Abstract
In this study, an optically active biodegradable poly(urethane-imide) (PUI) derived from L-tyrosine was synthesized. Since biopolyurethanes cannot be fully competitive with conventional thermoplastics and are sometimes too weak for practical use, these biopolymers have been formulated and associated with nano-sized fillers. For the preparation of PUI/titanium dioxide (TiO2) bionanocomposites (BNC)s, the high-intensity ultrasound was applied. The PUI/TiO2 BNCs were characterized by Fourier transform infrared spectroscopy, powder X-ray diffraction, transmission electron microscopy (TEM), scanning electron microscopy (SEM), atomic force microscopy, thermogravimetric analysis (TGA), differential scanning calorimetry (DSC), and UV–vis spectroscopy. The TEM and SEM results specified that the nanoparticles were dispersed homogeneously in PUI matrix on nanoscale. TGA/DSC confirmed that the heat stability of the novel BNCs was improved. UV–vis studies indicated that the resulting BNCs had high absorbance value than those values of pure PUI.
1. Introduction
Recently, many studies have been devoted to the biopolymers and biodegradable polymers, because of increasing environmental concern and decreasing fossil resources. Biologically functional polymers have attracted large interest both in academic and industrial fields for pharmaceutical, biomedical, agricultural, and environmental applications Citation[1–3].
Bionanocomposites (BNC)s are organic/inorganic hybrid biomaterials, which are produced by incorporating nano-sized fillers into a biopolymer matrix. Depending on the nanofiller chosen, the nanocomposite materials could show drastic modifications in their properties, like improved mechanical properties, barrier properties, or a change in their thermal and electrical conductivity Citation[4]. They exhibit improved properties with preservation of the material biodegradability, without ecotoxicity. Such materials are mostly intended to biomedical applications and different short-term applications, e.g. packaging, hygiene, or agriculture devices Citation[5–7]. Polymer nanocomposite materials could be fabricated by incorporating different inorganic nanoparticles (NPs) or fillers such as ZnO, SiO2, carbon nanotubes, and other metal NPs into the polymer matrix Citation[8–11]. Titanium dioxide (TiO2) is a nontoxic, cheap, and photostable material, which has been applied in food packaging material, cosmetics, and in environmental treatments such as water and air purification, water disinfection and sterilization because of its unique properties such as good stability, high refractive index, UV resistance, strong photocatalytic activity, and chemical stability Citation[12–14]. Polymer optical materials have been extensively utilized in optical fibers, optical waveguides, lenses, optical coatings, and optoelectronic devices as substitutes for inorganic optical materials. Since the refractive index of the polymers is usually in the range of 1.3–1.7, the application of polymers as high refractive index materials is limited Citation[15–19]. Lately, the preparation of inorganic/organic hybrid materials with high refractive index has attracted substantial interest Citation[20–28]. A technique for producing high refractive index nanocomposites and transparency in the fields of clear coatings, optical waveguides, and anti-reflection coatings for optical components was developed by incorporating premade inorganic colloidal particles into a polymer matrix. This method provides full synthetic control over the particle size, size distribution, and the surface properties of NPs Citation[29–31]. Due to the high surface-area/particle-size ratio, there is a strong tendency for NPs to aggregate, therefore decreasing the resultant mechanical properties of the nanocomposite materials Citation[32]. Some studies have shown that fully and uniformly dispersing NPs in the polymer matrix can noticeably improve the properties of materials, such as mechanical and thermal properties, barrier performance, and flame retardancy Citation[33–35]. Consequently, it is necessary to disperse the NPs without aggregation in organic binders. One approach is breaking down the agglomerated NPs applying mechanical shear forces such as ultrasonic irradiation Citation[36–38]. Ultrasonic has been broadly used in medicine, jointing, cleaning, machining, chemistry, and preparing nano-sized materials Citation[39–41]. Lately, ultrasonic irradiation has been used to synthesize the nanocomposites Citation[38]. Nevertheless, this approach will be restricted because of the limited interaction between the inorganic materials and the polymeric matrix, compared with the very strong interaction between individual NPs. In order to improve homogeneous dispersion of NPs and better compatibility between the NPs and polymer matrix, many researches were focused upon the surface modification of NPs and the use of different coupling agents such as trialkoxy silanes was suggested. Citation[29,30,37,42,43] The objective of this study is the efficient preparation of PUI/TiO2 BNC using TiO2 NPs modified by KH550 (with high refractive index, 2.5, in the crystal form of anatase and some small amount of rutile form), via ultrasonic irradiation process. The influence of interface structure on the thermal and the UV–vis absorber properties of the BNC are also discussed with the aid of thermogravimetric analysis (TGA), differential scanning calorimetry (DSC), and UV–vis spectrometric instrument.
