Abstract
Six dicarboxylic acids 3a–f were synthesized by the reaction of 3,3′,4,4′-benzophenonetetracarboxylic dianhydride 1 with L-aminoacids 2a–f in a solution of glacial acetic acid/pyridine (Py) at refluxing temperature. Then six new poly(amide-imide)s PAIs were synthesized by direct polycondensation reaction of [N,N′-(4,4′-carbonyldiphtaloyl)-bis-L-amino diacid]s with 2,5-bis(4-aminobenzilidene)cyclopentanone. Due to the presence of dibenzalacetone moiety in the polymer chain, the PAIs have photosensitive properties. Also these PAIs are optically active and soluble in various organic solvents. These resulting new polymers can be used in column chromatography for the separation of enantiomeric mixtures. The resulted polymers were fully characterized by means of FTIR, 1H NMR spectroscopy, elemental analyses, inherent viscosity, solubility tests, UV–vis spectroscopy, thermogravimetric analysis, and derivative of thermogravimetric.
1. Introduction
Since their development in the 1960s, polyimides have become an increasingly important class of polymers, finding a wide range of applications in the aerospace and microelectronics industries. Aromatic polyimides are well-known high-performance polymers that have excellent thermal, mechanical, and electrical properties Citation[1,2]. However, polyimides are often insoluble and intractable resulting in processing difficulties which limit their applications Citation[3].
Modification of high-performance materials by increasing the solubility and lowering the transition temperatures while maintaining thermal stability is of particular interest. Copolycondensation is one of the possible ways for modification of polymer properties. Thus, processing of polyimides and many copolyimides, such as poly(amide-imide)s PAIs, poly(ester-imide)s, and other copolymers have been prepared Citation[4–9]. The synthesis of PAIs is more attractive than the other methods of copolymerization, because solubility and processability can be improved without significantly sacrificing the thermal and mechanical properties, they are useful in numerous applications in electrical wire enamel, adhesives, and injection-molding and extrusion products Citation[10–12]. Photosensitivity in polymers means that polymers are sensitive to the irradiated light leading to some change in properties or structure.
We describe here the preparation and basic characterization of photosensitive and thermally stable PAIs 5a–f by the direct polycondensation reaction of [N,N′-(4,4′-carbonyldiphtaloyl)-bis-L-amino acids with 2,5-bis(4-aminobenzilidene) cyclopentanone (ABCP) in a medium consisting of N-methyl-2-pyrrolidone (NMP), triphenyl phosphite (TTP), calcium chloride (CaCl2), and pyridine (Py). As reported previously, ABCP has been synthesized through a two-step reaction starting from 4-nitrobenzaldehyde and cyclopentanone in ethanol/water and finally by a catalytic reduction of 2,5-Bis (4-nitrobenzylidene) cyclopentanone using Na2S, NaHCO3 in methanol Citation[13,14].
2 Experimental
2.1 Materials
3,3′,4,4′-benzophenonetetracarboxylic dianhydride (1; Aldrich), L-alanine (2a), L-valine (2b), L-leucine (2c), L-isoleucine (2d), L-phenylalanine (2e), and L-2-aminobutyric acid (2f) (Merck) were used without previous purification. Diamine ABCP (mp = 285–287 °C) was prepared according to our previous work. Solvents NMP (Fluka), Py (Acros), and TPP (Merck) were used as received. Commercially available CaCl2 (Merck) was dried under vacuum at 150 °C for 6 h.
2.2 Techniques
1H NMR spectra were recorded on a Bruker 300 MHz instrument (Germany). Fourier transform infrared (FTIR) spectra were recorded on Galaxy series FTIR 5000 spectrophotometer (England). Spectra of solid were performed by using KBr pellets. Vibration transition frequencies were reported in wave number (cm−1). Band intensities were assigned as weak (w), medium (m), shoulder (sh), strong (s), and broad (br). Inherent viscosities were measured by a standard procedure by using a Technico Regd Trad Mark Viscometer. UV–vis absorptions were recorded at 25 °C in the 190–790 nm spectral regions with a Perkin-Elmer Lambda 15 spectrophotometer on DMF solution by using cell lengths of 1 cm. Specific rotations were measured by an A-Kruss polarimeter. Thermal Gravimetric Analysis (TGA and DTG) data of polymers were taken on a Mettler TA4000 System under N2 atmosphere at rate of 10 °C/min. Elemental analyses were performed by Vario EL equipment in Arak University.
