?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
In this work, streptokinase (SK) coenzyme was coupled onto functionalized graft copolymer poly(vinyl chloride)-g-poly(ethylene glycol)methacrylate (PEGMA) using the water soluble carbodiimide 1-ethyl-3-(3-dimethyl aminopropyl carbodiimide hydrochloride) and sulfo-N-Hydroxysulfo succinimide. Different graft values of PEGMA copolymers were prepared via preirradiation method in air by using ultraviolet light. SK was coupled onto graft copolymer and the enzyme activity was determined before and after immobilization. Surface morphology, grafting, and enzymatic activity were studied by using infrared spectroscopy, atomic force microscopy, and fluorescence microscopy. Results revealed that graft value of 0.15 provide optimum conditions to retain original SK activity, the enzyme distribution was 2.8 μg cm−2 of copolymer surface and kinetic parameters, K m and V max were 0.14 μM and 29.8 μM min−1, respectively.
Introduction
Streptokinase (SK) is an effective and inexpensive human plasminogen activator used routinely in medical treatments for thrombolysis medication in some cases of myocardial infarction (heart attack) and pulmonary embolism [Citation1]. This coenzyme is a single chain polypeptide made up of 414 amino acid residue and has a molar mass of 47KD [Citation2]. SK tends to dissolve thrombus [Citation3] by binding with blood-free plasminogen to become plasmin [Citation4-6].
Potential applications, in bioprocess engineering, can be developed for SK if coupled onto a suitable polymer substrate as poly(vinyl chloride) (PVC). PVC exhibits a good price/performance balance that allows many applications such as pipes, packaging foils, and medical products [Citation7-9]. PVC has many applications due to its attractive physical characteristics such as flexibility, softness, and transparency [Citation10,Citation11]. Some of its surface characteristics such as wettability and biocompatibility within biological environments can be modified by different methods [Citation12,Citation13].
In the last decade, numerous amounts of enzyme immobilization methods have been developed [Citation14-16] and their advantages, as compared to conventional methods, are: high catalytic activity, substrate specificity, stereoselectivity, and regiospecificity. They deliver high performance at standard temperature and pressure. Although they offer advantages, their use has not become generalized because, in several cases, they lose their original activity after immobilization or in the case of neat organic liquids; they are orders of magnitude less active than in the aqueous phase [Citation17]. Also, large-scale syntheses imply several purification steps that could reduce purity.
SK immobilization onto polymeric soft surfaces as poly(ethylene glycol) (PEG) has been developed by researchers [Citation18-20] in organic media; however, the mechanical properties are very poor. Immobilizing SK onto PVC can combine the enzymatic properties of the SK and the excellent mechanical properties of the PVC using poly(ethylene glycol) methacrylate (PEGMA) as linker.
There is no data reported about immobilization of this protein onto PVC, probably because when the routine methods for protein immobilization are applied to PVC-SK system, the polymer substrate tends to lose weight due to those methods involve organic media or organic compounds, which tend to dissolve the additives contained into PVC substrate bulk losing their mechanical properties.
In the present work, we describe a method to immobilize functional SK on a PVC substrate using a PEGMA linker without losing SK activity or polymer substrate weight. The method requires the use of the water soluble carbodiimide 1-ethyl-3-(3-dimethyl aminopropyl carbodiimide hydrochloride) (EDC) and sulfo-N-Hydroxysulfo succinimide (sulfo-NHS) and UV light.
Experimental
Grafting of PEGMA onto PVC
PVC films were washed and dried with methanol and then irradiated in air at different exposure times with a 400W medium-pressure mercury lamp at room temperature. Dose rate of 17.30 quantum cm−2 s−1 was used to irradiate polymeric films.
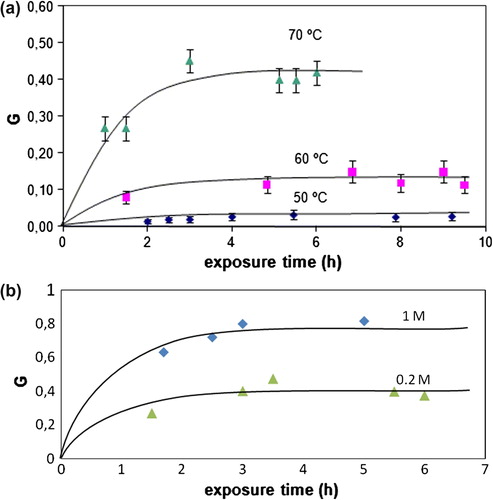
The ferric oxalate actinometry method was used to calibrate dose rate. After exposure time, PVC films were placed in glass tubes containing aqueous solutions of PEGMA (Mn 360) at two different monomer concentrations (0.2 and 1.0 mol L−1); the ampoules were sealed off in vacuum after degassing by repeated freeze/thaw cycles and then put in a water bath at different temperatures (50, 60, and 70 °C). Twenty-three hours of reaction time was used to insure complete reaction.
