Abstract
The generation of supramolecular architectures with well-defined structures and functionalities is recently garnering attraction. Self-assemblage of amphiphilic polymers leads to the formation of polymeric micelles that demonstrate unique set of characteristics such as excellent biocompatibility, low toxicity, enhanced blood circulation time, and solubilization of poorly water-soluble drugs. In this article, we provide an up-to-date review on important aspects of polymeric micelles. Critical factors for solubilization of hydrophobic drugs in the micellar core are discussed. Polymeric micelles can be used as ‘smart’ drug carriers for targeting certain areas of the body. Here, we have especially emphasized on the recent developments in the targetability of certain tissues such as cancerous tissues using polymeric micelles. Different stimuli exploited for creating stimuli-sensitive micelles are discussed comprehensively. Application of polymeric micelles in the photodynamic therapy is also meticulously described.
Introduction
Mysterious are the nature’s ways of creating the materials of great complexicity and functionality from simple ones with variety of arrangements starting from the biological membrane to nacre. With the hope to use nature’s tricks to an advantage, the very large effort in polymer science has been applied to get the molecules which will assemble spontaneously or with stimulus to structures that can be employed in science. One such assembly of interest is a micelle and more particularly a polymeric micelle. The field of polymeric micelles is expanding its roots from academic settings into industrial one adopting it for preclinical and clinical drug development. With more players embracing this technology, more innovative research on polymeric micelles spanning basic science as development of newer polymers and their micelles with characterization to application part as for drug solubilization, drug targeting via various routes of drug administration and targeting of nucleic acid drugs is expected.
Micelles: formation and features
The amphiphilic molecules or surfactant monomers that possess a polar head and a lipophilic tail show concentration dependent variation in the physicochemical properties. The changes in physicochemical properties are associated with the orientation and aggregation of amphiphilic molecules in solution resulting in the formation of structures called micelles. Micelles are dynamic structures that are in continuous equilibrium with free monomers, wherein monomers are constantly exchanged between micelles and intermicellar solution. Due to this to and fro motion of molecules or the exchange phenomenon, surfactant molecules reside in the micelle form for a definite time called the surfactant residence time Citation[1]. An average number of monomers forming micelle at any given time is termed as the aggregation number. Micelles are generally made up of 50–200 monomers. The radius of a spherical micelle is almost the same as the length of a fully extended surfactant monomer, which mostly is 1–3 nm, and thus micelles lie in the colloidal range. Molecular size and geometrical features of the surfactants determine size of the micelle Citation[2]. Micelle formation in aqueous solution is mainly governed by the effective interaction between the hydrophobic parts of the surfactants. The major driving force behind self-association is the decrease of free energy of the system. Decrease in energy of the system is a result of removal of hydrophobic fragments from the aqueous surroundings with the formation of a micelle core stabilized with hydrophilic blocks exposed into water. The change in free energy for the micellization process is described as:
where R is the gas constant, T is the temperature of the system and CMC is the critical micelle concentration Citation[3].
The effective interactions resulting in micellization are opposed by repulsive interactions between the head groups and an interaction associated with residual alkyl chain-water molecule contacts at the micelle surface.
The most important factor affecting the process of micelle formation or self-assembly is the size of the hydrophobic domain in the amphiphilic molecule Citation[4]. Other factors of importance are the conditions of the system as solvent, concentration of amphiphiles, temperature, etc. These micelles are microheterogeneous, in that they internally have a hydrophobic core and externally a hydrophilic surface. The assembly formation starts only when a certain minimum concentration is crossed by the amphiphilic molecules, called as critical micelle concentration. At low concentrations in a medium, the amphiphilic molecules exist separately, and are so small that they appear to be subcolloidal. Below the CMC, the concentration of amphiphile undergoing adsorption at the air–water interface increases as the total concentration of the amphiphile is increased. Finally at CMC, the interface as well as the bulk phase is saturated with monomers. Any further amphiphile added in excess of CMC results in the aggregation of monomers in the bulk phase, such that the free energy of the system is reduced Citation[1,5]. The temperature below which amphiphilic molecules exist as unimers and above which as aggregates is the critical micellization temperature (CMT). This self-assembly is initiated either at a given temperature by increasing the concentration beyond the CMC or at a given concentration by increasing the temperature beyond the CMT.
Polymeric micelles
Amphiphilic block or graft copolymers behave in the same manner as that of conventional amphiphiles. In solution, attachment of a water-soluble polymer to an insoluble polymer leads to the formation of micelles of amphiphilic block copolymers resulting in structural and flow characteristics of the polymer that differ from either parent polymer. A major difference between the micelles of conventional surfactant monomers and polymeric surfactants is that there usually is a covalent linkage in individual polymeric surfactant molecules within the hydrophobic core that does not allow dynamic exchange of monomers between free solution and the micellar pseudo-phase. This confers rigidity and stability to the polymeric micelles Citation[6]. The diameter of polymeric micelle ranges from 10 to 100 nm.
These supramolecular structures are generated as a result of delicate balance between strong covalent bonds that hold the molecular building blocks together and the reversible intermolecular forces that assemble them. Factors controlling the size of the polymeric micelles include molecular weight of the amphiphilic block copolymer, aggregation number of the amphiphiles, relative proportion of hydrophilic and hydrophobic chains, quantity of solvent trapped inside the micellar core, and the preparation process Citation[7,8]. The radius of an entire micelle is designated as R m and that of the core as R c.
In aqueous medium, amphiphilic block copolymers can principally self-assemble into spherical micelles, worm-like or cylindrical micelles, and polymer vesicles or polymersomes. Main factor governing the morphology of micelles is the hydrophilic–hydrophobic balance of the block copolymer defined by the hydrophilic volume fraction, f. For amphiphilic block copolymers with value of f ∼ 35%, polymer vesicles are formed, whereas for value of f > 45%, spherical micelles are formed. Other controlling experimental factors are degree of swelling of the corona, concentration, temperature, pH, ionic strength, and sample preparation Citation[9–11]. For convenience, amphiphilic diblock polymers are said to be those polymers for which the molecular mass is in the range 5000–30,000 Da in contrast with surfactants for which the molecular mass is in the range 100–500 Da Citation[12]. Besides higher molecular weights, amphiphilic block copolymers are complex structures. Thus, in dilute solutions they yield monomolecular micelles and micelles of various shapes at different concentrations Citation[13]. By using amphiphiles of more complicated molecular design, e.g. star copolymers or by varying the experimental conditions for self-assembly more complex morphologies such as crew-cut micelles, multicompartment micelles, toroids, etc. may be obtained which may have great influence on their application performance of interfacial activity, viscosity, and emulsification Citation[14–17].
Attractive features of polymeric micelles
Polymeric amphiphiles have received increasing attention because of their special physicochemical and morphological characteristics in water, and possibility to generate ‘application suitable’ polymers. Suitable amphiphilic block copolymers are easily obtainable via controlled synthesis by varying the block ratio, the total molecular weight, the chemical structure, and conjugation with biomolecules. Size and morphology of the polymeric micelles developed from amphiphilic polymers can readily be controlled through adjusting the structure of amphiphilic copolymers as the factors controlling size of the polymeric micelles include molecular weight of amphiphilic block copolymer, aggregation number of amphiphiles, relative proportion of hydrophilic and hydrophobic chains, quantity of solvent trapped inside the micellar core, and the preparation process.
The colloidal dimensions of micelles render them suitable for sterilization by simple filtrative process with no special aseptic processing. The micellar core produces a hydrophobic domain. Thus, polymeric micelles are used for solubilization of hydrophobic moieties in the core region through hydrophobic interactions and/or ionic interactions. Most of the drugs being poorly water-soluble can be easily incorporated into the core of polymeric micelles to overcome solubility problems Citation[18,19]. Solubility enhancement usually is associated with betterment of oral bioavailability of the hydrophobic drugs Citation[20,21]. Incorporation of the drug into block copolymer micelles results in the in vitro stabilization of drug as it is protected from various destructive agents in aqueous environment. Also, the hydrophilic shell of polymeric micelles is thought to disguise the drug in vivo and prevent its interaction with blood proteins, cells, and tissues which otherwise might lower the plasma drug concentration Citation[22]. Mostly, the hydrophobic segments exhibit low glass transition temperatures (ca. 40 °C). This feature allows incorporation of thermolabile agents into polymeric micelles at compatible temperatures Citation[23]. Surfactant micelles tend to disintegrate upon dilution triggering lysis of cell membranes. Polymeric micelles are considerably more stable toward dilution than surfactant micelles and hence, exhibit minimal cytotoxicity. The hydrophilic shell and the nanoscopic size prevent mechanical clearance of micelles by filtration or in the spleen Citation[24]. This is beneficial for prolonging the blood circulation of drug. Also, the shell stabilizes the micelle, interacts with the plasma proteins and cell membranes and its nature controls biodistribution of the carrier. Mostly, the shell is made up of chains of hydrophilic, biocompatible polymers such as poly(ethylene oxide) (PEO), etc. Citation[25]. It also favors the particular absorption in gastrointestinal system. Along with these features, low toxicity and faster rate of clearance of polymeric micelles from the body make them suitable for intravenously administered drug delivery systems Citation[26]. Additionally, there is no need of modification of chemical structure of the drugs Citation[27]. Thus, owing to the exciting facets offered by polymeric micelles, they are potential drug delivery carriers, especially when the micelles are made from suitable biodegradable polymers having low risk of chronic accumulation in the body Citation[28,29].
The size of polymeric micelles is typical of a virus. Nanoscopic size minimizes the risk of embolism in capillaries, contrary to larger drug carriers Citation[30]. It also avoids renal filtration and reticuloendothelial system (RES) uptake, and so can circulate in the blood for long periods of time, eventually passing through capillaries that are disrupted near tumor growth Citation[31–34]. Polymeric micelles thus provide targeting of the loaded drug. End-functionalization of block copolymers with sugars and peptides on the periphery yield an array of micelles that can be used for the receptor-mediated targeted drug and gene delivery. Immunomicelles, another means of targeting, are prepared by covalently attaching monoclonal antibody molecules to a surfactant or polymeric micelles demonstrate high binding specificity and targetability Citation[35,36]. Polymeric micelles are employed as ‘intelligent drug carriers’ through use of stimuli-sensitive (pH and temperature) copolymers, etc. and are investigated for controlled drug delivery Citation[37].
Limitations
The industrial growth of polymeric micelles is hindered by high cost of preparation and the difficulty in drug loading Citation[38]. When the polymer is sufficiently hydrophilic it can be dissolved directly along with the drug to yield drug-loaded polymeric micelles. But this method, which is suitable for highly hydrophilic polymers, usually is associated with low drug loading. For most other amphiphilic polymers, an organic solvent is used to solubilize polymer and poorly water-soluble drug. Drug loading in polymeric micelles is then effected by emulsification or dialysis techniques. However, emulsification usually involves use of chlorinated solvents which are not safe. Dialysis process often requires more than 36 h for efficient loading and replenishment of water at regular intervals. Nevertheless, the above-mentioned limitations can be overcome by employing a simple and cost-effective method in which water/tert-butanol mixture is used for dissolving drug as well as polymer and then lyophilizing it. Drug-loaded polymeric micelles are obtained by redispersing lyophilized product in a suitable vehicle Citation[39–41].
Owing to extreme dilutions by blood upon intravenous injections of micellar solution, polymeric micelles are prone to deformation and disassembly which may lead to leakage and burst release of loaded drugs. However, this limitation can now be overcome by improved interaction of the drug and polymer via chemical conjugation or by cross-linking of the shell Citation[42,43]. The loss of hydrophilic and hydrophobic balance upon increased loading of hydrophobic drug into the core region also has been related to decreased stability of the polymeric micelles. Drugs or copolymers prone to hydrolytic cleavage in aqueous systems may as well pose stability problems. However, lyophilized polymeric micelle formulations have shown to possess improved long-term stability for intravenously administered preparations Citation[44–46].
Polymers used for micelle preparation
Polymers for preparation of micelles can either be amphiphilic or nonamphiphilic in nature. Moreover, amphiphilic copolymers can be block copolymers or graft copolymers. These amphiphilic block or graft copolymers are usually biodegradable and also offer excellent biocompatibility. From the era of emergence of polymeric micelles, most of the research focus have been on the synthesis of diblock and triblock copolymers Citation[22]. Recently, some studies have been conducted for producing tetrablock and pentablock copolymers also. A block copolymer molecule contains two or more polymer chains attached at their ends. Linear block copolymers comprise two or more polymer chains in sequence, whereas a starblock copolymer comprises more than two linear block copolymers attached at a common branch point Citation[47,48]. Polymers containing at least three homopolymers attached at a common branching point have been termed mixed arm block copolymers, although they can also be viewed as multigraft copolymers Citation[47]. Synthesis of block copolymers is reviewed by Zhou et al. Citation[49]. Relatively fewer studies concerning the micellization of graft copolymers have been conducted. A graft copolymer is one which comprises a polymer chain as a backbone and another polymer chain as side ‘grafted’ part. These copolymers usually demonstrate properties of both, i.e. polymeric backbones as well as of the grafts. Copolymerization of styrene with a PEO macromonomer in water to form a unimolecular graft copolymer having tendency to micellize is reported Citation[50]. Synthesis of polystyrene-graft-polyolefin elastomer copolymer, having micellization ability, through Friedel–Crafts alkylation reaction was reported by Guo and Fang Citation[51]. In a recent report, amylopectin was hydrophobically modified by grafting poly (lactic acid) chains to provide a biodegradable amphiphilic polysaccharide with micellization properties Citation[52]. Table shows different possible structures of amphiphilic copolymers with representative example of each class.
Table 1. Possible structures of amphiphilic copolymers.
Usually when the length of a hydrophilic block exceeds to some extent than that of a hydrophobic one, spherical micelles are formed from self-assemblage of amphiphilic diblock or triblock copolymers in aqueous solutions. Whereas if the length of a hydrophilic block is too large, copolymers exist in water as individual molecules (unimers) and molecules with lengthy hydrophobic blocks develop various structures. Different backbone structures that are used for constructing amphiphilic polymers have been presented in Figure and their examples in Table . Certain other types of polymers have also found applications in micelle preparation. Dendritic macromolecules are investigated by some researchers for generation of micelles. Dendrimers are nanoscale macromolecules with high density of surface functionalities, well-defined structures, and molecular weights Citation[96]. Inner core cavity and a great number of end groups justify the development of dendritic molecules as a new model of potential amphiphilic polymers for micelle preparation Citation[97]. On this basis, a series of drug-incorporated dendritic micelles from linear-dendritc macromolecules having increased biocompatibility and biodegradability have been reported Citation[98]. Yet another variant class of polymer for micelle preparation is a new group of polymers, viz. hyperbranched polymers. Hyperbranched polymers are highly active nano-structured materials with a large number of end groups whose properties can be tailored for different applications Citation[99,100]. Amin and others prepared polyesteramide and poly(urea-urethane) hyperbranched amphiphilic polymers that could be used as carriers for hydrophobic drugs Citation[101]. Of late, a multifunctional unimolecular micelle made of a hyperbranched amphiphilic block copolymer was synthesized and characterized for cancer-targeted drug delivery and imaging Citation[102].
Table 2. Various polymers used for micelle preparation.
Interesting studies over nonamphiphilic block copolymers have revealed that such polymers can also be used for micelle preparation based on an indirect method of micellization. In this, molecularly dissolved nonamphiphilic copolymers are converted into amphiphilic copolymers in situ by certain stimuli as temperature, pressure, pH, or salt formation Citation[103]. In this context, Yoshida reported micelle preparation from poly(vinylphenol)-b-polystyrene in 1,4-dioxane (a non-selective solvent) in the presence of 1,4-butanediamine Citation[103]. Micelles with UV absorbents at their coronas prepared using poly(vinylphenol)-b-poly{4-(2-hydroxybenzophenoxymethyl)styrene-co-styrene} di-block copolymer were reported by Yoshida and Ohta Citation[104]. The polymer did not self-assemble in 1,4-dioxane, but in the presence of α,ω-diamine formed micelles with an UV absorbent at the corona. The micelles were formed by the hydrogen bond cross-linking between the poly(vinylphenol) blocks via the diamine.
Methods of preparation
Polymeric micelles are generally prepared by either of the two methods – direct dissolution of the polymer in an appropriate solvent (direct dissolution is usually followed by stepwise dialysis) or addition of a precipitating solvent for one block. Micellization leads to the formation of ordered structures in which the contact between the insoluble block and the solvent is minimized.
The first method is most commonly used for the formation of micelles of block copolymers whose total molecular weight is low and the length of the insoluble block is short. To facilitate dissolution, stirring, thermal, or ultrasound treatments have been used. Block copolymers in a selective solvent (a thermodynamically good solvent for one block, but a nonsolvent for the other) form a micellar structure through the association of the insoluble segments Citation[105]. Cao and others prepared polystyrene-poly(methacrylic acid) (PS-PMA) block copolymer micelles by directly adding the polymer to 80:20 v/v dioxane:water followed by a stepwise dialysis to pure aqueous buffer Citation[106].
The micelles become spherical and stable with a monodisperse type of size distribution when an appropriate solvent for a particular polymer and processing condition is chosen. Improper solvent combination and/or processing conditions may lead to a precipitate or an uncontrolled growth of structures that will eventually aggregate and come out of the solution.
The second method depends on formation of molecularly dissolved chains of polymer in a nonselective solvent. To induce micellization in the molecularly dissolved chains, a selective solvent for one of the blocks and precipitant for the other may be added. In nonselective solvents, the polystyrene–polybutadiene (PS–PB) chains were molecularly dissolved and both blocks adopted a stretched conformation due to intersegmental repulsion Citation[107]. In n-decane, a selective solvent for the PB block, aggregated PS–PB chains forming spherical micelles were observed above the CMC. But, addition of a precipitating solvent does not imply literally, rather it indicates changing the system such that the selective solvent conditions for a particular polymer are produced. Change in temperature, ionic strength, or pH of the system can be used for this purpose. In this context, formation of poly(2-vinylpyridine)-b-PEO micelles in aqueous solution by titration from highly acidic to highly alkaline pH has been demonstrated Citation[108].
The search for effective micellization conditions depends upon solubility of the individual block of the copolymer. The size of final micelle may depend on the preparation protocol for a given polymer. The selection of solvents, employment of dialysis procedure, thermal treatment, etc. may all influence the formation of micelles. For a series of PEO-b-poly(ϵ-caprolactone) (PCL) samples, it was observed that spherical micelles were formed upon self-assembly induced from either dimethylformamide or tetrahydrofuran Citation[109]. Whereas for the same set of polymer precursors, large wormlike micelles were formed in solutions prepared from acetone. Therefore, simple modification in the experimental method of micelle preparation may be used to alter the shape of the micelles. As per Tian and his group, the micelle properties are very stable once the micelle resides in a solvent that is a strong nonsolvent for the core Citation[110].
Drug loading into micelles
Ringsdorf’s group in early 1980s first proposed the use of block copolymer micelles as drug delivery vehicles Citation[111,112]. Since then many researchers have emphasized on the development of polymeric micellar systems for drug delivery using different techniques.
Both, chemical conjugation and physical entrapment techniques can be used for loading of drugs into the core or the shell region of polymeric micelles. Incorporation of drug in the outer shell is usually avoided as the drug molecules (mostly hydrophobic) might interact with the outer shell and lead to undesired aggregate formation. Also the minimization of hydrophobic interactions between drug carriers and components of the biological system (such as proteins and cells) is an important key to targeting, since these hydrophobic interactions may considerably reduce contribution of diffusive and convectional transport through intracellular channels or intercellular junctions of endothelia on which the enhanced permeability and retention (EPR) effect is based (discussed later). The outer shell serves the function of shielding and prevents the hydrophobic interactions that would occur if the drug is incorporated in the outer shell Citation[113].
Factors that influence drug loading
The loading efficiency of the micellar carrier is an indication of the amount of drug that can be incorporated per micelle. The factor of prime importance is the compatibility between the drug and the core-forming block. But stability and degradation of micelles in aqueous medium also affects the drug loading efficiency and drug release characteristics, which are very important for the application of amphiphilic block copolymer micelles in drug delivery Citation[114,115]. The length and nature of the core and corona forming blocks also is an important parameter Citation[116]. The larger is the hydrophobic block, the larger is the core size and greater is its ability to entrap hydrophobic drugs. While an increase in the length of the hydrophilic block is associated with an increase in the CMC. Thus, the quantity of the drug entrapped in micelle drops.
Several other factors that control the drug loading efficiency into the polymeric micelles include molecular weight of the copolymer, concentration of the copolymer, the nature and concentration of the drug, and finally, the method of preparation of the polymeric micellar drug delivery system Citation[117,118].
Different approaches for preparation of drug-loaded polymeric micelles
Drug-loaded polymeric micelles can be prepared mainly by three common approaches: direct dissolution, solvent evaporation, and dialysis. Direct dissolution of the amphiphilic copolymer and drug in water is the simplest technique of preparing drug-loaded polymeric micelles. At or above CMC, copolymer and the drug self-assemble in water to form drug-loaded micelles. To enhance drug loading, this technique can be combined with an increase in temperature or alternately a thin evaporated film of drug can be prepared before the addition of copolymer.
In solvent evaporation or solution-casting technique, volatile organic solvents like methanol, ethanol, acetone, acetonitrile, or others are used to dissolve the copolymer and the drug. A thin film of copolymer and drug is obtained after the solvent is removed by evaporation (mostly by rotary evaporator). Drug-loaded polymeric micelles are obtained by reconstitution of film with water or aqueous buffers. Methoxy PEO-b-PCL micelles were used for the encapsulation of cyclosporine A (CyA) by a cosolvent evaporation method Citation[119]. The cosolvent composition was varied by changing the type of organic cosolvent (using acetone, acetonitrile, and tetrahydrofuran), the ratio of organic to aqueous phase, and their order of addition. Manipulation of the self-assembly conditions such as organic to aqueous phase ratio and order of phase addition in this method may be used to improve the efficiency of hydrophobic drug encapsulation in polymeric nanocarriers and average diameter of assembled structures. Addition of acetone to water at low organic to aqueous phase ratio leads to a smaller average diameter for self-assembled structures and is shown to be more efficient for CyA encapsulation. The higher encapsulation capacity for CyA despite smaller size may be attributed to the formation of compact micelles under this condition.
When the core forming blocks are long and more hydrophobic, the two above mentioned techniques are unsuitable. Micelles from such copolymers have more potential to solubilize large amounts of poorly water-soluble drugs. In these cases, the dialysis technique has been used to prepare drug-loaded micelles. Solutions of the drug and the polymer in organic solvent are placed in the dialysis bag and the solvent is exchanged with water by immersing bag into water, inducing micelle assembly Citation[120,121]. Although highly effective, dialysis is a time-consuming process for preparation of polymeric micelles.
Table summarizes some studies demonstrating use of different preparation approaches of polymeric micelles.
Table 3. Examples of preparation of polymeric micelles by different approaches.
Properties and characterization of micelles
Critical micelle concentration
CMC is the key parameter for the formation and the static stability of polymeric micelles. In aqueous media, amphiphilic polymers can exist in the form of micelles when the concentration is higher than CMC, and when diluted below this concentration, the micelles may collapse. Thus, CMC is said to be an apparent measure of polymeric micellar thermodynamic stability Citation[134,135]. At CMC or slightly above the CMC, loose aggregates of micelles containing a little water in the core are formed Citation[136]. With an increase in polymer concentration above CMC, the residual solvent is excluded from the core; the micellar structure becomes more compact with reduction in the micelle size, and develops into stable structures. The CMC of the polymeric solution and the kind of aggregates the copolymers can form is dependent on the competition between the enthalpic interaction and the entropic effect in the solution Citation[137,138]. Below CMC, the entropic effects dominate over the enthalpic interaction, whereas the enthalpic contributions are dominant over the entropic effects above CMC, which makes the rapid aggregation of copolymers to form aggregates with particular properties.
To discriminate CMC of polymeric micelles from the CMC of surfactant micelles, the term critical association concentration (CAC) is sometimes employed for polymeric micelles. The enhanced stability of polymeric micelles as compared with conventional surfactants (with CMC 10−3–10−4 M) in water is owing to their lower CMC values (10−6–10−7 M) Citation[136,139]. Conventional surfactants have much higher CMC values as compared with polymeric surfactants and the resultant micelles may be destabilized earlier and collapse. In order to obtain stable polymeric micelles, the degree of hydrophobicity for the core-forming block must be controlled.
Factors affecting CMC are the hydrophilic and hydrophobic block length of the copolymer, branching parameter, temperature, salt concentration, pH, etc. The CMC of the drug loaded micelles is also influenced by the drug solubility, drug interaction with polymers, and the drug loading content Citation[140].
Generally, an increase in the number of hydrophilic units in the block copolymers leads to an increase in the CMC Citation[141,142]. An increase in the hydrophilicity improves the aqueous solubility of polymer and hence lowers the tendency for the polymeric surfactants to form micelles and thereby increases the CMC Citation[143].
Usually, the CMC is found to increase as the length of the core forming block decreases Citation[116]. Soga et al. observed that the CMC as well as the CMT decreased with increasing hydrophobic block lengths in poly(N-(2-hydroxypropyl) methacrylamide lactate)-b-PEG copolymer micelles, which can be attributed to the greater hydrophobicity of the block with increasing molecular weight Citation[144]. Similarly, Gadelle et al. and Kozlov et al. also have demonstrated that as the length of the hydrophobic block increases, the CMC decreases Citation[145,146]. With an effort to determine the effect of hydrophobic tail architecture on self-assembling behavior, different architectures of linear, branched, starlike, and dendritic tails were selected for comparison by Cheng and Cao Citation[147]. They used the branching parameter of the tail to characterize the tail architectures and showed that the self-assembly of linear tail copolymer had the lowest CMC, with almost a spherical shape of the micelle. It was found that the CMC is inversely proportional to the branching parameter and, as CMC is a result of competition between entropy and enthalpy contributions, tails with a low branching parameter have lower configurational entropy and lose less configurational entropy in the process of aggregation.