2.3 Monomer synthesis:
2.3.1 [N, N′-(4,4′-carbonyldiphtaloyl)-bis-L-amino diacid]s 3a–f
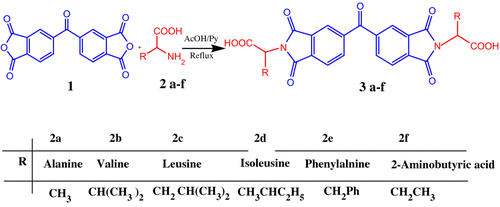
4.36 g (20.00 mmol) of 3,3′,4,4′-benzophenonetetracarboxylic dianhydride 1, 40.00 mmol of L-amino acids 2a–f, 80 mL of mixture of acetic acid/pyridine, and a stirring bar were placed into a 250 mL round-bottomed flask. The mixture was stirred at room temperature overnight and refluxed for 4–10 h. The solvent was removed under reduced pressure, and the residue was dissolved in 100 mL of cold acidic water. A white to cream precipitate was formed, filtered off, and dried to give compounds [N,N′-(4,4′-carbonyldiphtaloyl)-bis-L-amino diacid]s 3a–f Citation[15–17].
2.4 Polymer synthesis
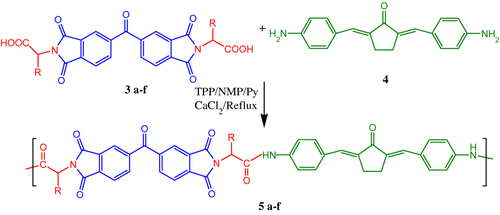
A mixture of diacid 3a–f (1 mmol), diamine ABCP 4 (1 mmol), calcium chloride (0.350 g), TPP (0.8 ml), pyridine (1.2 ml), and NMP (4.0 ml) was refluxed for 10 h. After cooling, the reaction mixture was poured into methanol (50 ml) to precipitate the corresponding polymer. The precipitated polymer was then separated by vacuum filtration and washed with methanol (30 ml), hot water (100 ml), and dried at 120 °C under vacuum for 24 h. IR and NMR spectroscopic results of the obtained polymers will be discussed in the Results and discussion section.
3 Results and discussion
3.1 Monomer synthesis
The asymmetric diacids 3a–f were synthesized by the condensation reaction of 3,3′,4,4′-benzophenonetetracarboxylic dianhydride 1 with two equimolars of L-alanine 2a, L-valine 2b, L-leucine 2c, L-isoleucine 2d, L-phenyl alanine 2e, and L-2-aminobutyric acid 2f in an acetic acid/pyridine solution. In this work, we used six diacids 3a-f for direct polycondensation (Scheme ). The diacids 3a, 3c, and 3e were synthesized previously Citation[18–20]. The yields and some physical properties of these compounds are shown in Table . The chemical structure and purity of the optically active diacids 3a–f were proved by using elemental analysis, FTIR and 1H NMR spectroscopic techniques.
Table 1. Synthesis of chiral diacid 3a-f.
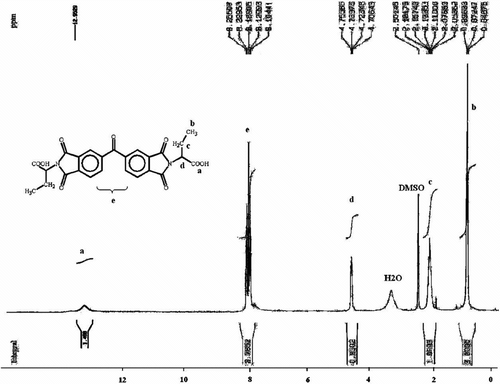
As an example, the 1H NMR spectrum of diacid 3f showed peaks between 0.84 and 0.89 ppm as a triplet, which was assigned for two CH3 (b); and peaks between 2.05 and 2.18 ppm as a multiplet, which was assigned to the CH2 (c); and peaks between 4.70 and 4.75 ppm as a doublet of doublet, which was assigned to the CH (d) protons, which are chiral centers. The peak at 8.1–8.25 ppm was assigned to aromatic protons (e). Also a broad peak in 12.95 ppm was assigned to COOH groups (Figure ).The measured results in elemental analyses of these compounds were closely corresponded to the calculated ones, demonstrating that the expected compounds were obtained.
3.2 Polymer synthesis
The direct polycondensation of a dicarboxylic acid and diamine is one of the well-known methods for PAI synthesis. In this work, we synthesized PAIs 5a–f containing dibenzalacetone moiety by direct polycondensation reactions of six chiral N,N′-(4,4′-carbonyldiphtaloyl)-bis-L-amino acids 3a–f with 2,5-bis(4-aminobenzylidene) cyclopentanone 4 by using triphenyl phosphite (TPP) and pyridine as condensing agents (Scheme ).
