Abstract
In a biological and phytochemical study on the leaves of Psychotria spectabilis. Steyerm., seven compounds were isolated and identified from the CHCl3/MeOH (2:1, v/v) and MeOH extracts. Among the isolates were two diterpenes, solidagenone and deoxysolidagenone; three coumarins, coumarin, umbelliferone, and psoralene; and two flavonols, quercetin and quercetrin. Biological evaluations showed that diterpenes and coumarins exhibited antifungal activity against the filamentous fungi Cladosporium cladosporioides. (Fresen) de Vries and C. sphaerospermum. Penzig. Solidagenone and psoralene also displayed selective cytotoxic activity against Rad 52Y mutant yeast strain of Saccharomyces cerevisiae.. In this paper, the isolation, structure elucidation, and bioactivity results of these compounds are reported.
Introduction
The Pantropical tribe Psychotrieae belongs to the family Rubiaceae and covers approximately 2000 species. Most of these are currently classified in Psychotria. L., the largest genus in the family, and with perhaps 1000 species (Hamilton, Citation1989) one of the largest genera of flowering plants. In addition, Psychotria. species are well represented in the New World with ca. 600 species (Taylor, Citation1996).
Plant species of Psychotria. have long been used in folk medicine to treat, among other ailments, intestinal infections, cough, and respiratory and stomach disorders. Data available in the literature strongly support the idea that the extract and some constituents isolated from these plants, including indole, quinoline and isoquinoline alkaloids, flavonoids, quinoids, and terpenes, account for their reported cytotoxic, analgesic, hypothermic, antiviral, antifungal, antispasmodic, and central nervous system activities.
Psychotria spectabilis. Steyerm. (Rubiaceae) is a woody shrub, endemic in the eastern Amazon of Brazil (Amapá, Pará except the Southern Serras, Maranhão). In accord with our search for biologically active compounds from the Brazilian flora (Benevides et al., Citation2001; da Silva, 2002), CH2Cl2/MeOH (2:1, v/v) and methanolic extracts of the leaves of this plant were selected for phytochemical investigation due to their activity against mutant strains of Saccharomyces cerevisiae. (Rad+, Rad 52Y, and 321N) that showed growth inhibition by DNA-damaging agents (Gunatilaka et al., Citation1992) and against the filamentous fungi Cladosporium cladosporioides. and C. sphaerospermum. (Homans & Fuchs, Citation1970).
In this paper, we describe the isolation, identification, and evaluation of the activity of coumarins and diterpenes as inhibitors of the growth of the filamentous fungi C. sphaerospermum. and C. cladosporioides.. These compounds were also assayed against mutant strains of S. cerevisiae..
Materials and Methods
General experimental procedures
NMR spectra in CDCl3 (1, 2, 5), CD3OD (3, 4, 6) or DMSO-d.6/CDCl3 (7) () were recorded on Bruker spectrometers (300 and 500MHz for 1H and 75 and 125MHz for 13C, respectively), and tetramethylsilane (TMS) was used as internal standard. Electron Impact Mass Spectra (EI-MS) were measured at 70eV on HP 5988A, Mat 90 Finnigan and Shimadzu Gas Chromatography-Mass Spectra (CG-MS) QR 5050A spectrometers. IR spectra were recorded on a Fourier-transform infrared (FTIR) 1750 Perkin Elmer and FTIR 510 Nicolet. UV spectra were recorded on a Perkin Elmer UV-Vis Hitachi U-300. Silica gel (Merck 230–400 mesh) and Sephadex LH-20 (Pharmacia) were used for column chromatography and Kielselgel 60 F254 (Merck) prepared plates for thin-layer chromatography (TLC).
Plant material
Psychotria spectabilis. Steyerm. (Rubiaceae) was collected near Barragem do Dormente, Amapá, Brazil, and identified by botanist Marina Thereza V. de A. Campos. Voucher specimens [Lopes 058 (10/96)] were deposited in the Herbário Maria Eneida P.K. Fidalgo of Botanical Garden, São Paulo, SP, Brazil.