The thermodynamics of micelle formation have been obtained from the temperature dependence of the CMC Citation[148,149]. Thurn et al. were able to demonstrate the temperature-dependent micellization of Pluronic F127 Citation[150]. CMC of Pluronic F127 decreased largely on increasing temperature due to the temperature-dependent difference in the solvation of ethylene oxide and propylene oxide blocks.
The effect of salt concentration on CMC has been studied by Elisseeva et al. Citation[151]. CMC of Pluronic F127 was strongly influenced by the salt concentration and the presence of 0.1 M sodium chloride decreased the CMC of Pluronic F127 by a factor of 2. Sodium chloride because of the salting-out effect decreases the solvent quality of the polymer aqueous solution with respect to the ethylene oxide blocks of the F127 molecules.
The dependence of the conformation and hydrophilicity of the Tetronic T904 unimers and micelles on CMC has been shown by Alvarez-Lorenzo and others Citation[152]. For Tetronic at pH < 5.8, the diprotonated form predominates over the nonprotonated one. The deprotonization of the central diamine group was essential for micellization, which was an endothermic entropy-driven process owing to hydrophobic interactions between the poly(propylene oxide) chains. With decrease in pH of the solutions, the CMC values were elevated and size of the micelles decreased as the positively charged amine groups repelled each other making the aggregation more difficult.
Different methods can be employed for the determination of CMC, and it has been frequently observed that the reported CMC value is influenced by the choice of the method. Therefore, a more feasible definition of CMC than the earlier one would be the concentration at which a sufficient number of micelles are formed and detected by a given method Citation[120,153]. Most commonly used methods include surface tensiometry and fluorescent probe techniques. Some other methods include conductivity, solubilization experiments, osmotometry, differential scanning calorimetry, chromatography, small angle neutron scattering (SANS), small angle X-ray scattering, and nuclear magnetic resonance (NMR). Recently, a method has been developed to determine the CMC of polydisperse block copolymer micelles of low CMC by static light scattering Citation[4,154]. Few of these methods are discussed.
Surface tensiometry
In surface tensiometry, surface tension of aqueous solutions is measured over a wide range of concentration. The method detects completion of the Gibb’s monolayer at the air/water interface, and is a secondary indicator of the onset of micellization. CMC is located as the point at which the surface tension becomes essentially independent of concentration. Hence, CMC can be given as the value at which the surface tension stops decreasing and reaches a plateau when a graph of surface tension is plotted as a function of the logarithm of concentration. The surface tension measurements are very sensitive to the presence of hydrophobic impurities, and only an impurity level of the order of 0.1% of the surfactant may well cause a drastic deviation from the normally obtained curve. In the case of polydisperse block copolymers, more difficulty arises in the determination of an effective CMC value as the polydisperse copolymers show a more gradual decrease in the surface tension when plotted against concentration.
El-Ghazawy et al. Citation[155] used drop volume tensiometry to measure the surface tension of aliphatic polyester surfactants. The surface tension isotherms were then used to determine CMC of these surfactants.
Fluorescence probe
The hydrophobic fluorescence probes that are sensitive to changes in the vicinal polarity are used to determine CMC. Amongst various fluorescent probes used, pyrene (a highly hydrophobic condensed aromatic hydrocarbon) is the most widely used molecule. Pyrene is sensitive to the polarity of the surrounding environment and its partitioning into the hydrophobic core is observed upon steadily increasing the polymer concentration from extremely low to high Citation[156]. The fluorescence spectrum of pyrene at the low concentration possesses a vibrational band structure that exhibits a strong sensitivity to the polarity of the pyrene environment Citation[156,157]. As pyrene partitions preferentially toward the hydrophobic core it experiences a nonpolar environment and results in an increase in the fluorescence intensity, a red shift in the excitation spectra and a change in the vibrational fine structure of the emission spectra. Based upon these changes, pyrene was used to measure the CAC for poly(styrene sulfonate)/dodecyltrimethylammonium bromide through quenching, vibrational fine structure shifts (I1/I3), and time-dependent fluorescence Citation[158,159]. For CMC determination, the I1/I3 ratio (the intensity ratio between the first and third highest energy emission peaks, which is measured at a constant excitation wavelength and variable emission wavelengths corresponding to I1 and I3) can be used. A drastic change in the slope of the plot of the fluorescence of pyrene, the I1/I3 ratio from emission spectra against concentration signifies the onset of micellization Citation[160]. Colombani et al. characterized poly(n-buyl acrylate)-b-poly(acrylic acid) diblock copolymer micelles in aqueous solution using fluorescence correlation spectroscopy (FCS) and pyrene fluorescence spectroscopy Citation[161]. FCS and steady-state pyrene fluorescence spectroscopy revealed a very low apparent CMC (nearly 10−8 mol/L), in the absence and presence of added salt at high pH.
During the determination, concentration of pyrene used should be very low (10−7 M) so that a change in slope can be precisely detected Citation[162]. Changes in anisotropy of fluorescent probes have also been associated with the onset of micellization Citation[163,164]. Pyrene has been used by many research groups for CMC determination of the polymeric micelles; few examples include micelles formed from methoxy PEG-PCL, Citation[165] β-Cyclodextrin-poly(γ-benzyl L-glutamate), Citation[166] β-cyclodextrin-poly(L-leucine), Citation[167] PEG-b-poly(2-hydroxyethyl methacrylate-g-PCL), Citation[168] PEG-b-PCL, Citation[169], and Tetronic-PCL-heparin Citation[170].
Other fluorescent probes used to determine CMC are naphthalene, Citation[171] phenanthrene, Citation[172] 9-chloromethyl anthracene, Citation[173] Nile red, Citation[174], and 1,6-diphenyl-1,3,5-hexatriene Citation[175].
Light scattering
Light scattering (static or dynamic) can be used to detect the start of micellization only if the CMC falls within the sensitivity of the scattering method which is unusual for aqueous polymeric solutions Citation[176]. In static light scattering, for a series of samples of varying concentrations, the scattering intensity at different scattering angles is collected. Under favorable circumstances, the concentration dependence of excess light scattering intensity (excess over copolymer solution) can be used to determine the CMC. The results are recapitulated in a so-called Zimm-plot and from reciprocal of the scattering intensity extrapolated to zero concentration, the angular dependence of scattering intensity, and concentration dependence of scattering intensity information about the molecular weight, size, and intermolecular interactions (via a second virial coefficient B), respectively, is extracted. For a given system, static and dynamic light scattering (DLS) experiments can be combined together which reveals information about the micellar size, shape, and aggregation number, N agg. The surfactant theory in small molecules can be used to relate N agg to the concentration from:
where C is the surfactant/copolymer concentration Citation[177]. This equation is valid for simple micelle morphologies like spheres, rods, and vesicles.
Chen et al. Citation[178] studied the effect of the architecture of graft copolymers on CAC and determined CAC of the copolymers by light intensity measurement. CAC of the copolymer was defined as the concentration at which the light intensity abruptly increased.
Pyrene method or light scattering may yield different results for the CMC of drug loaded micelles owing to the formation of a secondary micelle structures during drug loading (association of few primary micelles). Usually, only primary micelles are formed as the polymer concentration is gradually increased from low to high concentration in the pyrene method. Conversely, secondary micelle structures can be formed during drug loading by dialysis methods. Due to additional interactions between polymers, the CMC of these secondary micelles may be lower than the values obtained from the pyrene method.
Conductivity measurement
Poly(aspartic acid)-g-octadecylamine-g-polyethylene glycol (PASP-g-OD-g-PEG) solutions with an increasing concentration were prepared and used to measure the conductivity Citation[129]. In deionized water, the copolymer possessed a negative charge as a result of partial ionization of the carboxylic acid groups of PASP. Hence, a correlation between the conductivity of the PASP-g-OD-g-PEG solution with the concentration of the solution was observed. At a specific copolymer concentration, a change in slope was detected in the conductivity curves. The conductivity value increased with the increase in polymer concentration on the whole; however, it increased more gradually when the concentration was above the specific concentration. The copolymer concentration corresponding to the turning point in plots was noted as CMC.
Small angle neutron scattering (SANS)
The polymer volume fractions in the core and the corona can be calculated using SANS and the core-corona form factor can then be used to extract information about the micelle size and micelle aggregation number Citation[179,180]. Ramzi et al. used neutron experiments to derive information on change in the size of the core and the micelle’s aggregation number as a function of time Citation[181].
Gel permeation chromatography (GPC)
If the polymeric micelles are sufficiently stable to travel through the size exclusion column during their elution, then GPC can be employed for CMC determination of such systems. As single polymer chains and micellar copolymer chain fractions produce distinct elution volumes in aqueous milieu, GPC can be suitably used for measurements of CMC alongwith molecular weight and aggregation number of the micellar system Citation[182]. Yang and others studied the aggregation behavior of the PLA/PEG diblock copolymers in aqueous medium with aqueous GPC Citation[183].
Size, shape, and polydispersity index (PDI) determination
Size of polymeric micelles falls in the colloidal range. DLS/photon correlation spectroscopy affords characterization of micellar size (hydrodynamic diameters) and PDI Citation[184]. DLS offers the R h from the mode corresponding to micellar diffusion obtained from the intensity distribution of relaxation times and the time dependence of the light intensity fluctuations is analyzed in order to yield information about the diffusion coefficient. The R h can then be calculated using the diffusion coefficient from the Stokes-Einstein equation. By DLS, change in the size of micelles can also be determined. It was shown that the addition of a low molecular weight surfactant such as sodium dodecyl sulfate (1% w/v) can destroy the polymeric micelle structure and bring about a complete shift of the mean diameter from approximately 50–3 nm Citation[185].
Static light scattering also provides the data on association number of the micelles Citation[186]. Static light scattering experiments provide information for the thermodynamic radius of micelles.
PDI indicates the degree of the dispersity of the prepared polymer micelles Citation[187]. PDI of micelles is obtained by examining the micellar solution with quasi-elastic light scattering technique. Monodisperse micelles produce blue color from light scattering which indicates good micellar preparation, as contrasted with the white color shown by aggregates Citation[188]. PDI of the micelles is also obtained from DLS measurements. Accordingly, PDI of the disulfide-linked dextran-b-PCL diblock copolymer micelles was found to be 0.1–0.2, signifying almost monodisperse micelle preparation, when determined by DLS by Zhong and his coworkers Citation[189].
Other methods that are also frequently employed for determination of micellar size and shape include scanning electron microscopy (SEM) Citation[190], transmission electron microscopy (TEM) Citation[191,192], and atomic force microscopy (AFM) Citation[193]. Although SEM enjoys high resolution, its use is hindered by the fact that the sample should be able to withstand high vacuum and be conductive (which is done by coating gold on their surface). However, aqueous samples cannot withstand the high vacuum of an electron microscope and water loss occurs leading to microstructure changes. Therefore, sample preparation necessitates some special treatment before it is subjected to electron microscopy examination. Freeze fracture has shown promise to overcome these problems Citation[194]. AFM permits the visualization of polymeric micelles at atmospheric pressure without gold coating and hence overcomes the limitations of SEM Citation[195]. SEM or AFM reveal information regarding size distribution when micelles attached chemically to surfaces are presented. Direct visualization of block copolymer micelles either in the dried state or directly in situ within a liquid cell can be achieved by AFM. The microstructure of colloidal systems can be visualized with the high-magnification power of the electron microscope.
More recently developed cryo-TEM technique has increasingly started gaining importance for characterization of shape of polymeric micelles in aqueous medium. Cryo-TEM imaging of poly(n-butyl acrylate)-b-poly(acrylic acid) at different salt concentrations revealed spherical shape of these micelles Citation[70]. Size characterization of drug-loaded polymeric micelles was done using asymmetrical flow field-flow fractionation and the structure of assemblies was determined by SANS Citation[196].
Microviscosity of the micellar core
The micellar core viscosity can affect the micellar physical stability and drug release from the micelles. Fluorescence probe techniques have played a crucial role in obtaining microstructural information in micelles Citation[197]. Microviscosity (intrinsic viscosity or microfluidity) defines the viscosity of the probe environment in the interior of aggregate and is different from that of the bulk solvent medium. Microviscosity of the hydrophobic core can be determined by using fluorescent probes such as dipyme (1,1′-dipyrenyl methyl ether), DPH (1,6-diphenyl-1,3,5-hexatriene), and others. For example, the intramolecular excimer formation of dipyme is an attractive tool for studying hydrophobic microenvironments and the extent of excimer emission is dependent on the local friction imposed by the environment. As a result, measurement of the monomer to excimer intensity ratio, I M/I E provides information about the microviscosity experienced by the probe. Dipyme is sensitive to both polarity and viscosity changes in its local environment Citation[198]. The micellar microviscosity afforded by Pluronic and Tetronic PEO-PPO block copolymer aqueous solutions has been investigated by fluorescence spectroscopy by Nivaggioli et al. Citation[199].
1H NMR also provides information on the viscosity of the micellar core. The copolymers are usually dissolved in D2O and in a solvent where micelle formation is not expected, and where all the peaks proper to the hydrophilic and hydrophobic part of the polymer can be detected (e.g. CDCl3). In D2O, the presence of micelles with highly inner viscous state results in a restricted motion of the protons within the micellar core as demonstrated by the weak signals associated with the hydrophobic part of the copolymer Citation[200]. Highly viscous states were found to exist in PEO-poly(DL-lactic acid) Citation[163] and PEO-poly(β-benzyl L-aspartate) micelles Citation[134].
DSC experiments showed that methoxy PEG-poly(hexyl-substituted lactides) (mPEG-PHLA) block copolymer presents a bulk microstructure containing mPEG domains segregated from the PHLA domains Citation[174].
Stability of polymeric micelles
The stability of polymeric micelles can be defined in terms of thermodynamic and kinetic stability. Polymeric micelles are said to be thermodynamically stable when the polymer concentration in water is above their CMC.
The exchange rate of single polymer chain between the micelles and bulk determines the kinetic stability of a micellar system Citation[116,201]. In vitro and in vivo stability of polymeric micelles depends on the CMC values of micelle forming polymers, the strength of van der Waals interactions between hydrophobic blocks forming the core, and the molecular size of the hydrophilic block of the polymer Citation[202].
Upon intravenous injection, polymeric micelles are subject to extreme dilution in the circulation and hence when these are to be used as drug delivery vehicles, it is important to know the CMC Citation[203]. The CMC value should be sufficiently low so that the polymeric micellar drug carriers remain stable during circulation in the bloodstream Citation[204]. This is important as the micellar drug carrier will get sufficient time for drug delivery and accumulate at the target site Citation[136]. Kinetic stability is also important as it reflects the rate at which a physically entrapped drug is released from the micellar carrier. Polymeric micelles from some block copolymers are said to be kinetically stable apparently because of the presence of multiple sites capable of hydrophobic interaction within each polymer molecule Citation[182]. However, it has been observed that most other polymeric micelles are often destabilized in the presence of blood components leading to premature drug release Citation[205]. The micellar disassembly is governed by the magnitude of the interactions in the micellar core which are dependent on the crystalline or amorphous state of the core-forming polymer, solvent in the micellar core, hydrophilic and hydrophobic balance of the copolymer, and presence of loaded hydrophobic compound Citation[113,201]. Therefore, to improve the thermodynamic and the kinetic stability of drug-loaded micelles several strategies have emerged that include enhanced compatibility of the drug and polymer Citation[74,206], cross-linking of the micelle core/corona Citation[207,208], preparation of stereocomplex micelles Citation[209,210], and reduction in CMC by altering the polymer Citation[211,212].
Improved stability of polymeric micelles has been reported after the introduction of aromatic groups that helps lowering the CMC and also strengthens the interactions inside the micellar core through π–π-stacking. Hennink et al. recently were able to show the effect of the presence of an aromatic end group on the micelle stability Citation[206]. They loaded docetaxel and paclitaxel into oligomeric micelles composed of methoxyPEG750-b-oligo(ϵ-caprolactone)5 having a hydroxyl, benzoyl, or naphthoyl end group. Both taxanes contain several aromatic rings, which may form π–π interactions with the aromatic rings in the micellar core. It was concluded that the presence of an aromatic end group on the core forming block is necessary to improve the loading and enhance the stability of the formulation of taxanes, indicating the importance of a good compatibility between the loaded drug and the micellar core.
The importance of core cross-linking of the polymeric micelles to enhance stability has been demonstrated by Bronich et al. wherein they prepared polymeric micelles with cross-linked ionic cores by using block ionomer complexes of PEO-b-PMA and divalent metal cations Citation[213]. The drug-loaded micelles were stable in aqueous dispersions exhibiting no aggregation or precipitation for a prolonged period of time.
The dissociation of self-assembled polymeric micelles into unimers below the CMC in body fluids accelerates the release of any incorporated drug, which results in drug loss at unwanted tissues or organs Citation[203]. To tackle this problem, cross-linking is a powerful approach to stabilize micelles because such structures can hold self-assembled polymeric micelles Citation[214,215]. Generally, two methods of cross-linking have been used: one is cross-linking of the micellar shells and the other is preparation of core cross-linked micelles. The shell cross-linked micelles lead to formation of stable nanosized hollow particles by removal of the cores after cross-linking and then hydrophilic drugs can be encapsulated inside Citation[216,217]. Hence, reversibly shell cross-linked micelles based on triblock copolymer composed of poly(aliphatic ester), polyphosphoester, and PEG demonstrating improved physical stability were reported Citation[218].
The core cross-linking can increase the stability of the micellar structures without affecting the drug loading capacity, leading to the sequential control of the hydrophobic drug release. Henselwood and Liu prepared poly(2-cinnamoylethyl methacrylate)-b-poly(acrylic acid) micelles with the cinnamoyl moieties cross-linked by UV irradiation Citation[219]. The introduction of thiol groups into the core blocks as effective means for preparing the core cross-linked micelles using PEG-b-poly(L-lysine) was demonstrated by Kataoka and Harada Citation[220].
The stereocomplexes are characterized by higher physical and chemical stabilities Citation[221,222]. It has been reported that the kinetic stability of polymeric micelles greatly improved through the formation of stereocomplex cores. On this basis, monodisperse stereocomplex PLA-PEG micelles through the self-assembly of equimolar mixtures of the block copolymer in water were prepared Citation[223]. It was demonstrated that in an aqueous environment, the core of PLA based polymeric micelles can crystallize in a stereocomplex configuration and that these polymeric micelles exhibited enhanced kinetic stability. Chen et al. synthesized the block copolymers of enantiomeric poly(L-lactide)-PEG and poly(D-lactide)-poly(ethylene–glycol) and prepared a series of stereocomplex micelles of enantiomeric PLA-PEG copolymers loaded rifampin and having different length of PLA chains Citation[210]. The release rate of rifampin decreased as contents of PLA segment were increased due to higher stereocomplex crystallization and better stability of the polymer micelles.
Reduction in CMC has been correlated to improved stability of polymeric micelles. The reduction in CMC can be achieved by either of the two approaches: adjusting the sizes of the blocks such that the polymer becomes more hydrophobic or adjusting the nature of the hydrophobic block. Higher hydrophobicity can be achieved with introduction of a larger hydrophobic block or a smaller hydrophilic block, thereby resulting in a reduced CMC value Citation[224]. Alteration in the nature of the hydrophobic block is possible by means of chemical modification of the hydrophobic block, e.g. by introducing aromatic groups on the block Citation[225].
Reulen and Merkx have shown the potential of using Förster resonance energy transfer imaging technique in assessing stability of micelles Citation[226]. In their study, Förster resonance energy transfer between the fluorescent proteins ECFP and EYFP was used to investigate the lipid exchange behavior of protein-functionalized micelles of cysteine-functionalized PEG2000-distearoylphosphatidyl-ethanolamine. It was concluded that functionalization of PEGylated phospholipids with donor and acceptor fluorescent domains provides a straightforward approach to study the stability of protein-functionalized micelles with respect to protein-lipid transfer.
Applications of polymeric micelles
Solubilization of drug molecules
The poorly water-soluble drugs or contrast agents may be entrapped within the hydrophobic core or linked covalently to the surface of polymeric micelles to improve their aqueous solubility. Solubilization is controlled by characteristics of the drug as well as those of the micellar systems. The molecular weight and partition coefficient of the drug are important parameters, while hydrophobic block length of the micelle is also equally important. Sometimes, steric hindrance and interaction of drug and polymer may lead to an unfavorable aggregation process. Thus, selection of an amphiphilic polymer for solubilization of drug is a critical issue and requires an indepth understanding for the selectivity of micellar systems which is achieved by studying various types of intermolecular interactions for solubilization of drug in a given micellar system Citation[227–229]. In pharmaceutical industry, micellar solubilization finds important application for enhancement of solubility and bioavailability of drugs. It is noted that nearly half of the approved active pharmaceutical ingredients are poorly water-soluble and show very low bioavailability. Polymeric micellar solubilization may realize their usage Citation[230,231] (Table ).
Table 4. Examples of improvement in solubility of drugs using polymeric micellar system.
Solubilization of drug in polymeric micelles is expressed by the partitioning of the drug described as the ratio of molar drug concentration in the micelle to the molar concentration of drug in the aqueous phase. The extent of solubilization depends upon the micellization process, the compatibility between the drug and the core-forming block, chain length of the hydrophobic block, concentration of polymer, and temperature Citation[248]. It is observed that amphiphilic polymers can solubilize drugs even when micelles are not formed. Above CMC, there is a sharp increase in the solubility of drug as it gets more space to occupy in the aggregates of the hydrophobic part of micelle. The occupancy of core region by drug leads to an increased Rc of the micelle. It is worth mentioning that the core region has limited capacity for accommodation, for instance, Pluronic P85 has a core region which is 13% of the whole micelle weight Citation[249]. An increase in solubility is usually observed when there is a high degree of compatibility between the drug and the core-forming block of the micelles Citation[250]. The influence on solubilization capacity of hydrophobic block length has been examined for griseofulvin in polyoxyethylene and polyoxybutylene copolymer micelles with varying number of hydrophobic block lengths and hydrophilic block lengths sufficient for formation of spherical micelles. It was found that the solubilization capacity was dependent on the hydrophobic block length upto a particular extent (15 units of hydrophobic block), after which the solubilization capacity became independent of the same Citation[251]. Zhang et al. showed that the chitosan derivatives of high methylation degree, medium-sized long-chain acyl group (C14), and large molecular weight had the best effect in loading CyA Citation[252]. The effect of hydrophobic block length of diblock and triblock polyurethane surfactants on solubilization of toluene has been reported by Dong and coworkers Citation[253].
A temperature dependent transition in micellar shape has been quoted in literature. In dilute aqueous solutions, compact micelles turned to wormlike micelles with an increase in temperature from 25 to 40 °C for polyoxyethylene-b-polyoxybutylene copolymers. This shape transition phenomenon was attributed to the increase in number of unimers per micelle. An investigation about the effect of micelle shape (spherical and wormlike) on the aqueous solubility of three drugs: griseofulvin, spironolactone, and carbamazepine was conducted by Attwood and his research team. Griseofulvin and spironolactone were solubilized to a greater extent than the more water-soluble drug, carbamazepine. The change in shape was responsible for greater solubilization of the drugs Citation[254]. Contrarily, the process of solubilization can also influence micelle geometry. This feature has been elaborately discussed by Nagarajan in his review Citation[255]. Thus, designing block copolymers with proper composition and structure to form micelles with high solubilization capacity for poorly water-soluble drugs can lead to the formulation of efficient drug delivery systems. The potential of polymeric micelles as promising drug delivery systems for overcoming the problems of poor water solubility and poor bioavailabilty becomes quite evident.
Polymeric micelles may act as a carrier for transporting poorly water-soluble compounds across the intestinal mucosa by endocytosis. In general, cells take up materials such as micelles by folding the cell membrane inwardly, surrounding the materials to be ingested. The material is then engulfed in small bubble-like endocytic vesicles. This is called the endocytosis that allows supramolecular assemblies to sneak into intracellular regions avoiding the cell-membrane transporters. Another mechanism involves release of hydrophobic compound by the action of lipases on the polymeric micelles. The released compound is then transported across the mucosal barrier, with increased permeability, using the normal physiological constituents Citation[256–258].
A reverse transporter associated with P-glycoprotein may inhibit absorption of drugs by actively pumping drug out of gut wall cells back into the intestinal lumen. Inhibition of P-glycoprotein and of gut wall metabolism may lead to enhanced drug absorption. Kabanov et al. showed the effectiveness of Pluronic block copolymers as polymeric inhibitors of P-glycoprotein that sensitized multidrug resistant tumors to doxorubicin, paclitaxel, and vinblastine, and thereby led to efficient uptake of these drugs Citation[259]. The intestinal absorption of CyA was significantly improved by monomethoxy PEG-poly(lactide) micelles and was found comparable to that of Sandimmun Neoral® when a comparative study was conducted by Liu and his coworkers Citation[260]. A 15–250-fold higher uptake efficiency of particles ∼100 nm in diameter by the gastrointestinal tract was noted than that of the micrometer-sized particles Citation[132]. PEG-b-poly(alkyl acrylate-co-methacrylic acid) micelles entrapping fenofibrate exhibited enhanced oral bioavailability as compared to fenofibrate suspension Citation[261]. Han et al. studied the pharmacokinetics and biodistribution of Pluronic P123 micelles loaded with paclitaxel Citation[262]. The effective solubilization of paclitaxel by the micellar core resulted in an increased bioavailability of the drug. Our group has recently demonstrated the applicability of Pluronic L81 and P123 mixed micelles for effective solubilization of aceclofenac Citation[232]. The resultant mixed micelles bestowed very small sizes (around 20 nm) and high solubilization potential (about 4.7 mg/mL) making them potential candidates for passive targeting of drugs.
Polymeric micelles provide safer alternatives for parenteral administration of poorly water-soluble drugs. Aliabadi et al. evaluated PEO-b-PCL micelles to reduce the renal uptake and nephrotoxicity of CyA Citation[263]. Compared to Sandimmune® (Cremophor EL based commercial formulation), polymeric micelles reduced kidney uptake of CyA by 2.6-fold and increased CyA levels in blood by 2.1-fold Citation[264]. Hashida et al. demonstrated that all-trans retinoic acid (ATRA) incorporated in PEG-b-poly(aspartic acid) micelles showed the largest blood concentration when compared with inherent ATRA or ATRA in liposomes Citation[265,266]. Almost 2 mg/mL of paclitaxel was loaded in poly(N-(2-hydroxypropyl) methacrylamide lactate)-b-PEG micelles Citation[267]. The large solubilization capacity of micelles for paclitaxel, simple preparation method, size of around 60 nm, and size stability demonstrated make these micelles an attractive vehicle for parenteral paclitaxel delivery.