2 Experimental
2.1 Materials
Toluene 2,4-diisocyanate (TDI), dimethylformamide (DMF), coupling agent (γ-amidopropyltriethoxyl silicane) (APS), and NPs (TiO2) with average particle sizes of 35–50 nm were purchased from Merck chemical Co. (Darmstadt, Germany) and Nanosabz. Co. (Tehran, Iran) and were used without further purification.
2.2 Characterization
X-ray diffraction (XRD) measurements of the BNCs and its components were recorded using a Philips X’PERT MPD with a copper target at 40 kV and 30 mA. The powder samples were spread on a sample holder and the diffractograms were recorded in the range 5–80° at the speed of 0.05°/min. Fourier transform infrared spectroscopy (FTIR) were recorded on (Jasco-680, Japan) spectrophotometer. First, the samples were dried, and then mulled with KBr pellets. UV–vis spectra were recorded on a UV/vis/NIR spectrophotometer, JASCO, V-570 with solid pellets sample of BNCs. The surface morphology of the samples was monitored with transmission electron microscopy (TEM), scanning electron microscopy (SEM), and atomic force microscopy (AFM). TEM was performed on a Philips, CM120 (Netherland) microscope operating at an accelerating voltage of 100 kV. SEM was obtained using a Cambridge Oxford (UK) 7060 scanning electron microscope connected to a four-quadrant backscattered electron detector with resolution 1.38 eV. The samples were dusted on a double-sided carbon tape placed on a metal stub and coated with a layer of gold to minimize charging effects. AFM were obtained using a Multimode Nanoscope III instrument (Digital Instrument Inc., New York, USA) with noncontact tapping mode with a silica probe (NSC 11) and a frequency of 463 kHz. TGA and DSC data for the BNCs were recorded, on Perkin Elmer instrument at a heating rate of 10 and 20 °C/min, under nitrogen atmosphere by IPPI.
2.3 Apparatus
The reaction was performed on an Auburn Hills, MI, USA ultrasonic cell crusher. Ultrasonic irradiation was carried out with the probe of the ultrasonic horn immersed directly in the mixture solution system with frequency 2 × 108 Hz and high power level (100%).
2.4 Polymer synthesis
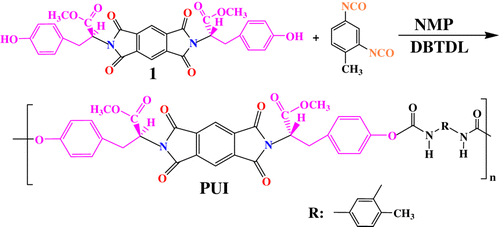
The PUI was synthesized from N,N′-(pyromellitoyl)-bis-L-tyrosine dimethyl ester as nontoxic aromatic diol (1) which was reported in our previous work Citation[44] with TDI under gradual heating Citation[45]. Here is a brief procedure. A three-necked flask containing diol 1 (0.10 g, 1.74 × 10−4 mol) and dibutyltin dilaurate (DBTDL) and 2 mL of DMF was magnetically stirred under nitrogen atmosphere at room temperature for 30 h. A solution of (0.04 g, 1.74 × 10−4 mol) of TDI in 1 mL of DMF was added dropwise through a dropping funnel over 15 min. The mixture was stirred at room temperature for 1 h and then it was heated at 80 °C for 8 h – then was heated gradually from 100 to 110 °C for 4 h. The mixture was cooled down to room temperature, poured into 15 mL of methanol to precipitate yellow flocculate. The product was filtered, washed with methanol several times, and dried at 80 °C (Scheme ).
2.5 In vitro toxicity and soil biodegradability test
In vitro toxicity of PUI derived from N,N′-(pyromellitoyl)-bis-L-tyrosine dimethyl ester was evaluated based on the growth of air-born spores on culture media Citation[44,45]. Also, the soil burial degradation test of PUI was conducted in aerobic soil condition in the presence of natural micro-organisms Citation[44,45].
