Abstract
Liquid crystalline behavior of polymeric materials is of considerable current interest in the last decades, but due to many different and distinct characteristics. Polyazomethines liquid crystalline polymers have received considerable attention due to their potential applications and considered as one of the most important liquid crystalline material produced. This review gives a simple introduction to liquid crystalline materials including definition and classification. Moreover, we will focus on the syntheses and properties of liquid crystalline polyazomethines with flexible spacers or hybrid liquid crystalline polyazomethines. Furthermore, give a general overview of thermotropic, mesophoric properties and texture observation for desired liquid crystalline polyazomethines were shown in details.
1. Introduction
Liquid crystalline materials have received significant attention in the past several decades. The combination of order and mobility in these systems leads to novel functional materials which have had a great impact on recent development of mobile information technologies. Although more than 100,000 liquid crystalline compounds have been synthesized thus far, their basic structures are quite similar. They represent either anisometric molecules with a specific rod-like or disk-like shape, or amphiphilic molecules, as well as oligomers, polymers, and dendrimers derived from these fundamental structures. With the beginning of the third millennium and the prosperity of industrial technology in many countries, the road became paved in front of developing countries to knock the ranks of the major industrialized ones. Liquid crystalline (LC) polymers are a class of materials that combine the properties of polymers with those of liquid crystals. These ‘hybrids’ (physico-chemical mixture) show the same mesophases characteristic of ordinary liquid crystals, yet retain many of the useful and versatile properties of polymers Citation[1]. Polymers that show liquid crystal behaviour are a new state of matter opening new approaches to new valuable applications and to processing methods which achieve unusual properties. These liquid crystals are candidates for electronic devices, such as hand held games, and ultra high strength materials Citation[2]. The idea is to combine the interesting properties of mesophase, found for low molecular-weight compounds, which can form films, fibres and molding. The anisotropy of liquid crystalline mesophase offers the possibility of production of novel high-performance materials, exhibiting excellent properties, due to proper arrangement macromolecules in the mesophase during the processing of applications in recent aspects. Liquid crystalline behaviour of polymeric materials is of considerable current interest, not only because of their potential as high-strength fibres Citation[3], plastics Citation[4], moldings Citation[5], etc. but due to many different and distinct characteristics. In 1998, Xu et al. Citation[6] prepared hybrid injection-molded binary and ternary blends of semi-aromatic liquid-crystalline polymers and study their morphologies and the mechanical properties. Furthermore, the effects of magnetic fields on polymer liquid crystals (PLCs) are analyzed by Lizuka in 2000 Citation[7], dealing in turn with isotropic solutions, lyotropic LCs (including polypeptides, polyribonucleotides and DNA) and thermotropic PLCs (including polyesters and polypeptides). In the last two decades, many studies have been reported on polymer films prepared by bulk polymerization of liquid-crystalline (LC) monomers such as acrylate Citation[8–14], methacrylate Citation[15–19], vinyl ether Citation[20,21], epoxide Citation[22,23], and styrene Citation[24,25] derivatives. Moreover, an epoxy monomer without liquid crystalline properties was transformed into liquid crystalline state by a curing reaction with 2,7-diaminofluorene to produce a liquid crystalline epoxy polymer based on biphenyl-type mesogenic compound giving a smectic-like structure in the temperature range between 132 and 162 °C Citation[26].
On the other hand, polyazomethines belong to a class of materials that are known for their excellent thermal stability, good mechanical strength, and environmental resistance Citation[27–29] and more particularly as promising materials with optoelectronic and photonic applications Citation[30]. Furthermore, polyazomethines are of particular interest due to their interesting properties such as syn-anti isomerism, nonlinear optical activity, ability to form metal chelates and supramolecular interactions with many electrophyles, fiber-forming ability, good thermal stability, and semiconductivity Citation[31,32]. Polyazomethines containing thermotropic liquid crystalline polymers have received considerable attention in the recent years due to their potential applications such as information storage, nonlinear optics, laminates and films with good thermal stability and chemical resistance Citation[5,33–37]. Moreover, high performance fiber and metal-chelating ability Citation[38] were also investigated for opto-electrical applications. Adams and his team Citation[39] synthesized the first polyazomethines from terephthalaldehyde and dianisidine. No solubility in common organic solvents were observed for those polymers. Number of polyazines and polyazomethines were prepared by Marvel et al. by the reaction of aromatic dialdehydes with hydrazine and o-phenylenediamine, and examined their complex-forming ability and thermal stability Citation[40]. Polyazomethines synthesized from benzidine and 4,4′-diformyl-α,ω-diphenoxydecane showed LC property above 300 °C. The higher thermal stability due to wholly aromatic polyazomethines was given by the greater number of aromatic rings present in the polymer backbone Citation[41]. Hydride siloxane-azomethine liquid crystal (LC) dimers and polymers of different mesogenic structure were synthesized and compared from the standpoint of thermotropic LC behavior like that reported by Raclesa et al. Citation[42].
2 Liquid crystal
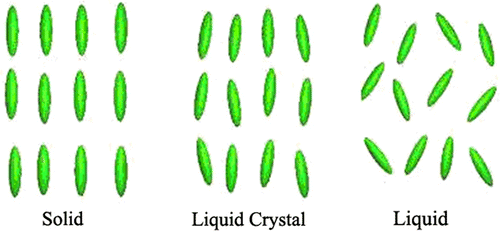
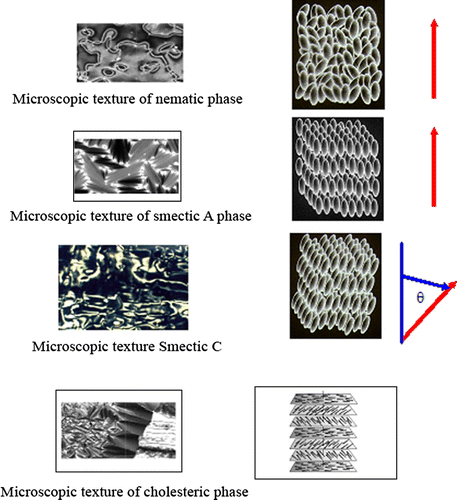
Liquid crystal materials generally have several common characteristics. Among these are rod-like molecular structures, rigidness of the long axis, and strong dipoles and/or easily polarizable substituents Citation[1]. Liquid crystal materials are unique in their properties and uses. As research into this field continues and as new applications are developed, liquid crystals will play an important role in modern technology Citation[1]. This tutorial provides an introduction to the science and applications of these materials. The distinguishing characteristic of the LC state is the tendency of the molecules (mesogens) to point along a common axis, called the director. This is in contrast to molecules in the liquid phase, which have no intrinsic order. In the solid state, molecules are highly ordered and have little translational freedom. The characteristic orientational order of the liquid crystal state is between the traditional solid and liquid phases and this is the origin of the term mesogenic state, used synonymously with liquid crystal state. Note the average alignment of the molecules for each phase in Figure .
It is sometimes difficult to determine whether a material is in a crystal or liquid crystal state. Crystalline materials demonstrate long-range periodic order in three dimensions. By definition, an isotropic liquid has no orientational order. Substances that are not as ordered as a solid yet have some degree of alignment are properly called liquid crystals, which are commonly found in computer monitors, digital clocks, and other read-out devices. The first reported liquid crystal behaviour was detected by Feinitzer Reintzer (an Austrian Biochemist) more than 100 years ago. He synthesized several esters of cholesterol. He observed that the cholesteryl ester formed opaque liquids, which on heating turned clear. We now know that, as a general rule, many materials are clear if they are anisotropic random, or if the materials are composed of ordered molecules or segments of molecules, whereas they are opaque if they contain a mixture of ordered and disordered regions. Lehman interpreted this behaviour as evidence of a third phase that exists between the solid and isotropic liquid states. This new phase named by Lehman the liquid crystal phase. Fiedel called this phase the mesophase, after the Greek word measos, meaning intermediate Citation[43]. Liquid crystal has been enormously extended in recent years, which is partly due to the detection of novel molecular types of liquid crystalline substances and partly due to the discovery of addition phase structures. Compared to isotropic liquids, liquid crystals represent a higher state of order which may be expressed in terms of order parameters. Compared to solid crystals, the liquid crystals have a higher intermolecular and intramolecular mobility, which means they may have several degrees of freedom of molecular rotation, translation, oscillation, and intramolecular conformational changes, some of these mobilities are also allowed in solids. Therefore, the exact delimitation between liquid crystals and solids is not clear Citation[44].
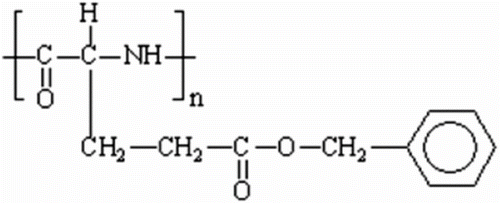
It was believed that the possibility of formation of liquid crystal phase in polymeric materials is difficult to achieve, because polymer chain unusually adopts a statistical coil conformation, whereas LC phases possess an orientational and/or a positional long-range order. The ability of polymers to exist in liquid crystal state and still retain a high degree of order was first suggested by Paul Flory in 1956 from his studies of the thermodynamic properties of solutions of polymers Citation[45]. In 1974, Flory received the Nobel Prize for chemistry as a result of his studies on relationships between polymer structure and properties. He predicted that the polymers which are composed of units having elongated rigid molecular structure should form anisotropic ordered solutions depending on the temperature and the length (diameter ratio of the rod-like section of the polymeric molecule). At the same time, investigations at the research laboratories of Courtaulds Ltd. in the UK observed that solutions of certain polypeptides should have such behaviour Citation[46]. In these polymers, the rod-like structures in solutions are formed by the stable helical conformation of the polymer molecules for poly(γ-benzyl-L-glutamate) 1 that polymer of this class which has been the subject of the most studies (cf. Figure ). The spontaneous packing of these stable helices imparts anisotropic liquid crystalline properties of their solutions as predicted by Flory. Now this kind of LC polymers is termed ‘Lyotropic’ to distinguish them from those that form LC melts and are termed ‘Thermotropic.’