After the completion of grafting, samples were exhaustively washed with distilled hot water and then dried in vacuum at room temperature until constant weight was achieved and the grafting yield was monitored through the relationship between the grafted and pregrafted substrates by the following Equation (Equation1(1)
(1) ):
where Wo and Wg are the weights of the PVC before and after grafting, respectively. Each experiment was repeated three times and the average value was used for plotting.
Fourier transform infrared spectroscopy (FTIR) analysis was performed using a Perking Elmer PARAGON 500 equipped with an attenuated total reflection (ATR) sample holder. For each spectrum, a total of 16 scans were acquired. The subsequent graft processes were followed by contact angle. Contact angle was measured at room temperature using the sessile drop method with an optical contact angle Krüss DSA 100 drop sharpe analysis system.
Surface topography was characterized by atomic force microscopy (AFM) using a digital instruments system in intermittent contact (tapping) mode. The scan rate was set at 0.5 Hz in order to scan over multiple 10 × 10 μm sampling areas.
Immobilization of SK
Immobilization of SK-FITC
Immobilization was carried out by dissolving 5 mg of SK in 1 ml of Na2HPO4 buffer solution pH 8.9 and then, in a darkened room, 251 μL of fluorescein isothiocyanate/dimethyl sulfoxide (FITC/DMSO) solution of 1.4 mg mL−1 were slowly added. After reacting for 2 h at 30 °C, the solution was dialyzed using a macromolecular membrane tubes with a 6.4 mm diameter and MWCO of 12–12,000 using NaH2PO4 buffer solution with pH 4.5.
Immobilization of SK-FITC onto graft copolymer in aqueous media
This step was performed by mixing different graft values of PVC-g-PEGMA copolymer with 1 mL of the previous dialyzed solution, 38.8 mg of EDC, and 1 mL of sulfo-NHS 2.3 mg mL−1 with stirring and reacted at 30 °C. After 3 h of reaction time, the samples were washed with NaH2PO4 buffer solution pH 7.5 and then, 2.5 mg of fresh solution of SK-FITC was added and reacted for 15 min more. The obtained samples were washed with distilled water for 1 h and dried in vacuum at room temperature. The samples were observed with an Olympus BX51 fluorescence microscope. Images were analyzed by means of QCapture Pro software from Qimaging.
Enzyme assay
SK assay was accomplished spectrophotometrically with H-D-Val-Leu-Lys-pNA × 2HCl (Chromogenix-Instrumentation Laboratory S.p.A. – Viale Monza, 338-20128 Milan) as substrate by continuous monitoring of the appearance of p-nitroaniline at 405 nm with a Beckman Coulter DU 800 UV/visible spectrophotometer as described by Ellen and Kenneth [Citation21,Citation22]. The reaction mixture comprised of 185 μL of substrate solution (1 mg mL−1), 25–562 μL of plasminogen from human plasma, and 3 μL of SK (0.02 u mL−1), or 2.8 μg cm−2 of immobilized enzyme. The final volume of this reaction mixture was made to 1 mL with 0.1 mM tris buffer with Triton X-100 pH 7.1. The test tubes were incubated at 37 °C under intermittent shaking and the absorbance converted to a concentration scale by a molar absorption coefficient of 9800 M−1 cm−1 for p-nitroaniline.
Kinetics parameters
The catalytic rate constant (kcat) and the apparent Michaelis constant (K m) were determined from Lineweaver–Burk plot using standard methods [Citation23-25]. This one-stage assay allows the determination of V max of SK to plasminogen and the K m of plasminogen activation.
Determination of SK immobilized
A study was conducted to find out the quantity of protein immobilized on the graft copolymer. The quantity of coupled and unbound SK was checked in the supernatant (which had already been withdrawn periodically) before and after coupling by standard methods.
Surface characterization
Surface topography was characterized by AFM using a multimode nanoscope (Veeco diMultiMode V) in intermittent contact (tapping) mode. The scan rate was set at 0.5 Hz in order to scan over multiple 10 × 10 μm sampling areas.