Recent developments in drug delivery reveal the applicability of polymeric micelles for water-soluble drugs as well. Trilayered polymeric micelles from PEG-b-PLA copolymer served as containers for hydrophilic compounds and were incorporated into hydrogels as cross-linking agents. Resulting hydrogel could be used for releasing hydrophilic compounds in a sustained manner owing to degradation of copolymer and collapse of the micelle Citation[268,269].
Targeting: general considerations and approaches
The site of action of the drug is mostly at distant locations from the site of administration. Drug has to take a complicated path to reach at the desired site, during which it might be destroyed or distributed to many unwanted tissues. This usually is associated with increased side-effects on the body. Also it results in subtherapeutic concentration of drug at target organs. Thus, drug targeting is a major issue for avoiding all the related problems.
Factors important for targeting related to polymeric micelles
Some of the factors of polymeric micelles that govern drug targeting include size, chain length, drug content, stability and degradation of micelles in aqueous solutions, and hydrophobic inner core.
Owing to their characteristic size, the polymeric micelles may represent suitable targeting vehicles utilizing EPR effect through passive targeting (discussed later) Citation[270]. Their size usually is small enough to extravasate the small gaps in endothelial lining of blood vessels. Usually, particles larger than 200 nm in diameter are hardly able to traverse the narrow gaps in the leaky vasculature. Also particles larger than 200 nm are immediately recognized as foreign products in blood and results in instant removal through the RES Citation[271]. Therefore, the particles should be small enough (<200 nm) to avoid such exclusion Citation[272]. Thus, size becomes an important parameter that needs consideration for targeting. Fenretinide, an anticancer agent, was encapsulated in PEG-poly(benzyl aspartate) block copolymer micelles by Hashida and his coworkers Citation[273]. They observed the mean particle size of drug encapsulated polymeric micelles to be around 173 nm. These micelles were able to accumulate in tumor and inhibit the tumor growth, providing promising and effective carrier of fenretinide for targeted cancer chemotherapy.
The chain length of each block is an important factor that determines micelle-forming characters such as the aggregation behavior of the polymer Citation[274]. Opanasopit et al. showed the importance of block chain length in their work, wherein PEG-poly(β-benzyl L-aspartate) block copolymer micelles were used for loading of camptothecin Citation[275]. They observed the chain lengths to influence the incorporation efficiency and stability of polymeric micelles. Several block copolymers were synthesized with variation in PEG and aspartic acid units and by esterification of these polymers with different groups as benzyl, lauryl, and n-butyl. In chain length variations, 5–27 Bz-69 coded polymer showed a higher camptothecin incorporation than 12–50 Bz-63, 12–26 Bz-64, and 5–52 Bz-67. (The block copolymers were coded by chain lengths of the PEG and polyaspartate, the name of the hydrophobic group, and degree of esterification. 5–27 Bz-69 implies a block copolymer composed of the PEG block of molecular weight of 5000, the polyaspartate block possessing 27 units of aspartic acid, and 69% of the aspartic acid residue that was esterified to the benzyl aspartate residue.) Among the higher camptothecin incorporations, 5–27 Bz-69 showed higher micelle stability than 12–50 Bz-63 and 12–26 Bz-64. This suggested that the balance between the hydrophobic and hydrophilic chains affected the stability of micelles.
The effect of drug content influencing the targeting efficacy has been demonstrated by Kataoka et al. for the adriamycin-incorporated polymeric micelles. The in vivo antitumor activities of two polymeric micelle samples composed of identical chain lengths of both the PEG and the poly(aspartic acid) chains, but different drug contents were compared Citation[113]. Adriamycin incorporated in the inner core (both by chemical conjugation and physical entrapment) was quantitatively measured using a synthetic method, and effects of the adriamycin contents on micelle stability and in vivo antitumor activity were analyzed. They found the physically entrapped adriamycin to be responsible for in vivo antitumor activity.
Kwon and Vakil have shown the effect of micellar stability for efficient targeting through the preparation of mixed polymeric micelles formed from PEG-b-PCL and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-methoxy PEG. They proposed the micelles to possess high thermodynamic stability and suitable as long circulating carriers in the context of antineoplastic and antibiotic drug delivery Citation[276]. The hydrolytic degradation of poly(lactic acid)-Pluronic F127-poly(lactic acid) nanoparticles has been reported by Xiong et al. Citation[277,278]. They noted that the hydrolytic degradation of the amphiphilic block copolymers affected the sizes and morphologies of these nanoparticles.
The extent of hydrophobic interactions of the core region of the polymeric micelles with the drug determines the strength with which the micelle system holds the drug within its core.
Different approaches toward targeting
Drug targeting can be classified as active targeting and passive targeting Citation[279]. Targeting via polymeric micelles is usually achieved by one of the following approaches; the EPR effect, stimuli-sensitivity, or by complexing specific targeting ligand molecules to the micelle surface Citation[36].
EPR effect
The greatest difficulty in effective treatment against cancer is the nonselective distribution of drug to healthy cells. Blood vessels of most of the normal tissues have an intact endothelial layer which restricts macromolecules or other nanoparticles from the tissue and only allows the diffusion of small molecules. Contrarily, because of the fast growth of tumor tissue, the endothelial layer of blood vessels in tumor is often porous, and both small and large molecules have access to the malignant tissue. Owing to their nanoscopic size, polymeric micelles passively accumulate at the interstitial spaces of various pathological sites by extravasating leaky capillaries (especially of solid tumors). They also have been shown to distribute to some of the cytoplasmic organelles and infarct tissues, infected areas, inflammatory sites that have compromised barrier function Citation[30,280]. This in turn helps in reducing the volume of distribution of the drug as the polymeric micellar drug carriers cannot pass through walls of normal blood vessels, thereby resulting in decreased side-effects of the drug. For effective therapy, drug carriers must be able to avoid uptake by the fixed macrophages. Polymeric micelles have the ability to escape uptake by the fixed macrophages of liver and spleen, i.e. by the mononuclear phagocyte system. In tumor neovasculature, there is a poorly developed lymphatic drainage system that leads to enhanced retention of polymeric micelles within the solid tumor as micelles are not efficiently cleared. This feature allows prolonged circulation of polymeric micelles in the circulatory system upon administration Citation[281]. Owing to these characteristics, it is possible to achieve passive drug targeting using polymeric micelles.
The hyperpermeability of tumors associated with the EPR effect is based on excessive production and secretion of vascular permeability factors stimulating extravasation within cancerous tissue. Commonly secreted chemicals include vascular endothelial growth factor bradykinins, nitric oxide, prostaglandins, enzyme collagenase, and peroxynitrite Citation[282,283].
EPR effect depends upon the pore cut-off size of blood vessels. It has been demonstrated that the pore cut-off size for different tumors is different. This leads to variable permeability through different tumors. The small size of polymeric micelles allows easy penetrability even through very small cut-off sizes of the tumors which could be of the order of even lesser than 200 nm Citation[139]. Efficacy of micelle accumulation depends on tumor type (cut-off size of tumor vasculature) and can be controlled by varying the molecular size of PEG blocks in PEG-PE conjugates Citation[270].
EPR effect is also associated with the concentration of drug in plasma and the molecular weight of polymers or drug-copolymer conjugates. The plasma drug concentration must always remain higher for effective therapy. It is well-established that the EPR effect is effective only with macromolecules which can avoid the renal clearance (generally larger than 40 kDa). It has been shown that there is an increase in EPR with an increase in molecular weight above some critical size, because it is usually hard to maintain the drug concentration in the tumor greater than the plasma drug concentration for long periods for low-molecular-weight drugs Citation[284].
Vetvicka and his associates formulated a micellar drug delivery system designed to prolong the blood circulation time and maximize the efficiency of the EPR effect. They prepared doxorubicin conjugated PEO-b-poly(allyl glycidyl ether) micellar system that circulated for long time and released doxorubicin efficiently at the tumor site because of the acidic pH prevailing at the tumor site. This also led to destabilization and disruption of the micellar system generating free diblock unimers that could be excreted Citation[285]. Watanabe et al. developed polymeric micelles composed of various PEG-poly(aspartate ester) block copolymers incorporating camptothecin. The stability of the formulation was found to strongly depend on the amount of benzyl esters and length of the PEG. The drug-loaded micelles delivered the drug to tumor sites owing to the EPR effect Citation[225]. The EPR effect has been exploited to advantage by other research groups also Citation[125,270].
Stimuli-sensitivity
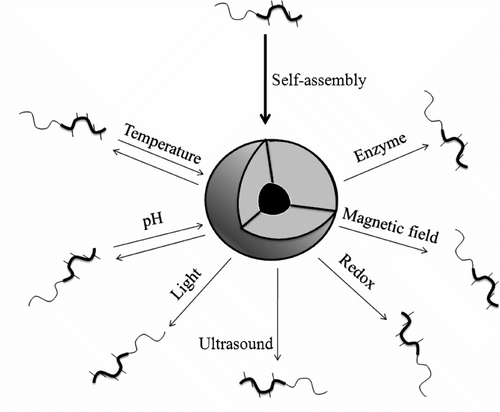
For ideal drug targeting, there should not be any drug release from the micelle during circulation. Only after the polymeric micelles accumulate at the targeted tissue, the drug should be released by means of some internal environmental trigger such as pH, particular enzyme, etc. or by an external trigger including temperature, light, or ultrasound (Figure ).
Depending on the stimulus applied varied responses may be observed including disruption of the structure, changes in shape, volume, permeation rates, hydration state, swelling/collapsing, hydrophilic/hydrophobic surface, or conformational changes. Destabilization of micelles as a result of stimulation by either physiological or external trigger is termed as ‘stimuli-sensitivity’ or ‘environmental sensitivity’ of the micelles Citation[286]. Release of drug from the micellar system is dependent on the exploitation of differences that exist in normal tissues and pathological tissues. Such a release mechanism from polymeric micelles is also termed as ‘ON-OFF release,’ ‘intelligent delivery,’ or ‘smart delivery’ by other researchers.
The origin of various stimuli for destabilization of micelles can be explained taking into consideration the pathophysiological changes that occur in a diseased state of body. Various stimuli-sensitive polymeric micellar systems used for targeting are discussed hereunder.
pH-sensitive polymeric micelles
There are a number of pH gradients that exist in normal and pathophysiological states inside the body. pH-sensitive polymeric micelles exploit these differences in pH for targeting. In tumors and inflammatory tissues a mildly acidic pH is encountered (pH around 6.8). This is a slightly low value as compared with the pH of blood and normal tissues (pH around 7.4). Micelles can also be taken up into the cell by the process of endocytosis and may as well enter cell organelles as endosomes, lysosomes, etc. The pH value inside these organelles is nearly 5.5 Citation[91].
The decreased pH in pathological areas such as tumors, infarcts, and inflammatory sites has been related to hypoxia, hydrolysis of ATP in hypoxic conditions and massive cell death, and decreased pH in endosomes and lysosomes to the change in proton concentration along with the presence of enzymes. Most of the tumors are poorly perfused and hence an altered metabolic pathway with subsequent glycolysis is being followed, leading to elevation in the levels of lactic acid within the interstitia and in effect lowering the pH value at that particular site Citation[287–289]. This served as the basis for the development of pH-sensitive polymeric micelles. For example, negatively charged oligo/poly(nucleic acids) can be delivered intracellularly by complexing them with cationic polymers. Once into endosomes, these are deprotonated causing disruption of endosomal membrane and releasing nucleic acids in the cytosol Citation[290].
In experimental animals and humans, pH 7.0–6.8 is the natural pH range used for targeting the solid tumors. Upon oral or intravenous administration of glucose, the extracellular tumor pH may reduce by 0.2–0.4 pH units. If required, sometimes, glucose can be administered for lowering pH for the treatment of patients Citation[291].
Two main approaches that have been used for developing pH-sensitive systems are: involvement of a titrable group into the copolymer and inclusion of labile linkages that are destabilized in acidic conditions. Incorporation of titrable groups such as amines and carboxylic acids into the backbone of the copolymer leads to an alteration of the solubility of the polymer upon protonation. This in effect may disrupt the micellar structure. Acid degradable linkages can be constructed using linkers such as hydrazone, acetal, imine, etc. that are cleaved at a pH nearly 5.5. Inclusion of acid-labile linkages, such as benzoic imine linkage, in polymeric structures has shown to cause change in micellar integrity or complete destruction of the micellar structure when these polymers encounter low-pH environment. However, the imine linkage is unstable at physiological conditions and is less frequently used in designing of drug delivery systems. To overcome the problem associated with imine linker it can be conjugated to other group. PEGylation via the benzoic imine linker has been successfully used for constructing self-assembling stealth amphiphilic polycation synthesized from poly-L-lysine-grafted-cholic acid. The benzoic imine group provided a physiologically stable pH responsive polymer that responded at very slight acidic condition, i.e. pH about 6.8 Citation[286,292].
Systems respond to pH changes if they have ionisable blocks with a pKa value between 3 and 10 in their backbone. Block copolymers having basic groups such as L-histidine, pyridine, tertiary amine, and acidic groups such as carboxylic acids and phosporic acids are pH sensitive Citation[290]. Many researchers are further trying to explore tumor-targetability via use of pH-responsive polymeric micelles. Some significant examples are shown in Table .
Table 5. Acid-sensitive drug-loaded polymeric micellar systems.
Thermosensitive polymeric micelles
Most of the primary tumors can directly be excised using heat such that the cancer cells are killed by the high temperatures (>43 °C) of sufficient time duration Citation[299]. Alternately, such tumors can indirectly be sensitized by mild hyperthermia Citation[300,301]. During the excision therapy, certain tissues experience only mild hyperthermia (40–43 °C), which is insufficient to cause tissue necrosis in that stipulated time interval. Thus, regions of tissue experiencing mild hyperthermia may be benefited from combined treatment with therapeutics that can respond to modest increases in temperature Citation[302]. Also, as most of the pathological areas (noticeably most of the tumors) demonstrate distinct hyperthermia the thought of developing temperature-sensitive micellar carriers for drug delivery has emerged as an interesting onset toward targeting.
Hyperthermia is thought to preferentially increase tumor blood flow and tumor microvascular permeability and thereby increase drug accumulation at the tumor tissue Citation[303]. Hyperthermia is associated with altered fluidity and stability of cellular membranes or inhibition of DNA-repair enzymes and thereby able to exert certain antitumor effects on tumors Citation[304]. When combined with chemotherapies, hyperthermia can synergetically kill malignant tumor cells Citation[305,306]. Temperature changes can be internal, e.g. hyperthermia during inflammation, or can be from an external source. Moreover, there exist various means to heat the required area in the body. Heat can be generated inside target tissues by locally applied ultrasound or by locally applied high frequency causing the oscillation of target-accumulated magneto-sensitive micelles.
The thermosensitivity of polymeric micelles is the phenomenon where the carrier undergoes a change in structure with an increase in temperature, leading to the deposition of the drug and easier drug absorption by cells. Temperature-sensitive polymeric micelles can be made by assembling them with amphiphilic copolymers, in which one of the blocks demonstrates thermosensitive properties. At a certain temperature, these polymers produce a volume phase transition associated with a sudden change in the solvation state. This transition temperature is termed as critical solution temperature. Polymers solubilized upon heating possess an upper critical solution temperature, and those which become insoluble possess lower critical solution temperature (LCST). With regard to the thermal targeting strategy, LCST is the most important physical parameter that governs the performance of a thermosensitive material for its application in drug delivery Citation[290]. Below the LCST, the polymer is well soluble in water due to extensive formation of hydrogen bonds between polymer and water molecules. However, the network of hydrogen bonds collapses to exclude water molecules from the polymer at a temperature above the LCST, eventually leading to aggregation and precipitation of the polymer Citation[307]. Exact properties of thermosensitive polymeric micelles can be adjusted by chemical modifications of both hydrophobic and hydrophilic blocks in such a way that the micelle can destabilize at temperatures above LCST and release the drug dissolved in its hydrophobic core The main mechanism of thermosensitive polymeric micelles is that during their circulation through the heated malignant tissues, where local temperature is above its LCST, the outer shell of the micelles transfers into a hydrophobic structure and is subsequently absorbed into cells mediated by hydrophobic interaction. Consequently, a high enough level of drug to kill the cancer cells can be achieved when the anticancer drug loaded inside the micelles accumulate at malignant tissues. Thermo-targeted polymeric micelles can be applied on a wide spectrum of tumors notably improving their clinical applications Citation[308]. One of the most widely studied polymer for thermoresponsiveness is poly(N-isopropylacrylamide) (PIPAAm). PIPAAm is well-known to exhibit a reversible phase transition across its LCST in aqueous medium. This polymer is water-soluble and hydrophilic, existing in an extended conformation, below its LCST but undergoes a phase transition to insoluble, hydrophobic aggregates above 32 °C Citation[309]. The thermoresponsive properties and structures of the polymeric micelles depend upon the molecular structure of a single modified PIPAAm chain that is the building block of the micellar assembly. The thermoresponsive character, especially the LCST, of polymeric micelles of the modified PIPAAm chains is not always consistent with that of PIPAAm. The LCST of PIPAAm is independent of the molecular weight and the concentration, but can be changed by shifting the hydrophilic/hydrophobic balance Citation[310].
On these backgrounds, many research groups have focused on the development of thermosensitve polymeric micelles. A block copolymer, poly(N-isopropylacrylamide-coacrylamide)-b-poly(D,L-lactide) with the LCST of 41 °C was synthesized and used as the carrier for delivery of docetaxel by Liu et al. Citation[311]. The polymer formed micelles and the hydrated outer shell prevented micelles from being aggregated and also enabled them to escape from nonselective scavenging by the RES to gain a longer plasma half-life at the physiological temperature. It was observed that hyperthermia greatly enhanced the targeting efficacy of drug-loaded micelles and also helped in reduction of toxicity of drug. They further compared the cytotoxicity of the docetaxel-loaded micelle with conventional docetaxel formulation as a control formulation in different cancer cell lines and the antitumor efficacy in Lewis lung carcinoma-bearing C57BL/6 mice Citation[312]. The results revealed reduced toxicity of docetaxel-loaded micelle and higher docetaxel concentration in tumor than that of the conventional docetaxel formulation. A significantly higher antitumor efficacy was observed in mice treated with docetaxel-loaded micelles accompanied by hyperthermia. Nakayama et al. demonstrated the effect of varying chain lengths and effects of terminal functional groups, especially surface polarity and hydrophobicity, on thermoresponses of the PIPAAm-b-poly(benzyl methacrylate) polymeric micelles Citation[67]. The micelle surface chemistry was found to significantly influence micellar thermoresponse, which depended on PIPAAm chain lengths. The LCST shifted drastically to lower temperatures with micelles having hydrophobic phenyl groups. The magnitude of these LCST shifts increased with decreasing molecular weight of the PIPAAm chains.
Santis et al. were the first to report the preparation of PEGylated and thermosensitive polyion complex micelles having a coacervate core formed by two strong oppositely and permanently charged polyelectrolyte block copolymers of poly(sodium 2-acrylamido-2-methylpropanesulfonate)-b-PIPAAm and poly[(3-acrylamidopropyl)-trimethylammonium chloride]-b-PEO under stoichiometric charge neutralization conditions and polyelectrolyte chain length matching Citation[313].
In general, incorporation of hydrophobic groups into PIPAAm chains decreases the LCST and hence, usually a slightly different value as compared with that of the PIPAAm is obtained Citation[314].
Besides PIPAAm, poly(N,N-diethylacrylamide) also possesses a LCST of about 32 °C and is considered as an alternative thermosensitive water-soluble polymer to replace PIPAAm due to its lower toxicity Citation[315]. Bian et al. synthesized poly(styrene-b-N,N-diethylacrylamide) with controlled molecular weight and narrow polydispersity Citation[316]. The aqueous micellar solution underwent a reversible aggregation transition at around the LCST of poly(N,N-diethylacrylamide) block chains comprising the outer shell.
Soga et al. studied thermosensitivity of poly(N-(2-hydroxypropyl) methacrylamide lactate)-b-PEG. Polymeric micelles were formed with approximately 50 nm diameter by heating aqueous polymer solution from below to above the CMT. With an increase in hydrophobic block length the CMC as well as the CMT decreased, which can be attributed to the greater hydrophobicity of the thermosensitive block with increasing molecular weight Citation[144]. It was found that micelles showed a controlled instability due to hydrolysis of the lactic acid side chains in the thermosensitive block. Rijcken et al. showed that poly(N-(2-hydroxyethyl)-methacrylamide-oligolactates)-b-PEG hydrolyzed more rapidly than poly(N-(2-hydroxypropyl)methacrylamide-oligolactates) Citation[317]. Thus, rapidly degrading thermosensitive polymeric micelles could possess attractive features for targeted drug delivery than slow degrading polymeric micelles. Of late, a new class of thermoresponsive polymer, 2-hydroxy-3-butoxypropyl starch was synthesized Citation[318]. The polymer self-assembled into micelles below the LCST, while above the LCST micelles aggregated into more polar and larger objects. Many other examples of PIPAAm and other polymers-based thermosensitive polymeric micelles have been reported. Some examples are depicted in Table .
Table 6. Examples of thermoresponsive polymeric micelles.
Dual-responsive polymeric micelles
To improve the targeting and treatment efficacy, efforts have been emphasized on the preparation of dual-responsive polymeric micelles, the micelles responding to temperature as well as pH changes. Accordingly, a pentablock copolymer was synthesized by the coupling of pH-sensitive poly(β-amino ester) to thermosensitive biodegradable PCL-PEG-PCL Citation[328]. An interesting dual-stimuli-responsive polymer has been based on acid-labile acetal linkages that provide an effective mechanism of polymer biodegradation in an acidic medium. Poly(N-(2-hydroxypropyl) methacrylamide dilactate)-b-PEG was synthesized by Soga et al. Citation[267]. This polymer formed polymeric micelles which gradually dissolved due to hydrolysis of the lactic acid side groups. The characteristic destabilization of the polymeric micelles was used for the generation of controlled release of paclitaxel. At pH 8.8 and 37 °C, paclitaxel-loaded micelles destabilized within 10 h due to the hydrolysis of the lactic acid side group of the copolymer.
Usually, the combination of a thermoresponsive monomer like IPAAm with a pH-responsive monomer yields double-responsive copolymers Citation[329]. Yin et al. synthesized a random copolymer of IPAAm and propylacrylic acid that showed a sharp phase transition in response to temperature and pH Citation[330]. Zhang et al. reported nanoparticles assembled from poly(IPAAm-co-acrylic acid)-b-PCL, which demonstrated dual-responsiveness to both thermo and pH in a suitable window for targeted anticancer drug delivery Citation[331]. An amphiphilic star block copolymer comprising of poly(methyl methacrylate)-b-poly(IPAAm-co-N,N-dimethylaminoethylmethacrylate) was synthesized and micelles were constructed Citation[332]. Dual-response originated from the thermo-sensitivity of PIPAAm and the pH-sensitivity from poly(N,N-dimethylaminoethylmethacrylate). In vitro drug release study revealed that methotrexate release was hastened by the thermo-trigger at pH 7.4, as well as the pH-trigger at 37 °C.
Block copolymers containing a hydrophilic N,N-dimethylacrylamide block and doubly responsive blocks of N-isopropylacrylamide and N-acryloylvaline were prepared Citation[333]. These copolymers demonstrated reversible pH- and temperature-induced unimer-to-micelle transition. Within a specified range of pH and temperature, the micelles could be ‘locked’ by interpolyelectrolyte complexation of anionic N-acryloylvaline segments with those of a cationic poly([ar-vinylbenzyl]trimethylammonium chloride). When the temperature was lowered to room temperature, the polymeric micelles remain ‘locked’ in their multimeric structures which remained stable in aqueous solution at temperature below CMT.
PEG-b-poly(trans-N-(2-ethoxy-1,3-dioxan-5-yl)acrylamide) dual-responsive micelles were constructed and loaded with hydrophobic Nile red by Huang and coworkers Citation[334]. The polymeric micelles were stable at pH 7.4 and the release of Nile red upon dissociation of the micelles was provoked by the acid-triggered hydrolysis of the orthoester groups in mildly acidic environment.
Other stimuli-sensitive polymeric micelles
Thiol-responsive (redox-responsive) systems
Redox sensitive systems are fascinating as these are susceptible toward disassembly in specific disease locations, where the redox environment is significantly different from the normal body cells. A redox potential difference exists between the reducing intracellular space and oxidizing extracellular space Citation[335,336]. The reducing environment near cancer cells due to the over-expression of several peptides like glutathione (the intracellular concentration of which is many folds greater than extracellular concentration) provides an opportunity to utilize these conditions for targeting Citation[337,338]. For example, the interconversion of thiols and disulfides has been exploited to synthesize various bioconjugated polymers. The disulfide bonds are sufficiently stable in the circulation and in the extracellular environment and are rapidly cleaved under a reductive environment through intracellular thiol-disulfide exchange reactions Citation[339,340]. The presence of reducing agents or the exchange of disulfide in the presence of other thiols can convert disulfide bonds reversibly to thiols. Thus, polymers containing disulfide bonds can be considered as thiol- as well as redox-responsive. Wang et al. recently reported synthesis of reversibly cross-linked triblock copolymer PCL-b-poly(2,4-dinitrophenylthioethyl ethylene phosphate)-b-PEG Citation[218]. After deprotection, core-shell-corona micelles were formed in aqueous milieu from the resultant triblock copolymer bearing thiol groups. The cross-linkages were cleaved intracellularly to result in enhanced drug release and cytotoxicity. Thayumanavan and others generated noncovalent polymeric assemblies between a surfactant complexed with a disulfide containing polyelectrolyte that could be disassembled via glutathione exposure Citation[341]. The disassembly was a result of the polyvalent interaction between the polyelectrolyte and surfactant changing to a monovalent interaction through a reductive disulfide bond cleavage reaction. PEG-poly(amino acid)s polymeric micelles with disulfide-cross-linked shells via thiol-reducible bonds were constructed Citation[342]. The reducing conditions prevalent in most of the cancerous tissues triggered the release of anticancer drugs preferentially at the tumor site. Redox-responsive core cross-linked PEO-b-poly(N-isopropylacrylamide-co-N-acryloxysuccinimide) micelles were developed by Zhang et al. Citation[343]. Recently, disulfide-linked star PEG-(N-acetyl cysteine) conjugates tailored to release the drug under intracellular glutathione levels were prepared Citation[344]. Terpolymers with thiol- and pH-responsive properties demonstrating membrane-disruptive characteristics were synthesized by copolymerization of a pyridyl disulfide containing acryloyl monomer with methacrylic acid and butyl acrylate by Bulmus et al. Citation[345]. The terpolymers were used in delivery of biomolecules.