All the polycondensations proceeded readily in a homogeneous solution. Tough and stringy precipitates formed when the viscous polymer solutions were trickled into the stirring methanol.
The syntheses and some physical properties of these new PAIs 5a–f are given in Table . All the polymers were obtained in high yields (83–94%), and the inherent viscosities were 0.30–0.47 dL/g which measured in DMF solutions. Due to the presence of chiral amino acid moieties 2a–f in the polymer backbone, the polymers 5a–f are optically active and specific rotations are given in Table . Also the resulting polymers have a range of color between cream and orange.
Table 2. Synthesis and some physical properties of PAIs 5a–f.
3.3 Polymer characterization
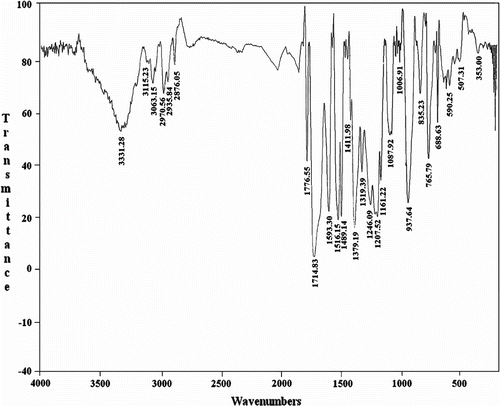
The structure of polymers was confirmed as PAIs by means of FTIR spectroscopy and elemental analyses the representative FTIR spectrum of PAI 5f was shown in Figure . The polymer showed the C=O asymmetric stretching of imide at 1776 cm−1, the C=O symmetric stretching of imide at 1714 cm−1, and C–N stretching at 1379 cm−1. The absorption bands of amide groups appeared at 3331 cm−1 (N–H stretching). All of these PAIs exhibited strong absorption around 1388 and 723 cm−1, which shows the presence of the heterocyclic imide groups. FTIR spectroscopy data for all of PAIs 5a–f are listed in Table .
Table 3. FTIR characterization of PAIs 5a-f.
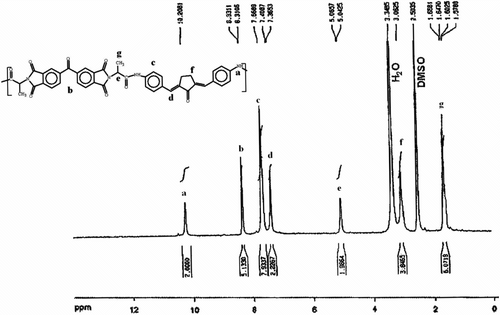
The 1H NMR spectrum of PAI 5a showed peaks that confirm its chemical structure (Figure ). The aromatic protons and two olefinic protons related to diamine 4 appeared in the region of 7.36–8.33 ppm and the peak in the region of 10.20 ppm is assigned for N–H amide groups in the polymer chain. Also the elemental analyses of the resulting PAIs 5a–f were in good agreement with the calculated values for the proposed structure (Table ).
Table 4. Elemental analysis of PAIs 5a-f.
3.4 Solubility of the PAIs
The solubility of PAIs 5a-f was investigated as 0.01 g of polymeric sample in 2 mL of solvent. These PAIs have good solubility in aprotic organic solvents. Remarkably, all of these PAIs were easily soluble at room temperature in aprotic polar solvents such as N-methyl-2-pyrrolidone (NMP), N,N-dimethylacetamide (DMAc), N,N-dimethylformamide (DMF), and insoluble in solvents such as chloroform, ethanol, and methanol (Table ).
Table 5. Solubility of PAIs 5a–f.
