Abstract
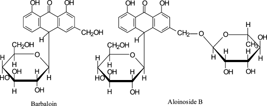
A compound was isolated from South African Cape aloes, and the structure was elucidated as 10-glucopyranosyl-1, 8-dihydroxy-3-(rhamnopyranosyl-hydroxymethyl)-9(10H.)-anthracenone (named aloinoside B) on the basis of various spectroscopic evidence (IR, MS, 1H NMR, 13C NMR, HMQC, HMBC, 1H–1H COSY). Aloinoside B was metabolized to barbaloin, isobarbaloin, and a hydroxyl metabolite by rat intestinal bacteria. The peak amount of barbaloin was reached about 3 h after oral administration.
Introduction
Aloes are obtained by evaporating the liquid that drains from the leaves of Aloe barbadensis. Miller or Aloe ferox. Miller (Liliaceae). Aloes have long been used in many countries around the world. The ancient Egyptians used aloes as a laxative some 5000 years ago. The Chinese made aloes into a traditional Chinese medicine (TCM) and have used it according to the TCM theory for more than 1000 years. Aloes used in China generally were imported, and Curacao aloes and Cape aloes were both used. Curacao aloes were made in Western Indian islands and Cape aloes were made in South Africa. Aloes were reported to have various pharmacological actions, including loosing the bowels, destroying parasites, and preventing fungi. Aloes were found listed in the U.S., British, Japanese, and Chinese pharmacopoeias as laxative. Many compounds have been separated from aloes, most of which are derivatives of hydroxyanthracene such as barbaloin, isobarbaloin, and homonataloin.
Many studies have shown that for generally orally administered TCM, it is common that the component compounds are metabolized by human intestinal bacteria to some activated forms (Eun-Ah Bae et al., Citation1999; Dong-Hyun Kim, Citation1998Citation1999aCitationbCitationc). Intestinal bacteria play a very important role in the effectiveness of many TCMs.
It has been demonstrated that barbaloin is a kind of pro-drug that is metabolized by intestinal bacteria to aloe-emodin-9-anthrone and aloe-emodin-9-anthrone as the ultimate laxative compounds. The mechanism of laxative effect is through increasing the intestinal water content and stimulating mucus secretion (Yasuko Ishii et al., Citation1990Citation1994aCitationb). A bacterium capable of cleaving the C.-glucosyl bond of barbaloin has been found in human feces, namely, Eubacterium. sp. strain BAR (Qing-Ming Che et al., Citation1991). Aloinoside B has a structure similar to that of barbaloin. We thought aloinoside B may be a kind of pro-drug that can be changed in animal gastrointestinal tract to be effective as a laxative.
Materials and Methods
Instruments
Melting points were determined on a Kofler micro-melting point apparatus (Vienna, Austria) and are uncorrected. IR spectra were recorded on a Shimadzu spectrometer (Kyoto, Japan) in KBr disks. UV spectra were obtained on a Shimadzu UV-2200 spectrometer. (Kyoto, Japan) 1H NMR, 13C NMR,1H-detected heteronuclear multiple-quantum correlation (HMQC), 1H-detected heteronuclear multiple-bond correlation (HMBC) and 1H–1H correlated spectroscopy (COSY) spectra were recorded on a Bruker ARX-300 spectrometer with tetramethylsilane (TMS) as an internal standard. A Finnigan TSQ triple quadrupole mass spectrometer (San Jose, CA, USA) equipped with an electrospray ionization (ESI) source was used for mass analysis and detection. An LC-10AD Shimadzu high-performance liquid chromatograph (HPLC) equipped with a SPD-10A UV detector was used.
Reagents
Methanol and acetonitrile were of HPLC grade, and water was distilled.
Medicine
Aloes were purchased from Tian-Yi-Tang Drug Store (Shenyang, China) and were identified by Professor Qishi Sun of Shenyang Pharmaceutical University as Cape (South Africa) aloes. Standard barbaloin was purchased from the National Institute of China for the Control of Pharmaceutical and Biological Products (batch no. 787-9001, with the purity higher than 98.0%).
Extraction and isolation
Crude powder of aloes (1 kg) was extracted three-times with n.-butanol to afford n.-butanol extract (600 g). The crude extract was subjected to SiO2 column chromatography (CC), eluted with CHCl3–MeOH (v:v) 50:1, 20:1, 15:1, 2:1 to yield compound 1 (500 mg) ().
