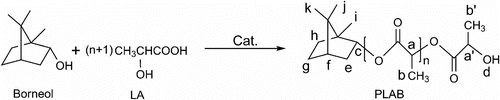
Abstract
Directly starting from D,L-lactic acid (LA) and L-borneol, novel biodegradable material poly(lactic acid-co-borneol) (PLAB) was synthesized via melt polycondensation. When the molar feed ratio LA/borneol is 64/1, the influences of different conditions including catalyst kinds and its dosage, copolymerization time, and temperature on the synthesis of the polymer are discussed. When the SnO was used as catalyst and the dosage was 0.3 wt% of the prepolymer, the 5 h direct melt polycondensation at 170 °C and absolute pressure of 70 Pa after prepolymerization gave the copolymer with the maximum intrinsic viscosity [η], 1.51 dL g−1. The structure of copolymer was confirmed by Fourier transform infrared spectroscopy (FTIR) and proton nuclear magnetic resonance (1H-NMR). According to the appropriate synthetic conditions, the copolymers with different molar feed ratios were synthesized and systematically characterized by [η], gel permeation chromatography (GPC), differential scanning calorimetry, thermogravimetric, and X-ray diffraction (XRD). The GPC results show that the maximum weight average molecular weight (M w) of PLAB is 11,900, when the molar feed ratio LA/borneol is 64/1. With the increase of the molar feed ratio of borneol, [η], M w and its polydispersity index (M w/M n) increase gradually. Due to the introduction of borneol into the copolymer, the glass-transition temperature (T g) decreases gradually with the increasing feed ratio of borneol. The direct melt copolycondensation is a cheap and practical method for the synthesis of borneol modified polylactic acid, a potential solid borneol flavor, polymeric drug, and functional drug carrier.
1. Introduction
As an important component of many traditional Chinese medicines, borneol has some special functions, such as refreshing, anti-inflammatory, and antibacterial. More importantly, borneol can enhance the absorption, distribution, permeability, and efficacy of other drugs Citation[1]. Moreover, due to the special scent in cool and refreshing, borneol is one of the oldest spices and commonly used in the field of cosmetics. In addition, borneol is also used as an important practical flavor additive in bathing agent, indoor fragrance agent, oral supplies, printing ink, nuts seasoning, and so on Citation[2]. However, easy sublimation or evaporation of borneol in the air not only makes it unstable as a medicine component in the process of preparation and storage, leading to the efficacy lowered Citation[3], but also limits its application scope as a spice.
In order to take full advantage of borneol whether as drug or spices, more and more attentions have attracted to the loading of borneol. Presently, there are two kinds of methods for the loading of borneol, physical method and chemical method. The former mainly is the inclusion of cyclodextrin or its derivative to borneol, which can increase the solubility of borneol in water, prevent its evaporation, and reduce its irritating Citation[4]. Usually, the researches on the inclusion of borneol with cyclodextrin or its derivative under certain conditions are advantageous for the application of borneol in pharmaceutical field Citation[5]. In contrast with physical method, chemical method via the esterification of borneol has more extensive application.
As a kind of fragrance component, borneol ester has been widely used in soap, cosmetics, edible essence, disinfectant, shower gels, air freshener, indoor spray perfume, bathing spices, talcum powder, etc., due to its special forest fresh aroma Citation[6]. At the same time, it is noteworthy that some borneol esters have certain cytotoxicity on human cancer cells Citation[7], or can inhibit cysteine protease Citation[8], or have certain analgesic effect but with lower human toxicity than borneol Citation[3], which make borneol ester widely used in the pharmaceutical industry also Citation[1]. However, as important loading method of borneol, usually borneol esters are organic small molecules, and the loading of borneol with polymeric material by esterification is rarely reported before.
As an inexpensive natural metabolite, lactic acid (LA) is safe for human. It can be easily absorbed, and directly take part in the metabolism, promote digestion, and suppress harmful intestinal bacteria. These make LA and its derivatives widely used in food industry, such as acidifier, preservative, antioxidant, stabilizer, bread improver, emulsifier, moisturizers, nutrition enhancer, thickener, and so on Citation[9,10]. In cigarette industry, LA can improve tobacco quality by keeping moisture and reducing harmful ingredient by neutralization with nicotine Citation[11]. When LA is polymerized into polylactic acid (PLA), PLA is usually used in biomedical fields, such as surgical suture, drug delivery carrier, and tissue engineering due to its nontoxicity and biodegradability. Furthermore, PLA is also an effective alternative to petroleum polymer. Therefore, there are more and more reports on the researches of PLA Citation[12–14]. However, there is no report on the modification of PLA by borneol.
