C. Reynolds (Eds.), Late-life mood disorders (pp. 559–571). Oxford University Press. https://doi.org/10.1093/med/9780199796816.001.0001
- Cubelli, R. (2017). Definition: Apraxia. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior, 93, 227. https://doi.org/10.1016/j.cortex.2017.03.012
- DeLuca, A. K., Lenze, E. J., Mulsant, B. H., Butters, M. A., Karp, J. F., Dew, M. A., Pollock, B. G., Shear, M. K., Houck, P. R., & Reynolds, C. F. (2005). Comorbid anxiety disorder in late life depression: Association with memory decline over four years. International Journal of Geriatric Psychiatry, 20(9), 848–854. https://doi.org/10.1002/gps.1366
- Dierckx, E., Engelborghs, S., De Raedt, R., De Deyn, P. P., D’Haenens, E., Verte, D., & Ponjaert-Kristoffersen, I. (2011). The 10-word learning task in the differential diagnosis of early Alzheimer’s disease and elderly depression: A cross-sectional pilot study. Aging & Mental Health, 15(1), 113–121. https://doi.org/10.1080/13607863.2010.505228
- Dierckx, E., Engelborghs, S., de Raedt, R., de Deyn, P. P., & Ponjaert-Kristoffersen, I. (2007). Differentiation between mild cognitive impairment, Alzheimer’s disease and depression by means of cued recall. Psychological Medicine, 37(5), 747–755. https://doi.org/10.1017/S003329170600955X
- Dorenkamp, M. A., Irrgang, M., & Vik, P. (2021). Assessment-related anxiety among older adults: Associations with neuropsychological test performance. Aging, Neuropsychology, and Cognition, 28(5), 781–795. https://doi.org/10.1080/13825585.2021.2016584
- Douglas, K. M., Gallagher, P., Robinson, L. J., Carter, J. D., McIntosh, V. V. W., Frampton, C. M. A., Watson, S., Young, A. H., Ferrier, I. N., & Porter, R. J. (2018). Prevalence of cognitive impairment in major depression and bipolar disorder. Bipolar Disorders, 20(3), 260–274. https://doi.org/10.1111/bdi.12602
- Dubois, B., Slachevsky, A., Litvan, I., & Pillon, B. (2000). The FAB: A Frontal Assessment Battery at bedside. Neurology, 55(11), 1621–1626. https://doi.org/10.1212/WNL.55.11.1621
- Eichenbaum, H. (2017). Prefrontal-hippocampal interactions in episodic memory. Nature Reviews. Neuroscience, 18(9), 547–558. https://doi.org/10.1038/nrn.2017.74
- Ferreira, M., do, C., Abreu, M. J., Machado, C., Santos, B., Machado, Á., & Costa, A. S. (2018). Neuropsychiatric profile in early- versus late-onset Alzheimer’s disease. American Journal of Alzheimer’s Disease and Other Dementias, 33(2), 93–99. https://doi.org/10.1177/1533317517744061
- Folstein, M. F., Folstein, S. E., & McHugh, P. R. (1975). “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–198. https://doi.org/10.1016/0022-3956(75)90026-6
- Fortier-Brochu, É., Beaulieu-Bonneau, S., Ivers, H., & Morin, C. M. (2012). Insomnia and daytime cognitive performance: A meta-analysis. Sleep Medicine Reviews, 16(1), 83–94. https://doi.org/10.1016/j.smrv.2011.03.008
- Foster, N. L., Chase, T. N., Patronas, N. J., Gillespie, M. M., & Fedio, P. (1986). Cerebral mapping of apraxia in Alzheimer’s disease by positron emission tomography. Annals of Neurology, 19(2), 139–143. https://doi.org/10.1002/ana.410190205
- Gass, C. S., & Patten, B. (2020). Depressive symptoms, memory complaints, and memory test performance. Journal of Clinical and Experimental Neuropsychology, 42(6), 602–610. https://doi.org/10.1080/13803395.2020.1782848
- Giannakopoulos, P., Duc, M., Gold, G., Hof, P. R., Michel, J. P., & Bouras, C. (1998). Pathologic correlates of apraxia in Alzheimer’s disease. Archives of Neurology, 55(5), 689–695. https://jamanetwork.com/ https://doi.org/10.1001/archneur.55.5.689