Ultrasound-sensitive systems
The application of the external ultrasound to control drug delivery and release from micellar carriers is based on accumulation of these systems in required areas and making the area leaky by local application of external ultrasound. Ultrasonic waves can be focused and transmitted through a medium, thus allowing the waves to be directed to and/or focused on a particular volume of tissue. The waves can induce either thermal or mechanical effects, while micellar systems can be designed to respond either to the elevation in temperature or to the mechanical effects of ultrasonic waves, or to both. Due to its noninvasive nature, and no need of surgery for insertion, these systems are currently gaining attraction. Ultrasound has been shown to facilitate the delivery of chemotherapeutic agents into tumors and facilitate the healing of wounds and bone fractures Citation[346–349].
Ultrasound has been exploited to trigger the release of drugs from micellar carriers. Doxorubicin encapsulated in micelles was released using ultrasound to provide high local concentrations at the tumor site Citation[350]. After systemic administration of micelle-encapsulated doxorubicin in rats bearing tumors and application of 70 kHz ultrasound, results showed that tumor volume was significantly reduced.
The group of Husseini has been engaged in the development of ultrasound-based polymeric micelles and their characterization to optimize the effects of drug for better efficiency. Polymeric micelles incorporating doxorubicin have been prepared that released the drug after ultrasonication Citation[351]. They showed that the DNA damage induced by doxorubicin delivered to human leukemia cells (HL-60) from Pluronic P105 micelles was at an optimum after cells were exposed to ultrasound, when comparatively studied with and without the application of ultrasound Citation[352]. The percentage of drug released was highest at 20 kHz ultrasound and decreased with increasing ultrasound frequency despite much higher power densities when an ultrasonic exposure chamber with real-time fluorescence detection was used to measure ultrasound-activated drug release from P105 micelles under continuous wave or pulsed ultrasound Citation[353]. The thermodynamic characteristics of ultrasound-activated release of doxorubicin from P105 micelles were observed Citation[354]. They further investigated the degradation kinetics of stabilized P105 micelles for several hours following a two-hour application of ultrasound at 70 and 476 kHz Citation[355]. At both frequencies, the degradation appeared to be at a statistically significantly higher rate compared to samples that were not exposed to ultrasound. Lately, the group presented an artificial neural network model that attempted to predict the dynamic release of doxorubicin from P105 micelles under different ultrasonic power densities at 20 kHz Citation[356]. The developed model could be used in optimizing the ultrasound application for targeted drug release at the tumor site by controlling power density and ultrasound duration via model predictive control. Their investigations over ultrasound-activated micellar drug delivery have been summarized in a recent review Citation[357].
Tumor imaging becomes quite essential when effective drug targeting via tumor irradiation by ultrasound is considered. Hence, multifunctional nanoparticles that could target tumor, act as long-lasting ultrasound contrast agents, and augment drug delivery of ultrasound-mediated carriers were developed Citation[358].
The mechanisms controlling acoustic activation of drug uptake from Pluronic micelles were described by Marin et al. from their study over Pluronic micelles Citation[359]. Acoustically-triggered drug release from micelles was related to higher concentration of free drug in the incubation medium and also increased uptake of the micellar-encapsulated drug because of disruption of cell membranes. Still further studies on ultrasound-sensitive systems and the mechanisms controlling drug release from such systems represent an important part of research on stimuli-sensitive micellar carriers. To design an ultrasound-sensitive system, assessing the destruction thresholds of echogenic carriers with clinical ultrasound is a major challenge Citation[360].
Magnetically-sensitive systems
Systems in which the rate of drug release is controlled by oscillating or heating the carrier in response to the external electromagnetic field are said to be magnetically responsive Citation[361]. Iron oxide nanoparticles possess specific magnetic properties in the presence of an external magnetic field making them an attractive platform as contrast agents for magnetic resonance imaging (MRI) (one of the best noninvasive methodologies in clinical science for evaluating anatomy and physiology of tissues) and because of their superparamagnetic properties they are commonly referred to as superparamagnetic iron oxide nanoparticles (SPIONs). The magnetic and optical properties of these magnetic particles, upon loading into micelles, were found to remain unchanged and these nanocomposites have shown good in vivo biocompatibility as well. The concept of magnetic targeting toward the intended tissues under the influence of external magnetic field using such nanocomposites has received increased consideration recently. Hong et al. constructed PEG-PCL micelles and folate was used as a targeting ligand to functionalize the micelles which contained the MRI contrast agent SPIONs and doxorubicin Citation[362]. These micelles demonstrated better targeting response to the hepatic carcinoma cells, Bel 7402 cells, in vitro than their nontargeted counterparts. Ai et al. also have shown great potential of combining MRI and drug delivery functions in cancer treatment Citation[363]. PEG-PLA polymeric micelles with a cyclic pentapeptide c(Arg-Gly-Asp-D-Phe-Lys) as a targeting ligand to detect the delivery of SPIONs and doxorubicin-loaded micelles into the tumor vascular endothelia cells were formulated. Gang et al. targeted gemcitabine-loaded magnetic PCL nanoparticles in pancreatic cancer xenograft mouse model using external magnets Citation[364]. Manganese ferrite-SPIONs were synthesized and solubilized with the help of methoxy PEG-b-poly(ϵ-caprolactone) micelles in water Citation[365]. Mn-SPIONs inside polymeric micelles were found to strongly improve the contrast between small lesions and normal tissues. Guthi et al. have developed a multifunctional micelle system to which a lung cancer-targeting peptide has been attached, and encapsulated with SPIONs and doxorubicin for MRI and therapeutic delivery, respectively Citation[366]. The multifunctional micelles modified with targeting peptide demonstrated improved lung cancer targeting. Hence, the usefulness of magneto-responsive micellar systems not only in targeted drug delivery but also for diagnostic applications like imaging has been very well established.
Photo-responsive systems
Photo-responsive systems comprise of macromolecules that change their properties when irradiated with light of suitable wavelength Citation[367,368]. Frequently, an incorporated chromophore into the structure of the hydrophobic block along the polymer backbone or side chains undergo structural alterations in response to light of particular wavelength, disturbing the hydrophilic/lipophilic balance of the polymer and ultimately leading to micellar disruption Citation[369,370]. An important advantage of photo-sensitive micellar systems is that light-irradiation is a relatively straight-forward, noninvasive mechanism to induce responsive behavior. Light-responsive systems are attractive as materials responding to electromagnetic radiations, mainly to the UV, visible and near-infrared range, can be developed and applied at well enclosed sites in the body Citation[371]. Various synthetic approaches to photoresponsive polymers and their properties have been reviewed by Yu and Kobayashi Citation[372].
Decorated shell cross-linked reverse polymer micelles constituted of poly(dimethylaminoethyl methacrylate)-b-poly(methyl methacrylate-random-coumarin methacrylate) and responsive to light were reported by Babin et al. Citation[373]. Jiang et al. prepared amphiphilic block copolymer micelles whose core-shell structure was disrupted upon irradiation with different wavelengths of light Citation[374,375]. Jing et al. reported a facile approach for the preparation of light-responsive monomethoxy PEG-b-poly(5-methyl-5-(2-nitro-benzoxycarbonyl)-1,3-dioxan-2-one) copolymer micelles containing a light-sensitive linkage Citation[376]. Photolysis of the 2-nitrobenzyl ester side-groups on the hydrophobic block of these micelles could be dissociated by UV irradiation to release their payload. Photosolvolysis of hydrophobic groups has been used in hydrophilic PEO copolymer micellar solutions with polymethacrylate bearing pyrene moiety in the side group as hydrophobic block Citation[377]. Pyrene from the copolymer was then being separated using UV-light irradiation that increased the hydrophilicity of the hydrophobic block by formation of PMA causing micellar dissociation.
Triple-responsive (multi-responsive) polymeric micelles
Newer strategies combine sensitivities to different stimuli in designing a highly efficient multistimuli responsive targeted polymeric micellar system. Klaikherd and his group reported a triple stimuli sensitive block copolymer assembly that could respond to changes in temperature, pH, and redox potential Citation[378]. The block copolymer comprised of an acid-sensitive tetrahydropyran-protected 2-hydroxyethyl methacrylate as the hydrophobic part and a temperature-sensitive PIPAAm as the hydrophilic part with an intervening disulfide bond. The developed structures were sensitive not only to a single stimulus, but responded simultaneously to the presence of multiple stimuli.
Less frequently used stimuli
Some reports over materials that change on receiving stimuli from electric field (electro-responsive) Citation[379], changes in glucose concentration (sugar-responsive) Citation[380], and presence of enzymes Citation[381] as well have been reported. Examples of stimuli-sensitive nanocarriers for targeting are reviewed by Torchilin Citation[382], and stimuli-sensitive materials by Roy et al. Citation[383].
Complexing targeting ligand molecules to micelles
One of the best approaches to enhance cellular internalization of polymeric micelles at desired target tissue is attachment of cell-specific ligands on the surface of these polymeric micelles. Thus, covalent attachment of cell specific ligands, e.g. monoclonal antibodies Citation[384], sugars Citation[385], folate residues Citation[386], and peptides Citation[387] on the surface of polymeric micelles has been pursued to enhance drug delivery to various cells.
Monoclonal antibodies
Immunomicelles can be prepared by covalently attaching an antibody to a surfactant or polymeric micelles. Attachment of antibodies to micelle surface provides the broadest opportunities in terms of diversity of targets. Thus, many groups are trying to exploit these opportunities by preparing ‘immunomicelles’ Citation[36,136]. To demonstrate the effectiveness of using immunomicelles in tumor targeting, Sawant et al. solubilized paclitaxel and camptothecin in mixed micelles of PEG-PE and vitamin E Citation[388]. The mixed micelles were modified with antinucleosome monoclonal antibody 2C5 (mAb 2C5), which can specifically bring micelles to tumor cells in vitro. These mixed micelles and mAb 2C5-immunomicelles demonstrated significantly higher in vitro cytotoxicity against various cancer cell lines.
Lee et al. prepared PEG-PE-based immunomicelles modified with monoclonal antibodies by using PEG-PE conjugates with the free PEG terminus activated with p-nitrophenylcarbonyl group Citation[389]. Targeted immunomicelles were prepared by incubating the corresponding antibody with doxorubicin-loaded p-nitrophenylcarbonyl-PEG-PE-containing micelles at pH around 8. The lipid fragments of this PEG derivative could firmly incorporate into the micelle core, while the p-nitrophenylcarbonyl group could interact, in response to pH, with amino-groups of various antibodies, their fragments, or other peptides, thus increasing tumor specificity. In most AB-type block copolymer syntheses that have the PEG chain for polymeric micelles, a chemically unreactive functional group such as methoxy is used as the PEG terminal. Synthesis of aldehyde group-terminated (on the PEG side) PEG-PLA block copolymers has been reported by Scholz et al. Citation[33]. Polymeric micelles that had antibodies as targeting ligands on their surface by utilizing this aldehyde group shown potential for active targeting of tumors.
Gao et al. have demonstrated the cytotoxicity of micellar paclitaxel and free drug using a standard MTS test Citation[390]. At paclitaxel concentration of 40 ng/ml, free drug killed <2% of cancer cells in the culture, whereas activity of paclitaxel in plain micelles was slightly higher killing about 5% of cancer cells. Paclitaxel-loaded immunomicelles killed more than 50% of cancer cells. Thus, paclitaxel-loaded 2C5-immunomicelles provided more efficient killing of cancer cells compared to the free drug or paclitaxel in plain micelles.
Sugars
One of the key players in cell-involved biorecognition is sugar, and perhaps one of the most fascinating routes in cellular-specific drug targeting comes via sugar-mediated delivery through glyco-receptors on the cellular plasma membrane Citation[391,392]. Glycoreceptor binding to a particular sugar often occurs in a regioselective manner Citation[393]. Thus, a block copolymer having a glucose or galactose residue at the chain end of one of the block in a regioselective manner was synthesized by Yasugi et al. Citation[85]. PEG-PLA block copolymer having a site specifically protected-sugar group at the PEG chain end using a metalated protected sugar as an initiator was synthesized. Polymer micelles having sugar residues on the surface were then prepared by dialyzing an N,N-dimethylacetamide solution of the sugar-bearing PEG-PLA block copolymer against water. A galactose-bearing PEG-PLA micelle was confirmed to selectively attach to RCA-1 lectin (RCA-1 is one of the well-studied plant lectins which specifically recognize a β-D-galactose residue). Nagasaki et al. in another study synthesized several types of sugar-installed PEG-PLA block copolymers which formed polymeric micelles in aqueous media Citation[385]. Specific recognition of lectin proteins with the sugar molecules on the micelle surface was observed. Both the galactose- and lactose-installed micelles specifically interacted with RCA-1; on the other hand, the mannose-installed micelle interacted specifically with Con A. These polymer micelles were expected to have wide utility in the field of drug targeting as glyco-receptor-directed carrier systems.
Folate residues
Membrane folate receptors (FRs), including FR-α and FR-β, are glycosylphosphatidylinositol-anchored glycoproteins. FR-α expression is amplified in over 90% of ovarian carcinomas and at varying frequencies in other epithelial cancers. FR-β is expressed in a nonfunctional form in neutrophils and in a functional form in activated macrophages and in myeloid leukemias Citation[394]. In contrast, most normal tissues lack expression of either of the FR isoform. Folate is a high affinity ligand for the FRs upon derivatization via one of its carboxyl group and has being widely studied due to its small size and ease of availability for tumor-specific drug delivery Citation[395]. Upon attaching the folate molecules onto the surface layer of polymeric micelles, FR-mediated endocytosis of micelles is attainable. In this regard, Kim et al. prepared poly(His-co-Phe)-b-PEG and PLLA-b-PEG-folate pH-sensitive micelles Citation[396]. Accelerated doxorubicin release from these polymeric micelles triggered by an early endosomal pH of 6.0 was observed. When this triggered release was combined with active targeting via FR-mediated endocytosis, the polymeric micelles were able to effectively kill drug-sensitive ovarian cancer cells as well as drug-resistant counterpart cells. Recently, micellar docetaxel formulation using folic acid-conjugated D-α-tocopheryl polyethylene glycol succinate 2000 for targeted delivery was reported and showed an enhanced cellular uptake with in vitro therapeutic effects Citation[397]. Amphiphilic hyperbranched block copolymer with a dendritic Boltorn® H40 core, a hydrophobic poly(L-lactide) inner shell, and a hydrophilic methoxy PEG and folate-conjugated PEG outer shell was synthesized by Prabaharan et al. Citation[398]. Doxorubicin encapsulated polymeric micelles were prepared and it was observed that the cellular uptake of the doxorubicin-loaded and folate-conjugated micelles was higher than doxorubicin-loaded, folate-free micelles because of the folate-receptor mediated endocytosis. It resulted in higher cytotoxicity against the 4T1 mouse mammary carcinoma cell line. Yang et al. synthesized the folate-conjugated copolymer, folate-PEG-PCL, and fabricated micelles of the same with the encapsulation of a potent multidrug resistance modulator, FG020326 Citation[399]. Cytotoxicity studies indicated that folate-functionalized and FG020326-loaded micelles resensitized human KBv200 cells treated with vincristine approximately five times more than their folate-free counterparts. Similarly, folate-modified chitosan micelles with enhanced tumor targeting were also recently developed Citation[400].
In a little different study, Bae et al. Citation[401] were able to significantly increase cancer treatment efficacy and safety of the polymeric micelles by optimizing the number of ligands on the micelle surface. Using precise synthesis, folate concentration on the surface of the micelles was controlled for two different amphiphilic block copolymers that self-assembled into spherical micelles, folate-PEG-poly(aspartate-hydrazone-adriamycin) with γ-carboxylic acid activated folate and methoxy PEG-poly(aspartate-hydrazone-adriamycin) without folate. Interestingly, folate conjugation could not significantly improve the tumor accumulation of the micelles as liver accumulation was seen. However, when folate concentration was adjusted to achieve minimum ligand–receptor interaction, folate-conjugated micelles showed an effective cancer treatment efficiency that was 5-fold broader than free adriamycin as well as the micelles without folate conjugation.
Many studies have been performed in which folic acid has been applied as a targeting ligand in both the tumor imaging diagnosis and cancer chemotherapy. But the combination of two strategies is little reported. In a more advanced study, Hong et al. accommodated doxorubicin and MRI contrast agent superparamagnetic iron oxide in the core of PEG-PCL micelles with a folate targeting ligand attached to the distal ends of PEG Citation[362]. The prepared micelles served dual purpose with better targeting tropism, in vitro, toward the hepatic carcinoma cells than their nontargeting counterparts, and showed a great potential in diagnostic imaging.
Peptides
For tumor targeting, cancer-specific peptides are also very appropriate, as peptides can easily be derivatized and engineered to achieve better in vivo stability and tissue specificity. Xiong et al. conjugated an arginine-glycine-aspartic acid (RGD) containing peptide as a ligand to recognize adhesion molecules overexpressed on the surface of metastatic cancer cells, over the surface of PEO-b-PCL micelles Citation[387]. These micelles proved to be good ligand-targeted carriers for enhanced drug delivery to metastatic tumor cells. Musacchio et al. used the overexpression of Peripheral Benzodiazepine Receptor (PBR) in certain cancers for targeting such cancers Citation[402]. Selective ligands to the PBR may induce apoptosis and cell cycle arrest. Hence, PBR-targeted PEG-PE micellar drug delivery system loaded with paclitaxel was prepared that demonstrated significantly enhanced toxicity against some cancerous cells. Lately, multifunctional RGD-functionalized polymeric micelles coencapsulating doxorubicin and combretastatin A4 were shown to have prolonged blood circulation and preferential accumulation in solid tumor Citation[403]. In B16-F10 tumor-bearing mice, these micelles demonstrated excellent antitumor efficacy and low side-effects.
Photodynamic therapy
Photodynamic therapy (PDT) is a minimally invasive treatment, considered as an alternative to classical therapies such as surgery, radiotherapy, and chemotherapy, that combines a photosensitiser or a photosensitising agent with photoirradiation by a specific type of light to kill cancer cells (particularly tumors of oesophagus, bladder, and melanoma). After the administration of the photosensitiser, the directed nonthermal light (635–760 nm) onto the abnormal tissue where the drug has preferentially accumulated leads to the formation of an excited photosensitizer. Upon activation, the photosensitiser transfers its excess energy to molecular oxygen, directly or through an indirect mechanism via formation of intermediate radical species, to produce the highly reactive cytotoxic singlet oxygen that causes irreversible oxidative destruction at the target site Citation[404–407]. PDT induces both apoptosis and necrosis leading to light-induced cell death of the subcellular organelles and other biomolecules. Although PDT was originally developed for cancer therapy, its potential for application has been substantially extended to many other clinical applications such as the treatment of hardening arteries, age-related macular degeneration, and sun induced precancerous skin lesions and wound infections Citation[408,409].
Some desirable properties of a photosensitizer molecule are its nontoxic nature in the absence of light irradiation, photostability, ability to absorb in the red region of spectrum with high extinction molar coefficient, target specificity, minimal tendency toward self-aggregation, and capability to be rapidly eliminated from the body. The pharmacokinetic properties of the photosensitizer play a critical role in achieving the desired biological response. Photosensitizers such as phthalocyanine and porphyrin derivatives have been mostly explored for systemic administration in PDT Citation[410].
pH-responsive micelles of N-isopropylacrylamide, methacrylic acid, and octadecyl acrylate copolymers were prepared and loaded with aluminum chloride phthalocyanine (AlClPc) by Taillefer et al. Citation[411]. The micelles exhibited higher cytotoxicity against EMT-6 mouse mammary cells in vitro than control Cremophor EL formulation. However, the polymeric micelles showed rapid clearance and relatively poor retention in blood because of the amphiphilic nature of the PIPAAm corona with relatively marginal stealth properties. To minimize clearance, Taillefer and coworkers in their further investigation increased the hydrophilicity of N-isopropylacrylamide copolymer by synthesizing different pH-sensitive copolymers bearing N-vinyl-2-pyrrolidone (VP) Citation[412]. AlClPc was loaded in the PIPAAm copolymeric micelles by dialysis. On comparing the biodistribution and in vivo photodynamic activity of the copolymer micelles and control Cremophor EL formulations in Balb/c mice bearing intradermal EMT-6 tumors, similar AlClPc tumor uptake was observed. However, the micelles exhibited greater activity in vivo than Cremophor EL formulations at an AlClPc subtherapeutic dose. The decrease in clearance of the micelles was accompanied by preferential accumulation of the drug at the target site. Sibata et al. verified complete loading of zinc(II) phthalocyanine (ZnPc) in PEG-5000-distearoyl-phosphatidylethanolamine micelles by absorbance measurements, steady state, and time-resolved fluorescence measurements Citation[413]. This system provided a better stability of the incorporated drug, with a narrow size distribution pattern of its particles and a lower photobleaching quantum yield. Silicon phthalocyanine Pc 4, a highly hydrophobic second-generation photosensitizer, was encapsulated in biocompatible micelles of PEG-b-poly-ϵ-caprolactone and in vitro PDT studies in MCF-7c3 human breast cancer cells were conducted Citation[414]. Studies revealed efficient intracellular uptake of the micelle-formulated Pc 4 in cells, and significant cytotoxic effect of the formulation upon photoirradiation.
Hioka et al. solubilized a benzoporphyrin derivative using Pluronic P123 for PDT Citation[415]. The behavior of the photosensitizer varied with differing polymer concentration. Above the CMC of the polymer, the photosensitizer was present in its monomeric form in the core of the micelle while aggregates were formed in water below the CMC. Aggregated structures possess low quantum yields of light absorption and cause significantly less singlet oxygen production. Li et al. investigated the formulation of hydrophobic protoporphyrin IX (PpIX) with methoxy PEG-b-PCL micelles and compared their PDT response to that of free PpIX Citation[416]. Fluorescence microscopy revealed that the subcellular localization of free PpIX and PpIX formulated in micelles was similar. However, the cellular uptake and photocytotoxicity of PpIX in RIF-1 cells from micelles was markedly increased than free PpIX signifying the potential of these micelles in drug delivery systems for hydrophobic photodynamic sensitizers.
Conclusions and future perspectives
Owing to their salient properties, polymeric micelles are emerging as important pharmaceutical drug carriers. The most pertinent trait of block copolymer micelles for drug delivery is their ability to form prominent core-shell structures. Poorly water-soluble drugs can easily be loaded in the hydrophobic core of the polymeric micelles, thus providing an opportunity to enhance bioavailability of such drugs. Importantly, stable polymeric micelles possessing an excellent ability to carry a variety of poorly water-soluble drugs can effectively be used to target certain pathological areas in the body with compromised vasculature such as tumors and infarcts owing to their size and the EPR effect. Targeting can also be achieved by attaching specific ligands or specific antibodies onto their surface. Thus, widespread use of polymeric micelles can be expected, particularly in the field of drug delivery for the cytotoxic agents.
So long as the mystery about micelle stability in the blood is resolved, formulation of polymeric micelles to maximize drug efficacy will remain challenge. Still, when compared with other novel drug delivery systems, the development of polymeric micelles appears to be quite promising. The development of stimuli-sensitive micelles exploiting the properties of polymeric micelles to improve the selective drug delivery via physiological triggers appears to be quite exhilarating field of research. Intensive efforts in this field of research would definitely foster polymeric micelles as one of the major vehicles in the field of site-specific drug delivery.
References
- Zana , R . 2005 . “ Introduction to surfactants and surfactant self-assemblies ” . In Dynamics of surfactant self-assemblies – micelles, microemulsions, vesicles, and lyotropic phases , Edited by: Zana , R . 2 – 36 . Boca Raton , FL : Taylor & Francis .
- Rangel-Yagui , CO , Pessoa , JA and Tavares , LC . 2005 . Micellar solubilization of drugs . Journal of Pharmacy and Pharmaceutical Sciences , 8 : 147 – 163 .
- Adams , ML , Lavasanifar , A and Kwon , GS . 2003 . Amphiphilic block copolymers for drug delivery . Journal of Pharmaceutical Sciences , 92 : 1343 – 1355 .
- M. Malmsten, Micelles. In: Surfactants and Polymers in Drug Delivery. Marcel Dekker, New York (2002).19–50.
- Y. Moroi, Dissolution of amphiphiles. In: Micelles: Theoretical and Applied Aspects, Plenum Press, New York (1992).25–40.
- Hickok , RS , Wedge , SA , Hansen , AL , Morris , KF , Billiot , FH and Warner , IM . 2002 . Pulsed field gradient NMR investigation of solubilization equilibria in amino acid and dipeptide terminated micellar and polymeric surfactant solutions . Magnetic Resonance in Chemistry , 40 : 755 – 761 .
- Jones , MC and Leroux , JC . 1999 . Polymeric micelles a new generation of colloidal drug carriers . European Journal of Pharmaceutics and Biopharmaceutics , 48 : 101 – 111 .
- Trubetskoy , VS and Torchilin , VP . 1996 . Polyethyleneglycol-based micelles as carriers for delivery of therapeutic and diagnostic agents . STP Pharma Sciences , 6 : 79 – 86 .
- Zarafshani , Z , Akdemir , O and Lutz , JF . 2008 . A ‘‘click’’ strategy for tuning in situ the hydrophilic-hydrophobic balance of AB macrosurfactants . Macromolecular Rapid Communications , 29 : 1161 – 1166 .