Herein, we designed to synthesize a novel functional biodegradable material, poly(lactic acid-co-borneol) (PLAB) via the direct melt polycondensation of LA and borneol (Scheme ). According to the design, once the polymer is degraded slowly, the function of the released LA and borneol may make PLAB a multifunctional additive in many fields, especially cosmetics, medicine, food, and tobacco. At the same time, as a preliminary investigation, the modified PLA was systematically characterized by Fourier transform infrared spectroscopy (FTIR), proton nuclear magnetic resonance (1H-NMR), gel permeation chromatography (GPC), differential scanning calorimetry (DSC), thermogravimetric (TG), and X-ray diffraction (XRD) in this article.
2 Experimental
2.1 Materials
D,L-LA was purchased from Guangzhou Chemical Reagent Factory (Guangzhou, China) and L-borneol was purchased from J&K Scientific Ltd. (Beijing, China). All other chemicals including p-toluenesulfonic acid (TSA), stannous chloride (SnCl2), stannous oxide (SnO), zinc chloride (ZnCl2), and zinc oxide (ZnO) were commercially available as analytical grades from Guangzhou Chemical Reagent Factory (Guangzhou, China). All these materials were used without further purification.
2.2 Preparation of PLAB
According to the previous works on direct melt homo/co polymerization of LA Citation[14–18], LA and borneol should be prepolymerized before copolymerization. After LA and borneol were uniformly mixed as preplanned molar feed ratio, the mixture was directly dehydrated for 5 h at 140 °C under 4000 Pa in a three-necked flask equipped with a mechanical stirring device and a thermometer. After prepolymerization, the selected catalyst was added as predetermined weight percentage of dehydrated reactants (wt%), then melt copolymerization was carried out at a certain temperature (140–180 °C) and an absolute pressure of 70 Pa for 3–11 h. When the reaction finished, the crude product was dissolved in CHCl3. The filtrate was subsequently precipitated by CH3OH. After drying in vacuo to constant weight, the simple purification ordinarily produced a white (or yellowish) powder. The yield was within the range of 31–51%, and in most cases it was above 40%.
2.3 Characterization
The intrinsic viscosity ([η]) of the copolymer PLAB was determined with Ubbelohde viscometer (Cannon-Ubbelohde, State College, PA) using CHCl3 as solvent at 25 °C. The relative molecular weight and molecular weight distribution of the polymer were determined by gel permeation chromatography (Waters 1515 pump, Torrance, CA) with tetrahydrofuran as solvent at 35 °C and a flow velocity 1 mL min−1. Three Styragel HR columns from Japan covering a molecular weight range of 1 × 103–106 Da were used and calibrated using five polys
tyrene narrow standards from BF Goodrich (Richfield, OH). Molecular weight distributions for the samples were calculated using the Millennium 2010 software from Waters and were reported as polystyrene equivalent values.
IR spectra were obtained from an FTIR spectrometer (Bruker Vector 33, Ettlingen, Germany) by the KBr salt slice method. 1H-NMR spectra were recorded with a Varian NMR system 400 MHz (USA) with CDCl3 as the solvent and tetramethylsilane (TMS) as internal standard. With a wavelength of 1.5406 × 10−10 m and a scanning scope of 2θ from 5 to 50° with Cu Kα radiation, Bruker D8 Advance X-ray diffractometer (Bruker, Germany) was used to investigate the crystallinity of the polymer.
The thermal properties of the polymer were measured by DSC and TG analysis with a PerkinElmer DSC7 thermal analyzer (PerkinElmer, Cetus Instruments, Norwalk, CT). The samples for DSC measurements (an average weight of 4 mg) were scanned at a heating rate of 10 °C·min−1 under a nitrogen atmosphere (flow velocity 20 mL min−1), and then they were cooled to −50 °C for 5 min and heated again to 200 °C. TG was performed at a heating rate of 10 °C min−1 under a nitrogen atmosphere (flow velocity 20 mL min−1) also.
3 Results and discussion
Using LA and borneol as starting materials, the copolymer PLAB with different molar feed ratios (LA/borneol = 15/1, 32/1, 64/1, 128/1) were directly synthesized via melt copolycondensation after the synthetic conditions were discussed. The structure and properties of these PLAB were characterized by FT-IR, 1H-NMR, GPC, DSC, XRD, TG, and viscosity [η] measurements.