- Graff-Radford, J., X Yong, K. X., Apostolova, L. G., Bouwman, F. H., Carrillo, M., Dickerson, B. C., Rabinovici, G. D., Schott, J. M., Jones, D. T., & Murray, M. E. (2021). New insights into atypical Alzheimer’s disease in the era of biomarkers. The Lancet Neurology, 20(3), 222–234. https://doi.org/10.1016/S1474-4422(20)30440-3
- Grossi, G., Perski, A., Osika, W., & Savic, I. (2015). Stress-related exhaustion disorder - clinical manifestation of burnout? A review of assessment methods, sleep impairments, cognitive disturbances, and neuro-biological and physiological changes in clinical burnout. Scandinavian Journal of Psychology, 56(6), 626–636. https://doi.org/10.1111/sjop.12251
- Hammers, D. B., & Weisenbach, S. (2020). Questioning the effort hypothesis that depressed patients perform disproportionately worse on effortful cognitive tasks. Perceptual and Motor Skills, 127(2), 401–414. https://doi.org/10.1177/0031512519898356
- Heidler-Gary, J., & Hillis, A. E. (2007). Distinctions between the dementia in amyotrophic lateral sclerosis with frontotemporal dementia and the dementia of Alzheimer’s disease. Amyotrophic Lateral Sclerosis, 8(5), 276–282. https://doi.org/10.1080/17482960701381911
- Hendriks, S., Peetoom, K., Bakker, C., van der Flier, W. M., Papma, J. M., Koopmans, R., Verhey, F. R. J., de Vugt, M., Köhler, S., Withall, A., Parlevliet, J. L., Uysal-Bozkir, Ö., Gibson, R. C., Neita, S. M., Nielsen, T. R., Salem, L. C., Nyberg, J., Lopes, M. A., Dominguez, J. C., … Ruano, L. (2021). Global prevalence of young-onset dementia: A systematic review and meta-analysis. JAMA Neurology, 78(9), 1080–1090. https://doi.org/10.1001/jamaneurol.2021.2161
- Ishibashi, R., Pobric, G., Saito, S., & Lambon Ralph, M. A. (2016). The neural network for tool-related cognition: An activation likelihood estimation meta-analysis of 70 neuroimaging contrasts. Cognitive Neuropsychology, 33(3–4), 241–256. https://doi.org/10.1080/02643294.2016.1188798
- Jacobs, H. I. L., van Boxtel, M. P. J., Jolles, J., Verhey, F. R. J., & Uylings, H. B. M. (2012). Parietal cortex matters in Alzheimer’s disease: An overview of structural, functional and metabolic findings. Neuroscience and Biobehavioral Reviews, 36(1), 297–309. https://doi.org/10.1016/j.neubiorev.2011.06.009
- Jagust, W. (2018). Imaging the evolution and pathophysiology of Alzheimer’s disease. Nature Reviews Neuroscience, 19(11), 687–700. https://doi.org/10.1038/s41583-018-0067-3
- Johnen, A., Brandstetter, L., Kärgel, C., Wiendl, H., Lohmann, H., & Duning, T. (2016). Shared neural correlates of limb apraxia in early stages of Alzheimer’s dementia and behavioural variant frontotemporal dementia. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior, 84, 1–14. https://doi.org/10.1016/j.cortex.2016.08.009
- Johnen, A., Frommeyer, J., Modes, F., Wiendl, H., Duning, T., & Lohmann, H. (2016). Dementia Apraxia Test (DATE): A brief tool to differentiate behavioral variant frontotemporal dementia from Alzheimer’s dementia based on apraxia profiles. Journal of Alzheimer’s Disease, 49(3), 593–605. https://doi.org/10.3233/JAD-150447
- Johnen, A., Reul, S., Wiendl, H., Meuth, S. G., & Duning, T. (2018). Apraxia profiles—A single cognitive marker to discriminate all variants of frontotemporal lobar degeneration and Alzheimer’s disease. Alzheimer’s & Dementia (Amsterdam, Netherlands), 10, 363–371. https://doi.org/10.1016/j.dadm.2018.04.002