2.6 Preparation of PUI/TiO2 BNCs
Preparation of PUI/TiO2 BNCs was carried out according to literature procedure in an ultrasonic wave irradiation process Citation[29,37]. Here is the brief procedures. NPs were firstly treated with 10 wt.% of silane coupling agent (APS). Different amounts of TiO2 (5, 10, 15, and 20 wt.%) modified NPs were mixed with the PUI and the mixture was dispersed in 20 mL of absolute ethanol, followed by irradiation with high-intensity ultrasonic wave for 4 h. After irradiation, the resulting suspension was cooled to room temperature and then centrifuged, and the precipitate was washed twice with absolute ethanol and distilled water, respectively. The solid was dried in vacuum at room temperature for 8 h. The obtained product was kept for further characterization.
PUI/TiO2 (10 wt.%) BNC spectroscopic data: ν max (KBr)/cm−1: 3380, 2928, 2870, 1722, 1631, 1535, 1123, 1060, 756.6, 655, and 542.
3 Results and discussion
3.1 In vitro toxicity and soil biodegradability test of polymer
According to former works Citation[44,45], it could be concluded that, PUI derived from N,N′-(pyromellitoyl)-bis-L-tyrosine dimethyl ester as nontoxic diphenolic monomer, is biologically active and biodegradable under microbial growth and soil burial test due to microbial invasion. Therefore, this polymer may be classified as biologically active and biodegradable material.
3.2 FT-IR spectroscopy
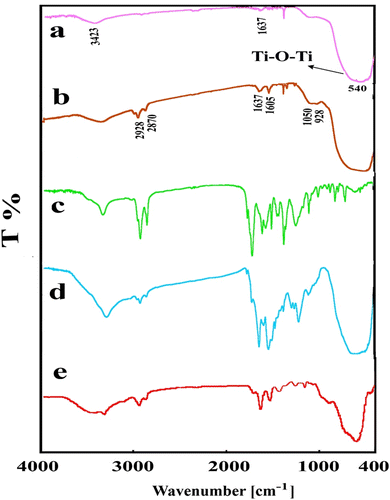
From the FT-IR spectra of the pure TiO2, TiO2 modified with APS, pure PUI, PUI/TiO2 BNCs, and TiO2 NP after removal of PUI by dissolving BNC in solvent (Figure ), it can be seen that in pure TiO2 (Figure ), ν(OH) stretching band and bending band are viewed at 3423 and 1637 cm−1, respectively. Based on Bezrodna et al. reported paper Citation[46], the observed broad bands at 3426–3500 cm−1 were ascribed to hydroxyl groups and some varying interactions between hydroxyl groups on TiO2, respectively. The bands at 643 and 540 cm−1 are assigned to the anatase nano-TiO2 Citation[47]. In the spectrum of TiO2 modified with APS (Figure ), the bands at 2928 and 2870 cm−1 were assigned to alkyl groups [–(CH2) n –], however, their intensity are weak. The N–H bending vibration of primary amine is observed at around 1605 cm−1. The appearance of these bands indicates that functional groups in modifier (aminopropyl group) are existed on the surface of modified TiO2. Furthermore, the peak at around 925 cm−1 appeared in both spectra which indicate that the condensation reaction between silanol groups and surface hydroxyl group took place. Li et al. Citation[48] showed the FT-IR spectrum of titania–silica composite and assigned the peak at around 960–910 cm−1 to the stretch vibration band of Ti–O–Si. In addition, the broad band at around 1050 cm−1 corresponded to Si–O–Si bond is observed which indicates the condensation reaction happened between silanol groups. The carbonyl group of urethane is shown in the region of 1725 cm−1 (Figure ). In the spectrum of 10 wt.% PUI/TiO2 BNC (Figure ), the characteristic peaks of pure PUI and TiO2 are still maintained, but it could be concluded that the structure of PUI may be affected by the presence of the modified TiO2. In order to understand the possible reactions between functional groups of modified TiO2 and the PUI matrix, TiO2 NP was isolated from the final BNC by dissolving BNC polymer in N,N-DMF and filtering it, following by precipitation and washing with DMF two times. The solid was dried in vacuum at 100 °C for 8 h and then was characterized by FT-IR. From this data (Figure ), it is implied that the NH2 groups from modified TiO2 may be reacted with the methyl ester pendant group of PUI.