3.1 Appropriate synthetic conditions
Firstly, different catalysts were screened. When the direct melt copolycondensation was carried out for 5 h at 140 °C, absolute pressure 70 Pa, with the catalyst quantity of 0.3 wt%, and the molar feed ratio LA/borneol of 64/1, the influences of catalyst types on the [η] of PLAB are shown in Table . It can be observed that the reaction catalyzed by SnO gave higher [η] than other familiar catalysts, such as SnO, ZnCl2, ZnO, and TSA used in the direct melt homo/co polymerization of LA Citation[14–24]. This may be related to its good dispersability in the reaction system. Therefore, SnO was selected as the catalyst in the following experiments.
Table 1. The influences of different catalysts on the reaction.Footnote a
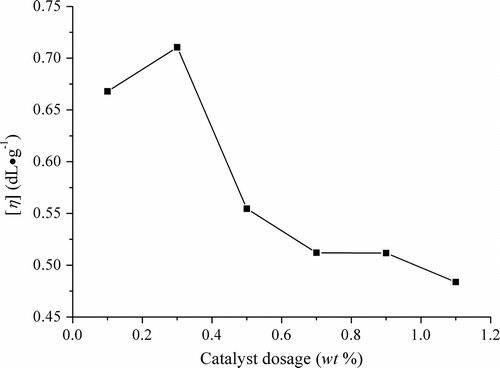
When the direct melt copolycondensation was carried out at 140 °C and absolute pressure 70 Pa for 5 h with the molar feed ratio LA/borneol of 64/1, the influences of catalyst SnO dosage on the [η] of PLAB are shown as Figure . It is clear that the [η] reached a maximum value when the weight percent of catalyst SnO quantity was 0.3 wt% of the prepolymer. Once the quantity was too small, the reaction was so insufficient after a certain time that the [η] was not high. When the quantity of SnO was excessive, short-chain molecule was apt to be formed through the degradation of polymer, which also was catalyzed by the metal catalyst Citation[22,23]. Therefore, the suitable dosage of catalyst SnO was 0.3 wt%.
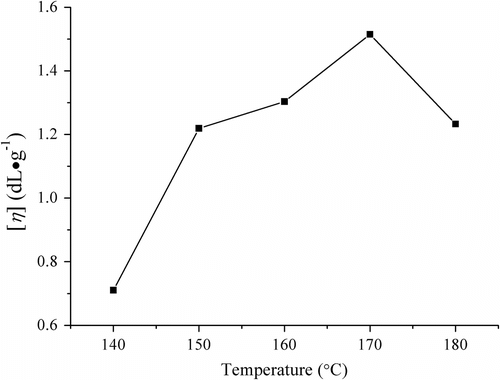
When the melt copolymerization was carried out respectively at different temperatures for 5 h under the conditions of the molar feed ratio LA/borneol of 64/1, absolute pressure 70 Pa, and catalyst SnO quantity 0.3 wt%, the [η] of the resulting polymer is shown in Figure . It is evident that the appropriate higher temperature was advantageous to increase molecular chain of the copolymer. However, when the temperature was too high, the side reactions, such as thermal degradation and oxidation markedly took place. Thus, the appropriate temperature should be 170 °C.
Figure 2 Influences of melt polymerization temperature on the viscosity of PLAB (conditions: 5 h and catalyst SnO dosage 0.3 wt%).
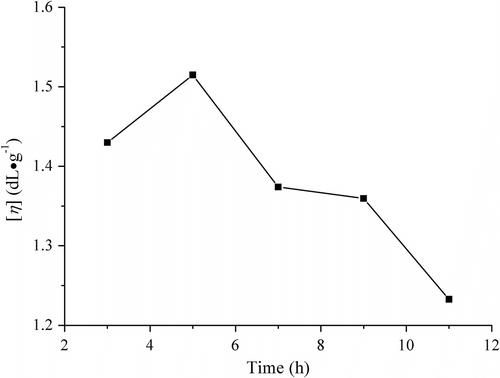
When the molar feed ratio LA/borneol was 64/1, the catalyst SnO quantity was 0.3 wt% and the melt copolycondensation was carried out at 170 °C and absolute pressure 70 Pa, the influences of the melt polymerization time on the [η] of PLAB are shown as Figure . It is obvious that the [η] reached a maximum value after the reaction lasted for 5 h. When the time was too short, the polymerization was insufficient. However, once the reaction time was longer than 5 h, the oxidation and thermal degradation of polymer became serious. So, the [η] dropped, the color of the purified product became deeper. Thus, the appropriate time may be 5 h.