Extraction and isolation
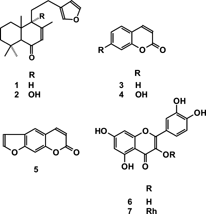
The dried and powdered leaves (125g) of P. spectabilis. were successively extracted by maceration with organic solvents at room temperature. The solvents were removed under vacuum to yield 8.0g of CHCl3/MeOH (2:1, v/v) extract and 19.0g of MeOH extract. The CHCl3/MeOH bioactive crude extract was separately dissolved in 80% aqueous MeOH and defatted with hexane. The aqueous MeOH fractions were diluted with H2O until 60% aqueous MeOH mixture and then partitioned with CHCl3 and EtOAc, affording 120mg and 900mg of the bioactive fractions, respectively. Bioactivity-guided fractionation of the EtOAc soluble fraction, using silica gel column chromatography and hexane/MeOH (with increasing polarity) as eluents, afforded 30 subfractions, whose bioactivities were detected only in the 12 and 13 subfractions. The F-12 was separated by preparative TLC using Si gel eluted with hexane-CH2Cl2 (7:3) to obtain deoxysolidagenone (1) (4.5mg). The F-13 was rechromatographed over silica gel (230–400 mesh) using as eluents CH2Cl2/MeOH (9.5:0.5) to give the compounds (in eluting order) solidagenone (2) (9.0mg), coumarin (3) crystallized from MeOH/Et2O (17.0mg), psoralene (4) (10.0mg), and the flavonol quercetin (5) (7.0mg). Part of the residual bioactive aqueous fractions MeOH (7.0g) resulting from partitioning procedures was chromatographed on Sephadex LH-20 using the solvents H2O/MeOH and MeOH. The fractions eluted with MeOH were further purified on a silica gel column using CH2Cl2/MeOH with increasing polarity. The active fractions were separated by preparative TLC (Si gel, CH2Cl2/EtOAc, 75:15) to obtain umbelliferone (6) crystallized from MeOH/Et2O (6.0mg), psoralene (4) (4.3mg), and quercetrin (7) (6.3mg).
The isolated compounds deoxysolidagenone (1), solidagenone (2), coumarin (3), umbelliferone (4), psoralene (5), and the flavonoids quercetin (6) and quercetrin (7) presented spectral data in agreement with literature.
15,16-Epoxy-labdan-7,13,14-triene (deoxysolidagenone; compound 1.)
White amorphous solid, (CHCl3: c=0.2). C20H28O2 requires 79.96% C, 9.39% H, 10.65% O; found 78.12% C, 8.96% H, 12.42% O. UV
nm (ε): 215 (3.8), 240 (4.03). IR
cm−1: 2990, 2920, 2902, 1676, 1506, 1462, 1450, 1383, 1168, 874, 778. MS m./z. (rel. int. %): [M]+ 300(1), 285(1), 220(1), 219(4.2), 205(2), 176(6.1), 149(20.1), 135(11), 109(20), 95(17), 83(11), 82(100), 81(25), 41(11), 40(1). 1H NMR (300MHz, CDCl3) and 13C NMR (75MHz, CDCl3) according to literature (Torres et al., Citation1989).
9α-Hydroxy-15,16-epoxy-labdan-7,13,14-triene (solidagenone; compound 2.)
Yellowish solid, . (CHCl3: c=1.0). C20H28O3 requires 75.91% C, 8.92% H, 15.17% O; found 75.08% C, 8.56% H, 15.01% O. UV
nm (ε..): 225 (4,03). IR
cm−1: 3443, 2938, 2870, 1718, 1666, 1460, 1380, 1234, 1069, 1068, 980, 874, 757. MS m./z. (rel. int. %): 316(3) [M]+; 303(1), 298(1), 192(5), 192(10), 124(1), 123(6,1), 111(20,1), 83(11), 82(100), 81(33), 43(15), 41(11). 1H NMR (300MHz, CDCl3) and 13C NMR (75MHz, CDCl3) according to literature (Torres et al., Citation1989).