Results and discussion
Graft copolymer
First, we measured the action of PVC without the action of UV light. Grafting was not detectable over a period of several hours of reaction. However, with UV light exposure there is a detectable graft even at 1 h. During UV exposure, free radicals are formed at the PVC surface and then react with the oxygen from the air forming peroxides and hydroperoxides [Citation13,Citation25,Citation26]. The peroxides concentration could be measured by weight difference between the PVC film before and after grafting. During grafting, peroxide bonds are broken due to the temperature, producing free radicals and reacting with PEGMA molecules.
Grafting could be up by increasing temperature (Figure ) or monomer concentration (Figure ). Figure shows the increase in grafting as a function of exposure time at different reaction temperatures. The weight gain for PVC films is due to the growing of PEGMA onto its surface when are reacted in a PEGMA solution of 0.2 mol L−1 and dose rate of 17.3 × 1017 quantum cm−2 s−1. PEGMA grafting onto PVC surface is increased with the dose, up to a constant value. Increasing temperature develop this tendency from 50 to 70 °C. In the same way, monomer concentration (Figure ) exhibits a gradual increase of peroxide concentration tending to be constant. Also an increase of monomer concentration just permits to clarify this tendency.
Physical characterization of the graft copolymer
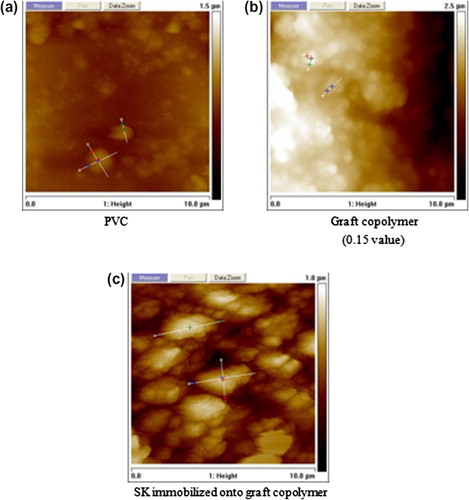
Although grafting is a direct measure of PEGMA binding onto the film, visual examination of the surface is always important. AFM pictures of the original and the grafted films at different stages are shown in Figure .
Figure 2. AFM at different graft of a partially graft was very important to have an overview of the process.
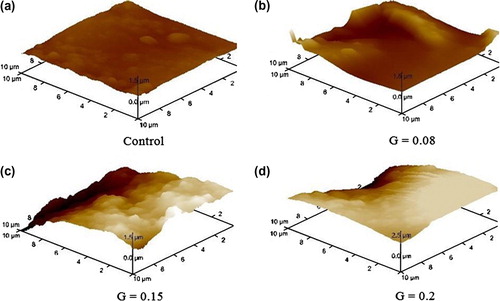
A nonirradiated film has a smooth appearance except for a few microscopic protuberances (Figure ). With short UV light exposure, there are certain places where the graft begins to grow meaning the free radicals were formed at specific areas of the polymer substrate (Figure ). While UV light exposure increases, new free radicals are formed in other areas (Figure ), this tendency remains constant until the entire polymer substrate is completely covered (Figure ). Based on grafting data (Figure ), we can say that the free radicals are formed continuously until 3 h of exposure, before this time, there is no formation of new free radicals and the concentration of the PEGMA onto PVC surface remains constant.
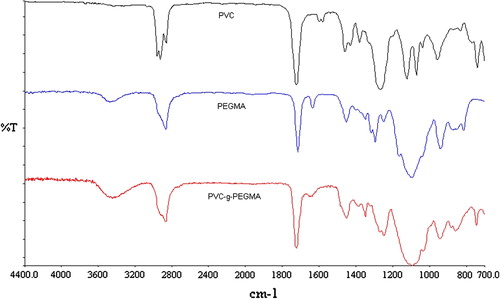
FTIR spectroscopy was conducted in the ATR mode and the spectrum of the PVC-g-PEGMA is showed in Figure . PVC and PEGMA spectra are included for comparison purposes.
The absorption peaks between 2800 and 2950 cm−1 represent C–H stretching vibrations. The peak around 1430 cm−1 represents C–C–Cl bond and the peak at 1267 cm−1 represents H–C–Cl bond. The peak close to 737 cm−1 corresponds to benzene ring o-disubstituted of the plasticizer contained in PVC films. The peak at 1727 cm−1 corresponds to carbonyl (C=O) stretch. The peak around 3400–3500 cm−1 corresponds to surface hydroxyl groups with typical polymeric association, while that around 1100 cm−1 is also indicative of primary alcohol O–H bending.