Figure 3 Influences of melt polymerization time on viscosity of PLAB (conditions: 170 °C and catalyst SnO dosage 0.3 wt%).
Therefore, PLAB with different molecular weights could be obtained via the control of synthetic conditions. In order to get higher [η], when the molar feed ratio LA/borneol was 64/1, the appropriate conditions for the synthesis of the copolymer PLAB via direct melt copolycondensation were as follows: catalyst SnO quantity 0.3 wt%, reaction temperature 170 °C, absolute pressure 70 Pa, and reaction time 5 h. In this case, the maximum [η] was 1.51 dL g−1 and the corresponding weight-average molecular weight (Mw ) was 11,900 Da (Table ).
Table 2. The influences of molar feed ratios on yield, [η], and M n of the copolymers.Footnote a
3.2 Structural characterization of PLAB
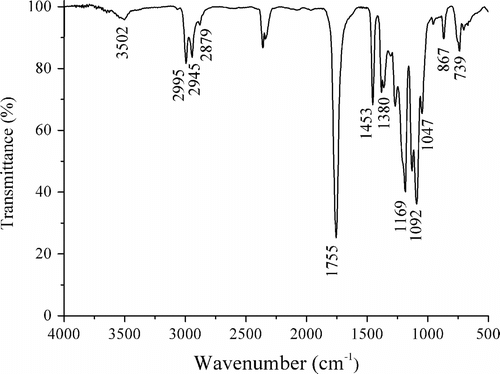
The structure of PLAB synthesized as the molar feed ratio LA/borneol 64/1 under the above appropriate synthetic condition was characterized by FTIR and 1H-NMR spectroscopy. Compared with the homopolymer poly(D,L-lactic acid) (PDLLA) synthesized via direct melt polycondensation Citation[15], it is elucidated that these compounds show many similar absorptions in their FTIR spectra, e.g. the weak absorption of terminal OH group at 3502 cm−1, the absorption of saturated C–H at 2995, 2945, 2879, 1453, and 1380 cm−1, the strongest absorption of ester carbonyl at 1755 cm−1, and the stronger absorption of C–O at 1169 and 1092 cm−1 (Figure ).
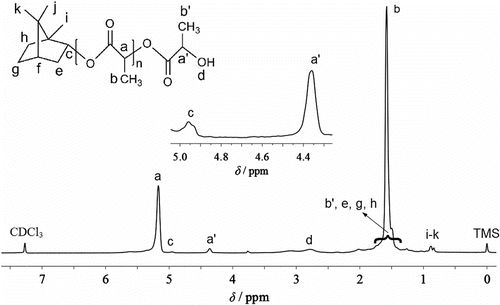
The data of 1H-NMR spectrum of PLAB synthesized as the molar feed ratio LA/borneol 64/1 were obtained as follows (Figure ). 1H-NMR (CDCl3 as solvent and TMS as internal reference), δ, ppm: 0.798–0.963 (H i – k , CH3 in borneol segment; 0.83–0.99, the literature data of CH3 in borneol segment Citation[25,26]), 1.265–2.058 (H b,b’ , CH3 in PLA segment, and other CH, CH2 in borneol segment except H i-k and H c ), 2.778–2.800 (H d , terminal OH of PLA chain), 4.286–4.453 (H a’ , CH in the terminal PLA segment), 4.901–5.048 (H c , CH–O in borneol segment; 4.88–5.14, the literature data of CH–O in borneol’s ester Citation[25,26]), 5.049–5.391 (H a , CH in PLA chain).
Especially, the data of H c as a characteristic data of borneol’s ester and the data of H i – k as other characteristic data of borneol segment are similar to the results in literature Citation[25,26]. When the molar feed ratio LA/borneol was different, the results were similar. Therefore, the data from FTIR and 1H-NMR indicated that the direct melt copolycondensation of LA and borneol indeed gave the copolymer PLAB (Scheme ).