- Erhardt , R , Boker , A , Zettl , H , Kaya , H , Pyckhout-Hintzen , W , Krausch , G , Abetz , V and Muller , AHE . 2001 . Janus micelles . Macromolecules , 34 : 1069 – 1075 .
- Forster , S and Plantenberg , T . 2002 . From self-organizing polymers to nanohybrid and biomaterials . Angewandte Chemie International Edition , 41 : 688 – 714 .
- Jacquin , M , Muller , P , Cottet , H , Crooks , R and Theodoly , O . 2007 . Controlling the melting of kinetically frozen poly(butyl acrylate-b-acrylic acid) micelles via addition of surfactant . Langmuir , 23 : 9939 – 9948 .
- Cao , Y and Li , H . 2003 . Micellar solutions of CMC amphipathic copolymers . Polymer International , 52 : 869 – 875 .
- Lutz , JF , Pfeifer , S and Zarafshani , Z . 2007 . In situ functionalization of thermoresponsive polymeric micelles using the “click” cycloaddition of azides and alkynes . QSAR & Combinatorial Science , 26 : 1151 – 1158 .
- Liu , H , Xu , J , Jiang , J , Yin , J , Narain , R , Cai , Y and Liu , S . 2007 . Syntheses and micellar properties of well-defined amphiphilic AB2 and A2B Y-shaped miktoarm star copolymers of ϵ-caprolactone and 2-(dimethylamino)ethyl methacrylate . Journal of Polymer Science Part A: Polymer Chemistry , 45 : 1446 – 1462 .
- Zhang , YX , Liu , SP , Du , LB , Zhang , DQ , Chen , JY and Jiang , M . 2002 . Synthesis and characterization of fluorocarbon-containing, hydrophobically associative poly (acrylic acid-co-Rf-poly (ethylene glycol) macromonomer) . Journal of Applied Polymer Science , 84 : 1035 – 1047 .
- Kline , SR . 1999 . Polymerization of rodlike micelles . Langmuir , 15 : 2726 – 2732 .
- Miyata , K , Christie , RJ and Kataoka , K . 2011 . Polymeric micelles for nano-scale drug delivery . Reactive & Functional Polymers , 71 : 227 – 234 .
- Mourya , VK , Inamdar , NN , Nawale , RB and Kulthe , SS . 2011 . Polymeric micelles: general considerations and their applications . Indian Journal of Pharmaceutical Education and Research , 45 : 128 – 138 .
- Varma , MV and Panchagnula , RV . 2005 . Enhanced oral paclitaxel absorption with vitamin E-TPGS: effect on solubility and permeability in vitro, in situ and in vivo . European Journal of Pharmaceutical Sciences , 25 : 445 – 453 .
- Bromberg , L . 2008 . Polymeric micelles in oral chemotherapy . Journal of Controlled Release , 128 : 99 – 112 .
- Kabanov AV, Alakhov VY. Micelles of amphiphilic block copolymers as vehicles for drug delivery. In: Amphiphilic block copolymers: self-assembly and applications. Alexandris P, Lindman B, editors. Amsterdam: Elsevier Science; 2000. 352–376.
- Elsabahy , M , Perron , M , Bertrand , N , Yu , G and Leroux , JC . 2007 . Solubilization of docetaxel in poly(ethylene oxide)-block-poly(butylene/styrene oxide) micelles . Biomacromolecules , 8 : 2250 – 2257 .
- Peng , X and Zhang , L . 2007 . Formation and morphologies of novel self-assembled micelles from chitosan derivatives . Langmuir , 23 : 10493 – 10498 .
- Yokoyama , M . 1998 . “ Novel passive targetable drug delivery with polymeric micelles ” . In Biorelated polymers and gels: controlled release and applications in biomedical engineering , Edited by: Okano , T . 193 – 231 . San Diego , CA : Academic Press .
- Zhang , C , Qu , G , Sun , Y , Wu , BX , Yao , Z , Guo , Q , Ding , Q , Yuan , S , Shen , Z , Ping , Q and Zhou , H . 2008 . Pharmacokinetics, biodistribution, efficacy and safety of N-octyl-O-sulfate chitosan micelles loaded with paclitaxel . Biomaterials , 29 : 1233 – 1241 .
- Nishiyama , N , Okazaki , S , Cabral , H , Miyamoto , M , Kato , Y , Sugiyama , Y , Nishio , K , Matsumura , Y and Kataoka , K . 2003 . Novel cisplatin-incorporated polymeric micelles can eradicate solid tumors in mice . Cancer Research , 63 : 8977 – 8983 .
- Jiang , M , Wu , Y , Heb , Y and Nie , J . 2009 . Micelles formed by self-assembly of hyperbranched poly[(amine-ester)-co-(D, L-lactide)] (HPAE-co-PLA) copolymers for protein drug delivery . Polymer International , 58 : 31 – 39 .
- Mahmud , A , Xiong , XB , Aliabadi , HM and Lavasanifar , A . 2007 . Polymeric micelles for drug targeting . Journal of Drug Targeting , 15 : 553 – 584 .
- Kwon , GS and Okano , T . 1996 . Polymeric micelles as new drug carriers . Advanced Drug Delivery Reviews , 21 : 107 – 116 .
- Harada , A and Kataoka , K . 1995 . Block copolymer micelles as long-circulating drug vehicles . Advanced Drug Delivery Reviews , 16 : 295 – 309 .
- Harada , A and Kataoka , K . 1995 . Formation of polyion complex micelles in an aqueous milieu from a pair of oppositely-charged block copolymers with poly(ethylene glycol) segments . Macromolecules , 28 : 5294 – 5299 .
- Scholz , C , Iijima , M , Nagasaki , Y and Kataoka , K . 1995 . A novel reactive polymeric micelle with aldehyde groups on its surface . Macromolecules , 28 : 7295 – 7297 .
- Juillerat-Jeanneret , L and Schmitt , F . 2007 . Chemical modification of therapeutic drugs or drug vector systems to achieve targeted therapy: Looking for the grail . Medicinal Research Reviews , 27 : 574 – 590 .
- Kataoka , K , Harada , A and Nagasaki , Y . 2001 . Block copolymer micelles for drug delivery: Design, characterization and biological significance . Advanced Drug Delivery Reviews , 47 : 113 – 131 .
- Torchilin , VP . 2004 . Targeted polymeric micelles for delivery of poorly soluble drugs . Cellular and Molecular Life Sciences , 61 : 2549 – 2559 .
- Seo , DH , Jeong , Y , Kim , DG , Jang , MJ , Jang , MK and Nah , JW . 2009 . Methotrexate-incorporated polymeric nanoparticles of methoxy poly(ethylene glycol)-grafted chitosan . Colloids and Surfaces B: Biointerfaces , 69 : 157 – 163 .
- Jiang , GB , Quan , D , Liao , K and Wang , H . 2006 . Novel polymer micelles prepared from chitosan grafted hydrophobic palmitoyl groups for drug delivery . Molecular Pharmaceutics , 3 : 152 – 160 .
- Fournier , E , Dufresne , MH , Smith , DC , Ranger , M and Leroux , JC . 2004 . A novel one-step drug-loading procedure for water-soluble amphiphilic nanocarriers . Pharmaceutical Research , 21 : 962 – 968 .
- Kwon , GS , Naito , M , Yokoyama , M , Okano , T , Sakurai , Y and Kataoka , K . 1995 . Physical entrapment of adriamicin in AB block copolymer micelles . Pharmaceutical Research , 12 : 192 – 195 .
- La , SB , Okano , T and Kataoka , K . 1996 . Preparation and characterization of the micelle-forming polymeric drug indomethacin-incorporated poly(ethylene oxide)-poly(β-benzyl l-aspartate) block copolymer micelles . Journal of Pharmaceutical Sciences , 85 : 85 – 90 .
- Ye , YQ , Yang , FL , Hu , FQ , Du , YZ , Yuan , H and Yu , HY . 2008 . Core-modified chitosan-based polymeric micelles for controlled release of doxorubicin . International Journal of Pharmaceutics , 352 : 294 – 301 .
- Hu , FQ , Wu , XL , Du , YZ , You , J and Yuan , H . 2008 . Cellular uptake and cytotoxicity of shell crosslinked stearic acid-grafted chitosan oligosaccharide micelles encapsulating doxorubicin . European Journal of Pharmaceutics and Biopharmaceutics , 69 : 117 – 125 .
- Yang , ZL , Li , XR , Yang , KW and Liu , Y . 2008 . Amphotericin B-loaded poly(ethylene glycol)-poly(lactide) micelles: Preparation, freeze-drying, and in vitro release . Journal of Biomedical Materials Research , 85A : 539 – 546 .
- Huh , KM , Lee , SC and Cho , YW . 2005 . Hydrotropic polymer micelle system for delivery of paclitaxel . Journal of Controlled Release , 101 : 59 – 68 .
- Burkersroda , FV , Gref , R and Gopferich , A . 1997 . Erosion of biodegradable block copolymers made of poly(DL-lactic acid) and poly(ethylene glycol) . Biomaterials , 18 : 1599 – 1607 .
- Hamley , IW . 2004 . “ Introduction to block polymers ” . In Developments in block copolymer science and technology , Edited by: Hamley , IW . 1 – 30 . Chichester : Wiley .
- Erdogan , T , Bernaerts , KV , Van Renterghem , LM , Du Prez , FE and Goethals , EJ . 2005 . Preparation of star block co-polymers by combination of cationic ring opening polymerization and atom transfer radical polymerization . Designed Monomers and Polymers , 8 : 705 – 714 .
- Zhou , J , Wang , L and Ma , J . 2009 . Recent research progress in the synthesis and properties of amphiphilic block co-polymers and their applications in emulsion polymerization . Designed Monomers and Polymers , 12 : 19 – 41 .
- Maniruzzaman , M , Kawaguchi , S and Ito , K . 2000 . Micellar copolymerization of styrene with a poly(ethylene oxide) macromonomer in water to a unimolecular graft copolymer micelle . Designed Monomers and Polymers , 3 : 255 – 261 .
- Guo , Z and Fang , Z . 2009 . Effects of the competition between grafting reaction and decomposition on the miscibility and rheological behaviors of PS/POE blends in the presence of AlCl3 . Polymer-Plastics Technology and Engineering , 48 : 1185 – 1190 .
- Lu , HW , Zhang , LM , Wang , C and Chen , RF . 2011 . Preparation and properties of new micellar drug carriers based on hydrophobically modified amylopectin . Carbohydrate Polymers , 83 : 1499 – 1506 .
- Bronstein , LM , Chernyshov , DM , Timofeeva , GI , Dubrovina , LV , Valetsky , PM and Khokhlov , AR . 2000 . Interaction of polystyrene-block-poly(ethylene oxide) micelles with cationic surfactant in aqueous solutions. Metal colloid formation in hybrid systems . Langmuir , 16 : 3626 – 3632 .
- Michels , B , Waton , G and Zana , R . 1997 . Dynamics of micelles of poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) block copolymers in aqueous solutions . Langmuir , 13 : 3111 – 3118 .
- Oh , KT and Lee , ES . 2008 . Cancer-associated pH-responsive tetracopolymeric micelles composed of poly(ethylene glycol)-b-poly(L-histidine)- b-poly(L-lactic acid)-b-poly(ethylene glycol) . Polymers for Advanced Technologies , 19 : 1907 – 1913 .
- Hadjiantoniou , NA , Christoforou , TK , Loizou , E , Porcar , L and Patrickios , CS . 2010 . Alternating amphiphilic multiblock copolymers: Controlled synthesis via raft polymerization and aqueous solution characterization . Macromolecules , 43 : 2713 – 2720 .
- Wei , H , Zhang , XZ , Chen , WQ , Cheng , SX and Zhuo , RX . 2007 . Self-assembled thermosensitive micelles based on poly(L-lactide-star block-N-isopropylacrylamide) for drug delivery . Journal of Biomedical Materials Research , 83A : 980 – 989 .
- Fattahi , A , Golozar , MA , Varshosaz , J , Sadeghi , HM and Fathi , M . 2012 . Preparation and characterization of micelles of oligomeric chitosan linked to all-trans retinoic acid . Carbohydrate Polymers , 87 : 1176 – 1184 .
- Li , Y , Zhang , S , Meng , X , Chen , X and Ren , G . 2011 . The preparation and characterization of a novel amphiphilic oleoyl-carboxymethyl chitosan self-assembled nanoparticles . Carbohydrate Polymers , 83 : 130 – 136 .
- Chen , XG , Lee , CM and Park , HJ . 2003 . O/W emulsification for the self-aggregation and nanoparticle formation of linoleic acid modified chitosan in the aqueous system . Journal of Agriculture and Food Chemistry , 51 : 3135 – 3139 .
- Zhang , C , Ding , Y , Ping , Q and Yu , L . 2006 . Novel chitosan-derived nanomaterials and their micelle-forming properties . Journal of Agriculture and Food Chemistry , 54 : 8409 – 8416 .
- Tan , YL and Liu , CG . 2009 . Self-aggregated nanoparticles from linoleic acid modified carboxymethyl chitosan: Synthesis, characterization and application in vitro . Colloids and Surfaces B: Biointerfaces , 69 : 178 – 182 .
- Huo , M , Zhang , Y , Zhou , J , Zou , A and Li , J . 2011 . Formation, microstructure, biodistribution and absence of toxicity of polymeric micelles formed by N-octyl-N,O-carboxymethyl chitosan . Carbohydrate Polymers , 83 : 1959 – 1969 .
- Duan , K , Zhang , X , Tang , X , Yu , J , Liu , S , Wang , D , Li , Y and Huang , J . 2010 . Fabrication of cationic nanomicelle from chitosan-graft-polycaprolactone as the carrier of 7-ethyl-10-hydroxy-camptothecin . Colloids and Surfaces B: Biointerfaces , 76 : 475 – 482 .
- Hu , FQ , Liu , LN , Du , YZ and Yuan , H . 2009 . Synthesis and antitumor activity of doxorubicin conjugated stearic acid-g-chitosan oligosaccharide polymeric micelles . Biomaterials , 30 : 6955 – 6963 .
- Qu , G , Yao , Z , Zhang , C , Wu , X and Ping , Q . 2009 . PEG conjugated N-octyl-O-sulfate chitosan micelles for delivery of paclitaxel: In vitro characterization and in vivo evaluation . European Journal of Pharmaceutical Sciences , 37 : 98 – 105 .
- Nakayama , M and Okano , T . 2005 . Polymer terminal group effects on properties of thermoresponsive polymeric micelles with controlled outer-shell chain lengths . Biomacromolecules , 6 : 2320 – 2327 .
- Kriz , J , Pletil , J , Tuzar , Z , Pospisil , H , Brus , J , Jakes , J , Masar , B , Vlcek , P and Doskocilova , D . 1999 . Interface affected polymer dynamics: NMR, SANS, and DLS study of the influence of shell-core interactions on the core chain mobility of poly(2-ethylhexyl acrylate)-block-poly(acrylic acid) micelles in water . Macromolecules , 32 : 397 – 410 .
- Prochazka , K , Martin , TJ , Munk , P and Webber , SE . 1996 . Polyelectrolyte poly(tert-butyl acrylate)-block-poly(2-vinylpyridine) micelles in aqueous media . Macromolecules , 29 : 6518 – 6525 .
- Colombani , O , Ruppel , M , Burkhardt , M and Drechsler , M . 2007 . Structure of micelles of poly(n-butyl acrylate)-block-poly(acrylic acid) diblock copolymers in aqueous solution . Macromolecules , 40 : 4351 – 4362 .
- Kriz , J , Masar , B , Pletil , J , Tuzar , Z , Pospisil , H and Doskocilova , D . 1998 . Three-layer micelles of an ABC block copolymer: NMR, SANS, and LS study of a poly(2-ethylhexyl acrylate)-block-poly(methyl methacrylate)-block-poly(acrylic acid) copolymer in D2O . Macromolecules , 31 : 41 – 51 .
- Liu , S , Weaver , JVM , Tang , Y , Billingham , NC , Armes , SP and Tribe , K . 2002 . Synthesis of shell cross-linked micelles with pH-responsive cores using ABC triblock copolymers . Macromolecules , 35 : 6121 – 6131 .
- Geng , Y and Discher , DE . 2005 . Hydrolytic degradation of poly(ethylene oxide)-block-polycaprolactone worm micelles . Journal of the American Chemical Society , 127 : 12780 – 12781 .
- Soo , PL , Luo , L , Maysinger , D and Eisenberg , A . 2002 . Incorporation and release of hydrophobic probes in biocompatible polycaprolactone-block-poly(ethylene oxide) micelles: Implications for drug delivery . Langmuir , 18 : 9996 – 10004 .
- Soo , PL , Lovric , J , Davidson , P , Maysinger , D and Eisenberg , A . 2005 . Polycaprolactone-block-poly(ethylene oxide) micelles: A nanodelivery system for 17β-estradiol . Molecular Pharmaceutics , 2 : 519 – 527 .
- Allen , C , Yu , Y , Maysinger , D and Eisenberg , A . 1998 . Polycaprolactone-b-poly(ethylene oxide) block copolymer micelles as a novel drug delivery vehicle for neurotrophic agents FK506 and L-685,818 . Bioconjugate Chemistry , 9 : 564 – 572 .
- Hu , Y , Zhang , L , Cao , Y , Ge , H , Jiang , X and Yang , C . 2004 . Degradation behavior of poly(ϵ-caprolactone)-b-poly(ethylene glycol)-b-poly(ϵ-caprolactone) micelles in aqueous solution . Biomacromolecules , 5 : 1756 – 1762 .
- Gong , CY , Shi , S , Dong , PW , Kan , B , Gou , ML , Wang , XH , Li , XY , Luo , F , Zhao , X , Wei , YQ and Qian , ZY . 2009 . Synthesis and characterization of PEG-PCL-PEG thermosensitive hydrogel . International Journal of Pharmaceutics , 365 : 89 – 99 .
- Lee , SC , Kim , KJ , Jeong , YK , Chang , JH and Choi , J . 2005 . pH-induced reversible complexation of poly(ethylene glycol) and poly(ϵ-caprolactone)-b-poly(methacrylic acid) copolymer micelles . Macromolecules , 38 : 9291 – 9297 .
- Wang , YC , Liu , XQ , Sun , TM , Xiong , MH and Wang , J . 2008 . Functionalized micelles from block copolymer of polyphosphoester and poly(ϵ-caprolactone) for receptor-mediated drug delivery . Journal of Controlled Release , 128 : 32 – 40 .
- Zhou , J , Wang , L , Ma , J , Wang , J , Yu , H and Xiao , A . 2010 . Temperature- and pH-responsive star amphiphilic block copolymer prepared by a combining strategy of ring-opening polymerization and reversible addition- fragmentation transfer polymerization . European Polymer Journal , 46 : 1288 – 1298 .
- Huang , Y , Li , L and Fang , Y . 2010 . Self-assembled particles of N-phthaloylchitosan-g-polycaprolactone molecular bottle brushes as carriers for controlled release of indometacin . Journal of Materials Science: Materials in Medicine , 21 : 557 – 565 .
- Danhier , F , Magotteaux , N , Ucakar , B , Lecouturier , N , Brewster , M and Preat , V . 2009 . Novel self-assembling PEG-p-(CL-co-TMC) polymeric micelles as safe and effective delivery system for paclitaxel . European Journal of Pharmaceutics and Biopharmaceutics , 73 : 230 – 238 .
- Yang , Z , Xie , J , Zhou , W and Shi , W . 2009 . Temperature sensitivity and drug encapsulation of star-shaped amphiphilic block copolymer based on dendritic poly(ether-amide) . Journal of Biomedical Materials Research , 89 : 988 – 1000 .
- Yasugi , K , Nakamura , T , Nagasaki , Y , Kato , M and Kataoka , K . 1999 . Sugar-installed polymer micelles: Synthesis and micellization of poly(ethylene glycol)-poly(d,l-lactide) block copolymers having sugar groups at the PEG chain end . Macromolecules , 32 : 8024 – 8032 .
- Jule , E , Nagasaki , Y and Kataoka , K . 2002 . Surface plasmon resonance study on the interaction between lactose-installed poly(ethylene glycol)-poly(d,l-lactide) block copolymer micelles and lectins immobilized on a gold surface . Langmuir , 18 : 10334 – 10339 .
- Arimura , H , Ohya , Y and Ouchi , T . 2004 . The formation of biodegradable polymeric micelles from newly synthesized poly(aspartic acid)-block-polylactide AB-type diblock copolymers . Macromolecular Rapid Communications , 25 : 743 – 747 .
- Stepaek , M , Uchman , M and Prochazka , K . 2009 . Self-assemblies formed by four-arm star copolymers with amphiphilic diblock arms in aqueous solutions . Polymer , 50 : 3638 – 3644 .
- Xun , W , Wang , HY , Li , ZY , Cheng , SX , Zhang , XZ and Zhuo , RX . 2011 . Self-assembled micelles of novel graft amphiphilic copolymers for drug controlled release . Colloids and Surfaces B: Biointerfaces , 85 : 86 – 91 .
- Benahmed , A , Ranger , M and Leroux , JC . 2001 . Novel polymeric micelles based on the amphiphilic diblock copolymer poly(N-vinyl-2-pyrrolidone)-block-poly(D,L-lactide) . Pharmaceutical Research , 18 : 323 – 328 .
- Yin , H and Bae , YH . 2009 . Physicochemical aspects of doxorubicin-loaded pH-sensitive polymeric micelle formulations from a mixture of poly(L-histidine)-b-poly(L-lactide)- b-poly(ethylene glycol) . European Journal of Pharmaceutics and Biopharmaceutics , 71 : 223 – 230 .
- Huang , CK , Lo , CL , Chen , HH and Hsiue , GH . 2007 . Multifunctional micelles for cancer cell targeting, distribution imaging, and anticancer drug delivery . Advanced Functional Materials , 17 : 2291 – 2297 .
- Bhattarai , N , Bhattarai , SR , Yi , HK , Lee , JC , Khil , MS , Hwang , PH and Kim , HY . 2003 . Novel polymeric micelles of amphiphilic triblock copolymer poly(p-dioxanone-co-L-lactide)-block-poly(ethylene glycol) . Pharmaceutical Research , 20 : 2021 – 2027 .
- Martinez-Castro , N , Zhang , M , Pergushov , DV and Müller , AHE . 2006 . Anionic polymerization of N,N-dimethylacrylamide with thienyllithium and synthesis of block co-polymers of isobutylene and N,N-dimethylacrylamide by site transformation of chain ends . Designed Monomers and Polymers , 9 : 63 – 79 .
- Volpert , E , Selb , J and Candau , F . 1998 . Associating behaviour of polyacrylamides hydrophobically modified with dihexylacrylamide . Polymer , 39 : 1025 – 1033 .
- Quanming , L , Guohua , J and Kangkang , T . 2010 . Dendrimers in drug-delivery applications . Designed Monomers and Polymers , 13 : 301 – 324 .
- Chang , Y and Kim , C . 2001 . Synthesis and Photophysical Characterization of Amphiphilic Dendritic–Linear–Dendritic Block Copolymers . Journal of Polymer Science Part A: Polymer Chemistry , 39 : 918 – 926 .
- Namazi , H and Jafarirad , S . 2011 . Application of hybrid organic/inorganic dendritic ABA type triblock copolymers as new nanocarriers in drug delivery systems . International Journal of Polymeric Materials , 60 : 603 – 619 .
- Gao , C and Yan , D . 2004 . Hyperbranched polymers: from synthesis to applications . Progress in Polymer Science , 29 : 183 – 275 .
- Guohua , J , Wenxing , C and Wei , X . 2008 . Environmental-sensitive hyperbranched polymers as drug carriers . Designed Monomers and Polymers , 11 : 105 – 122 .
- Amin , A , Rehim , MA and Mohamed , H . 2008 . Modification of some hyperbranched polyols with oligo (ethyleneoxide) units on the way to new applications . Polymer-Plastics Technology and Engineering , 47 : 1250 – 1255 .
- Xiao , Y , Hong , H , Javadi , A , Engle , JW , Xu , W , Yang , Y , Zhang , Y , Barnhart , TE , Cai , W and Gong , Shaoqin . 2012 . Multifunctional unimolecular micelles for cancer-targeted drug delivery and positron emission tomography imaging . Biomaterials , 33 : 3071 – 3082 .
- Yoshida , E . 2007 . TEM observation of nonamphiphilic copolymer micelles . Colloid and Polymer Science , 285 : 941 – 945 .
- Yoshida , E and Ohta , M . 2005 . Preparation of micelles having a UV absorbent at their coronas using a ’non-amphiphilic’ di-block co-polymer . Designed Monomers and Polymers , 8 : 501 – 513 .
- Bates , FS and Fredrickson , GH . 1990 . Block copolymer thermodynamics: theory and experiment . Annual Review of Physical Chemistry , 41 : 525 – 557 .
- Cao , T , Munk , P , Ramireddy , C , Tuzar , Z and Webber , SE . 1991 . Fluorescence studies of amphiphilic poly(methacrylic acid)-block-polystyrene-block-poly-(methacrylic acid) micelles . Macromolecules , 24 : 6300 – 6305 .
- Tsunashima , Y , Hirata , M and Kawamata , Y . 1990 . Diffusion motions and microphase separation of styrene-butadiene diblock copolymer in solution. 1. Extremely dilute solution region . Macromolecules , 23 : 1089 – 1096 .
- Prochazka , K , Martin , TJ , Webber , SE and Munk , P . 1996 . Onion type micelles in aqueous media . Macromolecules , 29 : 6526 – 6530 .
- Giacomelli , C and Borsali , R . 2006 . Morphology of poly(ethylene oxide)-block-polycaprolatone block copolymer micelles controlled via the preparation method . Macromolecular Symposium , 245–246 : 147 – 153 .
- Tian , M , Qin , A , Ramireddy , C , Webber , SE , Munk , P , Tuzar , Z and Prochazka , K . 1993 . Hybridization of block copolymer micelles . Langmuir , 9 : 1741 – 1748 .