3.3 XRD data
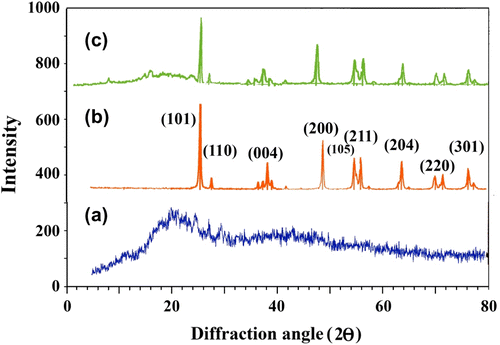
Figure shows the powder XRD patterns of the PUI (a), NP TiO2 (b), and (10 wt.%) PUI/TiO2 BNC (c). From the spectra, it can be observed that the pure PUI is the amorphous polymer and exhibit no anisotropic behavior, which may be due to the presence of the aromatic structures in the main chain, thus limiting the molecular mobility of the polymer. From Figure , it was observed that the most of the characteristic peaks at 2θ values of anatase TiO2 are kept intact (compared with Figure ). The average crystalline size of nano-TiO2, which is determined from the half-width of the diffraction using the Debye-Scherrer equation, is approximately 30–50 nm. Meanwhile, it is interesting to note that all the diffraction peaks become broad in these cases comparing with the XRD spectrum of TiO2, which should be attributed to the presence of the small-sized NPs Citation[49]. The findings indicate that ultrasonic irradiation reduces the crystallite size of anatase due to the generation of many localized hot spots in the solution by the ultrasound irradiation, which further gives rise to the homogeneous formation of a large number of seed nuclei, leading to a smaller particle size Citation[39–41].
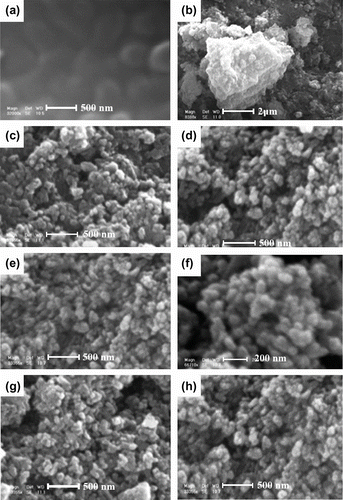
3.4 SEM and TEM observations
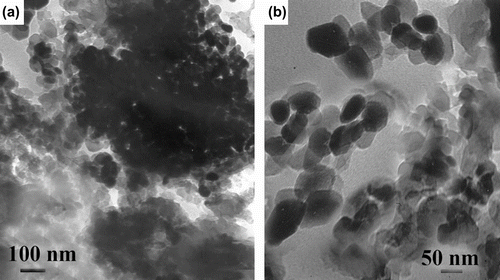
The direct verifications of the formation of BNCs are provided by TEM investigation. Figure show the morphology of 5% unmodified TiO2/PUI and 5% modified TiO2/PUI BNCs. The micrographs confirmed that the titania particles were well dispersed in the PUI matrix (Figure ). As can be seen, the average size of PUI/TiO2 BNCs is 35–50 nm. Moreover, this result was consistent with the XRD result mentioned previous. According to the SEM micrograph, as shown in Figure , the morphology of pure PUI does not show any nano-sized distribution of polymer (Figure ). The dimension of agglomerates for the unmodified-TiO2 filler composite is about 3 μm in average (Figure ), and that of the modified counterpart is about 50–100 nm (Figure ). The surface pretreatment with coupling agent on the nano-sized TiO2 fillers could really decrease the tendency of aggregation of the NP TiO2 fillers in the PUI matrix which indicates the formation of a BNC.
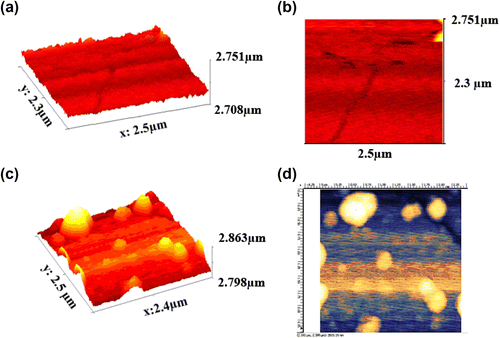
3.5 AFM observation
The topographic morphology of PUI surfaces was scanned by AFM (Figure ). Compared the surface morphology of pure PUI with BNC, a number of small bulges are observed on the interfaces. The bulges should be TiO2 particles because the size of the bulge varies with the particle size of TiO2 and so is close to the particle size of the used TiO2, indicating that some nano titania particles exist at the surface of PUI. AFM also reveals the size of the filler particles is about 50–150 nm, which is in the range of small aggregates of primary particles of 50 nm.