Table shows the contact angle for PVC films with different graft values. The contact angle decreases rapidly due to the presence of hydrophilic polymer chains (PEGMA) that cover the PVC surface, as presented in Figure . Contact angle remains constant at certain graft values because the surface is completely covered.
Table 1. Contact angle of PVC-g-PEGMA vs. graft value. All measures were taken in water at 26 °C and pH 7.
Immobilization of SK onto graft copolymer
Fluorescence microscope
In order to follow the immobilization of SK to PVC-g-PEGMA copolymer, FITC was binding with SK previously as a first step and then coupled onto graft copolymer in aqueous media as a second step, as described previously. Samples were then analyzed with fluorescence microscope.
Isothiocyanate group from FITC reacted with amines contained at SK molecules producing a stable bond. Figure shows the graft copolymer before and after SK-FITC immobilization. Figure has some bright parts in correspondence with SK-FITC molecules coupled onto graft copolymer surface (gray background).
Figure 4. Fluorescence microscope picture of (a) graft copolymer alone and (b) SK previously coupled with FITC and immobilized onto graft copolymer.
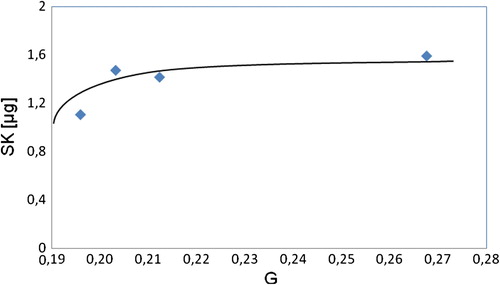
SK (total protein 5.0 μg) when incubated with polymer substrate support (0.5 cm2) showed constant binding (1.4 μg) between 20 and 27 graft percentage (Figure ). The immobilization kinetics revealed that the SK was optimally immobilized onto graft copolymer at around 0.3 graft value. Our results indicate that SK molecules might be immobilized on the exposed surface and within the upper layer of the support by the chemisorption (adsorption) as well as both chemisorption and chemical activation might be increased with the increase of PEGMA grated onto PVC surface at a certain value when SK binding remains constant. Pizzo [Citation20] reported a similar tendency when SK was immobilized on PEG alone.
K m and V max of the immobilized SK
The apparent Michaelis constant, K m, for immobilized SK was 0.14 μM and the corresponding value of velocity, V max, was 29.8 μM min−1. The V max of immobilized SK was found to be higher than that of free enzyme (13.8 μM min−1).
The comparable apparent Michaelis constant value for both immobilized and free SK (0.13 μM; experimentally determined) indicates practically the same affinity toward the substrate. Both V max and the apparent Michaelis constant values for free SK correspond with those reported earlier by Pizzo [Citation20] and Kenneth [Citation22].
Physical characterization of the film
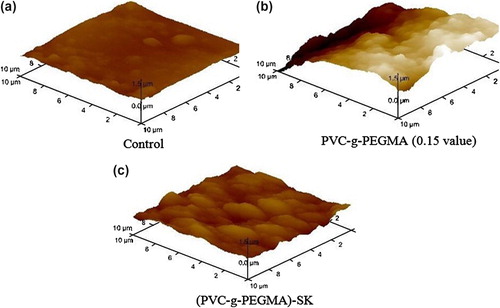
Examination of a partially modified surface is very important to have an overview of the molecules attaching process. AFM pictures of the PVC, PVC-g-PEGMA, and (PVC-g-PEGMA)-SK are shown in Figure .
PVC film has a smooth appearance except for a few microscopic protuberances Figure . Growing of PEGMA onto PVC surface is revealed in Figure . Figure shows clearly protein immobilization and there are certain places where the protein is located due to its size and binding. Figure shows the difference in radius and height among surfaces.
Conclusions
In the present work, we develop a functionalized graft copolymer PVC-g-PEGMA using the water soluble EDC and sulfo-NHS as a substrate for SK immobilization. In the first part of the work, the graft copolymer (PVC-g-PEGMA) was obtained by preirradiation method in air using UV light that reduces purification steps and increase purity. The maximum graft value obtained was 0.8 at a concentration of 1 mol L−1, reaction temperature 70 °C, preirradiation time of 3 h, and dose rate of 17.3 × 1017 quantum cm−2 s−1.