Elucidation
Compound 1 was a yellowish powder (MeOH), with melting point (m.p.) of 223–225°C, 31.0° (c. = 0.5, DMSO). The UV absorption (UV
nm: 221, 259, 267, 294, 359) and the IR spectrum ( IR
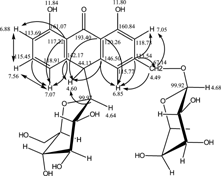
cm−1: 3516, 3216, 2937, 1636, 1618, 1487, 1456, 1276, 1035, 1003) implied that it was an anthranone. Acid hydrolysis revealed glucose and rhamnose. The ESI-MS spectrum gave its negative ion at m./z. 563 (M+–H). The molecular formula of C27H32O13 for compound 1 was established on the basis of electrosprayionzation mass spectrum (ESI-MS), 1H NMR, 13C NMR, and IR analysis. 1H NMR and 13C NMR and 2D NMR spectra data are shown in Tables . The 13C NMR spectrum gave the signal at δ 61.45 (C-6 signal of glucose) and δ 17.96 (C-6 signal of rhamnose), which indicated it had the glycosyl and rhamnosyl moieties. Signals between δ 113.55 and 161.07 implied that it had two aromatic rings. All these, with the 1H NMR spectrum, suggested that compound 1 was a double-sugar glycoside. The HMQC experiment of compound 1 revealed correlations of C3–CH2– (δ 67.14) and H (δ 4.49). The HMBC spectrum of compound 1 exhibited correlation peaks between C3–CH2 (δ 4.49) and rham-C1 (δ 99.92). Therefore, the rham links to C3–CH2–. Moreover, the linkage of the glucose moieties was deduced from the HMQC and HMBC experiments. The HMQC spectrum of compound 1 exhibited correlation peaks between H10 (δ 4.60) and C10 (δ 44.13). The HMBC spectrum of compound 1 exhibited correlation peak between H-10 (δ 4.60) and glc-C1 (99.70). Therefore, the glucose links to C-10. In addition, the 1H–1H COSY spectrum of compound 1 revealed correlations of H-6 (7.56) with H-5 (7.07) and H-7 (6.88), respectively. The HMBC correlation of compound 1 is shown in .
Table 1.. 13C-NMR spectral data for compound 1 (in DMSO, TMS).
Table 2.. HMQC spectral data for compound 1 (in DMSO, TMS).
Table 3.. HMBC spectral data for compound 1 (in DMSO, TMS).
Table 4 1H − 1H COSY spectral data for compound 1 (in DMSO, TMS).
The above evidence allowed us to establish unambiguously the structure of compound 1 as 10-glucopyranosyl-1,8-dihydroxy-3-(rhamnopyranosyl-hydroxymethyl)-9(10H.)-anthracenone, named as aloinoside B.
Animals
Adult Wistar rats of either sex (weighing 220–240 g) were provided by the Experimental Animal Center of Shenyang Pharmaceutical University and divided in groups of 6 animals. All animals were housed under standard conditions (room temperature 24±1°C, relative air humidity 50±2%) with food and water available and maintained on 12L:12D cycle.
Drug Preparation
Aloinoside B was made into a suspension of 10 mg/ml with 0.3% tragacanth.
Preparation of analytical solutions
Aloinoside B suspension (1.0 ml) was administered orally to each rat. The rats were sacrificed after 0.5, 11, 2, 3, 5, 9, 15 h according to the allocated group. The gastrointestinal tracts were cut open and extracted with methanol four times. The methanol extract was evaporated to nearly dryness, and the residue was resolved with methanol to 25.0 ml. The solution was filtered with an 0.45-µm microspore filter membrane to be ready for HPLC analysis and HPLC-MS analysis.
HPLC analysis
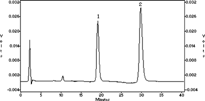
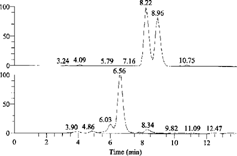
Separations were carried out with a Hypersil C18 reversed-phase column (particle size of the packing 5 µm, 200 × 4.6 mm i.d.) purchased from Dalian Elite Analytical Instruments Co., Ltd (Dalian, P.R. China) with a mobile phase of methanol-acetonitrile-water-acetic acid glacial (7:18:75:0.15, v/v/v/v), a detection wavelength of 359 nm, and a flow rate of 0.9 ml/min at room temperature. The retention time of aloinoside B was about 30 min, and the retention time of the metabolite barbaloin was about 19.5 min. The chromatogram is shown in .
HPLC-MS analysis
The same column described above was used with a mobile phase of methanol:water (60:40, v/v), a detection wavelength of 359 nm, and a flow rate of 0.5 ml/min at room temperature. The mass spectrometer was operated in a negative ion mode with the spray voltage set at 4.5 kV. Nitrogen was used both as the sheath gas (0.6 Mpa) and auxiliary gas (3 l/min) for nebulization. The temperature of the heated capillary was set at 200°C.