- Hirano , T , Klesse , W and Ringsdorf , H . 1979 . Polymeric derivatives of activated cyclophosphamide as drug delivery systems in anti-tumor chemotherapy- Pharmacologically active polymers . Macromolecular Chemistry and Physics , 180 : 1125 – 1131 .
- Gros , L , Ringsdorf , H and Schupp , H . 1981 . Polymeric antitumor agents on a molecular and on a cellular level? . Angewandte Chemie (International Edition in English) , 20 : 305 – 325 .
- Yokoyama , M , Fukushima , S , Uehara , R , Okamoto , K , Kataoka , K , Sakurai , Y and Okano , T . 1998 . Characterization of physical entrapment and chemical conjugation of adriamycin in polymeric micelles and their design for in vivo delivery to a solid tumor . Journal of Controlled Release , 50 : 79 – 92 .
- He , F , Li , SM , Vert , M and Zhuo , RX . 2003 . Enzyme-catalyzed polymerization and degradation of copolymers prepared from ϵ-caprolactone and poly(ethylene glycol) . Polymer , 44 : 5145 – 5151 .
- Chen , HL , Bei , JZ and Wang , SG . 2000 . Hydrolytic degradation of polyester-polyether block copolymer based on polycaprolactone/poly(ethylene glycol)/polylactide . Polymers for Advanced Technologies , 11 : 180 – 184 .
- Allen , C , Maysinger , D and Eisenberg , A . 1999 . Nano-engineering block copolymer aggregates for drug delivery . Colloids and Surfaces B: Biointerfaces , 16 : 3 – 27 .
- Bromberg , L and Magner , E . 1999 . Release of hydrophobic compounds from micellar solutions of hydrophobically modified polyelectrolytes . Langmuir , 15 : 6792 – 6798 .
- Patrick , LS , Laibin , L , Maysinger , D and Eisenberg , A . 2002 . Incorporation and release of hydrophobic probes in biocompatible polycaprolactone-block-poly(ethylene oxide) micelles: Implications for drug delivery . Langmuir , 18 : 9996 – 10004 .
- Aliabadi , HM , Elhasi , S , Mahmud , A , Gulamhusein , R , Mahdipoor , P and Lavasanifar , A . 2007 . Encapsulation of hydrophobic drugs in polymeric micelles through co-solvent evaporation: The effect of solvent composition on micellar properties and drug loading . International Journal of Pharmaceutics , 329 : 158 – 165 .
- Gohy , JF . 2005 . Block copolymer micelles . Advances in Polymer Science , 190 : 65 – 136 .
- Jette , KK , Law , D , Schmitt , EA and Kwon , GS . 2004 . Preparation and drug loading of poly(ethylene glycol)-block-poly(-caprolactone) micelles through the evaporation of a cosolvent azeotrope . Pharmaceutical Research , 21 : 1184 – 1191 .
- Park , KM , Bae , JW , Joung , YK , Shin , JW and Park , KD . 2008 . Nanoaggregate of thermosensitive chitosan-Pluronic for sustained release of hydrophobic drug . Colloids and Surfaces B: Biointerfaces , 63 : 1 – 6 .
- Gou , M , Zheng , X , Men , K , Zhang , J , Wang , B , Lei , L , Wang , X , Zhao , Y , Luo , F , Chen , L , Zhao , X , Wei , Y and Qian , Z . 2009 . Self-assembled hydrophobic honokiol loaded MPEG-PCL diblock copolymer micelles . Pharmaceutical Research , 26 : 2164 – 2173 .
- Chiappetta , DA , Degrossi , J , Teves , S , D’Aquino , M , Bregni , C and Sosnik , A . 2008 . Triclosan-loaded poloxamine micelles for enhanced topical antibacterial activity against biofilm . European Journal of Pharmaceutics and Biopharmaceutics , 69 : 535 – 545 .
- Nishiyama , N , Kato , Y , Sugiyama , Y and Kataoka , K . 2001 . Cisplatin-loaded polymer-metal complex micelle with time-modulated decaying property as a novel drug delivery system . Pharmaceutical Research , 18 : 1035 – 1041 .
- Xu , L , Chen , H , Xu , H and Yang , X . 2008 . Anti-tumour and immuno-modulation effects of triptolide-loaded polymeric micelles . European Journal of Pharmaceutics and Biopharmaceutics , 70 : 741 – 748 .
- Vakil , R , Knilans , K , Andes , D and Kwon , GS . 2008 . Combination antifungal therapy involving amphotericin B, rapamycin and 5-fluorocytosine using PEG-phospholipid micelles . Pharmaceutical Research , 25 : 2056 – 2064 .
- Ma , Z , Haddadi , A , Molavi , O , Lavasanifar , A , Lai , R and Samuel , J . 2008 . Micelles of poly(ethylene oxide)-b-poly(e-caprolactone) as vehicles for the solubilization, stabilization, and controlled delivery of curcumin . Journal of Biomedical Materials Research , 86A : 300 – 310 .
- Jiang , T , Wang , Z , Tang , L , Mo , F and Chen , C . 2006 . Polymer micellar aggregates of novel amphiphilic biodegradable graft copolymer composed of poly (aspartic acid) derivatives: Preparation, characterization, and effect of pH on aggregation . Journal of Applied Polymer Science , 99 : 2702 – 2709 .
- Jiang , T , Wang , Z , Chen , C , Mo , F , Xu , Y , Tang , L and Liang , J . 2006 . Poly(aspartic-acid) derivatives as polymeric micelle drug delivery systems . Journal of Applied Polymer Science , 101 : 2871 – 2878 .
- Bian , F , Jia , L , Yu , W and Liu , M . 2009 . Self-assembled micelles of N-phthaloylchitosan-g-polyvinylpyrrolidone for drug delivery . Carbohydrate Polymers , 76 : 454 – 459 .
- Francis , MF , Cristea , M , Yang , Y and Winnik , FM . 2005 . Engineering polysaccharide-based polymeric micelles to enhance permeability of cyclosporin A across Caco-2 cells . Pharmaceutical Research , 22 : 209 – 219 .
- Savi , R , Azzam , T , Eisenberg , A and Maysinger , D . 2006 . Assessment of the integrity of poly(caprolactone)-b-poly(ethylene oxide) micelles under biological conditions: A fluorogenic-based approach . Langmuir , 22 : 3570 – 3578 .
- Kwon , GS , Naito , M , Yokoyama , M , Okano , T , Sakurai , Y and Kataoka , K . 1993 . Micelles based on AB block copolymers of poly(ethylene oxide) and poly(β-benzyl L-aspartate) . Langmuir , 9 : 945 – 949 .
- Dowling , KC and Thomas , JK . 1990 . A novel micellar synthesis and photophysical characterization of water-soluble acrylamide-styrene block copolymers . Macromolecules , 23 : 1059 – 1064 .
- Torchilin , VP . 2001 . Structure and design of polymeric surfactant-based drug delivery systems . Journal of Controlled Release , 73 : 137 – 172 .
- Bourov , GK and Bhattacharya , A . 2005 . Brownian dynamics simulation study of self-assembly of amphiphiles with large hydrophilic heads . Journal of Chemical Physics , 122 : 044702
- Suek , NW and Lamm , MH . 2008 . Computer simulation of architectural and molecular weight effects on the assembly of amphiphilic linear-dendritic block copolymers in solution . Langmuir , 24 : 3030 – 3036 .
- Gao , Z , Anatoly , NL , Singhal , A and Torchilin , VP . 2002 . Diacyllipid-polymer micelles as nanocarriers for poorly soluble anticancer drugs . Nano Letters , 2 : 979 – 982 .
- Bae , YH and Yin , H . 2008 . Stability issues of polymeric micelles . Journal of Controlled Release , 131 : 2 – 4 .
- Schick , MJ and Gilbert , AH . 1965 . Effect of urea, guanidinium chloride, and dioxane on the c.m.c. of branched-chain nonionic detergents . Journal of Colloid Science , 20 : 464 – 472 .
- Rosen , MJ , Cohen , AW , Dahanayake , M and Hua , X . 1982 . Relationship of structure to properties in surfactants. 10. Surface and thermodynamic properties of 2-dodecyloxypoly(ethenoxyethanol)s, C12H25(OC2H4)xOH, in aqueous solution . Journal of Physical Chemistry , 86 : 541 – 545 .
- Al-Sabagh , AM . 1998 . Surface and thermodynamic properties of p-alkylbenzene polyethoxylated and glycerated sulfonate derivative surfactants . Colloids and Surfaces A: Physicochemical and Engineering Aspects , 134 : 313 – 320 .
- Soga , O , Nostrum , CF , Ramzi , A , Visser , T , Soulimani , F , Frederik , PM , Bomans , PH and Hennink , WE . 2004 . Physicochemical characterization of degradable thermosensitive polymeric micelles . Langmuir , 20 : 9388 – 9395 .
- Gadelle , F , Koros , WJ and Schechter , RS . 1995 . Solubilization of aromatic solutes in block copolymers . Macromolecules , 28 : 4883 – 4892 .
- Kozlov , MY , Melik-Nubarov , NS , Batrakova , EV and Kabanov , AV . 2000 . Relationship between Pluronic block copolymer structure, critical micellization concentration and partitioning coefficients of low molecular mass solutes . Macromolecules , 33 : 3305 – 3313 .
- Cheng , L and Cao , D . 2009 . Effect of tail architecture on self-assembly of amphiphiles for polymeric micelles . Langmuir , 25 : 2749 – 2756 .
- Quintana , JR , Villacampa , M , Munoz , M , Andrio , A and Katime , IA . 1992 . Micellization of a polystyrene-block-poly(ethylene/propylene) copolymer in n-alkanes. 1. Thermodynamic study . Macromolecules , 25 : 3125 – 3129 .
- Quintana , JR , Villacampa , M and Katime , IA . 1993 . Micellization of a polystyrene-b-poly(ethylene/propylene) block copolymer in n-dodecane/1,4-dioxane mixtures. 1. Thermodynamics of micellization . Macromolecules , 26 : 601 – 605 .
- Thurn , T , Couderc , S , Sidhu , J , Bloor , DM , Penfold , J , Holzwarth , JF and Wyn-Jones , E . 2002 . Study of mixed micelles and interaction parameters for ABA triblock copolymers of the type EOm-POn-EOm and ionic surfactants: Equilibrium and structure . Langmuir , 18 : 9267 – 9275 .
- Elisseeva , OV , Besseling , NA , Koopal , LK and Cohen , MA . 2005 . Influence of NaCl on the behavior of PEO-PPO-PEO triblock copolymers in solution, at interfaces, and in asymmetric liquid films . Langmuir , 21 : 4954 – 4963 .
- Alvarez-Lorenzo , C , Gonzalez-Lopez , J , Fernandez-Tarrio , M , Sandez-Macho , I and Concheiro , A . 2007 . Tetronic micellization, gelation and drug solubilization: Influence of pH and ionic strength . European Journal of Pharmaceutics and Biopharmaceutics , 66 : 244 – 252 .
- Elias , HG . 1973 . Nonionic micelles . Journal of Macromolecular Science-Chemistry , 7 : 601 – 622 .
- Khougaz , K , Gao , Z and Eisenberg , A . 1994 . Determination of the critical micelle concentration of block copolymer micelles by static light scattering . Macromolecules , 27 : 6341 – 6346 .
- El-Ghazawy , RA , Raheem , RA and Al-Sabagh , AM . 2004 . Surface activity-thermodynamic properties and light scattering studies for some novel aliphatic polyester surfactants . Polymers for Advanced Technologies , 15 : 244 – 250 .
- Kalyanasundaram , K and Thomas , JK . 1977 . Environmental effects on vibronic band intensities in pyrene monomer fluorescence and their application in studies of micellar systems . Journal of the American Chemical Society , 99 : 2039 – 2044 .
- Dong , DC and Winnik , MA . 1984 . The Py scale of solvent polarities . Canadian Journal of Chemistry , 62 : 2560 – 2565 .
- Abuin , EB and Scaiano , JC . 1984 . Exploratory study of the effect of polyelectrolyte surfactant aggregates on photochemical behavior . Journal of the American Chemical Society , 106 : 6274 – 6283 .
- Almgren , M , Hansson , P , Mukhtar , E and Van Stam , J . 1992 . Aggregation of alkyltrimethylammonium surfactants in aqueous poly(styrenesulfonate) solutions . Langmuir , 8 : 2405 – 2412 .
- Shin , IL , Kim , SY , Lee , YM , Cho , CS and Sung , YK . 1998 . Methoxy poly(ethylene glycol)-ϵ-caprolactone amphiphilic block copolymeric micelle containing indomethacin 1. Preparation and characterization . Journal of Controlled Release , 51 : 1 – 11 .
- Colombani , O , Ruppel , M , Schubert , F , Zettl , H , Pergushov , DV and Muller , AHE . 2007 . Synthesis of poly(n-butyl acrylate)-block-poly(acrylic acid) diblock copolymers by ATRP and their micellization in water . Macromolecules , 40 : 4338 – 4350 .
- Chen , YW , Alexandridis , P , Su , CK , Patrickios , CS , Hertler , WR and Hatton , TA . 1995 . Effect of block size and sequence on the micellization of ABC triblock methacrylic acid polyampholytes . Macromolecules , 28 : 8604 – 8611 .
- Zhang , X , Jackson , JK and Burt , HM . 1996 . Development of amphiphilic diblock copolymers as micellar carriers of taxol . International Journal of Pharmaceutics , 132 : 195 – 206 .
- Zhang , X , Jackson , JK and Burt , HM . 1996 . Determination of surfactant micelle concentration by a novel fluorescence depolarization technique . Journal of Biochemical and Biophysical Methods , 31 : 145 – 150 .
- Shi , B , Fang , C , Xian , M , Yan , Y , Shoukuan , Z , Yuan , F and Pei , Y . 2005 . Stealth MePEG-PCL micelles: Effects of polymer composition on micelle physicochemical characteristics, in vitro drug release, in vivo pharmacokinetics in rats and biodistribution in S180 tumor bearing mice . Colloid and Polymer Science , 283 : 954 – 967 .
- Wu , QH , Liang , F , Wei , TZ , Song , XM , Liu , DL and Zhang , GL . 2010 . Synthesis and micellization of an amphiphilic biodegradable β-cyclodextrin/poly(γ-benzyl L-glutamate) copolymer . Journal of Polymer Research , 17 : 183 – 190 .
- Zhang , G , Liang , F , Song , X , Liu , D , Li , M and Wu , Q . 2010 . New amphiphilic biodegradable b-cyclodextrin/poly(L-leucine) copolymers: Synthesis, characterization, and micellization . Carbohydrate Polymers , 80 : 885 – 890 .
- Zhang , W , Li , Y , Liu , L , Sun , Q , Shuai , X , Zhu , W and Chen , Y . 2010 . Amphiphilic toothbrushlike copolymers based on poly(ethylene glycol) and poly(ϵ-caprolactone) as drug carriers with enhanced properties . Biomacromolecules , 11 : 1331 – 1338 .
- Mohanty , AK , Dilnawaz , F , Mohanty , C and Sahoo , SK . 2010 . Etoposide-loaded biodegradable amphiphilic methoxy (poly ethylene glycol) and poly (e-caprolactone) copolymeric micelles as drug delivery vehicle for cancer therapy . Drug Delivery , 17 : 330 – 342 .
- Lee , JS , Go , DH , Bae , JW , Lee , SJ and Park , KD . 2007 . Heparin conjugated polymeric micelle for long-term delivery of basic fibroblast growth factor . Journal of Controlled Release , 117 : 204 – 209 .
- Eckert , AR and Webber , SE . 1996 . Naphthalene-tagged copolymer micelles based on polystyrene-alt-maleic anhydride-graft-poly(ethylene oxide) . Macromolecules , 29 : 560 – 567 .
- Teng , Y , Morrison , ME , Munk , P , Webber , SE and Prochazka , K . 1998 . Release kinetics studies of aromatic molecules into water from block polymer micelles . Macromolecules , 31 : 3578 – 3587 .
- Zhang , Y , Jin , T and Zhuo , R . 2005 . Methotrexate-loaded biodegradable polymeric micelles: Preparation, physicochemical properties and in vitro drug release . Colloids and Surfaces B: Biointerfaces , 44 : 104 – 109 .
- Trimaille , T , Mondon , K , Gurny , R and Moller , M . 2006 . Novel polymeric micelles for hydrophobic drug delivery based on biodegradable poly(hexyl-substituted lactides) . International Journal of Pharmaceutics , 319 : 147 – 154 .
- Mikhail , AS and Allen , C . 2010 . Poly(ethylene glycol)-b-poly(ϵ-caprolactone) micelles containing chemically conjugated and physically entrapped docetaxel: Synthesis, characterization, and the influence of the drug on micelle morphology . Biomacromolecules , 11 : 1273 – 1280 .
- Astafieva , I , Zhong , X and Eisenberg , FA . 1993 . Critical micellization phenomena in block polyelectrolyte solutions . Macromolecules , 26 : 7339 – 7352 .
- Zhang , L and Eisenberg , A . 1998 . Formation of crew-cut aggregates of various morphologies from amphiphilic block copolymers in solution . Polymers for Advanced Technologies , 9 : 677 – 699 .
- Yao , JH , Mya , KY , Li , X , Parameswaran , M , Xu , Q , Loh , KP and Chen , Z . 2008 . Light scattering and luminescence studies on self-aggregation behavior of amphiphilic copolymer micelles . Journal of Physical Chemistry B , 112 : 749 – 755 .
- Lin , Y and Alexandridis , P . 2002 . Small-angle neutron scattering characterization of micelles formed by poly(dimethylsiloxane)-graft-polyether copolymers in mixed polar solvents . Journal of Physical Chemistry B , 106 : 12124 – 12132 .
- Soni , SS , Sastry , NV , Aswal , VK and Goyal , PS . 2002 . Micellar structure of silicone surfactants in water from surface activity, SANS and viscosity studies . Journal of Physical Chemistry B , 106 : 2606 – 2617 .
- Ramzi , A , Rijcken , CJF , Veldhuis , T , Schwahn , D , Hennink , WE and Nostrum , C . 2008 . Core-shell structure of degradable, thermosensitive polymeric micelles studied by small-angle neutron scattering . Journal of Physical Chemistry B , 112 : 784 – 792 .
- Yokoyama , M , Sugiyama , T , Okano , T , Sakurai , Y , Naito , M and Kataoka , K . 1993 . Analysis of micelle formation of an adriamycin-conjugated poly(ethylene glycol)-poly(aspartic acid) block copolymer by gel permeation chromatography . Pharmaceutical Research , 10 : 895 – 899 .
- Yang , L , Qi , X , Liu , P , Ghazoui , AE and Li , S . 2010 . Aggregation behavior of self-assembling polylactide/poly(ethylene glycol) micelles for sustained drug delivery . International Journal of Pharmaceutics , 394 : 43 – 49 .
- Nagasaki , Y , Okada , T , Scholz , C , Iijima , M , Kato , M and Kataoka , K . 1998 . The reactive polymeric micelle based on an aldehyde-ended poly(ethylene glycol)/poly(lactide) block copolymer . Macromolecules , 31 : 1473 – 1479 .
- Yokoyama , M , Miyauchi , M , Yamada , N , Okano , T , Sakurai , Y , Kataoka , K and Inoue , S . 1990 . Characterization and anticancer activity of the micelle-forming polymeric anticancer drug adriamycin-conjugated poly(ethylene glycol)-poly (aspartic acid) block copolymer . Cancer Research , 50 : 1693 – 1700 .
- Booth , C and Attwood , D . 2000 . Effects of block architecture and composition on the association properties of poly(oxyalkylene) copolymers in aqueous solution . Macromolecular Rapid Communications , 21 : 501 – 527 .
- Cao , T , Yin , W and Webber , SE . 1994 . Poly(2-vinylnaphthalene-alt-maleic acid)-graft-polystyrene as a photoactive polymer micelle and stabilizer for polystyrene latexes . Macromolecules , 27 : 7459 – 7464 .
- Webber , SE . 1998 . Polymer micelles: An example of self-assembling polymers . Journal of Physical Chemistry B , 102 : 2618 – 2626 .
- Sun , H , Guo , B , Li , X , Cheng , R , Meng , F , Liu , H and Zhong , Z . 2010 . Shell-sheddable micelles based on dextran-ss-poly(ϵ-caprolactone) diblock copolymer for efficient intracellular release of doxorubicin . Biomacromolecules , 11 : 848 – 854 .
- Kim , SY , Shin , IG , Lee , YM , Cho , CS and Sung , YK . 1998 . Methoxy poly(ethylene glycol) and ϵ-caprolactone amphiphilic block copolymeric micelle containing indomethacin. II. Micelle formation and drug release behavior . Journal of Controlled Release , 51 : 13 – 22 .
- Yu , BG , Okano , T , Kataoka , K and Kwon , G . 1998 . Polymeric micelles for drug delivery: Solubilization and haemolytic activity of amphotericin B . Journal of Controlled Release , 53 : 131 – 136 .
- Del-real , A , Garcóa-Garduño , MV and Castaño , VM . 1998 . Transmission electron microscopy observations of micelle structure in a vinyl acetate-based microemulsion . International Journal of Polymeric Materials , 42 : 319 – 326 .
- Kohori , F , Sakai , K , Aoyagi , T , Yokoyama , M , Sakurai , Y and Okano , T . 1998 . Preparation and characterization of thermally responsive block copolymer micelles comprising poly(N-isopropylacrylamide-b-D,L-lactide) . Journal of Controlled Release , 55 : 87 – 98 .
- Mueller-Goymann , CC . 2001 . “ Drug delivery: Liquid crystals ” . In Encyclopedia of pharmaceutical technology , Edited by: Swarbrick , J . 1115 – 1131 . New York , NY : Informa Healthcare .
- Allemann , E , Leroux , JC and Gurny , R . 1998 . “ Biodegradable nanoparticles of poly(lactic acid) and poly(lactic-co-glycolic acid) for parenteral administration ” . In Pharmaceutical dosage forms: disperse systems , Edited by: Lieberman , H , Rieger , M and Banker , G . 163 – 193 . New York , NY : Marcel Dekker .
- Kang , DY , Kim , MJ , Kim , ST , Oh , KS , Yuk , SH and Lee , S . 2008 . Size characterization of drug-loaded polymeric core/shell nanoparticles using asymmetrical flow field-flow fractionation . Analytical and Bioanalytical Chemistry , 390 : 2183 – 2188 .
- Winnik , MA , Pekcan , O and Croucher , MD . 1990 . “ Fluorescence techniques in the study of polymer colloid ” . In Scientific methods for the study of polymer colloids and their applications , Edited by: Candau , F and Ottewill , RH . 225 – 246 . Dordrecht : Kluwer Academic .
- Winnik , FM , Davidson , AR , Hamer , GR and Kitano , H . 1992 . Amphiphilic poly(N-isopropylacrylamides) prepared by using a lipophilic radical initiator- synthesis and solution properties in water . Macromolecules , 25 : 1876 – 1880 .
- Nivaggioli , T , Tsao , B , Alexandridis , P and Hatton , TA . 1995 . Microviscosity in Pluronic and Tetronic poly(ethylene oxide)-poly(propylene oxide) block copolymer micelles . Langmuir , 11 : 119 – 126 .
- Bahadur , P , Sastry , NV , Rao , YK and Riess , G . 1988 . Interaction studies of styreneethylene oxide block copolymers with ionic surfactants in aqeuous solution . Colloids and Surfaces , 29 : 343 – 358 .
- Kwon , GS . 2003 . Polymeric micelles for delivery of poorly water-soluble compounds . Critical Reviews in Therapeutic Drug Carrier Systems , 20 : 357 – 403 .
- Torchilin , VP . 2006 . “ Polymeric micelles as pharmaceutical carriers ” . In Polymers in drug delivery , Edited by: Uchegbu , IF and Schatzlein , AG . 111 – 130 . Boca Raton , FL : Taylor & Francis .
- Liu , J , Zeng , F and Allen , C . 2007 . In vivo fate of unimers and micelles of a poly(ethylene glycol)-block-poly(caprolactone) copolymer in mice following intravenous administration . European Journal of Pharmaceutics and Biopharmaceutics , 65 : 309 – 319 .
- Yang , C , Attia , ABE , Tan , JPK , Ke , X , Gao , S , Hedrick , JL and Yang , YY . 2012 . The role of non-covalent interactions in anticancer drug loading and kinetic stability of polymeric micelles . Biomaterials , 33 : 2971 – 2979 .
- Lo , CL , Huang , CK , Lin , KM and Hsiue , GH . 2007 . Mixed micelles formed from graft and diblock copolymers for application in intracellular drug delivery . Biomaterials , 28 : 1225 – 1235 .
- Carstens , MG , Jong , PH , Nostrum , CF , Kemmink , J , Verrijk , R , Leede , LG , Crommelin , DA and Hennink , WE . 2008 . The effect of core composition in biodegradable oligomeric micelles as taxane formulations . European Journal of Pharmaceutics and Biopharmaceutics , 68 : 596 – 606 .
- Tao , Y , Xu , J , Chen , M , Bai , H and Liu , X . 2012 . Core cross-linked hyaluronan-styrylpyridinium micelles as a novel carrier for paclitaxel . Carbohydrate Polymers , 88 : 118 – 124 .
- Thurmond , KB II , Kowalewski , T and Wooley , KL . 1996 . Water-soluble knedel-like structures: the preparation of shell-cross-linked small particles . Journal of the American Chemical Society , 118 : 7239 – 7240 .
- Kataoka , K , Kwon , GS and Yokoy , M . 1993 . Block copolymer micelles as vehicles for drug delivery . Journal of Controlled Release , 24 : 119 – 132 .
- Chen , L , Xie , Z , Hu , J , Chen , X and Jing , X . 2007 . Enantiomeric PLA-PEG block copolymers and their stereocomplex micelles used as rifampin delivery . Journal of Nanoparticle Research , 9 : 777 – 785 .
- Gaucher , G , Dufresne , M , Sant , VP , Kang , N , Maysinger , D and Leroux , JC . 2005 . Block copolymer micelles: Preparation, characterization and application in drug delivery . Journal of Controlled Release , 109 : 169 – 188 .