Coumarin (compound 3.)
White crystals, m.p. 70°C. 1H, 13C NMR, and MS data according to literature (Austin et al., Citation1973; Chan et al., Citation1977; Hayakawa et al., Citation1984).
Umbelliferone (compound 4.)
White crystals, m.p. 230°C. 1H, 13C NMR, and MS data according to literature (Austin et al., Citation1973; Chan et al., Citation1977; Hayakawa et al., Citation1984).
Psoralene (compound 5.)
White crystals, m.p. 98–101°C. 1H, 13C NMR, and MS data according to literature (Austin et al., Citation1973; Chan et al., Citation1977; Hayakawa et al., Citation1984).
Quercetin (compound 6.)
Yellow amorphous solid. 1H and 13C NMR according to Markham et al. (Citation1978).
Quercetrin (compound 7.)
Yellow amorphous solid. 1H and 13C NMR according to Markham et al., Citation1978.
Bioassays
The crude extracts and isolates obtained from the leaves of P. spectabilis. were screened for DNA-damaging activity against mutant strains of Saccharomyces cerevisiae., according to established protocols (Gunatilaka et al., Citation1992). The IC12 values refer to the concentration in μg/ml required to produce an inhibition zone of 12-mm diameter around a 100-μl well after incubation for 48h at 30°C.
The microorganisms used in the bioautographic assays, Cladosporium cladosporioides. Fresen de Vries SPC 140 and C. sphaerospermum. Penzig SPC 491, have been maintained at the Instituto de Botânica, SMA/SP. For the antifungal assay, 10µl of the solutions of crude extracts, fractions, and pure compounds were prepared in different concentrations corresponding to 20, 10, 1, and 0.1μg for pure compounds and 100, 50, and 25μg for crude extracts or fractions, respectively. The samples were applied on TLC plates, and these were developed in an appropriate solvent system followed by complete removal of solvents. The chromatographic plates were sprayed with spore suspension of C. sphaerospermum. and C. cladosporioides. in a nutritive medium (Rahalison et al., Citation1994) and incubated for 48h at 28°C. After incubation, clear inhibition zones appeared against a dark background. Nystatin was used as positive control.
Results and discussion
All compounds isolated from P. spectabilis. in the current investigation have been previously reported from other plant species, and their molecular structures were determined by spectral data interpretation and comparison with literature values (see “Materials and Methods”). Two of the seven compounds obtained in this investigation are diterpenes of the labdane-type (1–2), three are coumarins (3–5), and two are flavonols (6–7). Diterpenes are not common in Rubiaceae where they were only reported in Coffea., which accumulates kaurane-type diterpenes. Coumarins have been isolated from innumerable plants of Angiospermae, especially from Apiaceae and Rutaceae families. However, in Rubiaceae they have not been reported until the current work.
summarizes the antifungal and cytotoxic activities of the compounds isolated from P. spectabilis. leaves. Among these compounds, the flavonols quercetin and quercetrin were completely inactive against all tested microorganisms. Diterpenes and coumarins displayed activity against both tested fungi. The activities of pure compounds were expressed as detection limits: minimum amounts required to inhibit growth of spores in the TLC bioassay (Rahalison et al., Citation1994). The lowest detection limits were observed for the diterpene solidagenone (0.25μg with C. cladosporioides., 0.5μg with C. sphaerospermum.) and psoralene (0.25μg against both microorganisms). These results are higher than the reference compound nystatin (1.0μg). For umbelliferone, this value was 2.5μg, whereas for coumarin and deoxysolidagenone 10.0 μg were required to inhibit growth of both filamentous fungi. Furthermore, their potential anticancer activities were also tested, using a mechanism-based yeast bioassay for DNA-modifying agents (Gunatilaka et al., Citation1994). Compounds 2 and 5 exhibited weak effect on DNA, as deduced by the selective inhibition of the mutant strain Rad 52Y, relative to the wild strain Rad+ ().