2.1 Classification of LC polymers
LC polymers macromolecules can be classified as proposed by Gray and Winsor Citation[46,47], according to the structure (amphiphilic or nonamphiphilic), the mesogenic groups, and the phase as follows:
2.1.1 According to the structure
2.1.1.1 Amphiphilic LC polymers
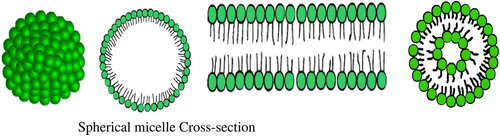
These contain lipophilic and hydrophilic groups, which adopt the solubility in organic solvents and/or water, respectively. They can be divided into: anionic, cationic, and nonionic amphiphiles. The amphiphilic molecules forming mesophases with the participation of water and/or organic solvent are defined as Lyotropic LC Polymers (cf. Figure ).
2.1.1.2 Nonamphiphilic LC polymers
These are nonpolar or moderately polar organic polymers exhibiting highly geometrical anisotropy as a consequence of rod-like or disk-like shape. The nonamphiphilic compounds form usually liquid crystalline phases after melting, and are defined as Thermotropic LC Polymers (cf. Figure ).
2.1.2 According to the kind of mesogenic groups
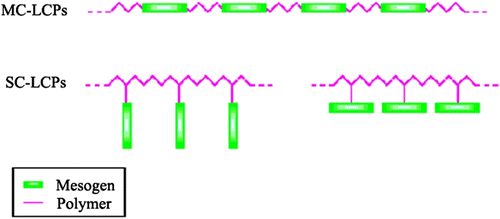
LC polymers are classified according to the kind of the mesogenic groups; the placement of the mesogens plays a large role in determining the type of LC polymers that formed. So, another two main types of LC polymers: main-chain liquid crystalline polymers or MC-LCPs which are formed when the mesogens are themselves part of the main chain of a polymer; conversely, side-chain polymer liquid crystals or SC-LCPs which are formed when the mesogens are connected as side chains to the polymer by a flexible ‘bridge’ (called the spacer) Citation[1,43] (cf. Figure ).
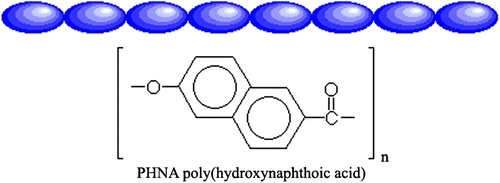
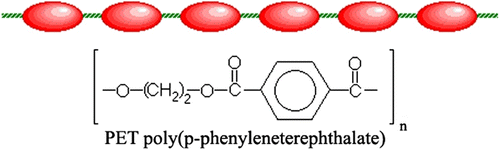
MC-LCPs are formed when rigid elements are incorporated into the backbone of normally flexible polymers. There are two distinct groups of MC-LCPs, differentiated by the manner in which the stiff regions are formed. The first group of MC-LCPs is characterized by stiff, rod-like monomers. These monomers are typically made up of several aromatic rings which provide the necessary size, for example, poly(hydroxynaphthonic acid) (cf. Figure ). The second and more prevalent group of MC-LCPs is different, because it incorporates a mesogen directly into the chain. The mesogen acts just like the stiff areas in the first group. Generally, the mesogenic units are made up of two or more aromatic rings which provide the necessary restriction on movement that allow the polymer to display liquid crystal properties. This group is different from the first, in that the mesogens are separated or ‘decoupled’ by a flexible bridge called a spacer. Decoupling of the mesogens provides for independent movement of the molecules which facilitates proper alignment, for example, poly(ethyleneterephthalate) (cf. Figure ).
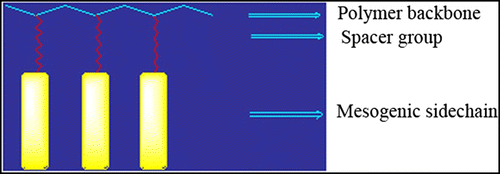
SC-LCPs have three major structural components: the backbone, the spacer, and the mesogen. The backbone of a SC-LCP is the element that the side chains are attached to. The structure of the backbone can be very important in determining whether the polymer shows liquid crystal behaviour or not. The most important part of a SC-LCP is the mesogen. It is the alignment of these groups that causes the liquid crystal behaviour. Usually, the mesogen is made up of a rigid core of two or more aromatic rings joined together by a functional group. Figure represents a typical repeating unit in a SC-LCP.
2.1.3 According to the phases
LC polymers can be divided into two large groups: thermotropic and lyotropic. Thermotropic liquid crystalline polymers are formed when pure molecules form ordered structures upon heating. When LC polymers occur through mixing with solvent, they are called lyotropic. Thermotropic LC polymers can be further divided into enanthiotropic materials, where the LC phases are formed on both heating and cooling cycles, and mesotropic materials, where the LC polymers are stable only on super cooling from the isotropic melt. The mesotropic LC polymers have been further divided into three groups: semectic, nematic, and cholesteric Citation[1,43] (cf. Figure ).
In the last few decades, many MC-LCPs and SC-LCPs have been synthesized and investigated Citation[48–60]. These polymers include: polyesters, polyethers, polyamides, polyurethanes, polysiloxanes, polyamines, polythioether, and polyazomethines. Furthermore, the hybrid polymers ‘which consists of two or more functional groups’ were synthesized including: poly(ether-ester)s, poly(amide-esters)s, poly(imide-ester)s, poly(azomethine-ether)s, poly(ether-sulphone)s, etc.
3 LC polymers with flexible spacers in the polymers backbone
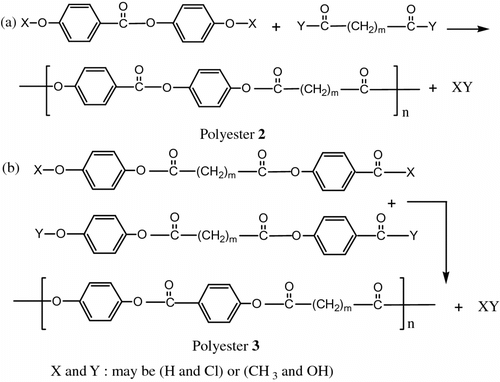
Chemical modification of a nonliquid crystalline polymer to liquid crystalline polymer and the synthesis of liquid crystalline polymers from proper monomers are two basic approaches which lead to the preparation of LC polymers with mesogenic groups and flexible spacer in the main chain, these methods are: (i) the first is characterized by the polycondensation of flexible spacer monomer with a monomer unit (cf. Figure equation a); (ii) the second one involves the formation of mesogenic units as a result of polycondensation reaction of two different monomers containing no typical rigid mesogens (cf. Figure equation b).
Great efforts have been concentrated on the synthesis of new polyazomethines with enhanced properties. Many of these polymers form mesomorphic phases on heating (thermotropic LC polymers). However, their high melting points and low solubility usually make them inaccessible for processing by conventional methods. In order to lower the transition temperatures and improve their solubility and process ability, several approaches, such as introduction of alkyl or alkoxy groups in the ortho position of the aromatic ring, were carried out Citation[38,61,62]. Cristofor and his research team synthesized shorter methylene chain containing azomethine polymers. The formed polymers showed higher thermal stability, poor solubility and did not show LC textures Citation[63]. Choi et al. Citation[64] synthesized banana-shaped mesogens containing main chain azomethine polymers. Those polymers were exhibited nematic LC texture with Tm = 120–224 °C. They were soluble in strong acids such as sulphuric acids and trifluoroacetic acids. Longer methylene chain containing poly(azomethine ether)s were synthesized and studied for their LC properties Citation[65]. Furthermore, the introductions of flexible aliphatic segments or bulky lateral substituents into the polymers main chain have been reported with higher solubility in a wide range of solvents Citation[66,67]. Selected LC polyazomethines with flexible aliphatic spacers in the polymers backbone were summarized as the following.
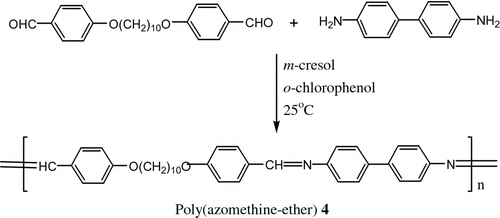
Guillion and Skoulios Citation[68] prepared the first poly (azomethine-ether)s 4 from 4,4′-diformyl-1,10-diphenoxydecane and benzidine (Figure ).
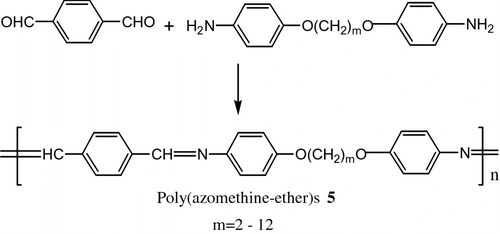
Also, terephthalaldehyde was reacted with 4,4′-diamino-α,ω-diphenoxyalkane to obtain poly (azomethine-ether)s 5 (Figure ).