3.5 UV–Vis absorption characteristics
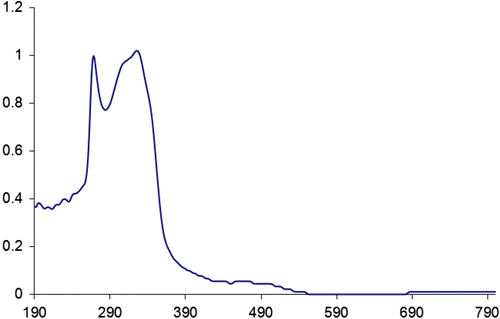
The photosensitive property of the new PAIs 5a–f in the DMF solution was studied by a UV spectrophotometer. All polymer solutions exhibit the same two positions of absorption maximum in UV–vis spectra at 325–335 nm and 260–270 nm. The absorption maximum at around 265 nm corresponds to π → π ∗ transition of the olefinic double bond was present in the dibenzalacetone moiety and carbon double bonds in aromatic rings in the polymer backbone. Also, the absorption maximum at around 390 nm corresponds to n → π ∗ transition of the nonbonding electrons which was present in nitrogen and oxygen atoms in the polymer backbone. The UV–vis absorption spectrum of PAI 5f in N,N′-dimethylformamide is shown in Figure . The spectrum of PAI 5f exhibited two typical peaks at 266.89 nm (π → π ∗) and 325 nm (n→ π ∗).
3.6 Thermal properties
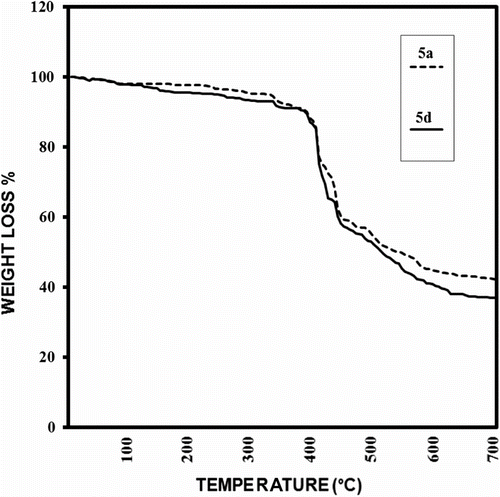
TGA and derivative of thermogravimetric (DTG) analysis at a rate of 10 °C min−1 in a nitrogen atmosphere were utilized to examine the thermal properties of these PAIs, and the obtained results are summarized in Table . Figure shows TGA results of PAIs 5a and 5d, respectively.
Table 6. Thermal behavior of PAIs 5a and 5d.
The thermal stability of the polymers was studied on the basis of 5 and 10% weight losses (T 5 and T 10, respectively) of the polymers and the residue at 700 °C (char yield). The results revealed that the PAIs were thermally stable up to 300 °C. TGA data showed that the resulting polymers were good thermally stable (Table ).
The char yield can be applied as a decisive factor for estimating the limited oxygen index (LOI) of polymers using Van Krevelen and Hoftyzer’s equation Citation[21]:
LOI = 17.5 + 0.4CR
Where CR is the char yield.
PAIs 5a and 5d had LOI values around 33, which were calculated from their char yield. On the basis of the LOI values, such macromolecules can be classified as self-extinguishing polymers.
4 Conclusion
In this article, we have successfully synthesized 6 dicarboxylic acid 3a–f containing ether and methylene groups. A series of new thermally stable PAIs 5a–f were prepared from chiral [N,N′-(4,4′-carbonyldiphtaloyl)-bis-L-amino acids 3a–f with 2,5-bis(4-aminobenzilidene) cyclopentanone 4 by direct polycondesation method. The results presented here also clearly demonstrate that incorporating the imide group into the polymer main chain as well as combination of the wholly aromatic backbone and several functional groups remarkably enhanced the thermal stability of the new polymers. These polymers are expected to have higher solubility due to the presence of the alkyl groups in the polymer chain. These properties could make these PAIs attractive for practical applications such as processable high-performance engineering plastics, column chromatography for the separation of the enantiomeric mixtures.
Notes
aMeasured at a concentration of 0.5 g /dL in DMF at 25 °C.
aMeasured at a concentration of 0.5 g /dL in DMF at 25 °C.
aTemperature at which 5% or 10% weight loss was recorded by TGA at a heating rate of 10 °C/min under N2.
bWeight percentage of material left after TGA analysis at a maximum temperature of 800 °C under N2.
cLimiting Oxygen Index.
References
- Wilson , D , Stenzenberger , HD and Hergenrother , PM . 1990 . Polyimides , Glasgow : Blackie .
- Ghosh , MK and Mittal , KL . 1996 . Polyimides: fundamentals and applications , New York , NY : Marcel Dekker .
- Cassidy , PE . 1980 . Thermally stable polymer , New York , NY : Marcel Dekker .
- Li , L , Jikei , M and Kakimoto , MA . 2003 . Synthesis and characterization of main chain degradable poly(amide-imide)s with acetal unit . High Performance Polymers , 15 : 131 – 141 .