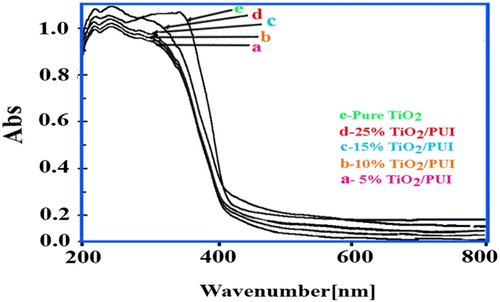
3.6 UV–vis spectroscopy
UV–vis spectroscopy of BNCs coatings containing various loadings of modified TiO2 NPs is shown in Figure . For comparison between different BNCs coatings, the UV–vis absorbance spectrum of the pure PUI has been used as a reference (i.e. made to have zero absorbance). It is clear from Figure that the increase in TiO2 NP loadings leads to notably increased absorption in UV-wavelength region.
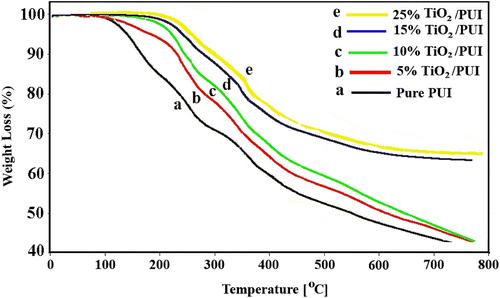
3.7 TGA/DSC thermal data
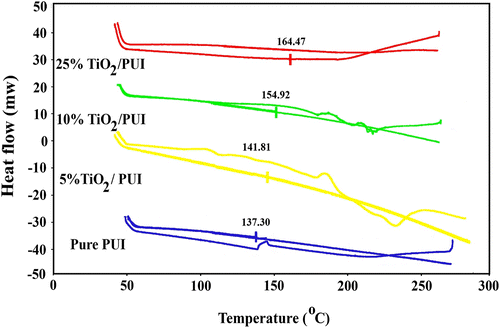
Figure showed the thermogravimetric curves of the pure PUI and PUI/TiO2 BNCs with the different NP contents. It was evident that the pure PUI had a low decomposition temperature of about 120 °C which was further improved when TiO2 was introduced. However, the initial temperature of the BNCs weight loss was increased with increasing TiO2 content until at a weight loss of 40%, this increase in the thermal stability may result from the high thermal stability of TiO2 network and the physical cross-link points of the TiO2 particles, which limited the movement of the molecular chain of PUI. After addition of TiO2 NPs with high-melting point to the polymer matrix, the TiO2 particles can serve as a good thermal cover layer, avoiding the direct thermal decomposition of polymer matrix by heat. In addition, the TiO2 is a nanoscale particle, which offers a larger surface area and improves the effect of thermal cover.
In order to estimate the effect of surface modification of NP TiO2 on the thermal stability of the PUI BNCs, thermal scans were carried out from 50 to 300 °C by the DSC method. As shown in Figure , the thermal stability of the PUI polymer can be significantly improved by the inclusion of TiO2 nanofillers (T g increased from 137.30 °C for pure PUI to 141.80, 154.92, and 164.47 °C for 5, 10, and 25%, respectively). As a result, mobility of the matrix molecules was restricted to certain extent, and creep resistance of the BNCs became much higher than the pure PUI.
3.8 Mechanism illustration for the preparation of BNCs
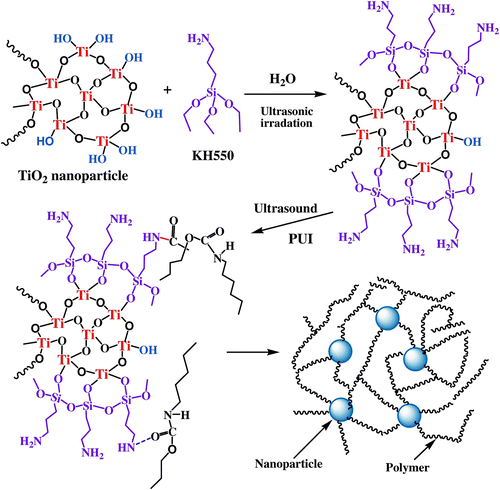
The surface modification of TiO2 NP and interaction with the PUI matrix are schematically illustrated in Figure . Under ultrasonic, silanol groups of APS generated by hydrolysis can interact with hydroxyl groups on the TiO2 surface and form the modification layer –NH2 groups. Generally, the main effects of sonication are due to cavitation or the growth and explosive collapse of microscopic bubbles on a microsecond time scale. At the same time, ultrasonic cavitation can generate a rigorous environment of local temperature up to 5000 K and local pressure up to 500 atm Citation[37]. Under such conditions, the modified NPs might be dispersed absolutely and will combine with PUI via the amino of coupling agent by physical and may be chemical absorption. The amine groups on APS surface may be reacted with methyl ester pendant groups of the PUI chains to form the interface structure based on covalent bonding. As a result, the dispersity of NPs increases with the decrease in surface energy of NPs, and the polymer on the NP surface has a steric dispersing and stabilizing effect, which can be observed from the photographs of TEM and SEM.