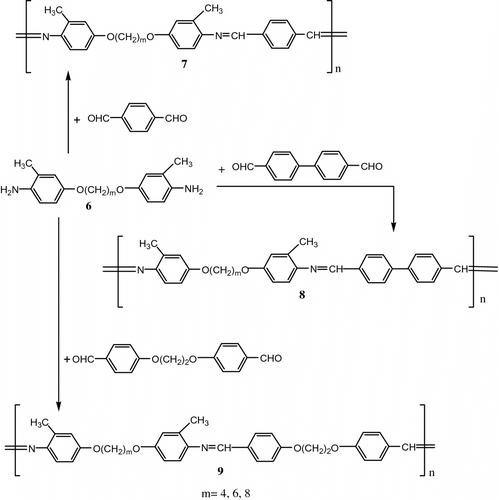
Poly (azomethine-ether)s 7–9 were also obtained from the polycondensation of 1,m-bis(4-amino-3-methylphenoxy)alkanes 6 with terephthalaldehyde; 1,2-bis(4-formylphenoxy)ethane or 4,4′-biphenyldicarboxaldehyde (Figure ).
The mesophoric properties were studied as a function of diphenoxyalkane spacers’ length. Analysis by differential scanning calorimetry (DSC) and optical polarized microscopy (OPM) demonstrated that the poly(azomethine-ether)s form nematic mesophases over wide temperature ranges. Both the Tm and Ti values increased as the length of the flexible aliphatic spacers decreased; and also decreased with introduction of the methoxy group as a substituent in the polymer main chain.
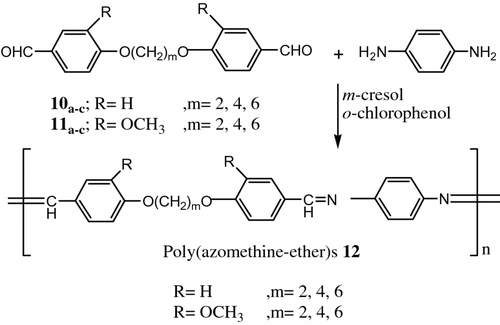
Li and Chang Citation[69] described the preparation of poly (azomethine-ether)s 12 with rod-like segments connected by a short alkaline oxide spacers or insertion of methoxy group at the ortho position of the phenyl group (Figure ).
The thermotropic liquid crystalline properties of the polymer were examined by DSC, OPM and thermogravimetric analysis (TGA).
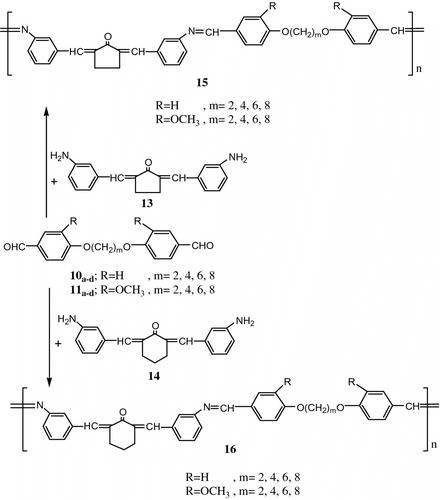
Aly and Ahmed Citation[70] synthesized a series of polyazomethines 15, 16 containing flexible spacers through polycondensation of diformyl-α,ω-diphenoxyalkanes 10a–d , 11a–d with 2,5-bis(m-aminobenzylidene)cyclopentanone 13 and 2,6-bis(m-aminobenzy-lidene)cyclohexanone 14 (Figure ).
The polymers were characterized by IR spectroscopy, viscosity measurements, DSC and OPM.
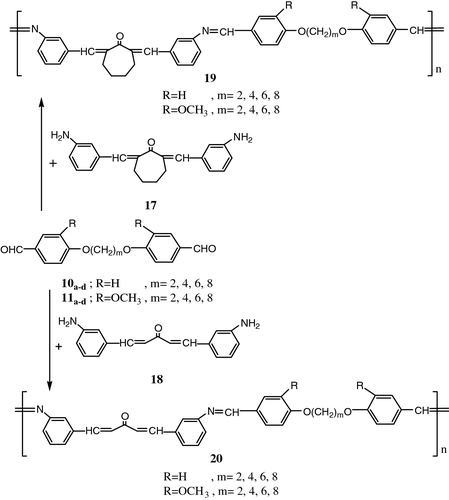
Also, Aly et al. Citation[71] synthesized a new homologous series of thermotropic LC poly (azomethine-ether)s based on dibenzylidene derivatives 19, 20 by solution polycondensation of various diformyl-α,ω-diphenoxyalkanes 10a–d , 11a–d with 2,7-bis(m-aminobenzylidene)cycloheptanone 17 and bis(m-amino-benzylidene)acetone 18 (Figure ).
The mesomorphic properties of these polymers were studied as a function of the diphenoxyalkanes space length. Analysis by DSC and OPM demonstrated that the poly (azomethine-ether)s form nematic mesophases over wide temperature ranges.
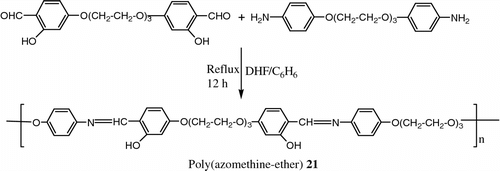
Jayanthi and Kishore Citation[72] synthesized a new hydroxy functionalized LC poly(azomethine-ether)s 21 by solution polycondensation between dialdehyde and diamine (Figure ).
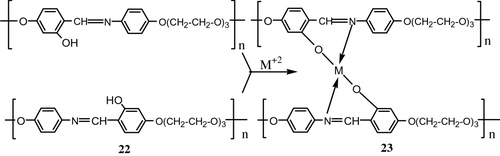
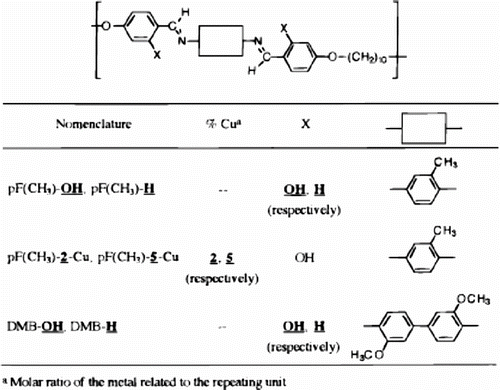
An attempt was made to synthesize metal-containing LC polymer networks based on a new hydroxyl functionalized thermotropic polymer 22, 23. This polymer exhibited nematic mesophase. DSC studies showed that the networks exhibited mesomorphic behaviour up to 30 mol% of Cu (II). Similar networks were also formed by complexation of the polymer with various other metals like Co, Ni, Cd and Zn (Figure ).
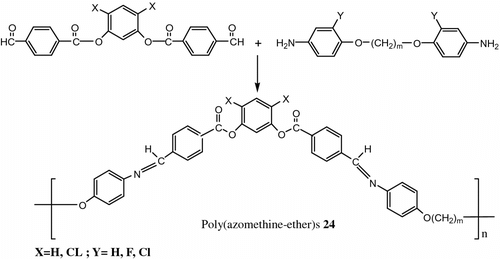
In 2004 Choi et al. Citation[64] synthesized twelve azomethine polymers 24 containing Banana-Shaped mesogen as a repeating unit by polycondensation between different bisaldehyde and bisamine molecules (Figure ).
The mesomorphic properties of these polymers were studied. The obtained polymers were characterized by FT/IR and NMR spectrophotometry, DSC, OPM and X-ray diffractometry.
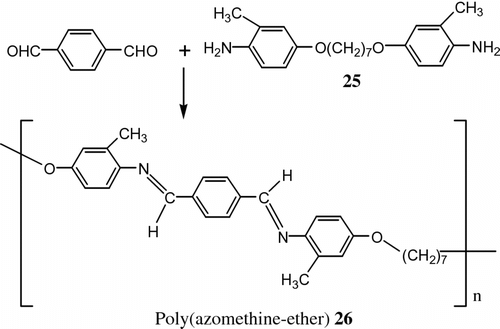
According to the importance of LC poly (azomethine-ether)s, Sauer et al. Citation[73] used temperature modulated DSC, variable heating rate DSC, and trapping atomic force microscopy (AFM) to study thermal and morphological properties of LC polymer including both polyester and azomethine groups 26. This polymer was prepared from terephthaldehyde and 1,7-bis (4-amino-3-methyl phenoxy)heptane 25 as reported previously Citation[74] (Figure ).
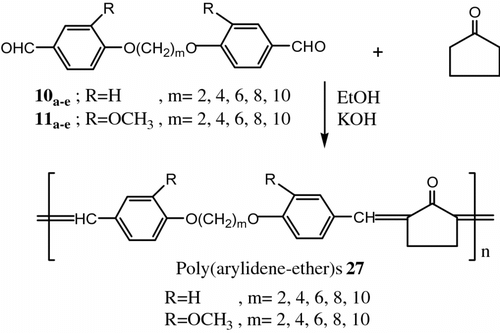
In 2000, Aly and Hammam Citation[75] synthesized a new series of LC poly(arylidene-ether)s 27 containing flexible spacers based on cyclopentanone. The polycondensation of 4,4′-diformyl-α,α′-diphenoxyalkanes 10a–e and 4,4′-diformyl-2,2′-diphenoxyalkanes 11a–e with cyclopentanone in ethanol and KOH as a catalyst (Figure ).