- Mahmud , A , Xiong , XB and Lavasanifar , A . 2006 . Novel self-associating poly(ethylene oxide)-block-poly(1-caprolactone) block copolymers with functional side groups on the polyester block for drug delivery . Macromolecules , 39 : 9419 – 9428 .
- Kim , JO , Kabanov , AV and Bronich , TK . 2009 . Polymer micelles with cross-linked polyanion core for delivery of a cationic drug doxorubicin . Journal of Controlled Release , 138 : 197 – 204 .
- Zhang , L , Bernard , J , Davis , TP , Barner-Kowollik , C and Stenzel , MH . 2008 . Acid-degradable core-crosslinked micelles prepared from thermosensitive glycopolymers synthesized via RAFT polymerization . Macromolecular Rapid Communications , 29 : 123 – 129 .
- Rosler , A , Vandermeulen , WM and Klok , HA . 2001 . Advanced drug delivery devices via self-assembly of amphiphilic block copolymers . Advanced Drug Delivery Reviews , 53 : 95 – 108 .
- Murthy , KS , Ma , Q , Clark , CG , Remsen , EE and Wooley , KL . 2001 . Fundamental design aspects of amphiphilic shell-crosslinked nanoparticles for controlled release applications . Chemical Communications , : 773 – 774 .
- Huang , H , Remsen , EE , Kowalewski , T and Wooley , KL . 1999 . Nanocages derived from shell cross-linked micelle templates . Journal of the American Chemical Society , 121 : 3805 – 3806 .
- Wang , YC , Li , Y , Sun , TM , Xiong , MH , Wu , J , Yang , YY and Wang , J . 2010 . Core–shell–corona micelle stabilized by reversible cross-linkage for intracellular drug delivery . Macromolecular Rapid Communications , 31 : 1201 – 1206 .
- Henselwood , F and Liu , G . 1997 . Water-soluble nanospheres of poly(2-cinnamoylethyl methacrylate)-block-poly(acrylic acid) . Macromolecules , 30 : 488 – 493 .
- Kakizawa , Y , Harada , A and Kataoka , K . 2001 . Glutathione-sensitive stabilization of block copolymer micelles composed of antisense DNA and thiolated poly(ethylene glycol)-block-poly(L-lysine): a potential carrier for systemic delivery of antisense DNA . Biomacromolecules , 2 : 491 – 497 .
- Hu , J , Tang , Z , Qiu , X , Pang , X , Yang , Y , Chen , X and Jing , X . 2005 . Formation of flower- or cake-shaped stereocomplex particles from the stereo multiblock copoly(rac-lactide)s . Biomacromolecules , 6 : 2843 – 2850 .
- Stager , J and Domb , A . 2003 . Biopolymer stereocomplexes . Advanced Drug Delivery Reviews , 55 : 549 – 583 .
- Kang , N , Perron , ME , Prud’homme , RE , Zhang , Y , Gaucher , G and Leroux , JC . 2005 . Stereocomplex block copolymer micelles: Core-shell nanostructures with enhanced stability . Nano Letters , 5 : 315 – 319 .
- Carstens , MG , Bevernage , JJL , Van Nostrum , CF , Van Steenbergen , MJ , Flesch , FM , Verrijk , R , De Leede , LGJ , Crommelin , DJA and Hennink , WE . 2007 . Small oligomeric micelles based on end group modified mPEG-oligocaprolactone with monodisperse hydrophobic blocks . Macromolecules , 40 : 116 – 122 .
- Watanabe , M , Kawano , K , Yokoyama , M , Opanasopit , P , Okano , T and Maitani , Y . 2006 . Preparation of camptothecin-loaded polymeric micelles and evaluation of their incorporation and circulation stability . International Journal of Pharmaceutics , 308 : 183 – 189 .
- Reulen , S and Merkx , M . 2010 . Exchange kinetics of protein-functionalized micelles and liposomes studied by Förster resonance energy transfer . Bioconjugate Chemistry , 21 : 860 – 866 .
- Sezgin , Z , Yuksel , N and Baykara , T . 2006 . Preparation and characterization of polymeric micelles for solubilization of poorly soluble anticancer drugs . European Journal of Pharmaceutics and Biopharmaceutics , 64 : 261 – 268 .
- Bugrin , VS , Kozlov , MY , Baskin , II and Melik-Nubarov , NS . 2007 . Intermolecular interactions governing solubilization in micelles of poly(alkylene oxide) surfactants . Polymer Science Series A , 49 : 463 – 472 .
- El-Saeed , SM , Farag , RK , Abdul-Raouf , ME and Abdel-Azim , A . 2008 . Synthesis and characterization of novel crude oil dispersants based on ethoxylated schiff base . International Journal of Polymeric Materials , 57 : 860 – 877 .
- Chiappetta , DA and Sosnik , A . 2007 . Poly(ethylene oxide)-poly(propylene oxide) block copolymer micelles as drug delivery agents: Improved hydrosolubility, stability and bioavailability of drugs . European Journal of Pharmaceutics and Biopharmaceutics , 66 : 303 – 317 .
- Chi , SC , Yeom , D , Kim , SC and Park , ES . 2003 . A polymeric micellar carrier for the solubilization of biphenyl dimethyl dicarboxylate . Archives of Pharmacal Research , 26 : 173 – 181 .
- Kulthe , SS , Inamdar , NN , Choudhari , YM , Shirolikar , SM , Borde , LC and Mourya , VK . 2011 . Mixed micelle formation with hydrophobic and hydrophilic Pluronic block copolymers: Implications for controlled and targeted drug delivery . Colloids and Surfaces B: Biointerfaces , 88 : 691 – 696 .
- Witt , KA , Huber , JD , Egleton , RD and Davis , TP . 2002 . Pluronic P85 copolymer enhances opioid peptide analgesia . Journal of Pharmacology and Experimental Therapeutics , 303 : 760 – 767 .
- Gao , Y , Li , LB and Zhai , G . 2008 . Preparation and characterization of Pluronic/TPGS mixed micelles for solubilization of camptothecin . Colloids and Surfaces B: Biointerfaces , 64 : 194 – 199 .
- Mondon , K , Zeisser-Labouèbe , M , Gurny , R and Möller , M . 2011 . Novel Cyclosporin A formulations using MPEG–hexyl-substituted polylactide micelles: A suitability study . European Journal of Pharmaceutics and Biopharmaceutics , 77 : 56 – 65 .
- Chiappetta , DA , Hocht , C , Taira , C and Sosnik , A . 2011 . Oral pharmacokinetics of the anti-HIV efavirenz encapsulated within polymeric micelles . Biomaterials , 32 : 2379 – 2387 .
- Kabanov , AV , Ckekhonin , VP , Alakhov , V , Batrakova , EV , Lebedev , AS , Melik-Nubarov , NS , Arzhakov , S , Levashov , AV , Morozov , GV , Severin , ES and Kabanov , VA . 1989 . The neuroleptic activity of haloperidol increases after its solubilization in surfactant micelles . FEBS Letters , 258 : 343 – 345 .
- Kadam , Y , Yerramilli , U , Bahadur , A and Bahadur , P . 2011 . Micelles from PEO–PPO–PEO block copolymers as nanocontainers for solubilization of a poorly water soluble drug hydrochlorothiazide . Colloids and Surfaces B: Biointerfaces , 83 : 49 – 57 .
- Yang , L , Kuang , J , Wang , J , Li , Z and Zhang , LM . 2008 . Loading and in vitro controlled release of indomethacin using amphiphilic cholesteryl-bearing carboxymethylcellulose derivatives . Macromolecular Bioscience , 8 : 279 – 286 .
- Alakhov , V , Pietrzynski , G , Patel , K , Kabanov , A , Bromberg , L and Hatton , TA . 2004 . Pluronic block copolymers and Pluronic poly(acrylic acid) microgels in oral delivery of megestrol acetate . Journal of Pharmacy and Pharmacology , 56 : 1233 – 1241 .
- Dumortier , G , Groissord , JL , Zuber , M , Couarraze , G and Chaumeil , JC . 1991 . Rheological study of a thermoreversible morphine gel . Drug Development and Industrial Pharmacy , 17 : 1255 – 1265 .
- Parekh , P , Singh , K , Marangoni , DG and Bahadur , P . 2011 . Micellization and solubilization of a model hydrophobic drug nimesulide in aqueous salt solutions of Tetronic® T904 . Colloids and Surfaces B: Biointerfaces , 83 : 69 – 77 .
- Croy , SR and Kwon , GS . 2004 . The effects of Pluronic block copolymers on the aggregation state of nystatin . Journal of Controlled Release , 95 : 161 – 171 .
- Mo , R , Jin , X , Li , N , Ju , C , Sun , M , Zhang , C and Ping , Q . 2011 . The mechanism of enhancement on oral absorption of paclitaxel by N-octyl-O-sulfate chitosan micelles . Biomaterials , 32 : 4609 – 4620 .
- Momot , KI , Kuchel , PW , Deo , P and Whittaker , D . 2003 . NMR study of the association of propofol with nonionic surfactants . Langmuir , 19 : 2088 – 2095 .
- Xiong , MP , Yanez , JA , Kwon , GS , Davies , NM and Forrest , ML . 2009 . A cremophor-free formulation for tanespimycin (17-AAG) using PEO-b-PDLLA micelles: Characterization and pharmacokinetics in rats . Journal of Pharmaceutical Sciences , 98 : 1577 – 1586 .
- El-Kamel , AH . 2002 . In vitro and in vivo evaluation of Pluronic F127- based ocular delivery system for timolol maleate . International Journal of Pharmaceutics , 241 : 47 – 55 .
- Lee , H , Zeng , F , Dunne , M and Allen , C . 2005 . Methoxy poly(ethylene glycol)-block-poly(δ-valerolactone) copolymer micelles for formulation of hydrophobic drugs . Biomacromolecules , 6 : 3119 – 3128 .
- Kabanov , AV and Alakov , VY . 2002 . Pluronic block copolymers in drug delivery: From micellar nanocontainers to biological response modifiers . Critical Reviews in Therapeutic Drug Carrier Systems , 19 : 1 – 73 .
- Liu , JB , Xiao , YH and Allen , C . 2004 . Polymer-drug compatibility: a guide to the development of delivery systems for the anticancer agent, ellipticine . Journal of Pharmaceutical Sciences , 93 : 132 – 143 .
- Ribeiro , ME , Cavalcante , IM , Ricardo , NM , Mai , SM , Attwood , D , Yeates , SG and Booth , C . 2009 . Solubilisation of griseofulvin in aqueous micellar solutions of diblock copolymers of ethylene oxide, 1,2-butylene oxide with lengthy B blocks . International Journal of Pharmaceutics , 369 : 196 – 198 .
- Chen , X , Ding , S , Qu , G and Zhang , C . 2008 . Synthesis of novel chitosan derivatives for micellar solubilization of cyclosporine A . Journal of Bioactive and Compatable Polymers , 23 : 563 – 578 .
- Dong , Y , Jin , Y and Wei , D . 2007 . Surface activity and solubilization of a novel series of functional polyurethane surfactants . Polymer International , 56 : 14 – 21 .
- Zhou , Z , Chaibundit , C , D’Emanuele , A , Lennon , K , Attwood , D and Booth , C . 2008 . Solubilisation of drugs in worm-like micelles of block copolymers of ethylene oxide and 1,2-butylene oxide in aqueous solution . International Journal of Pharmaceutics , 354 : 82 – 87 .
- Nagarajan , R . 1999 . Solubilization of hydrocarbons and resulting aggregate shape transitions in aqueous solutions of Pluronic (PEO-PPO-PEO) block copolymers . Colloids and Surfaces B: Biointerfaces , 16 : 55 – 72 .
- Bae , Y , Fukushima , S , Harada , A and Kataoka , K . 2003 . Design of environment-sensitive supramolecular assemblies for intracellular drug delivery: polymeric micelles that are responsive to intracellular pH change . Angewandte Chemie International Edition , 42 : 4640 – 4643 .
- Van Hasselt , PM , Janssens , GEPJ , Slot , TK , Van der Ham , M , Minderhoud , TC , Talelli , M , Akkermans , LM , Rijcken , CJF and Van Nostrum , CF . 2009 . The influence of bile acids on the oral bioavailability of vitamin K encapsulated in polymeric micelles . Journal of Controlled Release , 133 : 161 – 168 .
- Zastre , J , Jackson , J and Burt , H . 2004 . Pharmaceutical Research , 21 : 1489
- Sharma , AK , Zhang , L , Li , S , Kelly , DL , Alakhov , VY , Batrakova , EV and Kabanov , AV . 2008 . Prevention of MDR development in leukemia cells by micelle-forming polymeric surfactant . Journal of Controlled Release , 131 : 220 – 227 .
- Zhang , Y , Li , X , Zhou , Y , Wang , X , Fan , Y , Huang , Y and Liu , Y . 2010 . Preparation and evaluation of poly(ethylene glycol)-poly(lactide) micelles as nanocarriers for oral delivery of cyclosporine A . Nanoscale Research Letters , 5 : 917 – 925 .
- Sant , VP , Smith , D and Leroux , JC . 2005 . Enhancement of oral bioavailability of poorly water-soluble drugs by poly(ethylene glycol)-block-poly(alkyl acrylate-co-methacrylic acid) self-assemblies . Journal of Controlled Release , 104 : 289 – 300 .
- Han , LM , Guo , J , Zhang , LJ , Wang , QS and Fang , XL . 2006 . Pharmacokinetics and biodistribution of polymeric micelles of paclitaxel with Pluronic P123 . Acta Pharmacologica Sinica , 27 : 747 – 753 .
- Aliabadi , HM , Brocks , DR and Lavasanifar , A . 2005 . Polymeric micelles for the solublization and delivery of cyclosporine A: pharmacokinetics and biodistribution . Biomaterials , 26 : 7251 – 7259 .
- Aliabadi , HM , Elhasi , S , Brocks , DR and Lavasanifar , A . 2008 . Polymeric micellar delivery reduces kidney distribution and nephrotoxic effects of cyclosporine A after multiple dosing . Journal of Pharmaceutical Sciences , 97 : 1916 – 1926 .
- Kawakami , S , Opanasopit , P , Yokoyama , M , Chansri , N , Yamamoto , T , Okano , T , Yamashita , F and Hashida , M . 2005 . Biodistribution characteristics of all-trans retinoic acid incorporated in liposomes and polymeric micelles following intravenous administration . Journal of Pharmaceutical Sciences , 94 : 2606 – 2615 .
- Chansri , N , Kawakami , S , Yokoyama , M , Yamamoto , T , Charoensit , P and Hashida , M . 2008 . Anti-tumor effect of all-trans retinoic acid loaded polymeric micelles in solid tumor bearing mice . Pharmaceutical Research , 25 : 428 – 434 .
- Soga , O , Van Nostrum , CF , Fens , M , Rijcken , CJF , Schiffelers , RM , Storm , G and Hennink , WE . 2005 . Thermosensitive and biodegradable polymeric micelles for paclitaxel delivery . Journal of Controlled Release , 103 : 341 – 353 .
- Uchida , Y and Murakami , Y . 2010 . Trilayered polymeric micelle: A newly developed macromolecular assembly that can incorporate hydrophilic compounds . Colloids and Surfaces B: Biointerfaces , 79 : 198 – 204 .
- Uchida , Y and Murakami , Y . 2011 . Successful preferential formation of a novel macromolecular assembly—Trilayered polymeric micelle—That can incorporate hydrophilic compounds: The optimization of factors affecting the micelle formation from amphiphilic block copolymers . Colloids and Surfaces B: Biointerfaces , 84 : 346 – 353 .
- Lukyanov , AN , Gao , Z , Mazzola , L and Torchilin , VP . 2002 . Polyethylene glycol-diacyllipid micelles demonstrate increased acculumation in subcutaneous tumors in mice . Pharmaceutical Research , 19 : 1424 – 1429 .
- Grislain , L , Couvreur , P , Lenaerts , V , Roland , M , Deprez-Decampeneere , D and Speiser , P . 1983 . Pharmacokinetics and distribution of a biodegradable drug-carrier . International Journal of Pharmaceutics , 15 : 335 – 345 .
- Remant Bahadur , KC , Aryal , S , Bhattarai , SR , Khil , MS and Kim , HY . 2007 . Amphiphilic triblock copolymer based on poly(p-dioxanone) and poly(ethylene glycol): Synthesis, characterization, and aqueous dispersion . Journal of Applied Polymer Science , 103 : 2695 – 2702 .
- Okuda , T , Kawakami , S , Higuchi , Y , Satoh , T , Oka , Y , Yokoyama , M , Yamashita , F and Hashida , M . 2009 . Enhanced in vivo antitumor efficacy of fenretinide encapsulated in polymeric micelles . International Journal of Pharmaceutics , 373 : 100 – 106 .
- Sayyah , SM , Abd El-Salam , HM and Azzam , EMS . 2005 . Surface activity of monomeric and polymeric (3-alkyloxy aniline) surfactants . International Journal of Polymeric Materials , 54 : 541 – 555 .
- Opanasopit , P , Yokoyama , M , Watanabe , M , Kawano , K , Maitani , Y and Okano , T . 2004 . Block copolymer design for camptothecin incorporation into polymeric micelles for passive tumor targeting . Pharmaceutical Research , 21 : 2001 – 2008 .
- Vakil , R and Kwon , GS . 2006 . Poly(ethylene glycol)-b-poly(ϵ-caprolactone) and PEG-phospholipid form stable mixed micelles in aqueous media . Langmuir , 22 : 9723 – 9729 .
- Xiong , XY , Tam , KC and Gan , LH . 2003 . Synthesis and aggregation behavior of pluronic f127/poly(lactic acid) block copolymers in aqueous solutions . Macromolecules , 36 : 9979 – 9985 .
- Xiong , XY , Tam , KC and Gan , LH . 2004 . Hydrolytic degradation of Pluronic F127/poly(lactic acid) block copolymer nanoparticles . Macromolecules , 37 : 3425 – 3430 .
- Yokoyama , M and Okano , T . 1996 . Targetable drug carriers: Present status and a future perspective . Advanced Drug Delivery Reviews , 21 : 77 – 80 .
- Lukyanov , AN and Torchilin , VP . 2004 . Micelles from lipid derivatives of water-soluble polymers as delivery systems for poorly soluble drugs . Advanced Drug Delivery Reviews , 56 : 1273 – 1289 .
- Vlerken , LE , Vyas , TK and Amiji , MM . 2007 . Poly(ethylene glycol)-modified nanocarriers for tumor-targeted and intracellular delivery . Pharmaceutical Research , 24 : 1405 – 1414 .
- Maeda , H , Greish , K and Fang , J . 2006 . The EPR effect and polymeric drugs: A paradigm shift for cancer chemotherapy in the 21st century . Advances in Polymer Science , 193 : 103 – 121 .
- Matsumura , Y . 2008 . Poly(amino acid) micelle nanocarriers in preclinical and clinical studies . Advanced Drug Delivery Reviews , 60 : 899 – 914 .
- Jagur-Grodzinski , J . 2008 . Polymers for targeted and/or sustained drug delivery . Polymers for Advanced Technologies , 19 : 1 – 12 .
- Vetvicka , D , Hruby , M , Hovorka , O , Etrych , T , Vetrik , M , Kovar , L , Kovar , M , Ulbrich , K and Rihova , B . 2009 . Biological evaluation of polymeric micelles with covalently bound doxorubicin . Bioconjugate Chemistry , 20 : 2090 – 2097 .
- Gillies , ER and Fréchet , JM . 2004 . Development of acid-sensitive copolymer micelles for drug delivery . Pure and Applied Chemistry , 76 : 1295 – 1307 .
- Kim , MS , Hwang , SJ , Han , JK , Choi , EK , Park , HJ , Kim , JS and Lee , DS . 2006 . pH-responsive PEG-poly(b-amino ester) block copolymer micelles with a sharp transition . Macromolecular Rapid Communications , 27 : 447 – 451 .
- Rodriguez-Hernandez , J , Checot , F , Gnanou , Y and Lecommandoux , S . 2005 . Toward ‘smart’ nano-objects by self-assembly of block copolymers in solution . Progress in Polymer Science , 30 : 691 – 724 .
- Sethuraman , VA , Lee , MC and Bae , YH . 2008 . A biodegradable pH-sensitive micelle system for targeting acidic solid tumors . Pharmaceutical Research , 25 : 657 – 666 .
- Schmaljohann , D . 2006 . Thermo- and pH-responsive polymers in drug delivery . Advanced Drug Delivery Reviews , 58 : 1655 – 1670 .
- Lee , ES , Gao , Z , Kim , D , Park , K , Kwon , IC and Bae , YH . 2008 . Super pH-sensitive multifunctional polymeric micelle for tumor pHe specific TAT exposure and multidrug resistance . Journal of Controlled Release , 129 : 228 – 236 .
- Gu , J , Cheng , WP , Liu , J , Lo , SY , Smith , D , Qu , X and Yang , Z . 2008 . pH-triggered reversible “stealth” polycationic micelles . Biomacromolecules , 9 : 255 – 262 .
- Yoo , HS , Lee , EA and Park , TG . 2002 . Doxorubicin-conjugated biodegradable polymeric micelles having acid-cleavable linkages . Journal of Controlled Release , 82 : 17 – 27 .
- Mohajer , G , Lee , ES and Bae , YH . 2007 . Enhanced intercellular retention activity of novel pH-sensitive polymeric micelles in wild and multidrug resistant MCF-7 cells . Pharmaceutical Research , 24 : 1618 – 1627 .
- Oh , KT , Lee , ES , Kim , D and Bae , YH . 2008 . l-Histidine-based pH-sensitive anticancer drug carrier micelle: Reconstitution and brief evaluation of its systemic toxicity . International Journal of Pharmaceutics , 358 : 177 – 183 .
- Lin , J , Zhu , J , Chen , T , Lin , S , Cai , C , Zhang , L , Zhuang , Y and Wang , XS . 2009 . Drug releasing behavior of hybrid micelles containing polypeptide triblock copolymer . Biomaterials , 30 : 108 – 117 .
- Xiong , XB , Mahmud , A , Uludag , H and Lavasanifar , A . 2008 . Multifunctional polymeric micelles for enhanced intracellular delivery of doxorubicin to metastatic cancer cells . Pharmaceutical Research , 25 : 2555 – 2566 .
- Kim , S , Kim , JY , Huh , KM , Acharya , G and Park , K . 2008 . Hydrotropic polymer micelles containing acrylic acid moieties for oral delivery of paclitaxel . Journal of Controlled Release , 132 : 222 – 229 .
- Dewhirst , MW , Viglianti , BL , Lora-Michiels , M , Hanson , M and Hoopes , PJ . 2003 . Adverse temperature levels in the human-proceedings of the WHO conference . International Journal of Hyperthermia , 19 : 267 – 294 .
- Kampinga , HH . 2006 . Cell biological effects of hyperthermia alone or combined with radiation or drugs: A short introduction to newcomers in the field . International Journal of Hyperthermia , 22 : 191 – 196 .
- Falk , MH and Issels , RD . 2001 . Hyperthermia in oncology . International Journal of Hyperthermia , 17 : 1 – 18 .
- Dreher , MR , Liu , W , Michelich , CR , Dewhirst , MW and Chilkoti , A . 2007 . Thermal cycling enhances the accumulation of a temperature-sensitive biopolymer in solid tumors . Cancer Research , 67 : 4418 – 4424 .
- Chilkoti , A , Dreher , MR , Meyer , DE and Raucher , D . 2002 . Targeted drug delivery by thermally responsive polymers . Advanced Drug Delivery Reviews , 54 : 613 – 630 .
- Gaber , MH , Wu , NZ , Hong , K , Huang , SK , Dewhirst , MW and Papahadjopoulos , D . 1996 . Thermosensitive liposomes: Extravasation and release of contents in tumor microvascular networks . International Journal of Radiation Oncology Biology Physics , 36 : 1177 – 1187 .
- Riehemann , K , Schmitt , O and Ehlers , EM . 2005 . The effects of thermochemotherapy using cyclophosphamide plus hyperthermia on the malignant pleural mesothelioma in vivo . Annals of Anatomy , 187 : 215 – 223 .
- Dewhirst , MW , Prosnitz , L , Thrall , D , Prescott , D , Clegg , S , Charles , C , MacFall , J , Rosner , G , Samulski , T , Gillette , E and LaReu , S . 1997 . Hyperthermic treatment of malignant diseases: Current status and a view toward the future . Seminars in Oncology , 24 : 616 – 625 .
- Schild , HG . 1992 . Poly(N-isopropylacrylamide): Experiment, theory and application . Progress in Polymer Science , 17 : 163 – 249 .
- Kong , G and Dewhirst , MW . 1999 . Review- hyperthermia and liposomes . International Journal of Hyperthermia , 15 : 345 – 370 .
- Heskins , M and Guillet , JE . 1968 . Solution properties of poly(N-isopropylacrylamide) . Journal of Macromolecular Science-Chemistry , A2 : 1441 – 1455 .
- Fujishige , S , Kubota , K and Ando , I . 1989 . Phase transition of aqueous solutions of poly(N-isopropylacrylamide) and poly(N-isopropylmethacrylamide) . Journal of Physical Chemistry , 93 : 3311 – 3313 .
- Yang , M , Ding , YT , Zhang , LY , Qian , XP , Jiang , XQ and Liu , BR . 2007 . Novel thermosensitive polymeric micelles for docetaxel delivery . Journal of Biomedical Materials Research , 81A : 847 – 857 .
- Liu , B , Yang , M , Li , R , Ding , Y , Qian , X , Yu , L and Jiang , X . 2008 . The antitumor effect of novel docetaxel-loaded thermosensitive micelles . European Journal of Pharmaceutics and Biopharmaceutics , 69 : 527 – 534 .
- Santis , SD , Ladogana , RD , Diociaiuti , M and Masci , G . 2010 . Pegylated and thermosensitive polyion complex micelles by self-assembly of two oppositely and permanently charged diblock copolymers . Macromolecules , 43 : 1992 – 2001 .
- Takei , YG , Aoki , T , Sanui , K , Ogata , N , Okano , T and Sakurai , Y . 1993 . Temperature-responsive bioconjugates. 2. Molecular design for temperature-modulated bioseparations . Bioconjugate Chemistry , 4 : 341 – 346 .