According to the principle that the integral of proton peak area ratio reflects the amount of substance containing hydrogen group ratio, we could calculate the composition of the copolymer molar ratio of each unit using S H a +H a’ +H c /S H i – k as the standard (here, S is the integration area). The results of LA/borneol molar ratios are shown in Table also. It could be found that the content of LA was higher than that in the feed ratios. This may be related to the fact that, when LA escaped out of the reaction systems as lactide during the direct melt copolycondensation Citation[14–18], the escape of borneol with higher escape rate also occurred.
3.3 Molecular weight of PLAB
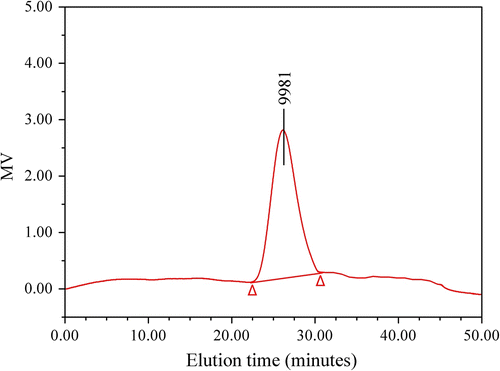
The influences of different molar feed ratios on yield, [η] and GPC results are shown in Table . Obviously, with the increase of the molar feed ratio of LA, not only [η] increased gradually, but also M n, M w, and PDI (M w/M n) had the trend of the corresponding increase. When the molar feed ratio LA/borneol was 128/1, the PDI of the copolymer was above 2, though it is normal according to the Carothers’ model, it is slightly higher than that reported before on the step-growth polymerization of LA Citation[15–18], which may be related to the partial formation of ether bond between the OH terminal polymers Citation[14]. Even so, all the GPC flow curves only showed a single peak (using PLAB synthesized as the molar feed ratio LA/borneol 64/1 as an example, its GPC curve is shown as Figure ). This also proved that the direct melt copolycondensation of LA and borneol indeed gave the copolymer PLAB.
At the same time, the minimum M w of serial PLAB was 3300 Da in this study (Table , Run 1). Generally, when the PLA biodegradable polymers were used as drug-delivery materials, the molecular weights were no more than 30,000 Da Citation[15,27,28]. As reported in the literatures Citation[29–31], the PLAs material with molecular weight of 1800 Da could be applied in drug delivery, even PLA copolymers with molecular weight of only 900 Da could be used as drug-delivery device. The molecular weight of PLAB synthesized here was overwhelmingly higher than 900 Da. Therefore, PLAB may be used as not only a potential solid borneol flavor, but also an important biodegradable and functional drug carrier for its molecular weight meets the requirement for drug-delivery applications.
3.4 Thermal properties of PLAB
The thermal properties of PLAB were characterized by DSC and TG, and the influences of different molar feed ratios are shown in Table . Firstly, the DSC data showed that the glass transition temperature (T g) of copolymers was lower than that of homopolymer PDLLA synthesized via the direct melt polycondensation Citation[15]. At the same time, the data of the melting temperature (T m) and heat were not detected. These indicated that the introduction of borneol made the structure changed. Especially, the decrease of structure regularity was advantageous for the movement of molecular chain, which was further demonstrated in the following analyzes of XRD characterization.
Table 3. The influences of molar feed ratio on the thermal properties of PLAB.Footnote a
On the other hand, there were different single and acute thermal decomposition peaks for different molar feed ratios (Table , and using PLAB synthesized as the molar feed ratio LA/borneol 64/1 as an example, its DTG curve is shown as Figure ). This indicated that, once the thermal decomposition started, the entire polymeric chain instantaneously split into small molecular fragments Citation[32]. At the same time, all thermal decomposition temperatures of PLAB were obviously different from those of homopolymer PDLLA synthesized via the direct melt polycondensation (Table ). In addition, this also proved that the copolymer PLAB was formed indeed from another side.
Figure 7 Differential thermogravimetry (DTG) curve of PLAB synthesized as the molar feed ratio 64/1 (LA/borneol).