4 Conclusions
In this investigation, novel PUI/TiO2 BNCs were prepared via ultrasonic process. The results showed that surface treatment of TiO2 NPs with APS improves NPs dispersion, thermal properties, and UV protection of BNCs coating. A possible mechanism of ultrasonic-induced composite reaction was proposed based on the experimental results. DSC studies indicated that the thermal stability of the BNCs was improved with increasing NPs contents. The UV–vis studies showed that the BNCs possessed high absorbance value than those of pure polymer.
Acknowledgments
We wish to express our gratefulness to the Research Affairs Division, Isfahan University of Technology (IUT), for partial financial support. Further partial financial support from National Elite Foundation (NEF), Centre of Excellency in Sensors and Green Chemistry (IUT), and Iran nanotechnology Initiative Council (INIC) is gratefully acknowledged.
References
- Naira , LS and Laurencin , CT . 2007 . Biodegradable polymers as biomaterials . Progress in Polymer Science , 32 : 762 – 798 .
- Wee , YJ , Kim , JN and Ryu , HW . 2006 . Biotechnological production of lactic acid and its recent applications . Food Technology and Biotechnology , 44 : 163 – 172 .
- Fan , Y , Kobayashi , M and Kise , H . 2001 . Synthesis and specific biodegradation of novel polyesteramides containing amino acid residues . Journal of Polymer Science Part A: Polymer Chemistry , 39 : 1318 – 1328 .
- Bordes , P , Pollet , E and Avérous , L . 2009 . Biodegradable polyester/nanoclay systems . Progress in Polymer Science , 34 : 125 – 155 .
- Chivrac , F , Pollet , E and Avérous , L . 2009 . Progress in nanobiocomposites based on polysaccharides and nanoclays . Materials Science and Engineering: R , 67 : 1 – 17 .
- Haq , M , Burgueno , R , Mohanty , AK and Misra , M . 2009 . Processing techniques for bio-based unsaturated-polyester/clay nanocomposites: Tensile properties, efficiency, and limits . Composites A , 40 : 394 – 403 .
- Azeredo , HMC . 2009 . Nanocomposites for food packaging applications . Food Research International , 42 : 1240 – 1253 .
- Shen , R , Du , P , Mu , B and Liu , P . 2011 . Biocompatible and biodegradable polymeric nanocapsules from poly(α-malic acid)-grafted nano-silica templates . Designed Monomers Polymers , 14 : 39 – 45 .
- Aminabhavi , TM and Patil , MB . 2010 . Nanocomposite membranes of poly(Vinyl alcohol) loaded with polyaniline-coated TiO2 and TiO2 nanoparticles for the pervaporation dehydration of aqueous mixtures of 1,4-dioxane and tetrahydrofuran . Designed Monomers Polymers , 13 : 497 – 508 .
- Mallakpour , S and Zeraatpisheh , F . 2011 . Construction and characterization of bionanocomposites based on optically active poly(ester-imide) containing L-amino acids using nano-ZnO surface-coupled by γ -methacryloxypropyl-trimethoxysilane . Designed Monomers Polymers , 14 : 487 – 498 .
- Mallakpour , S and Hatami , M . 2011 . Bionanocomposites preparation and characterization: dispersion of surface-modified ZnO nanoparticles in optically active poly(amide-imide) derived from 3,5-diamino-N-(4-hydroxyphenyl)benzamide and amino acid . Designed Monomers Polymers , 14 : 461 – 473 .
- Ansari Amin , S , Pazouki , M and Hosseinnia , A . 2009 . Synthesis of TiO2-Ag nanocomposite with sol-gel method and investigation of its antibacterial activity against E. coli . Powder Technology , 196 : 241 – 245 .