Table 1.. Antifungal and cytotoxic activities of compounds 1–7 from P. spectabilis.Footnotea..
In conclusion, P. spectabilis. is a good source of antifungal compounds, and its chemical profile is not in accordance with existing knowledge of the chemosystematic pattern in the Psychotrieae, suggesting that a more extensive chemical and biological study is required in order to identify new antifungal or other compounds that can substantiate systematic delimitations proposed to this genus (Taylor, Citation1996; Nepokroeff et al., Citation1999).
References
- Austin DJ, Brown SA (1973): Furanocoumarin biosynthesis in Ruta graveolens. cell cultures. Phytochemistry 12: 1657–1661.
- Benevides PJC, Young MCM, Giesbrect AM, Roque NF, Bolzani V da S (2001): Antifungal polysulphides from Petiveria alliacea. L. Phytochemistry 57: 743–747. [CROSSREF], [CSA]
- Chan KK, Giannini DD, Cain AH, Roberts JD, Porter W (1977): 13C Nuclear magnetic resonance studies of coumarin and related compounds. Tetrahedron 33: 899–902. [CROSSREF]
- Davis DL, Dinse GE, Hoel DG (1994): Decreasing cardiovascular disease and increasing cancer among whites in the United States from 1973 through 1987: Good news and bad news. JAMA 271: 431–437. [CROSSREF]
- Gunatilaka AAL, Samaranayake G, Kingston DGI, Hoffmann G, Johnson RK (1992): Bioative ergost-5-ene-3β, 7α-diol derivatives from Pseudobersama mossambicensis.. J Nat Prod 55: 1648–1654. [CROSSREF]
- Gunatilaka AAL, Kingston DGI, Johnson RK (1994): Mechanism-based isolation and structures of some anticancer active natural products. Pure Appl Chem 66: 2219–2222.
- Hamilton CW (1989): A revision of Mesoamerican Psychotria. subg Psychotria. (Rubiaceae). Part 1: Introduction and species 1–16. Ann Missouri Bot Garden 76: 67–111.
- Hayakawa K, Yodo M, Ohsuki S, Kanematsu K (1984): Novel bicyloannulation via tandem vinylation and intramolecular Diels Alder reaction of five membered: A new approach to construction of psoralen and azapsoralen. JACS 106: 6735–6740. [CROSSREF]
- Homans AL, Fuchs A (1970): Direct bioautography on thin layer chromatograms as a method for detecting fungitoxic substances. J Chromatogr 51: 327–329. [CROSSREF]
- Markham KR, Ternai B, Stanley R, Geiger H, Mabry TJ (1978): 13C NMR studies of flavonoids 3. Naturally occuring flavonoid glycosides and their acylated derivatives. Tetrahedron 34: 1389–1395. [CROSSREF]
- Mitscher LA, Drake S, Gollapudi SR, Okute SK (1997): A modern look at folkloric use of anti-infective agents. J Nat Prod 50: 1025–1040. [CROSSREF]
- Neprokoeff M, Bremer B, Sytsma KJ (1999): Reorganization of the genus Psychotria. and tribe Psychotrieae (Rubiaceae) inferred from ITS and rbcL. sequence data. Systematic Botany 24: 5–27.
- Rahalison L, Hamburger M, Monod M, Frenk E, Hostettmann K (1994): Antifungal tests in phytochemical investigations: Comparison of bioautographic methods using phytopathogenic and human pathogenic fungi. Planta Med 60: 41–44. [CSA]
- Taylor CM (1996): Overview of the Psychotrieae (Rubiaceae) in the neotropics. Opera Bot Belg 7: 261–270. [CSA]
- Torres LMB, Roque NF, Akisue MK, Roque NF (1989): Diterpenes from roots of Solidago microglossa.. Rev Latinoam Quim 20: 94–97.