- Kaltenboeck, A., & Harmer, C. (2018). The neuroscience of depressive disorders: A brief review of the past and some considerations about the future. Brain and Neuroscience Advances, 2, 2398212818799269. https://doi.org/10.1177/2398212818799269
- Kas, A., de Souza, L. C., Samri, D., Bartolomeo, P., Lacomblez, L., Kalafat, M., Migliaccio, R., Thiebaut De Schotten, M., Cohen, L., Dubois, B., Habert, M. O., & Sarazin, M. (2011). Neural correlates of cognitive impairment in posterior cortical atrophy. Brain: A Journal of Neurology, 134(Pt 5), 1464–1478. https://doi.org/10.1093/brain/awr055
- Kassambar, A. (2021). rstatix: Pipe-friendly framework for basic statistical tests. http://CRAN.R-project.org/package=rstatix
- Kleineberg, N. N., Dovern, A., Binder, E., Grefkes, C., Eickhoff, S. B., Fink, G. R., & Weiss, P. H. (2018). Action and semantic tool knowledge – Effective connectivity in the underlying neural networks. Human Brain Mapping, 39(9), 3473–3486. https://doi.org/10.1002/hbm.24188
- Lanza, C. E., Sejunaite, K., Steindel, C., Scholz, I., & Riepe, M. W. (2020a). On the conundrum of cognitive impairment due to depressive disorder in older patients. PLoS One, 15(4), e0231111. https://doi.org/10.1371/journal.pone.0231111
- Lanza, C., Sejunaite, K., Steindel, C., Scholz, I., & Riepe, M. W. (2020b). Cognitive profiles in persons with depressive disorder and Alzheimer’s disease. Brain Communications, 2(2), fcaa206. https://doi.org/10.1093/braincomms/fcaa206
- Lesourd, M., Le Gall, D., Baumard, J., Croisile, B., Jarry, C., & Osiurak, F. (2013). Apraxia and Alzheimer’s disease: Review and perspectives. Neuropsychology Review, 23(3), 234–256. https://doi.org/10.1007/s11065-013-9235-4
- Lesourd, M., Osiurak, F., Baumard, J., Bartolo, A., Vanbellingen, T., & Reynaud, E. (2018). Cerebral correlates of imitation of intransitive gestures: An integrative review of neuroimaging data and brain lesion studies. Neuroscience and Biobehavioral Reviews, 95, 44–60. https://doi.org/10.1016/j.neubiorev.2018.07.019
- Luan Phan, K. (2015). Neurobiology and neural circuitry of anxiety disorders. In K. Ressler, D. Pine, & B. Rothbaum (Eds.), Anxiety disorders (pp. 33–45). Oxford University Press. http://ebookcentral.proquest.com/lib/tampere/detail.action?docID=2012685
- Masse, C., Vandel, P., Sylvestre, G., Noiret, N., Bennabi, D., Mauny, F., Puyraveau, M., Barsznica, Y., Dartevelle, J., Meyer, A., Binetruy, M., Lavaux, M., Ryff, I., Giustiniani, J., Magnin, E., Galmiche, J., Haffen, E., & Chopard, G. (2021). Cognitive impairment in late-life depression: A comparative study of healthy older people, late-life depression, and mild Alzheimer’s disease using multivariate base rates of low scores. Frontiers in Psychology, 12, 724731. https://doi.org/10.3389/fpsyg.2021.724731
- McKhann, G. M., Knopman, D. S., Chertkow, H., Hyman, B. T., Jack, C. R., Kawas, C. H., Klunk, W. E., Koroshetz, W. J., Manly, J. J., Mayeux, R., Mohs, R. C., Morris, J. C., Rossor, M. N., Scheltens, P., Carrillo, M. C., Thies, B., Weintraub, S., & Phelps, C. H. (2011). The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia : The Journal of the Alzheimer’s Association, 7(3), 263–269. https://doi.org/10.1016/j.jalz.2011.03.005
- McMillan, T. M., Anderson, S., Baker, G., Berger, M., Powell, G., & Knight, R. (2009). Assessment of effort in clinical testing of cognitive functioning for adults. The British Psychological Society.