Results
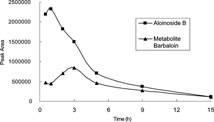
The time-course of aloinoside B and one of its metabolites, barbaloin, is shown in . About 3 h after oral administration of aloinoside B, the metabolite barbaloin reached the highest point. The content of barbaloin in the rat gastrointestinal tract declined, and 12 h later, the contents of both aloinoside B and barbaloin declined to a very low concentration due to their excretion and transformation to other forms.
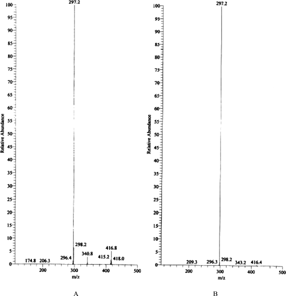
Three metabolites were found in the extract of orally administered rat gastrointestinal tract, the molecular weights of which were 580 (tR = 6.56 min) and 418 (tR = 8.22 and 8.96 min). The compound with a molecular weight of 418 was the de-rhamnosylated metabolite, barbaloin, and its isomer, isobarbaloin. The compound with a molecular weight of 580 was the hydroxyl metabolite of aloinoside B. Aloinoside B was metabolized to barbaloin and isobarbaloin. shows the chromatograms of de-rhamnose metabolites and hydroxyl metabolite. shows the full-scan MS-MS spectra of ion m./z. 417 (de-rhamnose metabolites) and standard barbaloin. The fragment ion of m./z. 417 was m./z. 297. shows the full-scan MS-MS spectrum of ion m./z. 579 (hydroxyl metabolite). The fragment ion of m./z. 579 was m./z. 416.
Discussion
It is known that barbaloin can be metabolized to aloe-emodin-9-anthrone (which is actually the compound accounting for the laxative effectiveness of aloes) by certain bacterial species that have been isolated from human feces. Because aloinoside B is metabolized to barbaloin and isobarbaloin, then barbaloin and isobarbaloin are metabolized to aloe-emodin-9-anthrone by some bacterial species in human intestine, it can be suggested that aloinoside B is one of the laxative components of aloes.
References
- Dong-Hyun Kim, Ki-Ung Yu, Eun-Ah Bae, Myung Joo Han (1998): Metabolism of puerarin and daidzin by human intestinal bacteria and their relation to in vitro. cytotoxicity. Biol Pharm Bull 21: 628–630. [CSA]
- Dong-Hyun Kim, Seung-Won Lee, Myung Joo Han (1999a): Biotransformation of glycyrrhizin to 18β-glycyrrhetinic acid-3-O.-β.-D-glucuronide by Streptococcus. LJ-22, a human intestinal bacterium. Biol Pharm Bull 22: 320–322. [CSA]
- Dong-Hyun Kim, Eun-Ah Bae, Myung Joo Han (1999b): Anti-Helicobacter pylori. activity of the metabolites of poncirin from Poncirus trifoliate. by human intestinal bacteria. Biol Pharm Bull 22: 422–424. [CSA]
- Dong-Hyun Kim, Suk-Young Kim, Sun-Young Park, Myung Joo Han (1999c): Metabolism of quercitrin by human intestinal bacteria and its relation to some biological activities. Biol Pharm Bull 22: 749–751. [CSA]
- Eun-Ah Bae, Myung Joo Han, Kyung-Tae Lee, Jong-Won Choi, Hee-Juhn Park, Dong-Hyun Kim (1999): Metabolism of 6″-O.-xylosyltectoridin and tectoridin by human intestinal bacteria and their hypoglycemic and in vitro. cytotoxic activities. Biol Pharm Bull 22: 1314–1318. [CSA]
- Qing-Ming Che, Teruaki Akao, Masao Hattori, Yoshisuke Tsuda, Tsuneo Namba, Kyochi Kobashi (1991): Barbaloin stimulates growth of Eubacterium. sp. strain BAR, a barbaloin-metabolizing bacterium from human feces. Chem Pharm Bull 39: 757–760. [CSA]
- Yasuko Ishii, Hisayuki Tanizawa, Yoshio Takino (1990): Studies of aloe. III. Mechanism of cathartic effect. (2). Chem Pharm Bull 38: 197–200.
- Yasuko Ishii, Hisayuki Tanizawa, Yoshio Takino (1994a): Studies of aloe. IV. Mechanism of cathartic effect. (3). Biol Pharm Bull 17: 495–497. [CSA]
- Yasuko Ishii, Hisayuki Tanizawa, Yoshio Takino (1994b): Studies of aloe. V. Mechanism of cathartic effect. (4). Biol Pharm Bull 17: 651–653. [CSA]