Thermotropic LC properties were examined by DSC, OPM, and TGA. Almost all the polymers exhibited thermotropic LC properties. In most cases the mesophase extended up to 358 °C, where the thermal decomposition prevented further observations. In addition the crystallinity of some polymers was examed by X-ray analysis, and showed some degree of crystallinity.
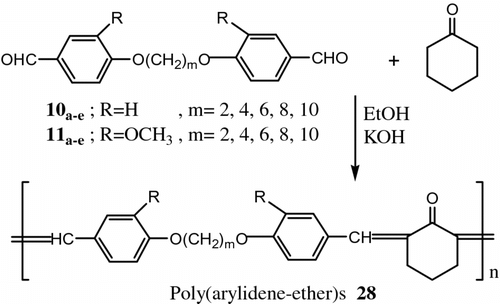
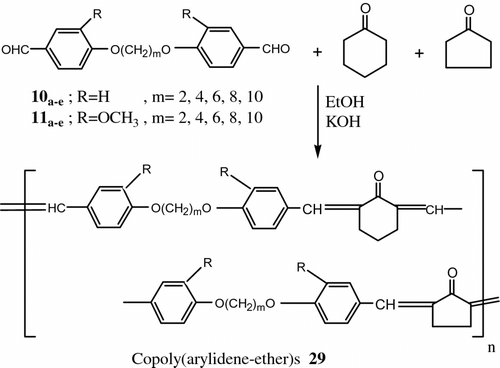
Also, Aly Citation[76] synthesized new LC polymers from another two new series of poly(arylidene-ether)s 28 and copoly(arylidene-ether)s 29 by polycondensation of 4,4′-diformyl-α,ω-diphenoxyalkanes 10a–e and 4,4′-diformyl-2,2′-dimethoxy-α,ω-diphenoxyalkanes 11a–e with cyclohexanone and/or cyclopentanone (Figures and 23).
All the polymers and copolymers exhibited thermotropic LC properties. In most cases the mesophase extended up to 310 °C where thermal decomposition prevented further observation. Methoxy substituents, on the benzene ring of these polymers lowered the transition temperature significantly.
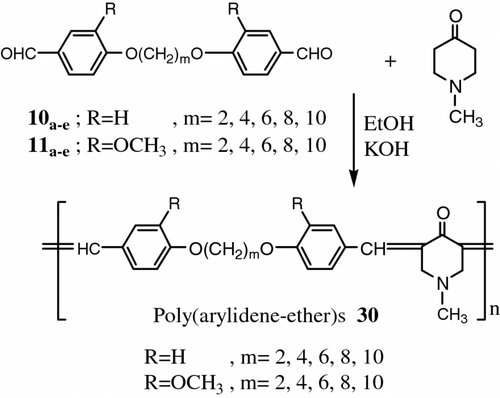
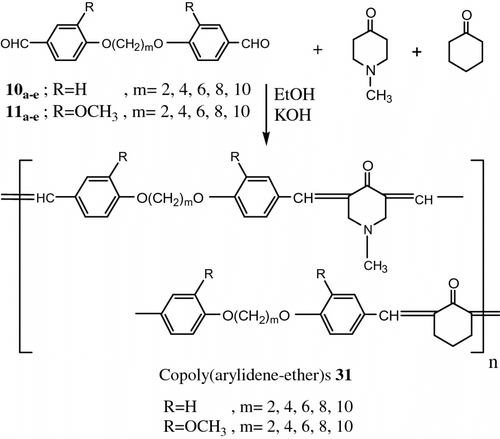
Moreover, Aly and El-Kashef Citation[77] synthesized two new series of two poly (arylidene-ether)s 30 and copoly(arylidene-ether)s 31 based on N-methylpiperidone and/or cyclohexanone. The first series (homopolymers) was derived from 10a–e , 11a–e and N-methylpiperidone. The second series (copolymers) was derived from the diphenoxyalkanes 10a–e , 11a–e and N-methylpiperidone and cyclohexanone (Figures and 25).
The polymers had been characterized by DSC measurements and microscope observation polarized light which demonstrated that this type of poly(arylidene-ether)s form nematic mesophase over a wide temperature range in contrast to the corresponding copoly(arylidene-ether)s. All of the polymers and copolymers exhibited thermotropic LC properties.
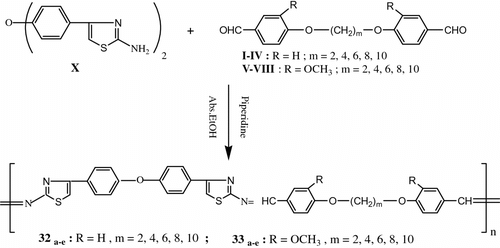
After that, Aly et al. Citation[78] synthesize a new homologous series of thermally stable thermotropic LC poly(azomethine – ether)s based on thiazole moiety using solution polycondensation of 4,4′-diformyl-α,ω-diphenoxyalkanes, or 4,4′-diformyl-2,2′-dimethoxy-α,ω-diphenoxyalkanes with the new bis(2-aminothiazole) monomer (Figure ).
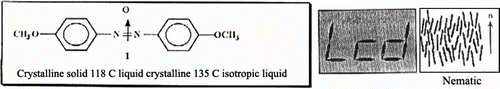
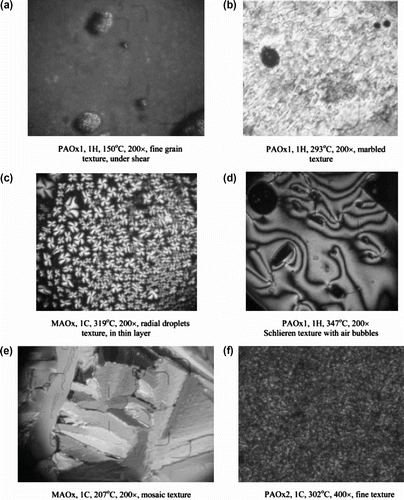
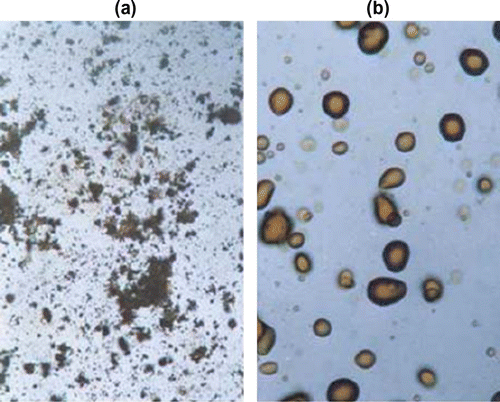
The inherent viscosities of the resulting polymers were in the range 0.43–1.34 dI/g. All the poly(azomethine–ether)s were insoluble in common organic solvents but dissolved completely in concentrated H2SO4 and formic acid. The mesomorphic properties of these polymers were studied as a function of the diphenoxyalkane space length. Their thermotropic LC properties were examined by DSC and OPM and demonstrated that the resulting polymers form nematic mesophases over wide temperature ranges. Optical microscopy showed that all the polymers melt yield viscous, birefringent liquid crystal phases except polymers 33a,b ; which did not show any birefringence, so providing direct evidence for lack of mesomorphic behavior for these polymers. The temperatures correspond roughly with those observed by DSC. Observation of poly(azomethine-ether) 32b under a polarizing microscope revealed that this polymer exhibited a threaded-schlieren texture of nematic phase with a thick dark rim in Figure (before melting), and the mesophase extend up to the isotropic at 270 °C (Ti). After cooling to room temperature a highly spheroidal texture appeared (cf. Figure ).
Figure 27 Photomicrographs of poly(azomethine-ether)s in the heating cycle at (a) 219 °C, (b) 270 °C (magnification X = 200).
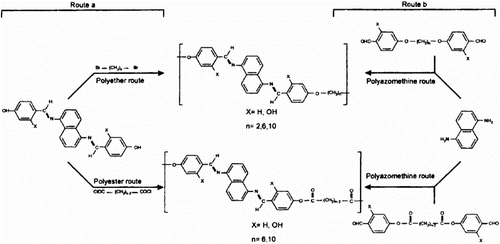
Cerrada et al. Citation[79] synthesized new semi-flexible LC polymers 34a–d based on Schiff bases as mesogenic cores by two different synthetic strategies. The first one (route a) involves the linking of the promesogenic unit by means of flexible spacers during the polymerization process. The second one (route b) leads to the final polymer by forming the potentially mesogenic cores during the polymerization. In this case, the flexible spacer is incorporated into the starting monomer. The mesogenic cores were derived from 1,5-naphthalenediamine linked to a polymethylenic spacer by means of ether or ester groups. The synthetic strategies differ in the disconnection of the molecules to design the synthesis of the repeating units. Polymers were synthesized either as polyazomethines (route b), where imine bonds are formed in the polymer synthesis or as polyethers or polyesters (route a), where the ether or ester groups are formed at the polymer-forming stage as illustrated in Figure .
The structure of all the synthesized polymers was confirmed by IR spectroscopy and elemental analysis. The influence of the synthetic method and the linking groups between mesogenic core and flexible spacer on the thermal and mesogenic properties had been analyzed by DSC, thermogravimetry, and optical microscopy. The anisotropic melt of the mesogenic polymers were characterized as nematic by optical microscopy. The polymers exhibited some typically developed Schlieren and threaded textures under annealing. On mechanical stress, marbled textures were observed. In all of these cases the mesophase temperature range determined under the microscope agrees with the DSC data.