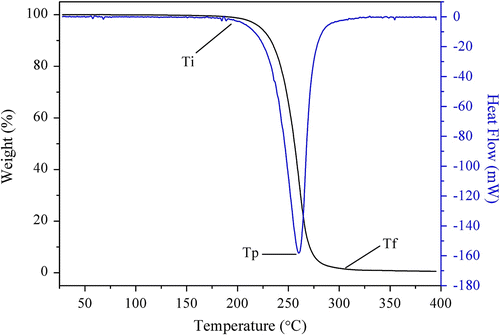
More importantly, in our experiments, the data of maximum weight loss rate temperature (T p) were between 254.5 and 276.5 °C (Table ), which was higher than the melting point of borneol (208 °C). Therefore, the functional polymer PLAB used to release borneol could improve the deficiency of borneol, especially its easy sublimation and thermal instability, which is advantageous for the application of borneol esters spices in the environment requiring heat processing. When borneol was loaded with cyclodextrin, decomposition temperature area from initial temperature (T i) to final temperature (T f) was 280–310 °C Citation[33]. Therefore, the decomposition temperature of PLAB was relatively smaller (Table ), which may be related to the different loading principle.
3.5 Crystallinity of PLAB
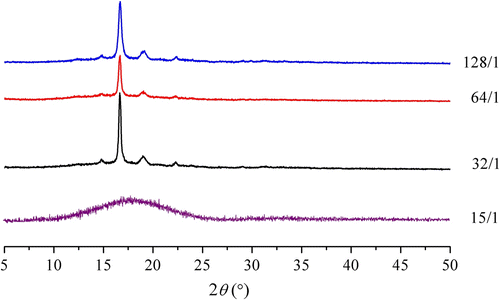
The crystallinity of PLAs material is crucial for their physical and biological properties, especially degradability. The results showed that the position of the XRD absorption peak of PLABs (Figure ) was basically the same as the position of PDLLA (2θ = 16.7°, 19.1°, respectively, Citation[16]). However, compared with the crystallinity of PDLLA (20.8% Citation[16]), the crystallinity of PLABs was lowered (Table ) due to the introduction of borneol. For the same reason, the crystallite size of PLABs markedly became bigger than that of PDLLA (Table ). Therefore, the XRD results further confirmed that the introduction of borneol made the structure changed.
Table 4. The influences of molar feed ratio on the XRD results of PLABFootnote a .
Fortunately, lower or no crystallinity is more beneficial for PLA biodegradable materials to be applied in the biomedical fields, especially drug delivery carrier materials, because there will be no residual microcrystalline after degradation in vivo Citation[27,34].
4 Conclusions
Directly using D,L-LA and borneol as the starting materials, a novel biodegradable material PLAB was synthesized via melt polycondensation as designed for the first time. The structure and properties of the modified PLA by flavor and drug borneol were systematically characterized by FTIR, 1H-NMR, GPC, DSC, TG, and XRD. As a borneol polymeric ester, PLAB could be a potential solid borneol spice, polymeric drug, and biodegradable functional drug carrier and its molecular weight could be controlled by synthetic conditions. Now, the functional modification of PLA has been an important direction in PLA researches Citation[35–39], this work will further promote its development in the future.
Acknowledgments
The authors are grateful to the 3rd Talents Special Funds of Guangdong Higher Education (grant number Guangdong-Finance-Education[2011]431), the Natural Science Foundation of Guangdong Province (grant number 5300082), and the Natural Science Foundation of China (grant number 20772035) for the financial support of this work.
Notes
aAll runs were polymerized with a polycondensation temperature of 140 °C, a polycondensation time of 5 h, and catalyst quantity of 0.3 wt%.
aAll runs were polymerized with a polycondensation temperature of 170 °C, a polycondensation time of 5 h, and catalyst SnO quantity of 0.3 wt%.
bTested by 1H-NMR, and for M NMR using 100 Da as unit.
aAll runs were polymerized with a polycondensation temperature of 170 °C, a polycondensation time of 5 h, and catalyst SnO quantity of 0.3 wt%.
bNot detected.
aAll runs were polymerized with a polycondensation temperature of 170 °C, a polycondensation time of 5 h, and catalyst SnO quantity of 0.3 wt%.