- Mallakpour , S and Barati , A . 2011 . Efficient preparation of hybrid nanocomposite coatings based on poly(vinyl alcohol) and silane coupling agent modified TiO2 nanoparticles . Progress in Organic Coatings , 71 : 391 – 398 .
- Feng , XX , Zhang , LL , Chen , JY , Guo , YH , Zhang , HP and Jia , CI . 2007 . Preparation and characterization of novel nanocomposite films formed from silk fibroin and nano-TiO2 . International Journal of Biological Macromolecules , 40 : 105 – 111 .
- Cui , Z , Lü , C , Yang , B , Shen , J , Su , X and Yang , H . 2001 . The research on syntheses and properties of novel epoxy/polymercaptan curing optical resins with high refractive indices . Polymer , 42 : 10095 – 10100 .
- Olshavsky , MA and Allcock , HR . 1995 . Polyphosphazenes with high refractive indices: Synthesis, characterization, and optical properties . Macromolecules , 28 : 6188 – 6197 .
- Ma , H , Jen , AKY and Dalton , LR . 2002 . Polymer-based optical waveguides: materials, processing, and devices . Advanced Materials , 14 : 1339 – 1365 .
- Burroughes , JH , Bradley , DDC , Brown , AR , Marks , RN , Mackay , K , Friend , RH , Burns , PL and Holmes , AB . 1990 . Light-emitting diodes based on conjugated polymers . Nature , 347 : 539 – 541 .
- Lorenz , Z , Weibel , M , Walter , C and Ulrich , SW . 1993 . High refractive index films of polymer nanocomposites . Journal of Materials Research , 8 : 1742 – 1748 .
- Papadimitrakopoulos , F , Wisniecki , P and Bhagwagar , DE . 1997 . Mechanically attrited silicon for high refractive index nanocomposites . Chemistry of Materials , 9 : 2928 – 2933 .
- Kyprianidou-Leodidou , T , Caseri , W and Suter , UW . 1994 . Size variation of PbS particles in high-refractive-index nanocomposites . Journal of Physical Chemistry , 98 : 8992 – 8997 .
- Weibel , M , Caseri , W , Suter , UW , Kiess , H and Wehrli , E . 1991 . Preparation of polymer nanocomposites with ultrahigh refractive index . Polymers for Advanced Technologies , 2 : 75 – 80 .
- Yoshida , M and Prasad , PN . 1996 . Sol-gel-processed SiO2/TiO2/poly(vinylpyrrolidone) composite materials for optical waveguides . Chemistry of Materials , 8 : 235 – 241 .
- Lee , LH and Chen , WC . 2001 . High-refractive-index thin films prepared from trialkoxysilane-capped poly(methylmethacrylate)-titania materials . Chemistry of Materials , 13 : 1137 – 1142 .
- Marques , PAAP , Trindade , T and Neto , CP . 2006 . Titanium dioxide/cellulose nanocomposites prepared by a controlled hydrolysis method . Composites Science and Technology , 66 : 1038 – 1044 .
- Wang , B , Wilkes , GL , Hedrick , JC , Liptak , SC and Mcgrath , JE . 1991 . New high refractive index organic/inorganic hybrid materials from sol-gel processing . Macromolecules , 24 : 3449 – 3450 .
- Chau , JLH , Tung , CT , Lin , YM and Li , AK . 2008 . Preparation and optical properties of titania/epoxy nanocomposite coatings . Materials Letters , 62 : 3416 – 3418 .
- Chen , XC . 2005 . Synthesis and characterization of ATO/SiO2 nanocomposite coating obtained by sol-gel method . Materials Letters , 59 : 1239 – 1242 .
- Chen , J , Zhou , Y , Nan , Q , Sun , Y , Ye , X and Wang , Z . 2007 . Synthesis, characterization and infrared emissivity study of polyurethane/TiO2 nanocomposites . Applied Surface Science , 253 : 9154 – 9158 .
- Chen , J , Zhou , Y , Nan , Q , Ye , X , Sun , Y , Zhang , F and Wang , Z . 2007 . Preparation and properties of optically active polyurethane/TiO2 nanocomposites derived from optically pure 1,10-binaphthyl . European Polymer Journal , 43 : 4151 – 4159 .
- Zan , L , Liu , Z , Zhong , J and Peng , Z . 2004 . Organic modification on TiO2 nanoparticles by grafting polymer . Journal of Materials Science , 39 : 3261 – 3264 .