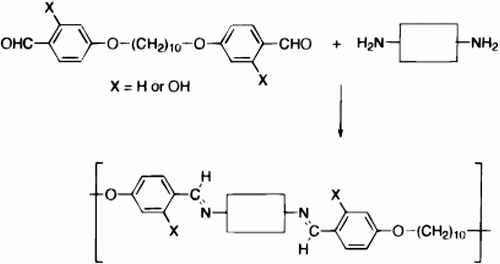
Furthermore three years more, the same group Citation[80] synthesized another semi-flexible LC polyazomethines 35a–c in good yields by solution polycondensation using freshly distilled N,N-dimethylacetamide and anhydrous LiCl to remove the water formed during the reaction. LC polyazomethines prepared by condensation of two dialdehyde monomers namely: (1,10-bis[(4-formyl-3-hydroxyphenyl)oxy]decane or 1,10-bis-[(4-formylphenyl)oxy]-decane) with two diamines (2-methyl-1,4-phenylenediamine or 3,3′-dimethoxy-benzidine) as shown in Figure .
Polyazomethines having a hydroxyl group at the ortho position of the imine bond have higher degrees of polymerization and a remarkable tendency to show an increase in their molecular weight upon postpolymerization thermal treatments. These hydroxy-functionalized polymers were coordinated with Cu (II) ions to gave rise to metallomesogenic cross-linked polymers (cf. Figure )
Fiber spinning of both organic and Cu (II)-complexed polymers was carried out, and the structure, orientation, and morphology of the fibers were studied by X-ray diffraction, electron paramagnetic resonance, and scanning electron microscopy. The mechanical properties had also been evaluated. Hydroxy functionalization and Cu (II) complexation are the key strategies to obtained highly oriented fibers with good mechanical properties and strong intermolecular cohesive forces.
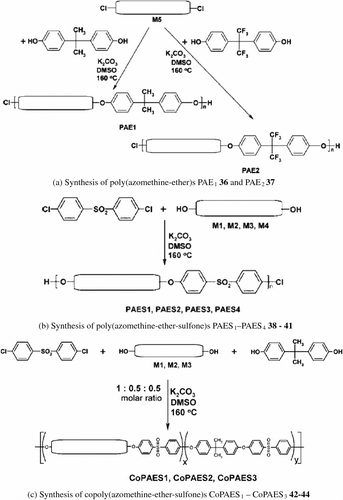
In 2006, Marin, Cozan and Bruma Citation[81] synthesized three series of novel modified polyazomethines with mesogenic units and varying kinking groups using the classical Williamson condensation reaction of aromatic dichlorides with bisphenols having various structures. The produced monomers including: 4,4′-bis(p-hydroxybenzylidene-iminophenoxy)biphenyl (M 1), 4,4′-bis(p-hydroxybenzylideneim-ino)diphenylether (M 2), 4,4′-bis(p-hydroxyaniline)terephthalaldehyde (M 3), 4-(p-hydroxybenzylideneimino)phenol (M 4), 4,4′-bis(p-chlorobenzylidene-iminophenoxy)-biphenyl (M 5). The first series namely: poly(azomethine-ether)s (PAE1) 36 and (PAE2) 37 were obtained by the reaction of M 5 monomer with bisphenol A or bisphenol F, respectively (Figure a). The second series namely: poly(azomethine-ether-sulfone)s (PAES1 – PAES4) 38 – 41 were obtained by the reaction of bis(p-chlorophenyl)sulfone with the corresponding monomers M 1–M 4 (Fig. b). The third series namely: copoly(azomethine-ether-sulfone)s (CoPAES1–CoPAES3) 42 – 44 were obtained by the reaction of bis(p-chlorophenyl) sulfone with a mixture of bisphenol A and a bisphenol containing azomethine group M 1–M 3 in 1:0.5:0.5 M ratio (Figure ).
The effect of the incorporation of the kinking groups on the thermotropic properties of the alternated and random polymers was studied. Structural elucidation was carried out by elemental analysis, spectral analyses (IR, 1H-NMR and UV–vis spectroscopy). Inherent viscosity of the polymers measured in DMF at 20 °C was in the range 0.15–0.44 dl/g. Their molecular weight and molecular weight distribution were determined using Gel Permeation Chromatography (GPC). Mesomorphic properties were studied by DSC, hot stage OPM and the thermal stability was determined TGA.
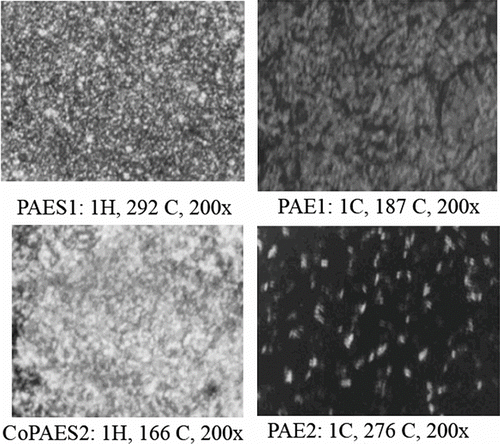
The polymers PAE1 and PAE2 showed a strong birefringence on both heating and cooling, while the polymers PAES1, CoPAES1, CoPAES2 only at first heating, probably due to the partial decomposition of the polymer near the clearing temperature. The thermotropic polymers (PAE1, PAE2, PAES1, CoPAES1, CoPAES2) when observed by OPM showed a fine texture (Figure ), similar to those reported for thermotropic polymers having stiff chains without flexible spacers Citation[82]
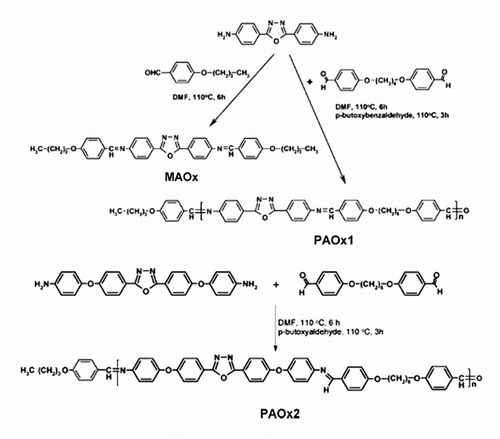
Recently, Marin et al. Citation[83] synthesized new thermotropc LC polyazomethines 45, 46 containing 1,3,4-oxadiazole luminescent mesogens by solution polycondensation technique. These polymers were semi-flexible LC compounds and obtained by the condensation of mesogenic aromatic diamines namely: 2,5-Bis(p-aminophenyl)-1,3,4-oxadiazole (AOx1), 2,5-Bis[4-(p-amino-phenoxy)-phenylene]-1,3,4-oxadiazole (AOx2) with a flexible 4,4′-diformyl-dipheno-xyhexane (A) in DMF, as shown in Figure . A model compound (MAOx) was performed by an acid-catalyzed condensation reaction of p-butoxy benzaldehyde and AOx1 taken in a 2/1 M ratio with the aim to accomplish the structural characterization of the desired polymers.
TGA, DSC, POM and wide angle X-ray diffraction (WAXD) were used to evaluate their thermal and mesomorphic properties. OPM was performed to confirm the transition temperatures obtained by DSC studies and to find out the type of mesophases present in the studied materials (cf. Figure ). The target polyazomethines present liquid crystalline behaviour with classical calamitic textures, large mesophase range, and high thermal stability. The investigation of the optical properties of the synthesized polymers, in solution and solid state, confirmed a blue fluorescence.
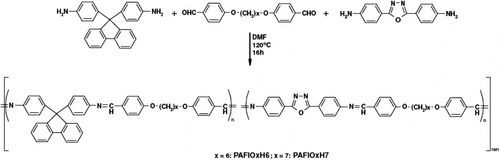
Moreover, Marin and co-workers Citation[84] synthesized luminescent thermotropic LC polyazomethines based on fluorene and/or 1,3,4-oxadiazole as chromophoric mesogen. The new thermotropic LC alternating polyazomethines PAOxH6, PAOxH7, PAFIH6 and PAFIH7 (47 – 50 respectively) were prepared by solution polycondensation of AOx1 and 9,9-bis(p-aminophenyl)-fluorene (AFl) with flexible dialdehydes 4,4′-diformyl-diphenoxyhexane and 4,4′-diformyl-diphenoxyheptane (H6 and H7 respectively) in DMF, as shown in Figure .
Figure 35 Synthesis of alternating poly(azomethine–oxadiazole)s 34, 35 and poly(azomethine–fluorene)s 36, 37.
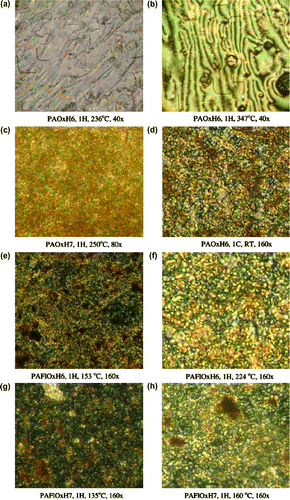
Random copolymers containing both chromophores PAFlOxH6 51 and PAFlOxH7 52 were synthesized by the reaction of the dialdehydes with a 1:1 mixture of AOx and AFl as shown in Figure .
The investigation of the thermotropic behaviour by three complementary methods of analyses, i.e. OPM, DSC and WAXD, indicated a nematic mesophase for the polymers containing oxadiazole units, either alternating or random ones.