- McWhirter, L., Ritchie, C. W., Stone, J., & Carson, A. (2020). Performance validity test failure in clinical populations - A systematic review. Journal of Neurology, Neurosurgery, and Psychiatry, 91(9), 945–952. https://doi.org/10.1136/jnnp-2020-323776
- Mendez, M. F., Lee, A. S., Joshi, A., & Shapira, J. S. (2012). Nonamnestic presentations of early-onset Alzheimer’s disease. American Journal of Alzheimer’s Disease and Other Dementias, 27(6), 413–420. https://doi.org/10.1177/1533317512454711
- Mendez, M. F., Moheb, N., Desarzant, R. E., & Teng, E. H. (2018). The progressive acalculia presentation of parietal variant Alzheimer’s disease. Journal of Alzheimer’s Disease, 63(3), 941–948. https://doi.org/10.3233/JAD-180024
- Merckelbach, H., Jelicic, M., & Jonker, C. (2012). Planting a misdiagnosis of Alzheimer’s disease in a person’s mind. Acta Neuropsychiatrica, 24(1), 60–62. https://doi.org/10.1111/j.1601-5215.2011.00586.x
- Mergl, R., Mavrogiorgou, P., Hegerl, U., & Juckel, G. (2005). Kinematical analysis of emotionally induced facial expressions: A novel tool to investigate hypomimia in patients suffering from depression. Journal of Neurology, Neurosurgery, and Psychiatry, 76(1), 138–140. https://doi.org/10.1136/jnnp.2004.037127
- Moritz, S., Xie, J., Penney, D., Bihl, L., Hlubek, N., Elmers, J., Beblo, T., & Hottenrott, B. (2022). The magnitude of neurocognitive impairment is overestimated in depression: The role of motivation, debilitating momentary influences, and the overreliance on mean differences. Psychological Medicine, 1–11. https://doi.org/10.1017/S0033291721004785
- Nelson, A., Gavelin, H. M., Boraxbekk, C. J., Eskilsson, T., Josefsson, M., Slunga Järvholm, L., & Neely, A. S. (2021). Subjective cognitive complaints in patients with stress-related exhaustion disorder: A cross sectional study. BMC Psychology, 9(1), 84. https://doi.org/10.1186/s40359-021-00576-9
- Niessen, E., Fink, G. R., & Weiss, P. H. (2014). Apraxia, pantomime and the parietal cortex. NeuroImage. Clinical, 5, 42–52. https://doi.org/10.1016/j.nicl.2014.05.017
- Olaya, B., Bobak, M., Haro, J. M., & Demakakos, P. (2017). Trajectories of verbal episodic memory in middle-aged and older adults: Evidence from the English Longitudinal Study of Ageing. Journal of the American Geriatrics Society, 65(6), 1274–1281. https://doi.org/10.1111/jgs.14789
- Olivieri, P., Lagarde, J., Hamelin, L., Niel, P., Bottlaender, M., Kas, A., Habert, M., & Sarazin, M. (2020). Occupational burnout‐like syndrome in early‐onset Alzheimer’s disease. Alzheimer’s & Dementia, 16(6), e041999. https://doi.org/10.1002/alz.041999
- Osiurak, F., Reynaud, E., Baumard, J., Rossetti, Y., Bartolo, A., & Lesourd, M. (2021). Pantomime of tool use: Looking beyond apraxia. Brain Communications, 3(4), fcab263. https://doi.org/10.1093/braincomms/fcab263
- Osterrieth, P. A. (1944). Le test de copie d’une figure complexe. Archives De Psychologie, 30, 286–356.
- Pavlidou, A., Viher, P. v., Bachofner, H., Weiss, F., Stegmayer, K., Shankman, S. A., Mittal, V. A., & Walther, S. (2021). Hand gesture performance is impaired in major depressive disorder: A matter of working memory performance? Journal of Affective Disorders, 292, 81–88. https://doi.org/10.1016/j.jad.2021.05.055
- Perin, S., Lai, J., Pase, M., Bransby, L., Buckley, R., Yassi, N., Pietrzak, R. H., Maruff, P., & Lim, Y. Y. (2022). Elucidating the association between depression, anxiety, and cognition in middle-aged adults: Application of dimensional and categorical approaches. Journal of Affective Disorders, 296, 559–566. https://doi.org/10.1016/j.jad.2021.10.007
- Pollet, M., Skrobala, E., Lopes, R., Kuchcinski, G., Bordier, C., Rollin-Sillaire, A., Bombois, S., Pasquier, F., & Delbeuck, X. (2021). A multimodal, longitudinal study of cognitive heterogeneity in early-onset Alzheimer’s disease. European Journal of Neurology, 28(12), 3990–3998. https://doi.org/10.1111/ene.15097