References
- Liu , D , Zheng , XH , Tang , YT , Zi , J , Nan , YF , Wang , SX , Xiao , CN , Zhu , JL and Chen , C . 2010 . Metabolism of tanshinol borneol ester in rat and human liver microsomes . Drug Metabolism and Disposition , 38 : 1464
- Ge , JY . 2011 . A synthetic method of S-(+)lactic acid with high optical purity and production ratio . Hubei Agricultural Sciences , 50 : 1671
- Wang , T , Chen , CB , Zhang , R , Wang , NS and Mi , SQ . 2011 . The pharmacokinetics of (+)-bornyl monomaleate in rat serum . Lishizhen Medicine and Materia Medica Research , 22 : 2300
- Guo , LR and Zhou , LL . 2011 . Study on preparation and stability of borneol β-CD and HP-β-CD inclusion complex . Chinese Journal of Experimental Traditional Medical Formulae , 17 : 7
- Guo , LR and Zhou , LL . 2011 . Comparative study on β-cyclodextrin inclusion preparation of bornel . Lishizhen Medicine and Materia Medica Research , 22 : 1462
- Chen , HZ , Yang , YW , Liu , YG , Wang , RF , Ge , JY and Liu , F . 2005 . Ester synthesis of borneol and isoborneol catalyzed by nanometer solid superacid . Chinese Journal of Organic Chemistry , 25 : 1490
- Zhang , Q , Chen , Y , Zheng , YQ , Xia , P , Xia , Y , Yang , ZY , Bastow , KF , Morris-Natschke , SL and Leeb , KH . 2003 . Synthesis and bioactivity of 4,10-dimethyl-pyridino[2,3-h]quinolin-2(1H)-one-9-carboxylic acid and its esters . Bioorganic & Medicinal Chemistry , 11 : 1031
- Ogungbea , IV , Croucha , RA , Haberb , WA and Setzera , WN . 2010 . Phytochemical investigation of verbesina turbacensis kunth: trypanosome cysteine protease inhibition by (-)-bornyl esters . Natural Products Communications , 5 : 1161
- Qian , ZL , Hu , J and Lei , ZZ . 2001 . The industrialization, application and markets of lactic acid . Industrial Microbiology , 31 : 49
- Narayanan , N , Roychoudhury , PK and Srivastava , A . 2004 . L-(+)-lactic acid fermentation and its product polymerization . Electronic Journal of Biotechnology , 7 : 167
- Wu , HH . 2010 . The progress on research and application of lactic acid and its derivatives . Journal of Northwest University for Nationalities (Natural Science) , 31 : 67
- Maharana , T , Mohanty , B and Negi , YS . 2009 . Melt-solid polycondensation of lactic acid and its biodegradability . Progress in Polymer Science , 34 : 99
- Rasal , RM , Janorkar , AV and Hirt , DE . 2010 . Poly(lactic acid) modifications . Progress in Polymer Science , 35 : 338
- Luo , SH , Wang , ZY , Mao , CX and Huo , JP . 2011 . Synthesis of biodegradable material poly(lactic acid-co-glycerol) via direct melt polycondensation and its reaction mechanism . Journal of Polymer Research , 18 : 2093
- Zhao , YM , Wang , ZY and Yang , F . 2005 . Characterization of poly(D,L-lactic acid) synthesized by direct melt polymerization and its application in Chinese traditional medicine compound prescription microspheres . Journal of Applied Polymer Science , 97 : 195
- Wang , ZY , Zhao , YM and Yang , F . 2006 . Syntheses of poly(lactic acid-co-glycolic acid) serial biodegradable polymer materials via direct melt polycondensation and their characterization . Journal of Applied Polymer Science , 99 : 244
- Wang , ZY , Zhao , HJ , Wang , QF , Ye , RR and Finlow , DE . 2010 . Synthesis of poly(D,L-lactic acid) modified by cholic acid via direct melt copolycondensation and its characterization . Journal of Applied Polymer Science , 117 : 1405
- Ye , RR , Wang , ZY , Wang , QF , Yang , K and Luo , SH . 2011 . Synthesis of biodegradable material poly(lactic acid-co-aspartic acid) via direct melt polycondensation and its characterization . Journal of Applied Polymer Science , 121 : 3662
- Moon , SI and Kimura , Y . 2003 . Melt polycondensation of L-lactic acid to poly(L-lactic acid) with Sn(II) catalysts combined with various metal alkoxides . Polymer International , 52 : 299
- Abe , H , Tetsuka , H and Doi , Y . 2005 . Synthesis and characterization of periodic co-polymers from L-lactic acid and L-alanine. Polymer Preprint Japan , 54 : 5243