- Liaw , DJ , Liaw , BY , Li , LJ , Sillion , B , Mercier , R , Thiria , R and Sekiguchi , H . 1998 . Synthesis and characterization of new soluble polyimides from 3,3‘,4,4‘-benzhydrol tetracarboxylic dianhydride and various diamines . Chemistry of Materials , 10 : 734
- Faghihi , Kh and Mozaffari , Z . 2008 . New polyamides based on 2,5-bis[(4-carboxyanilino) carbonyl] pyridine and aromatic diamines: synthesis and characterization . Journal of Applied Polymer Science , 108 : 1152
- Faghihi , Kh , Hajibeygi , M and Shabanian , M . 2009 . Novel flame-retardant and thermally stable poly(amide-imide)s based on bicyclo[2,2,2]oct-7-ene-2,3,5,6-tetracarboxylic diimide and phosphine oxide in the main chain: synthesis and characterization . Journal of the Chinese Chemical Society Taipei , 56 : 609
- Faghihi , Kh and Naghavi , H . 2008 . Synthesis and characterization of new polyamides containing p-phenylenediacryloyl moieties in the main chain . Journal of Applied Polymer Science , 108 : 1136
- Faghihi , Kh . 2006 . Synthesis and characterization of new flame-retardant poly(amide-imide)s containing phosphine oxide and hydantoin moieties in the main chain . Journal of Applied Polymer Science , 102 : 5062
- Faghihi , Kh and Moghanian , H . 2010 . Synthesis and characterization of new optically active poly(amide-imide)s based on N, N_-(pyromellitoyl)-bis-L-amino acids and 1,3,4-oxadiazole moieties . Designed Monomers and Polymers , 13 : 207
- Faghihi , Kh , Shabanian , M and Valikhani , N . 2011 . New poly(amide-imide)s based on 1,3-bis[4,4′-(trimellitimido) phenoxy] propane and hydantoin derivatives: synthesis and properties . Designed Monomers and Polymers , 14 : 109
- Mallakpour , S and Kolahdoozan , M . 2007 . A comparative study of two different methods for direct polyamidation of N-trimellitylimido-L-methionine with various aromatic diamines . Designed Monomers and Polymers , 10 : 439
- Gutch , PK , Banerjee , S and Jaiswal , DK . 2003 . Synthesis and properties of novel aromatic polyamides derived from 2,2-bis[4-(4-amino-2-fluorophenoxy) phenyl]hexafluoropropane and aromatic dicarboxylic acids . Journal of Applied Polymer Science , 89 : 691
- Mallakpour , S and Kowsari , E . 2006 . Synthesis of novel optically active poly(ester imide)s by direct polycondensation reaction promoted by tosyl chloride in pyridine in the presence of N, N-dimethyformamide . Journal of Applied Polymer Science , 101 : 455
- Faghihi , Kh , Feyzi , A and Nasr Isfahani , H . 2010 . Synthesis and characterization of new optically active poly(amide-imide)s based on N, N_-(bicyclo[2,2,2]oct-7-ene- 2,3,5,6-tetracarboxylic)-bis-L-2-aminobutyric acid . Designed Monomers and Polymers , 13 : 131
- Faghihi , Kh . 2004 . Microwave assisted rapid synthesis of novel optically active poly(amide-imide)s based on N-trimellitylimido-L-leucine diacid chloride and hydantoin derivatives . Macromolecular Research , 12 : 258
- Mehdipour-Ataei , S and Zigheimat , F . 2007 . Soluble poly(amide imide)s containing oligoether spacers . European Polymer Journal , 43 : 1020
- Mallakpour , S , Hajipour , AR and Faghihi , K . 2001 . Microwave-assisted synthesis of optically active poly(amide–imide)s with benzophenone and l-alanine linkages . European Polymer Journal , 37 : 119
- Mallakpour , S , Hajipour , AR and Zamanlou , MR . 2002 . Novel optically active poly(amide–imide)s from N, N′-(4,4′-carbonyldiphthaloyl)-bis-l-phenylalanine diacid chloride and aromatic diamines by microwave irradiation . European Polymer Journal , 38 : 475
- Mallakpour , S , Hajipour , AR and Zamanlou , MR . 2001 . Synthesis of optically active poly(amide-imide)s derived from N, N′-(4,4′-carbonyldiphthaloyl)-bis-L-leucine diacid chloride and aromatic diamines by microwave radiation . Journal of Polymer Science Part A: Polymer Chemistry , 39 : 177
- Van Krevelen , DW and Hoftyzer , PJ . 1976 . Properties of polymers , New York , NY : Elsevier .