In the second part of the work, the graft copolymer with a value of 0.15 provide optimum conditions to retain original SK activity, enzyme distribution of 2.8 μg cm−2 of functionalized surface and kinetic parameters, K m and V max of 0.14 μM and 29.8 μM min−1, respectively.
Acknowledgments
The authors wish to express their thanks to Hugo Galdamez from the University of California, Riverside for technical assistance. This work was financially supported by PROMEP Grant PROMEP/103-5/11/3713 and CONACyT.
References
- Dev B Rajendra N Chaudhari M Kadam S Plasminogen activators: A comparison. Vascular Pharmacology 2006 44 1 9
- Couto L Donato J Nucci G Analysis of five streptokinase formulations using the euglobulinlysis test and the plasminogen activation assay Brazilian Journal of Medical and Biological Research 2004 37 1889 1894
- Peter H , Thomas C , Joerg W , Karl-Heinz S , Marion K , Michael S , Michael T . Comparative analysis of the activity and content of different streptokinase preparations. European Heart Journal. 2005;26:933–40.
- Peter S Frans V Thrombolytic therapy. State of the art Thrombosis Research 2001 103 S71 9
- Dan LL Hematology and oncology McGraw-HillNew YorkNY 2010
- Banerjee A Chisti Y Banerjee U Streptokinase—a clinically useful thrombolytic agent Biotechnology Advances 2004 22 287 307
- Sung H Seung-Yeop K Takenori S Photocatalytic degradation of flexible PVC/TiO2 nanohybrid as an eco-friendly alternative to the current waste landfill and dioxin-emitting incineration of post-use PVC Polymer 2006 47 3005 3016
- Costa L Brunella V Paganini M Bacaro S Cecilia A Radical formation induced by γ radiation in poly(vinyl chloride) powder Nuclear Instruments and Methods in Physics 2004 215 471 8
- Feldman D Polymer history Designed Monomers and Polymers 2008 11 1 15
- Dietrich B Recycling of PVC Progress in Polymer Science 2002 27 2171 2195
- Baccaro S Brunella V Cecilia A Costa L γ irradiation of poly(vinyl chloride) for medical applications Nuclear Instruments and Methods in Physics 2003 208 195 198
- Wei Z Paul K Junhui J Yihe Z Zhimin J Effects of O2 and H2O plasma immersion ion implantation on surface chemical composition and surface energy of poly vinyl chloride Applied Surface Science 2006 252 7884 7889
- Arenas E Bucio E Burillo G Lopez G Radiation grafting of poly(ethylene glycol) methacrylate onto poly(vinyl chloride) tubes Designed Monomers and Polymers 2007 10 459 467
- Sven P Marcel G Enzyme technology and bioprocess engineering Current Opinion in Biotechnology 2002 13 111 116
- Goddard J Hotchkiss J Polymer surface modification for the attachment of bioactive compounds Progress in Polymer Science 2007 32 698 725
- Tiller J Klemm D Berlin P Designed aliphatic aminocellulose derivatives as transparent and functionalized coatings for enzyme immobilization Designed Monomers and Polymers 2001 4 315 328
- Lu Y Jonathan S Shekhar G Hydration of enzyme in nonaqueous media is consistent with solvent dependence of its activity Biophysical Journal 2004 87 812 821
- Abraham R Menahem G Sara R Studies on the activation of plasminogen I. Preparation and properties of an insoluble derivative of streptokinase Biochimica et Biophysica Acta 1962 73 301 310
- Leo C Kathleen E William A Johannes E Immobilization of the plasminogen activator streptokinase and its fibrinolytic effects in vivo Thrombosis Research 1978 13 931 940
- Pizzo S Preparation, in vivo properties and proposed clinical use of polyoxyethylene-modified tissue plasminogen activator and streptokinase Advanced Drug Delivery Reviews 1991 6 153 166
- Ellen S Stig E Kenneth H A chromogenic assay for the detection of plasmin generated by plasminogen activator immobilized on nitrocellulose using a para-nitroanilide synthetic peptide substrate Analytical Biochemistry 1989 177 78 84
- Kenneth C Louis S Robert C Human plasmin Methods in Enzymology 1981 80 379 387
- Francis J James R Human plasminogen Methods in Enzymology 1981 80 365 377
- Harvey W Douglas S Biochemical engineering Marcel Dekker New YorkNY 1996
- Anthony LA Physical properties of polymers handbook Springer Science+Business Media New YorkNY 2007
- Michele E Christopher M Norman S Rakel H Surface pinking in titanium dioxide/lead stabiliser filled PVC profiles Polymer Degradation and Stability 2010 95 2022 2040