- Takasu , A , Narukawa , Y and Hirabayashi , T . 2006 . Direct dehydration polycondensation of lactic acid catalyzed by water-stable lewis acids . Journal of Polymer Science Part A: Polymer Chemistry , 44 : 5247
- Duan , JF , Du , J and Zheng , YB . 2007 . Synthesis and characterization of a novel biodegradable polymer poly(lactic acid-glycolic acid-4-hydroxyproline) . Journal of Applied Polymer Science , 103 : 3585
- Lu , DD , Ren , ZL , Zhou , TH , Wang , SF and Lei , ZQ . 2008 . Synthesis and characterization of amphiphilic biodegradable poly(glutamic acid-co-lactic acid-co-glycolic acid) by direct polycondensation . Journal of Applied Polymer Science , 107 : 3638
- Wei , ZY , Liu , L , Qu , C , Liu , HZ and Qi , M . 2010 . Design, preparation and characterization of biodegradable poly(ester-amide) . Journal of Functional Materials , 41 : 656
- Paine , JB . 2008 . Esters of pyromellitic acid. Part II. Esters of chiral alcohols: para pyromellitate diesters as a novel class of resolving agents and use of pyromellitates as duplicands for chiral purification . Journal of Organic Chemistry , 73 : 4939
- Mino , T , Hasegawa , T , Shirae , Y , Sakamoto , M and Fujita , T . 2007 . N,O-ligand accelerated zinc-catalyzed transesterification of alcohols with vinyl esters . Journal of Organometallic Chemistry , 692 : 4389
- Zhou , SB , Deng , XM and Li , XH . 2004 . Synthesis and characterization of biodegradable low molecular weight aliphatic polyesters and their use in protein-delivery systems . Journal of Applied Polymer Science , 91 : 1848
- Yang , F , Song , FL , Pan , YF , Wang , ZY , Yang , YQ , Zhao , YM , Liang , SZ and Zhang , YM . 2010 . Preparation and characteristics of interferon-alpha poly(lactic-co-glycolic acid) microspheres . Journal of Microencapsulation , 27 : 133
- Wang , ZY , Hou , XN , Mao , ZZ , Ye , RR , Mo , YQ and Finlow , DE . 2008 . Synthesis and characterization of biodegradable poly(lactic acid-co-glycine) via direct melt copolymerization . Iranian Polymer Journal , 17 : 791
- Wang , N , Wu , XS , Lujan-Upton , H , Donahue , E and Siddiqui , A . 1997 . Synthesis, characterization, biodegradation, and drug delivery application of biodegradable lactic/glycolic acid oligomers. I. Synthesis and characterization . Journal of Biomaterials Science Polymer Edition , 8 : 905
- Wang , N and Wu , XS . 1998 . Synthesis, characterization, biodegradation, and drug delivery application of biodegradable lactic/glycolic acid oligomers: Part II. Biodegradation and drug delivery application . Journal of Biomaterials Science Polymer Edition , 9 : 75
- Apreutesei , D , Lisa , G , Hurduc , N and Scutaru , D . 2006 . Thermal behavior of some cholesteric esters . Journal of Thermal Analysis and Calorimetry , 83 : 335
- Song , HT , Guo , T , Zhao , MH , Zhang , RH , Li , X and Bi , KS . 2002 . Studies on the physicochemical properties of borneol beta-cyclodextrin inclusion complex . Journal of Shenyang Pharmaceutical University , 19 : 249
- Zhao , YM , Wang , ZY , Wang , J , Mai , HZ , Yan , B and Yang , F . 2004 . Direct synthesis of poly(D, L-lactic acid) by melt polycondensation and its application in drug delivery . Journal of Applied Polymer Science , 91 : 2143
- Ouchi , T , Seike , H , Miyazaki , H , Tasaka , F and Ohya , Y . 2000 . Synthesis of a block copolymer of L-lactide and depsipeptide with pendant thiol groups . Designed Monomers and Polymers , 3 : 279
- Ye , RR , Wang , ZY , Yang , K and Luo , SH . 2010 . Synthesis and characterization of a novel biodegradable material, poly(lactic acid-co-tryptophane) . Designed Monomers and Polymers , 13 : 415
- Pandey , A and Aswath , P . 2010 . Microwave-assisted in situ synthesis of poly L-lactic acid with nanoparticles of calcium phosphate . International Journal of Polymeric Materials , 59 : 911
- Helaly , MF , Essawy , HA and Shabana , MA . 2011 . Elastic polymeric network structure for slow release drug delivery systems . Polymer – Plastics Technology and Engineering , 50 : 438
- Balakrishnan , H , Hassan , A , Imran , M and Wahit , MU . 2012 . Toughening of polylactic acid nanocomposites: a short review . Polymer – Plastics Technology and Engineering , 51 : 175