- Pressman, P. S., Matlock, D., & Ducharme, S. (2021). Distinguishing behavioral variant frontotemporal dementia from primary psychiatric disorders: A review of recently published consensus recommendations from the Neuropsychiatric International Consortium for Frontotemporal Dementia. The Journal of Neuropsychiatry and Clinical Neurosciences, 33(2), 152–156. https://doi.org/10.1176/appi.neuropsych.20090238
- Randall, C., Mosconi, L., de Leon, M., & Glodzik, L. (2013). Cerebrospinal fluid biomarkers of Alzheimer’s disease in cognitively healthy elderly. Frontiers in Bioscience, 18(3), 1150–1173. https://doi.org/10.2741/4170
- Renftle, D., Becker, S., Brockmann, K., Gasser, T., Michaelis, K., Solbrig, S., Sulzer, P., Johnen, A., & Liepelt-Scarfone, I. (2022). Evaluation of the Dementia Apraxia Test in Parkinson’s disease patients. Dementia and Geriatric Cognitive Disorders, 51(3), 271–278. https://doi.org/10.1159/000525618
- Reynaud, E., Lesourd, M., Navarro, J., & Osiurak, F. (2016). On the neurocognitive origins of human tool use: A critical review of neuroimaging data. Neuroscience and Biobehavioral Reviews, 64, 421–437. https://doi.org/10.1016/j.neubiorev.2016.03.009
- Robin, X., Turck, N., Hainard, A., Tiberti, N., Lisacek, F., Sanchez, J.-C., & Müller, M. (2011). pROC: An open-source package for R and S + to analyze and compare ROC curves. BMC Bioinformatics, 12(1), 77. https://doi.org/10.1186/1471-2105-12-77
- Rock, P. L., Roiser, J. P., Riedel, W. J., & Blackwell, A. D. (2014). Cognitive impairment in depression: A systematic review and meta-analysis. Psychological Medicine, 44(10), 2029–2040. https://doi.org/10.1017/S0033291713002535
- Ross, S. J., Graham, N., Stuart-Green, L., Prins, M., Xuereb, J., Patterson, K., & Hodges, J. R. (1996). Progressive biparietal atrophy: An atypical presentation of Alzheimer’s disease. Journal of Neurology, Neurosurgery, and Psychiatry, 61(4), 388–395. https://doi.org/10.1136/jnnp.61.4.388
- Sachdev, P., Kalaria, R., O’Brien, J., Skoog, I., Alladi, S., Black, S. E., Blacker, D., Blazer, D. G., Chen, C., Chui, H., Ganguli, M., Jellinger, K., Jeste, D. V., Pasquier, F., Paulsen, J., Prins, N., Rockwood, K., Roman, G., & Scheltens, P, Internationlal Society for Vascular Behavioral and Cognitive Disorders. (2014). Diagnostic criteria for vascular cognitive disorders: A VASCOG statement. Alzheimer Disease and Associated Disorders, 28(3), 206–218. https://doi.org/10.1097/WAD.0000000000000034
- Salem, L. C., Andersen, B. B., Nielsen, T. R., Stokholm, J., Jørgensen, M. B., Rasmussen, M. H., & Waldemar, G. (2012). Overdiagnosis of dementia in young patients-a nationwide register-based study. Dementia and Geriatric Cognitive Disorders, 34(5–6), 292–299. https://doi.org/10.1159/000345485
- Sanin, G., & Benke, T. (2017). Bimanual gesture imitation in Alzheimer’s disease. Journal of Alzheimer’s Disease, 57(1), 53–59. https://doi.org/10.3233/JAD-160680
- Schmidt, R., Schmidt, H., Haybaeck, J., Loitfelder, M., Weis, S., Cavalieri, M., Seiler, S., Enzinger, C., Ropele, S., Erkinjuntti, T., Pantoni, L., Scheltens, P., Fazekas, F., & Jellinger, K. (2011). Heterogeneity in age-related white matter changes. Acta Neuropathologica, 122(2), 171–185. https://doi.org/10.1007/s00401-011-0851-x
- Sexton, C. E., McDermott, L., Kalu, U. G., Herrmann, L. L., Bradley, K. M., Allan, C. L., Le Masurier, M., MacKay, C. E., & Ebmeier, K. P. (2012). Exploring the pattern and neural correlates of neuropsychological impairment in late-life depression. Psychological Medicine, 42(6), 1195–1202. https://doi.org/10.1017/S0033291711002352
- Sheikh, J. I., & Yesavage, J. A. (1986). Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. Clinical Gerontologist, 5(1-2), 165–173. https://doi.org/10.1300/J018v05n01_09
- Smith, E. E., Saposnik, G., Biessels, G. J., Doubal, F. N., Fornage, M., Gorelick, P. B., Greenberg, S. M., Higashida, R. T., Kasner, S. E., & Seshadri, S, American Heart Association Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Functional Genomics and Translational Biology; and Council on Hypertension. (2017). Prevention of stroke in patients with silent cerebrovascular disease: A scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke, 48(2), e44–e71. https://doi.org/10.1161/STR.0000000000000116