- Ngo , VG , Bressy , C , Leroux , C and Margaillan , A . 2009 . Synthesis of hybrid TiO2 nanoparticles with well-defined poly(methyl methacrylate) and poly(tert butyldimethylsilyl methacrylate) via the RAFT process . Polymer , 50 : 3095 – 3102 .
- Zhou , TH , Ruan , WH , Yang , JL , Rong , MZ , Zhang , MQ and Zhang , Z . 2007 . A novel route for improving creep resistance of polymers using nanoparticles . Composites Science and Technology , 67 : 2297 – 2302 .
- Li , Y , Fu , SY , Li , YQ , Pan , QY , Xu , G and Yue , CY . 2007 . Improvements in transmittance, mechanical properties and thermal stability of silica-polyimide composite films by a novel sol-gel route . Composites Science and Technology , 67 : 2408 – 2416 .
- Jordan , J , Jacob , KI , Tannenbaum , R , Sharaf , MA and Jasiuk , I . 2005 . Experimental trends in polymer nanocomposites-a review . Materials Science and Engineering A , 393 : 1 – 11 .
- Xia , H and Wang , Q . 2003 . Preparation of Conductive polyaniline/nanosilica particle composites through ultrasonic irradiation . Journal of Applied Polymer Science , 87 : 1811 – 1817 .
- Chen , J , Zhou , YM , Nan , QL , Ye , XY , Sun , YQ , Wang , ZQ and Zhang , SM . 2008 . Synthesis and characterization of polyurethane/CdS-SiO2 nanocomposites via ultrasonic process . Applied Surface Science , 255 : 2244 – 2250 .
- Wang , Q , Xia , H and Zhang , C . 2001 . Preparation of polymer/inorganic nanoparticles composites through ultrasonic irradiation . Journal of Applied Polymer Science , 80 : 1478 – 1488 .
- Ghasemi , S , Mousavi , MF , Shamsipur , M and Karami , H . 2008 . Sonochemical-assisted synthesis of nano-structured lead dioxide . Ultrasonics Sonochemistry , 15 : 448 – 455 .
- Gallego-Juarez , JA , Riera , E , Acosta , V , Rodrguez , G and Blanco , A . 2010 . Ultrasonic system for continuous washing of textiles in liquid layers . Ultrasonics Sonochemistry , 17 : 234 – 238 .
- Mason , TJ . 1991 . Chemistry with ultrasound , London : Society of Chemical Industry by Elsevier Applied Science . 1
- Chen , L , Shen , H , Lu , Z , Feng , C , Chen , S and Wang , Y . 2007 . Fabrication and characterization of TiO2–SiO2 composite nanoparticles and polyurethane/(TiO2-SiO2) nanocomposite films . Colloid and Polymer Science , 285 : 1515 – 1520 .
- Mallakpour , S and Soltanian , S . 2010 . Studies on syntheses and morphology characteristic of chiral novel poly(ester-imide)/TiO2 bionanocomposites derived from L-phenylalanine based diacid . Polymer , 51 : 5369 – 5376 .
- Mallakpour , S , Tirgir , F and Sabzalian , MR . 2011 . Synthesis and structural characterization of novel biologically active and thermally stable poly(ester-imide)s containing different natural amino acids linkages . Journal of Polymer Research , 18 : 373 – 384 .
- Mallakpour , S , Tirgir , F and Sabzalian , MR . 2010 . Novel biobased polyurethanes synthesized from nontoxic phenolic diol containing L-tyrosine moiety under green media . Journal of Polymers and the Environment , 18 : 685 – 695 .
- Bezrodna , T , Puchkovska , G , Shymanovska , V , Baran , J and Ratajczak , H . 2004 . IR-analysis of H-bonded H2O on the pure TiO2 surface . Journal of Molecular Structure , 700 : 175 – 181 .
- Rong , JN , Zhao , JX , He , X and Chin , J . 2006 . Synthesis at low temperature and characterization . Inorganic Chemistry , 22 : 546 – 550 .
- Li , Z , Hou , B , Xu , Y , Wu , D , Sun , Y , Hu , W and Deng , F . 2005 . Comparative study of sol–gel-hydrothermal and sol-gel synthesis of titania-silica composite nanoparticles . Journal of Solid State Chemistry , 178 : 1395 – 1405 .
- Huang , W , Tang , X , Wang , Y , Koltypin , Y and Gedanken , A . 2000 . Selective synthesis of anatase and rutile via ultrasound irradiation . Chemical Communications , 15 : 1415 – 1416 .