All polymers containing oxadiazole units, either alternating or random, exhibit, in the first heating scan, the occurrence of a birefringent viscous fluid under shear, and the texture appears to be of a fine Schlieren type, which is often exhibited by stiff-chain thermotropic polymers Citation[85,86] In the case of the PAOxH6 alternating poly(azomethine–oxadiazole) containing an even-numbered spacer, a clear transition to the nematicmesophase is evidencedby the appearance of a fluid marble texture at 230 °C (Figure ), and a Schlieren texture at 310 °C (Figure ). The isotropization is higher than 350 °C for all the oxadiazole containing polymers and, upon cooling from the mesophase state, the fine textures freeze, giving a continuous birefringent film, without physical grain boundaries or cracks even after several months (Figure ). The poly(azomethine–fluorene)s PAFlH6 and PAFlH7 show rare nematic droplets in the heating and cooling scans which not coalesce to form a nematic texture. This suggest that the fluorene containing unit is not planar enough to facilitate self-assembling, the thermotropic behavior of random poly(azomethine–fluorene–oxadiazole)s being a result of the self-assembling of oxadiazole containing units.
Figure 37 Polarized optical micrographs of the synthesized polymers (C, cooling; H, heating; RT, room temperature).
A mesomorphic state maintaining the degree of order of the semi-crystalline state while being viscous fluid was evidenced too. The photoluminescence spectra recorded for both polymer solutions and films exhibited a blue light emission. These results point to the possibility of obtaining monodomain or multidomain ordered thin films without grain boundaries showing good mechanical and luminescent properties.
On the other hand, Schab-Balcerzak et al. Citation[87] synthesized a new type of thermotropic LC compounds containing azomethine linkages and naphthalene diimide moieties (AZ-NIs) via condensation of novel N,N′-bis(4-amino-2,3,5,6-tetramethylphenyl)naphthalene-1,4,5,8-dicarboximide (DANDI) with 4-(4,4,5,5,6,6,7,7,8,8,9,9,10,10,11,11,11-heptadecafluoroundecyloxy)benzaldehyde (HDFUB) and 4-octadecyloxy-benzaldehyde. HDFUB was utilized for preparation of compound AZ-NI-I 53 and 4-ctadecyloxybenzaldehyde for AZ-NI-II 54. DANDI was prepared from condensation reaction of excess of 2,3,5,6-tetramethyl-1,4-phenylenediamine with 1,4,5,8-naphthalenetetra-carboxylic dianhydride (NTDA) in the presence of imidazole in dry pyridine. The reaction mixture was refluxed under argon atmosphere as illustrated in Figure . While NTDA is only partially soluble in chloroform, synthesized DANDI was soluble in this solvent at room temperature. The presence of four methyl groups on the phenyl rings and incorporation of the imide rings increased the solubility. DANDI was applied for condensation with two aldehydes. The reaction route and chemical structure and the obtained azomethine-naphthalene diimides are presented in Figure .
Figure 38 Synthetic route and chemical structure of diamine (DANDI) and azomethine–naphthalene diimides (AZ-NIs) 40, 41.
The structures of compounds were characterized by means of FTIR, NMR spectroscopy and elemental analysis; the results showed an agreement with the proposed structure. Phase behaviors of the both compounds were investigated additionally by observation of the optical textures on a OPM equipped with hot-stage. Photomicrographs of the optical textures of mesophases obtained for the AZ-NI-I and AZ-NI-II are presented in Figure . The differences in the temperature of isotropisation and also kind of the mesophases of the investigated compounds were detected. This behavior indicates role of the aldehyde structure in creating their mesomorphic properties of azomethine–naphthalene diimides.
Figure 39 Photomicrographs of the optical textures of mesophases obtained for: (a) 40 (AZ-NI-I)(188, 205 °C) and (b) 41 (AZ-NI-II) (133, 195 °C).
Optical properties of the obtained AZ-NIs in solution and in solid state as thin films on the quartz substrate were tested by UV–vis absorption spectroscopy. The UV–vis absorption spectra were recorded both in chloroform solution and in solid state as thin films spin-coated on quartz substrate. The range of UV–vis measurements was limited by the transparency of the used solvent and the substrate. The representative absorption spectra of DANDI, AZ-NIs in solution and in AZ-NI-II film are depicted. Furthermore, the electrochemical behavior of AZ-NIs was studied by cyclic voltammetry and differential pulse voltammetry. The highest occupied molecular orbital and the lowest unoccupied molecular orbital energy levels, and electrochemical and optical band gap values were calculated using the results of electrochemical and UV/vis measurements, respectively. The electrical properties of the azomethine–naphthalene diimides were investigated by current–voltage (I–V) measurements. I–V characteristics were performed on ITO/compound/Al, and ITO/compound: PC61BM/Al devices in the dark and during irradiation with light (under illumination 1000 W/m2). Additionally, the azomethine–naphthalene diimides films were tested usingAFMtechnique. The mesomorphic behavior of the AZ-NIs was investigated via DSC and POM
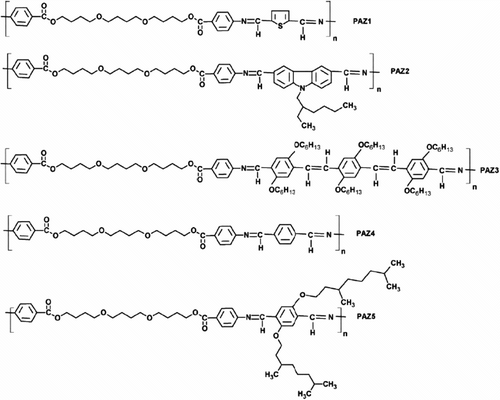
Iwana et al. Citation[88] synthesized new series of semiconducting materials based on aliphatic–aromatic poly(azomethine)s 55 PAZ1–5 as presented in Figure . The structures of polymers were characterized by means of FTIR, 1H, 13C-NMR spectroscopy, and elemental analysis. UV–vis properties of the thin films of the polymers were investigated on the quartz substrate. In addition, the opto(electronic) and LC properties for the resulting polymers had explored.
The lowest optical energy gap (Eg) at 2.28 eV was found. The polymers were irradiated with a test dose of 2 Gy Co-60 gamma-rays to detect their thermoluminescence properties in the temperature range 25–200 °C. Mesomorphic behavior was investigated via DSC and POM studies. Being into consideration backbone geometry, all polymers, excepted polymer PAZ2, obtained from poly(1,4-butanediol)bis(4-aminobenzoate) and 9-(2-ethylhexyl)carbazole-3,6-dicarboxalde-hyde, exhibited LC properties. Moreover, the electrical characterizations of bulk heterojunction and bilayer devices with the following architecture ITO/PEDOT/PAZ:TiO2/Al were investigated. Additionally, devices without and with TiO2 layer such as ITO/PAZ/Al and ITO/TiO2/PAZ/Al were prepared and investigation in the dark and during irradiation with light (under illumination 1000 W/m2). The sol–gel technique was applied to prepared TiO2 layers and powders. Moreover, impedance spectroscopy at different temperatures for electrical properties measurement was used.
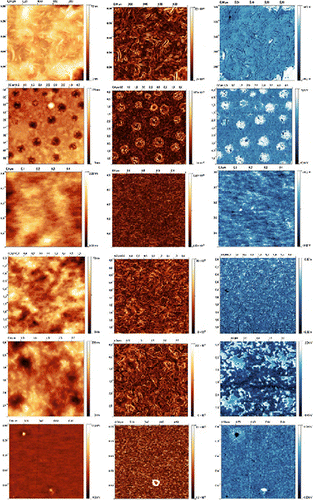
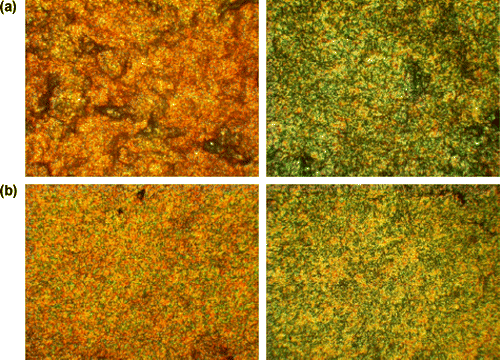
Moreover, the chemical structure of the poly(azomethine)s on the surface morphology of the materials was investigated by AFM. Films on the glass substrate were obtained by dissolving at room temperature the polymer in chloroform to form a homogenous solution. Residual solvent was removed by heating of the film. Figure showed an AFM images obtained for all polymers. The scan sizes were chosen in order to provide good view of the key features of specific sample. PAZ1 is presented on the two images. Films of the poly(azomethine)s obtained by casting from chloroform solution shows very interesting morphology, typical for systems capable for forming organized supramolecular structures in linear polymers with different type of sub-unit. Figure showed typical, planar view of the topography as well as inclination transformation, which we used to provide a better view of fine structures and features. Also Phase Image was placed, which shows maps of viscoelastic forces, useful for imaging inhomogeneous materials.
References
- Gordon W. Review (Virtual text book). Available from: http://PLC.cwru.edu/tutorial/enhanced/main.htm
- Khoo , IC and Wu , ST . 1993 . Optics and nonlinear optics of liquid crystals , Singapore : World Scientific Publishing .
- Chapoy , LL , ed. 1986 . Recent advances in liquid crystalline polymers , London : Elsevier .
- Blumstein , A , ed. 1985 . Polymeric liquid crystals , New York , NY : Plenum Press .
- Griffin , AG and Johnson , JF , eds. 1984 . Liquid crystals and ordered fluids , New York , NY : Plenum Press .