- Stegmayer, K., Moor, J., Vanbellingen, T., Bohlhalter, S., Müri, R. M., Strik, W., & Walther, S. (2016). Gesture performance in first-and multiple-episode patients with schizophrenia spectrum disorders. Neuropsychobiology, 73(4), 201–208. https://doi.org/10.7892/boris.83458
- Suhonen, N. M., Hallikainen, I., Hänninen, T., Jokelainen, J., Krüger, J., Hall, A., Pikkarainen, M., Soininen, H., & Remes, A. M. (2017). The Modified Frontal Behavioral Inventory (FBI-mod) for patients with frontotemporal lobar degeneration, Alzheimer’s disease, and mild cognitive impairment. Journal of Alzheimer’s Disease, 56(4), 1241–1251. https://doi.org/10.3233/JAD-160983
- Thiele, C., & Hirschfeld, G. (2021). Cutpointr: Improved estimation and validation of optimal cutpoints in R. Journal of Statistical Software, 98(11), 1–27. https://doi.org/10.18637/jss.v098.i11
- Tian, Q., Chastan, N., Thambisetty, M., Resnick, S. M., Ferrucci, L., & Studenski, S. A. (2019). Bimanual gesture imitation links to cognition and olfaction. Journal of the American Geriatrics Society, 67(12), 2581–2586. https://doi.org/10.1111/jgs.16151
- Vanbellingen, T., Kersten, B., van Hemelrijk, B., van de Winckel, A., Bertschi, M., Müri, R., de Weerdt, W., & Bohlhalter, S. (2010). Comprehensive assessment of gesture production: A new test of upper limb apraxia (TULIA). European Journal of Neurology, 17(1), 59–66. https://doi.org/10.1111/j.1468-1331.2009.02741.x
- Vanhoutte, M., Semah, F., Rollin Sillaire, A., Jaillard, A., Petyt, G., Kuchcinski, G., Maureille, A., Delbeuck, X., Fahmi, R., Pasquier, F., & Lopes, R. (2017). 18F-FDG PET hypometabolism patterns reflect clinical heterogeneity in sporadic forms of early-onset Alzheimer’s disease. Neurobiology of Aging, 59, 184–196. https://doi.org/10.1016/j.neurobiolaging.2017.08.009
- Voros, V., Fekete, S., Tenyi, T., Rihmer, Z., Szili, I., & Osvath, P. (2020). Untreated depressive symptoms significantly worsen quality of life in old age and may lead to the misdiagnosis of dementia: A cross-sectional study. Annals of General Psychiatry, 19(1), 52. https://doi.org/10.1186/s12991-020-00302-6
- Walther, S., Vanbellingen, T., Müri, R., Strik, W., & Bohlhalter, S. (2013). Impaired pantomime in schizophrenia: Association with frontal lobe function. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior, 49(2), 520–527. https://doi.org/10.1016/j.cortex.2011.12.008
- Wechsler, D. (2007). Wechsler memory scale III, käsikirja. Psykologien Kustannus.
- Wickham, H. (2016). Ggplot2: Elegant graphics for data analysis. Springer. https://doi.org/10.1007/978-3-319-24277-4
- Williams, M. W., Kueider, A. M., Dmitrieva, N. O., Manly, J. J., Pieper, C. F., Verney, S. P., & Gibbons, L. E. (2017). Anxiety symptoms bias memory assessment in older adults. International Journal of Geriatric Psychiatry, 32(9), 983–990. https://doi.org/10.1002/gps.4557
- Woolley, J. D., Khan, B. K., Murthy, N. K., Miller, B. L., & Rankin, K. P. (2011). The diagnostic challenge of psychiatric symptoms in neurodegenerative disease: Rates of and risk factors for prior psychiatric diagnosis in patients with early neurodegenerative disease. The Journal of Clinical Psychiatry, 72(2), 126–133. https://doi.org/10.4088/JCP.10m06382oli
- World Health Organization. (2016). International statistical classification of diseases and related health problems (10th ed.). World Health Organization. https://icd.who.int/browse10/2019/en#/
- Yliranta, A., Nuorva, J., Karjalainen, V. L., Ahmasalo, R., & Jehkonen, M. (2023). The dementia apraxia test can detect early-onset Alzheimer’s disease. Neuropsychology, 37(1), 44–51. https://doi.org/10.1037/neu0000873
- Yoshida, K., & Bartel, A. (2021). Create ‘Table1’ to describe baseline characteristics with or without propensity score weights. http://CRAN.R-project.org/package=tableone