- Luo , S , Ling , C , Hu , X , Liu , X , Chen , S , Han , M and Xia , J . 2011 . Thermoresponsive unimolecular micelles with a hydrophobic dendritic core and a double hydrophilic block copolymer shell . Journal of Colloid and Interface Science , 353 : 76 – 82 .
- Bian , F , Xiang , M , Yu , W and Liu , M . 2008 . Preparation and self-assembly behavior of thermosensitive polymeric micelles comprising poly(styrene-b-N,N-diethylacrylamide) . Journal of Applied Polymer Science , 110 : 900 – 907 .
- Rijcken , CJF , Veldhuis , TFJ , Ramzi , A , Meeldijk , JD , Nostrum , CF and Hennink , WE . 2005 . Novel fast degradable thermosensitive polymeric micelles based on PEG-block-poly(N-(2-hydroxyethyl)methacrylamide-oligolactates) . Biomacromolecules , 6 : 2343 – 2351 .
- Ju , B , Yan , D and Zhang , S . 2012 . Micelles self-assembled from thermoresponsive 2-hydroxy-3-butoxypropyl starches for drug delivery . Carbohydrate Polymers , 87 : 1404 – 1409 .
- Qiao , P , Niu , Q , Wang , Z and Cao , D . 2010 . Synthesis of thermosensitive micelles based on poly(N-isopropylacrylamide) and poly(l-alanine) for controlled release of adriamycin . Chemical Engineering Journal , 159 : 257 – 263 .
- Li , W , Tu , W and Cao , D . 2009 . Synthesis of thermoresponsive polymeric micelles of PNIPAAm-b-OMMA as a drug carrier for loading and controlled release of prednisolone . Journal of Applied Polymer Science , 111 : 701 – 708 .
- Li , G , Guo , L and Ma , S . 2009 . Self-assembly and drug delivery behaviors of thermo-sensitive poly(t-butyl acrylate)-b-poly (N-isopropylacrylamide) micelles . Journal of Applied Polymer Science , 113 : 1364 – 1368 .
- Qu , T , Wang , A , Yuan , J and Gao , Q . 2009 . Preparation of an amphiphilic triblock copolymer with pH- and thermo-responsiveness and self-assembled micelles applied to drug release . Journal of Colloid and Interface Science , 336 : 865 – 871 .
- Zhang , ZX , Liu , X , Xu , FJ , Loh , XJ , Kang , ET , Neoh , KG and Li , J . 2008 . Pseudo-block copolymer based on star-shaped poly(N-isopropylacrylamide) with β-cyclodextrin core and guest-bearing PEG: Controlling thermoresponsivity through supramolecular self-assembly . Macromolecules , 41 : 5967 – 5970 .
- Liu , Y , Wu , J , Meng , L , Zhang , L and Lu , X . 2008 . Self-assembled, fluorescent polymeric micelles of a graft copolymer containing carbazole for thermo-controlled drug delivery in vitro . Journal of Biomedical Materials Research Part B: Applied Biomaterials , 85B : 435 – 443 .
- Zhang , ZX , Qiu , LY , Zhu , KJ and Jin , Y . 2004 . Thermosensitive micelles self-assembled by novel N-isopropylacrylamide oligomer grafted polyphosphazene . Macromolecular Rapid Communications , 25 : 1563 – 1567 .
- Liyan , Q , Jianxiang , Z , Yi , J and Kangjie , Z . 2005 . Thermosensitive self-assembly behaviors of novel amphiphilic polyphosphazenes . Chinese Science Bulletin , 50 : 1453 – 1455 .
- Kim , IS , Jeong , Y , Lee , YH and Kim , SH . 2000 . Drug release from thermo-responsive self-assembled polymeric micelles composed of cholic acid and poly(N-isopropylacrylamide) . Archives of Pharmacal Research , 23 : 367 – 373 .
- Tomlinson , R , Klee , M , Garrett , S , Heller , J , Duncan , R and Brocchini , S . 2002 . Pendent chain functionalized polyacetals that display pH-dependent degradation: A platform for the development of novel polymer therapeutics . Macromolecules , 35 : 473 – 480 .
- Dong , LC and Hoffman , AS . 1991 . A novel-approach for preparation of pH-sensitive hydrogels for enteric drug delivery . Journal of Controlled Release , 15 : 141 – 152 .
- Yin , X , Hoffman , AS and Stayton , PS . 2006 . Poly(N-isopropylacrylamide-co-propylacrylic acid) copolymers that respond sharply to temperature and pH . Biomacromolecules , 7 : 1381 – 1385 .
- Zhang , LY , Guo , R , Yang , M , Jiang , XQ and Liu , BR . 2007 . Thermo and pH dual-responsive nanoparticles for anti-cancer drug delivery . Advanced Materials , 19 : 2988 – 2992 .
- Liu , Y , Cao , X , Luo , X , Le , Z and Xu , W . 2009 . Self-assembled micellar nanoparticles of a novel star copolymer for thermo and pH dual-responsive drug release . Journal of Colloid and Interface Science , 329 : 244 – 252 .
- Lokitz , BS , York , AW , Stempka , JE , Treat , ND , Li , Y , Jarrett , WL and McCormick , CL . 2007 . Aqueous RAFT synthesis of micelle-forming amphiphilic block copolymers containing N-acryloylvaline. Dual mode, temperature/ ph responsiveness, and “locking” of micelle structure through interpolyelectrolyte complexation . Macromolecules , 40 : 6473 – 6480 .
- Huang , X , Du , F , Cheng , J , Dong , Y , Liang , D , Ji , S , Lin , SS and Li , Z . 2009 . Acid-sensitive polymeric micelles based on thermoresponsive block copolymers with pendent cyclic orthoester groups . Macromolecules , 42 : 783 – 790 .
- Koo , AN , Min , KH , Lee , HJ , Lee , SU , Kim , K , Kwon , IC , Cho , SH , Jeong , SY and Lee , SC . 2012 . Tumor accumulation and antitumor efficacy of docetaxel-loaded core-shell-corona micelles with shell-specific redox-responsive cross-links . Biomaterials , 33 : 1489 – 1499 .
- Saito , G , Swanson , JA and Lee , KD . 2003 . Drug delivery strategy utilizing conjugation via reversible disulfide linkages: Role and site of cellular reducing activities . Advanced Drug Delivery Reviews , 55 : 199 – 215 .
- Li , J , Huo , M , Wang , J , Zhou , J , Mohammada , JM , Zhang , Y , Zhu , Q , Waddad , AY and Zhang , Q . 2012 . Redox-sensitive micelles self-assembled from amphiphilic hyaluronic aciddeoxycholic acid conjugates for targeted intracellular delivery of paclitaxel . Biomaterials , 33 : 2310 – 2320 .
- Meng , FH , Hennink , WE and Zhong , Z . 2009 . Reduction-sensitive polymers and bioconjugates for biomedical applications . Biomaterials , 30 : 2180 – 2198 .
- Raina , S and Missiakas , D . 1997 . Making and breaking disulfide bonds . Annual Review of Microbiology , 51 : 179 – 202 .
- Gilbert , HF . 1995 . Thiol/disulfide exchange equilibria and disulfide bond stability . Methods in Enzymology , 251 : 8 – 28 .
- Ghosh , S , Yesilyurt , V , Savariar , EN , Irvin , K and Thayumanavan , S . 2009 . Redox, ionic strength, and pH sensitive supramolecular polymer assemblies . Journal of Polymer Science Part A: Polymer Chemistry , 8 : 1052 – 1060 .
- Koo , AN , Lee , HJ , Kim , SE , Chang , JH , Park , C , Kim , C , Park , JH and Lee , SC . 2008 . Disulfide-cross-linked PEG-poly(amino acid)s copolymer micelles for glutathione-mediated intracellular drug delivery . Chemical Communications , 48 : 6570 – 6572 .
- Zhang , J , Jiang , X , Zhang , Y , Li , Y and Liu , S . 2007 . Facile fabrication of reversible core cross-linked micelles possessing thermosensitive swellability . Macromolecules , 40 : 9125 – 9132 .
- Navath , RS , Wang , B , Kannan , S , Romero , R and Kannan , RM . 2010 . Stimuli-responsive star poly(ethylene glycol) drug conjugates for improved intracellular delivery of the drug in neuroinflammation . Journal of Controlled Release , 142 : 447 – 456 .
- Bulmus , V , Woodward , M , Lin , L , Murthy , N , Stayton , P and Hoffman , A . 2003 . A new pH-responsive and glutathione-reactive, endosomal membrane-disruptive polymeric carrier for intracellular delivery of biomolecular drugs . Journal of Controlled Release , 93 : 105 – 120 .
- Mitragotri , S , Blankschtein , D and Langer , R . 1995 . Ultrasound-mediated transdermal protein delivery . Science , 269 : 850 – 853 .
- Rapoport , NY , Christensen , DA , Fain , HD , Barrows , L and Gao , Z . 2004 . Ultrasound-triggered drug targeting of tumors in vitro and in vivo . Ultrasonics , 42 : 943 – 950 .
- Mitragotri , S and Kost , J . 2004 . Low-frequency sonophoresis: A review . Advanced Drug Delivery Reviews , 56 : 589 – 601 .
- Mitragotri , S . 2005 . Healing sound: The use of ultrasound in drug delivery and other therapeutic applications . Nature reviews: Drug discovery , 4 : 255 – 260 .
- Nelson , JL , Roeder , BL , Carmen , JC , Roloff , F and Pitt , WG . 2002 . Ultrasonically activated chemotherapeutic drug delivery in a rat model . Cancer Research , 62 : 7280 – 7283 .
- Husseini , GA , Diaz de la Rosa , MA , Gabuji , T , Zeng , Y , Christensen , DA and Pitt , WG . 2007 . Release of doxorubicin from unstabilized and stabilized micelles under the action of ultrasound . Journal of Nanoscience and Nanotechnology , 7 : 1 – 6 .
- Husseini , GA , Fayoumi , RIE , O’Neill , KL , Rapoport , NY and Pitt , WG . 2000 . DNA damage induced by micellar-delivered doxorubicin and ultrasound: Comet assay study . Cancer Letters , 154 : 211 – 216 .
- Husseini , GA , Myrup , GD , Pitt , WG , Christensen , DA and Rapoport , NY . 2000 . Factors affecting acoustically triggered release of drugs from polymeric micelles . Journal of Controlled Release , 69 : 43 – 52 .
- Husseini , GA , Stevenson-Abouelnasr , D , Pitt , WG , Assaleh , KT , Farahat , LO and Fahadi , J . 2010 . Kinetics and thermodynamics of acoustic release of doxorubicin from non-stabilized polymeric micelles . Colloids and Surfaces A: Physicochemical and Engineering Aspects , 359 : 18 – 24 .
- Husseini , GA , Pitt , WG , Christensen , DA and Dickinson , DJ . 2009 . Degradation kinetics of stabilized Pluronic micelles under the action of ultrasound . Journal of Controlled Release , 138 : 45 – 48 .
- Husseini , GA , Abdel-Jabbar , NM , Mjalli , FS , Pitt , WG and Al-Mousa , A . 2011 . Optimizing the use of ultrasound to deliver chemotherapeutic agents to cancer cells from polymeric micelles . Journal of the Franklin Institute , 348 : 1276 – 1284 .
- Husseini , GA and Pitt , WG . 2008 . The use of ultrasound and micelles in cancer treatment . Journal of Nanoscience and Nanotechnology , 8 : 2205 – 2215 .
- Rapoport , N , Gao , Z and Kennedy , A . 2007 . Multifunctional nanoparticles for combining ultrasonic tumor imaging and targeted chemotherapy . Journal of the National Cancer Institute , 99 : 1095 – 1106 .
- Marin , A , Muniruzzaman , M and Rapoport , N . 2001 . Acoustic activation of drug delivery from polymeric micelles: Effect of pulsed ultrasound . Journal of Controlled Release , 71 : 239 – 249 .
- Smith , DA , Porter , TM , Martinez , J , Huang , S , MacDonald , RC , McPherson , DD and Holland , CK . 2007 . Destruction thresholds of echogenic liposomes with clinical diagnostic ultrasound . Ultrasound in Medicine and Biology , 33 : 797 – 809 .
- Sukhorukov , GB , Rogach , AL , Garstka , M , Springer , S , Parak , WJ , Munoz-Javier , A , Kreft , O , Skirtach , AG , Susha , AS , Ramaye , Y , Palankar , R and Winterhalter , M . 2007 . Multifunctionalized polymer microcapsules: novel tools for biological and pharmacological applications . Small , 3 : 944 – 955 .
- Hong , G , Yuan , R , Liang , B , Shen , J , Yang , X and Shuai , X . 2008 . Folate-functionalized polymeric micelle as hepatic carcinoma-targeted, MRI-ultrasensitive delivery system of antitumor drugs . Biomedical Microdevices , 10 : 693 – 700 .
- Ai , H , Flask , C , Weinberg , B , Shuai , XT , Pagel , MD , Farrell , JD and Gao , JM . 2005 . Magnetite-loaded polymeric micelles as ultrasensitive magnetic-resonance probes . Advanced Materials , 17 : 1949 – 1952 .
- Gang , J , Park , SB , Hyung , W , Choi , EH , Wen , J , Kim , HS , Shul , YG , Haam , S and Song , SY . 2007 . Magnetic poly epsilon-caprolactone nanoparticles containing Fe3O4 and gemcitabine enhance anti-tumor effect in pancreatic cancer xenograft mouse model . Journal of Drug Targeting , 15 : 445 – 453 .
- Lu , J , Ma , S , Sun , J , Xia , C and Liu , C . 2009 . Manganese ferrite nanoparticle micellar nanocomposites as MRI contrast agent for liver imaging . Biomaterials , 30 : 2919 – 2928 .
- Guthi , JS , Yang , SG , Huang , G , Li , S , Khemtong , C , Kessinger , CW , Peyton , M , Minna , JD , Brown , KC and Gao , J . 2009 . MRI-visible micellar nanomedicine for targeted drug delivery to lung cancer cells . Molecular Pharmaceutics , 7 : 32 – 40 .
- Dai , S , Ravi , P and Tam , KC . 2009 . Thermo- and photo-responsive polymeric systems . Soft Matter , 5 : 2513 – 2533 .
- Kumar , GS and Neckers , DC . 1989 . Photochemistry of azobenzene-containing polymers . Chemical Reviews , 89 : 1915 – 1925 .
- Irie , M . 1990 . Photoresponsive polymers . Advances in Polymer Science , 94 : 27 – 67 .
- Natansohn , A and Rochon , P . 2002 . Photoinduced motions in azo-containing polymers . Chemical Reviews , 102 : 4139 – 4175 .
- Alvarez-Lorenzo , C , Bromberg , L and Concheiro , A . 2009 . Light-sensitive intelligent drug delivery systems . Photochemistry and Photobiology , 85 : 848 – 860 .
- Yu , H and Kobayashi , T . 2010 . Photoresponsive block copolymers containing azobenzenes and other chromophores . Molecules , 15 : 570 – 603 .
- Babin , J , Lepage , M and Zhao , Y . 2008 . Decoration of shell cross-linked reverse polymer micelles using ATRP: A new route to stimuli-responsive nanoparticles . Macromolecules , 41 : 1246 – 1253 .
- Jiang , J , Tong , X and Zhao , Y . 2005 . A new design for light-breakable polymer micelles . Journal of the American Chemical Society , 127 : 8290 – 8291 .
- Jiang , J , Tong , X , Morris , D and Zhao , Y . 2006 . Toward photocontrolled release using light-dissociable block copolymer micelles . Macromolecules , 39 : 4633 – 4640 .
- Xie , Z , Hu , X , Chen , X , Mo , G , Sun , J and Jing , X . 2009 . A novel biodegradable and light-breakable diblock copolymer micelle for drug delivery . Advanced Engineering Materials , 11 : B7 – B11 .
- Tong , X , Wang , G , Soldera , A and Zhao , Y . 2005 . How can azobenzene block copolymer vesicles be dissociated and reformed by light? . Journal of Physical Chemistry B , 109 : 20281 – 20287 .
- Klaikherd , A , Nagamani , C and Thayumanavan , S . 2009 . Multi-stimuli sensitive amphiphilic block copolymer assemblies . Journal of the American Chemical Society , 131 : 4830 – 4838 .
- Bajpai , A , Shulka , S , Bhanu , S and Kankane , S . 2008 . Responsive polymers in controlled drug delivery . Progress in Polymer Science , 33 : 1088 – 1118 .
- Jin , Q , Lv , LP , Liu , GY , Xu , JP and Ji , J . 2010 . Phenylboronic acid as a sugar- and pH-responsive trigger to tune the multiple micellization of thermo-responsive block copolymer . Polymer , 51 : 3068 – 3074 .
- Carstens , MG , Nostrum , CF , Verrijk , R , De Leede , LGJ , Crommelin , DJA and Hennink , WE . 2008 . A mechanistic study on the chemical and enzymatic degradation of PEG-oligo(ϵ-caprolactone) micelles . Journal of Pharmaceutical Sciences , 97 : 506 – 518 .
- Torchilin , V . 2009 . Multifunctional and stimuli-sensitive pharmaceutical nanocarriers . European Journal of Pharmaceutics and Biopharmaceutics , 71 : 431 – 444 .
- Roy , D , Cambre , JN and Sumerlin , BS . 2010 . Future perspectives and recent advances in stimuli-responsive materials . Progress in Polymer Science , 35 : 278 – 301 .
- Vinogradov , S , Batrakova , E , Li , S and Kabanov , A . 1999 . Polyion complex micelles with protein-modified corona for receptor-mediated delivery of oligonucleotides into cells . Bioconjugate Chemistry , 10 : 851 – 860 .
- Nagasaki , Y , Yasugi , K , Yamamoto , Y , Harada , A and Kataoka , K . 2001 . Sugar-installed block copolymer micelles: Their preparation and specific interaction with lectin molecules . Biomacromolecules , 2 : 1067 – 1070 .
- Leamon , CP , Weigl , D and Hendren , RW . 1999 . Folate copolymer-mediated transfection of cultured cells . Bioconjugate Chemistry , 10 : 947 – 957 .
- Xiong , XB , Mahmud , A , Uludag , H and Lavasanifar , A . 2007 . Conjugation of arginine-glycine-aspartic acid peptides to poly(ethylene oxide)-b-poly(ϵ-caprolactone) micelles for enhanced intracellular drug delivery to metastatic tumor cells . Biomacromolecules , 8 : 874 – 884 .
- Sawant , RR , Sawant , RM and Torchilin , VP . 2008 . Mixed PEG–PE/vitamin E tumor-targeted immunomicelles as carriers for poorly soluble anti-cancer drugs: Improved drug solubilization and enhanced in vitro cytotoxicity . European Journal of Pharmaceutics and Biopharmaceutics , 70 : 51 – 57 .
- Lee , ES , Na , K and Bae , YH . 2005 . Doxorubicin loaded pH-sensitive polymeric micelles for reversal of resistant MCF-7 tumor . Journal of Controlled Release , 103 : 405 – 418 .
- Gao , Z , Lukyanov , AN , Chakilam , AR and Torchilin , VP . 2003 . PEG-PE/phosphatidylcholine mixed immunomicelles specifically deliver encapsulated taxol to tumor cells of different origin and promote their efficient killing . Journal of Drug Targeting , 11 : 87 – 92 .
- Goto , M , Yura , H , Chang , CW , Kobayashi , A , Shinoda , T , Maeda , A , Kojima , S , Kobayashi , K and Akaike , TJ . 1994 . Lactose-carrying polystyrene as a drug carrier-investigation of body distribution to parenchymal liver-cells using I-125 labeled lactose-carrying polystyrene . Journal of Controlled Release , 28 : 223 – 233 .
- Dwek , RA . 1996 . Glycobiology: toward understanding the function of sugars . Chemical Reviews , 96 : 683 – 720 .
- Mammen , M , Choi , SK and Whitesides , GM . 1998 . Polyvalent interactions in biological systems: Implications for design and use of multivalent ligands and inhibitors . Angewandte Chemie (International Edition in English) , 37 : 2754 – 2794 .
- Elwood , PC . 1989 . Molecular cloning and characterization of the human folate-binding protein cDNA from placenta and malignant tissue culture (KB) cells . Journal of Biological Chemistry , 264 : 14893 – 14901 .
- Kim , SH , Jeong , JH , Mok , H , Lee , SH , Kim , SW and Park , TG . 2007 . Folate receptor targeted delivery of polyelectrolyte complex micelles prepared from ODN-PEG-folate conjugate and cationic lipids . Biotechnology Progress , 23 : 232 – 237 .
- Kim , D , Lee , ES , Oh , KT , Gao , ZG and Bae , YH . 2008 . Doxorubicin-loaded polymeric micelle overcomes multidrug resistance of cancer by double-targeting folate receptor and early endosomal pH . Small , 4 : 2043 – 2050 .
- Mi , Y , Liu , Y and Feng , SS . 2011 . Formulation of Docetaxel by folic acid-conjugated D-a-tocopheryl polyethylene glycol succinate 2000 (Vitamin E TPGS2k) micelles for targeted and synergistic chemotherapy . Biomaterials , 32 : 4058 – 4066 .
- Prabaharan , M , Grailer , JJ , Pilla , S , Steeber , DA and Gong , S . 2009 . Amphiphilic multi-arm-block copolymer conjugated with doxorubicin via pH-sensitive hydrazone bond for tumor-targeted drug delivery . Biomaterials , 30 : 5757 – 5766 .
- Yang , X , Deng , W , Fu , L , Blanco , E , Gao , J , Quan , D and Shuai , X . 2008 . Folate-functionalized polymeric micelles for tumor targeted delivery of a potent multidrug-resistance modulator FG020326 . Journal of Biomedical Materials Research , 86A : 48 – 60 .
- Zhua , H , Liu , F , Guo , J , Xue , J , Qian , Z and Gu , Y . 2011 . Folate-modified chitosan micelles with enhanced tumor targeting evaluated by near infrared imaging system . Carbohydrate Polymers , 86 : 1118 – 1129 .
- Bae , Y , Nishiyama , N and Kataoka , K . 2007 . In vivo antitumor activity of the folate-conjugated pH-sensitive polymeric micelle selectively releasing adriamycin in the intracellular acidic compartments . Bioconjugate Chemistry , 18 : 1131 – 1139 .
- Musacchio , T , Laquintan , V , Latrof , A , Trapani , G and Torchilin , VP . 2009 . PEG-PE micelles loaded with paclitaxel and surface-modified by a PBR-ligand: Synergistic anticancer effect . Molecular Pharmaceutics , 6 : 468 – 479 .
- Wang , Y , Yang , T , Wang , X , Dai , W , Wang , J , Zhang , X , Li , Z and Zhang , Q . 2011 . Materializing sequential killing of tumor vasculature and tumor cells via targeted polymeric micelle system . Journal of Controlled Release , 149 : 299 – 306 .
- Wilson , BC . 2002 . Photodynamic therapy for cancer: Principles . Canadian Journal of Gastroenterology , 16 : 393 – 396 .
- Dougherty , TJ , Gomer , CJ , Henderson , BW , Jori , G , Kessel , D , Korbelik , M , Moan , J and Peng , Q . 1998 . Photodynamic therapy . Journal of the National Cancer Institute , 90 : 889 – 905 .
- Dolmans , DE , Fukumura , D and Jain , RK . 2003 . Photodynamic therapy for cancer . Nature Reviews Cancer , 3 : 380 – 387 .
- Bechet , D , Couleaud , P , Frochot , C , Viriot , ML , Guillemin , F and Barberi-Heyob , M . 2008 . Nanoparticles as vehicles for delivery of photodynamic therapy agents . Trends in Biotechnology , 26 : 612 – 621 .
- Meisel , P and Kocher , T . 2005 . Photodynamic therapy for periodontal diseases: state of the art . Journal of Photochemistry and Photobiology B: Biology , 79 : 159 – 170 .
- Demidova , TN and Hamblin , MR . 2004 . Photodynamic therapy targeted to pathogens . International Journal of Immunopathology and Pharmacology , 17 : 245 – 254 .
- Chen , JX , Wang , HY , Li , C , Han , K , Zhang , XZ and Zhuo , RX . 2011 . Construction of surfactant-like tetra-tail amphiphilic peptide with RGD ligand for encapsulation of porphyrin for photodynamic therapy . Biomaterials , 32 : 1678 – 1684 .
- Taillefer , J , Jones , MC , Brasseur , N , Lier , JE and Leroux , JC . 2000 . Preparation and characterization of pH-responsive polymeric micelles for the delivery of photosensitizing anticancer drugs . Journal of Pharmaceutical Sciences , 89 : 52 – 62 .
- Garrec , DL , Taillefer , J , Lier , JEV , Lenaerts , V and Leroux , JC . 2002 . Optimizing pH-responsive polymeric micelles for drug delivery in a cancer photodynamic therapy model . Journal of Drug Targeting , 10 : 429 – 437 .
- Sibata , MN , Tedesco , AC and Marchetti , JM . 2004 . Photophysicals and photochemicals studies of zinc(II) phthalocyanine in long time circulation micelles for photodynamic therapy use . European Journal of Pharmaceutical Sciences , 23 : 131 – 138 .
- Master , AM , Rodriguez , ME , Kenney , ME , Oleinick , NL and Gupta , AS . 2010 . Delivery of the photosensitizer Pc 4 in PEG–PCL micelles for in vitro PDT studies . Journal of Pharmaceutical Sciences , 99 : 2386 – 2398 .
- Hioka , N , Chowdhary , RK , Chansarkar , N , Delmarre , D , Sternberg , E and Dolphin , D . 2002 . Studies of a benzoporphyrin derivative with Pluronics . Canadian Journal of Chemistry , 80 : 1321 – 1326 .
- Li , B , Moriyama , EH , Li , F , Jarvi , MT , Allen , C and Wilson , BC . 2007 . Diblock copolymer micelles deliver hydrophobic protoporphyrin ix for photodynamic therapy . Photochemistry and Photobiology , 83 : 1505 – 1512 .