- Xu , Q-W , Man , H-C and Lau , W-S . 1998 . Study on the morphology and mechanical properties of binary and ternary blends of semiaromatic LCP/PP/PC . Polymer-Plastics Technology and Engineering , 37 : 253 – 259 .
- Lizuka , E . 2000 . Effects of magnetic fields on polymer liquid crystals . International Journal of Polymeric Materials , 45 : 191 – 238 .
- Broer , DJ , Finkelmann , H and Kondo , K . 1988 . In-situ photopolymerization of an oriented liquid-crystalline acrylate . Die Makromolekulare Chemie , 189 : 185 – 194 .
- Broer , DJ and Mol , GN . 1989 . In situ photopolymerization of an oriented liquid-crystalline acrylate 2 . Makromolekulare Chemie , 190 : 19 – 30 .
- Broer , DJ , Boven , J , Mol , GN and Challa , G . 1989 . In-situ photopolymerization of oriented liquid-crystalline acrylates, 3. Oriented polymer networks from a mesogenic diacrylate . Makromolekulare Chemie , 190 : 2255 – 2268 .
- Hikmet , RAM and Lub , J . 1995 . Anisotropic networks with stable dipole orientation obtained by photopolymerization in the ferroelectric state . Journal of Applied Physics , 77 : 6234 – 6238 .
- He , L , Zhang , S , Jin , S and Qi , Z . 1995 . In-situ photopolymerization of an oriented chiral liquid crystal acrylate in an electric field . Polymer Bulletin , 34 : 7 – 12 .
- Hoyle , CE , Mathias , LJ , Jariwala , C and Sheng , D . 1996 . Photopolymerization of a semifluorinated difunctional liquid crystalline monomer in a smectic phase . Macromolecules , 29 : 3182 – 3187 .
- Guymon , CA , Hoggan , EN , Clark , NA , Rieker , TP , Walba , DM and Bowman , CN . 1997 . Effects of monomer structure on their organization and polymerization in a smectic liquid crystal . Science , 275 : 57 – 59 .
- Broer , DJ , Mol , GN and Challa , G . 1991 . Temperature effects on the kinetics of photoinitiated polymerization of dimethacrylates . Polymer , 32 : 690 – 695 .
- Hoyle , CE , Kang , D , Jariwala , C and Griffin , AC . 1993 . Efficient polymerization of a semi-fluorinated liquid crystalline methacrylate . Polymer , 34 : 3070 – 3075 .
- Hoyle , CE and Watanabe , T . 1994 . Kinetics of polymerization of liquid-crystalline monomers: an exotherm and light scattering analysis . Macromolecules , 27 : 3790 – 3796 .
- Hoyle , CE , Watanabe , T and Whitehead , JB . 1994 . Anisotropic network formation by photopolymerization of liquid crystal monomers in a low magnetic field . Macromolecules , 27 : 6581 – 6588 .
- Sahlen , F , Trollsas , M , Hult , A and Gedde , UW . 1996 . Synthesis and characterization of bifunctional liquid-crystalline monomers showing smectic C phase. photopolymerization and cross-linking . Chemistry of Materials , 8 : 382 – 388 .
- Hellermark , C , Gedde , UW and Hult , A . 1992 . Synthesis and characterization of poly(11-(4′-cyano-trans-4-stilbenyloxy)undecanyl vinyl ether) . Polymer Bulletin , 28 : 267 – 274 .
- Andersson , H , Gedde , UW and Hult , A . 1992 . Preparation of ordered, crosslinked and thermally stable liquid crystalline poly(vinyl ether) films . Polymer , 33 : 4014 – 4018 .
- Broer , DJ , Lub , J and Mol , GN . 1993 . Synthesis and photopolymerization of a liquid-crystalline diepoxide . Macromolecules , 26 : 1244 – 1247 .
- Jahromi , S , Jub , J and Mol , GN . 1994 . Synthesis and photoinitiated polymerization of liquid crystalline diepoxides . Polymer , 35 : 622 – 629 .
- Andersson , H , Trollsas , M , Gedde , UW and Hult , A . 1995 . Preparation of ordered and crosslinked films from liquid crystalline p-vinylphenoxy-based monomers . Macromolecular Chemistry and Physics , 196 : 3667 – 3676 .
- Williamson , SE , Kang , D and Hoyle , CE . 1996 . Phase effects on the polymerization of a styrloxy cholesteric liquid crystalline monomer . Macromolecules , 29 : 8656 – 8665 .
- Mititelu , A and Cascaval , CN . 2005 . Liquid crystalline epoxy thermoset obtained from biphenyl mesogen . Polymer-Plastics Technology and Engineering , 44 : 151 – 162 .
- Morgan , PW , Kwolek , SL and Plecher , TC . 1987 . Aromatic azomethine polymers and fibers . Macromolecules , 20 : 729 – 739 .
- Wojkowski , PW . 1987 . Aromatic-aliphatic azomethine ether polymers and fibers . Macromolecules , 20 : 740 – 748 .
- Yeakel , KG , Mani , RS , Allen , RD and Mohanty , DK . 1993 . Synthesis and characterization of processable aromatic poly(azomethine)s with ether linkages . Macromolecular Chemistry and Physics , 194 : 2779 – 2787 .
- Yang , CJ and Jenekhe , SA . 1995 . Conjugated aromatic polyimines. 2. Synthesis, structure, and properties of new aromatic polyazomethines . Macromolecules , 28 : 1180 – 1196 .
- Iwan , A and Sek , D . 2008 . Processible polyazomethines and polyketanils: from aerospace to light-emitting diodes and other advanced applications . Progress in Polymer Science , 33 : 289 – 345 .
- Grigoras , M and Catanescu , CO . 2004 . Imine oligomers and polymers . Journal of Macromolecular Science: Part C: Polymer Reviews , 44 : 131 – 173 .
- Chapoy , L.L. , ed. 1986 . Recent advances in liquid crystalline polymers , London : Elsevier .
- Blumstein , A , ed. 1985 . Polymeric liquid crystals , New York , NY : Plenum .
- (a) Yang CY, Jenekhe SA. Conjugated aromatic poly(azomethines) 1. Characterization of structure, electronic spectra, and processing of thin films from soluble complexes. Chemistry of Materials. 1991; 3: 878–887; (b) Kannan P et al. Synthesis and characterization of thermotropic liquid crystalline poly(azomethine ether)s. Polymer. 2004; 45: 7895–7902.
- Luzny , W , Pomarzanska , SE and Pron , A . 1999 . Structural properties of selected poly(azomethines) . Polymer , 40 : 6611 – 6614 .
- Shiota , A and Ober , CK . 1997 . Rigid rod and liquid crystalline thermosets . Progress in Polymer Science , 22 : 975 – 1000 .
- Higuchi , M , Tsuruta , M , Chiba , H , Shiki , S and Yamamoto , K . 2003 . Control of stepwise radial complexation in dendritic polyphenylazomethines . Journal of the American Chemical Society , 125 : 9988 – 9997 .
- Adams , R , Bullock , RE and Wilson , WC . 1923 . Contribution to the structure of benzidine . Journal of the American Chemical Society , 45 : 521 – 527 .
- Marvel , CS and Hill , HW . 1950 . Polyazines . Journal of the American Chemical Society , 72 : 4819 – 4820 .
- Spiliopoulos , IK and Mikroyannidis , JA . 1996 . Soluble, rigid-rod polyamide, polyimides, and polyazomethine with phenyl pendent groups derived from 4,4‘‘-diamino-3,5,3‘‘,5‘‘-tetraphenyl-p-terphenyl . Macromolecules , 29 : 5313 – 5319 .
- Racleşa , C , Cazacua , M , Vasiliua , M and Cozana , V . 2005 . Structure – LC properties relationship in siloxane–azomethine compounds . Polymer-Plastics Technology and Engineering , 44 : 1049 – 1058 .
- Carracher , CE Jr . 2000 . Polymer chemistry , 5th ed. , New York , NY : Marcel Dekker .
- Stegemeyer H. Topic in physical chemistry. In Liquid crystals, Vol. 3. New York NY: Springer, 1994.
- Gray GW, Winsor PA. Liquid crystals and plastic crystals. London: Ellis, Horwood, Vol. 1; 1974.
- Robinson , C . 1956 . Liquid-crystalline structures in solutions of a polypeptide . Transactions of the Faraday Society , 52 : 571 – 592 .
- Gray , GW . 1987 . Thermotropic liquid crystals , New York , NY : Willy .
- Barcel , M , Zigen , M and Malavasic , T . 1998 . Side chain liquid crystal polyurethanes with azobenzene mesogenic moieties: Influence of spacer length on hydrogen bonding at different temperatures . Journal of Polymer Science Part A: Polymer Chemistry , 36 : 2135 – 2146 .
- Barcon , F , Guittard , F , Givernchy , F and Cambon , A . 1999 . Highly fluorinated monomers precursors of side-chain liquid crystalline polysiloxanes . Journal of Polymer Science Polymer Chemistry Edition , 37 : 4487 – 4496 .
- Dong , D , Zhuang , H , Lig , NY and Ding , M . 1999 . Synthesis and characterization of thermotropic liquid crystalline poly(ester imide)s . Journal of Polymer Science Part A: Polymer Chemistry , 37 : 211 – 218 .
- Sudha , JH . 2000 . Synthesis and characterization of hydrogen-bonded thermotropic liquid crystalline aromatic-aliphatic poly(ester–amide)s from amido diol . Journal of Polymer Science Part A: Polymer Chemistry , 38 : 2469 – 2486 .
- Kricheldorf , HR , Greken , A and Karhinen , H . 1998 . LC-polyimides XXXIV. Noncrystalline thermotropic copoly(ester-imide)s based on PET . Journal of Polymer Science Part A: Polymer Chemistry , 36 : 1813 – 1820 .
- Sun , SJ , Lio , YC and Chang , TC . 2000 . Studies on the synthesis and properties of thermotropic liquid crystalline polycarbonates. VII. Liquid crystalline polycarbonates and poly(ester-carbonate)s derived from various mesogenic groups . Journal of Polymer Science Polymer Chemistry Edition , 38 : 1852 – 1860 .
- Kricheldof , HR , Greken , A , Yulchibaev , B and Friedrich , C . 2000 . New polymer syntheses. 102. Aspirin-modified LC polyesters based on PET and 4-hydroxybenzoic acid . Journal of Polymer Science Part A: Polymer Chemistry , 38 : 2013 – 2022 .
- Karayannidis , GR and Psalida , EA . 2000 . Chain extension of recycled poly(ethylene terephthalate) with 2,2′-(1,4-phenylene)bis(2-oxazoline) . Journal of Applied Polymer Science , 77 : 2206 – 2211 .
- Jung , HC , Kang , SJ , Kim , WN , Lee , YB , Choe , KH , Hong , SH and Kimg , SB . 2000 . Properties of crosslinked polyurethanes synthesized from 4,4′-diphenylmethane diisocyanate and polyester polyol . Journal of Applied Polymer Science , 78 : 624 – 630 .
- Kannan , P , Raja , S and Sakthivel , P . 2004 . Synthesis and characterization of thermotropic liquid crystalline poly(azomethine ether)s . Polymer , 45 : 7895 – 7902 .
- Mehdipour-Ataei , S , Maleki-Moghaddam , R and Nami , M . 2005 . Synthesis and characterization of heat resistant, pyridine-based polyimides with preformed ether and ester groups . European Polymer Journal , 41 : 1024 – 1029 .
- Cho , I and Jo , S-Y . 1999 . New side-chain liquid crystalline polymer with flexible spacer backbone . Macromolecules , 32 : 521 – 523 .
- Zhang , D , Liu , Y , Wan , X and Zhou , Q-F . 1999 . Synthesis of a new side-chain type liquid crystal polymer poly[dicyclohexyl vinylterephthalate] . Macromolecules , 32 : 4494 – 4496 .
- Al-Dujaili , AH , T-Atto , A and Al-Kurde , AM . 2001 . Synthesis and liquid crystalline properties of models and polymers containing thiazolo[5,4-d]thiazole and siloxane flexible spacers . European Polymer Journal , 37 : 927 – 932 .
- Aharoni , SM . 1988 . Hydrogen-bonded highly regular strictly alternating aliphatic-aromatic liquid-crystalline poly(ester amides) . Macromolecules , 21 : 1941 – 1961 .
- Otilia , C , Mircea , G , Georgiana , C , Alina , D , Nicolae , H and Cristofor , IS . 2001 . Radical polymerization of vinyl acetate using p-acetylbenzylidene triphenylarsonium ylide as an initiator . European Polymer Journal , 37 : 2213 – 2218 .
- Choi , EJ , Ahn , JC , Chien , LC , Lee , CK , Zin , WC , Kim , DC and Shin , ST . 2004 . Main chain polymers containing banana-shaped mesogens: synthesis and mesomorphic properties . Macromolecules , 37 : 71 – 78 .
- Li , CH and Chang , TC . 1990 . Studies on the thermotropic liquid crystalline polymer. I. Synthesis and properties of polyamide-azomethine-ether . Journal of Polymer Science Part A: Polymer Chemistry , 28 : 3625 – 3638 .
- Barbera , J , Oriol , L and Serrano , JL . 1992 . Hydroxy-functionalized liquid-crystalline polyazomethines I. Synthesis, characterization and structure-mesogenic behaviour relationship . Liquid Crystals , 12 : 37 – 47 .
- Parks , SB , Kim , H , Zin , WC and Jung , JC . 1993 . Synthesis and properties of polyazomethines having flexible (n-alkyloxy)methyl side chains . Macromolecules , 26 : 1627 – 1632 .
- Guillion , D and Skoulios , A . 1978 . Mesophases Smectiques Thermotropes de Polybases de Schiff . Molecular Crystals and Liquid Crystals , 49 : 119 – 123 .
- Li , CH and Chang , TC . 1991 . Studies on thermotropic liquid crystalline polymers—Part II. Synthesis and properties of poly(azomethine-ether) . European Polymer Journal , 27 : 35 – 39 .
- Aly , KI and Ahmed , RA . 2000 . Liquid crystalline polymers V. Thermotropic liquid crystalline poly(azomethine-ether)s containing a cycloalkanone moiety in the polymer backbone . Liquid Crystals , 27 : 451 – 458 .
- Aly , KI , Khalaf , AA and Alkaskas , IA . 2003 . Liquid crystalline polymers VII. Thermotropic liquid crystalline poly(azomethine-ether)s containing dibenzylidene derivatives in the main chain . European Polymer Journal , 39 : 1035 – 1044 .
- Jayanthi , GS and Kishore , K . 1996 . Synthesis and mesogenic behavior of metal-containing liquid crystalline networks . Journal of Applied Polymer Science , 60 : 791 – 798 .
- Sauer , BB , Kampert , WG and Mclean , RS . 2003 . Thermal and morphological properties of main chain liquid crystalline polymers . Polymer , 44 : 2721 – 2738 .
- Biswas , A , Gardner , KH and Wojtkowshi , PW . 1990 . “ Liquid crystalline polymers ” . In ACS Symposium Series 435 , Edited by: Weiss , RA and Ober , CK . Washington , DC : American Chemical Society .
- Aly , KI and Hammam , AS . 2000 . Liquid crystalline polymers: I. Main chain thermotropic poly(arylidene-ether)s containing cyclopentanone moiety linked with polymethylene spacers . European Polymer Journal , 36 : 1933 – 1942 .
- Aly , KI . 2000 . Liquid crystalline polymers. 3. Synthesis and liquid crystal properties of thermotropic poly(arylidene-ether)s and copolymers containing cycloalkanone moiety in the polymer backbone . Journal of Macromolecular Science Part A: Pure and Applied Chemistry , 37 : 93 – 115 .
- Aly , KI and El-Kashef , HM . 2001 . Liquid crystalline polymers. IV. Thermotropic liquid crystalline poly(arylidene-ether)s and copolymers containing n-methylpiperidone linked to the main chain through spacers of various lengths . Journal of Macromolecular Science Part A: Pure and Applied Chemistry , 38 : 785 – 806 .
- Aly , KI , Abbady , MA , Mahgoub , SA and Hussein , MA . 2007 . Liquid crystalline polymers. IX. Main chain thermotropic poly (azomethine – ether)s containing thiazole moiety linked with polymethylene spacers . Journal of Express Polymer Letters , 1 : 197 – 207 .
- Cerrada , P , Oriol , L , Plnol , M and Serrano , JL . 1996 . Alternative synthetic strategies for Schiff base derived liquid crystal polymers: A comparative study . Journal of Polymer Science Part A: Polymer Chemistry , 34 : 2603 – 2611 .
- Cerrada , P , Oriol , L , Pinol , M , Serrano , JL , Alonso , PJ , Puertolas , JA , Iribarren , I and Munoz Guerra , S . 1999 . Munoz Guerra S. Influence of hydroxy functionalization and metal cross-linking on fiber properties of liquid-crystalline polyazomethines . Macromolecules , 32 : 3565 – 3573 .
- Marin , L , Cozan , V and Bruma , M . 2006 . Comparative study of new thermotropic polyazomethines . Polymers for Advanced Technologies , 17 : 664 – 672 .
- Windle , AH . 1994 . “ Structure of thermotropic main-chain polymers ” . In Liquid crystalline and mesomorphic polymers , Edited by: Shibaev , VP and Lam , L . 42 Berlin : Springer-Verlag .
- Marin , L , Damaceanu , MD and Timpu , D . 2009 . New thermotropc liquid crystalline polyazomethines containing luminescent mesogens . Soft Materials , 7 : 1 – 20 .
- Marin , L , Perju , E and Damaceanu , MD . 2011 . Designing thermotropic liquid crystalline polyazomethines based on fluorene and/or oxadiazole chromophores . European Polymer Journal , 47 : 1284 – 1299 .
- Demus , D , Goodby , J , Spiess , GW and Vill , V , eds. 1998 . Handbook of liquid crystals, high molecular weight liquid crystals, Vol. 3 , Weinheim : VCH-Wiley .
- Windle , AH . 1994 . “ Structure of thermotropic main chain polymers ” . In Liquid crystalline and mesomorphic polymers , Edited by: Shibaev , VP and Lam , L . New York , NY : Springer-Verlag .
- Schab-Balcerzak , E , Iwanc , A , Krompiecb , M , Siwya , M , Tapaa , D , Sikorac , A and Palewiczc , M . 2010 . Study of charge transport in blends of natural rubber and poly(o-methoxyaniline) based on a resistor network statistical model . Synthetic Metals , 160 : 2208 – 2210 .
- Iwana , A , Palewicza , M , Sikoraa , A , Chmielowieca , J , Hreniaka , A , Pasciaka , G and Bilski , P . 2010 . Aliphatic–aromatic poly(azomethine)s with ester groups as thermotropic materials for opto(electronic) applications . Synthetic Metals , 160 : 1856 – 1867 .