Abstract
The methanol extract of Cassia nigricans. Vahl. (Caesalpinaceae) was investigated for its antiplasmodial activity against chloroquine-resistant strains of Plasmodium falciparum.. The in vitro. activity against P. falciparum. strain K1 was assessed using the parasite lactate dehydrogenase assay method. The main antiplasmodial principle, 1,3,8-trihydroxy-6-methyl-9,10-anthracenedione, has been isolated from C. nigricans. (whole plant) for the first time. This was found to have in vitro. activity against P. falciparum.. This finding supports the traditional use of the plant for the treatment of malaria.
Introduction
Cassia nigricans. Vahl. (Caesalpinaceae) is an herbaceous plant that grows widely in the savannah grasslands of West Africa. The plant parts have been used medicinally in Senegal and Guinea as vermifuge and as a substitute for quinine in the treatment of fever. The pulverized leaves added to food improve appetite and acts as a febrifuge. Also, a decoction of the dried aerial parts is used as a wash and fumigation in fever and to treat purulent sores in Guinea-Bissau (Irvine, Citation1961; Silva et al., 2001). In northern Nigeria, an infusion of the dried leaf is used for stomach ulcers (Akah et al., Citation1998).
The antiulcer properties of the aqueous and methanol extracts of C. nigricans. have been reported (Akah et al., Citation1998; Nwafor & Okwuasaba, Citation2001). There are a few reports on the antimicrobial and pharmacological activities (Chidume et al., Citation2002; Silva et al., 2001) but no report on the antiplasmodial profile of this widely used plant. The current study was therefore undertaken to evaluate the antiplasmodial effects of the methanol extract of C. nigricans. on Plasmodium falciparum.. This note describes the isolation and activities of 1,3,8-trihydroxy-6-methyl-9,10-anthracenedione, the major antimalarial principle of the plant.
Materials and Methods
Plant material
The whole plant of Cassia nigricans. Vahl. (Caesalpinaceae) was kindly supplied by Mr. S.D. Fumen (Kaduna State, Nigeria) in September 2001. The plant was identified and authenticated by Mr. Abraham O. Ohaeri (taxonomist), Department of Medicinal Plant Research and Traditional Medicine, National Institute for Pharmaceutical Research and Development (NIPRD), Abuja. A voucher specimen was deposited at the NIPRD herbarium and assigned the voucher specimen no. 3833.
Extraction and isolation
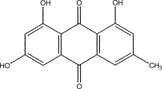
The dried powdered whole plant of C. nigricans. (80 g) was exhaustively extracted with 90% methanol using a Soxhlet apparatus. The extract was concentrated to a small volume in vacuo. and this gave a yield of 20.28% w/w. Analytical silica gel 150 A° (Whatman) 250 µm thick was activated at 80–100°C. The solvent system used was hexane:ethyl acetate (80:20). The crude methanol extract (4 g) was made into a slurry with silica gel (20 g), dried in an oven, and fractionated using accelerated gradient chromatography (AGC) for gradient elution as follows: hexane, hexane:ethyl acetate, ethyl acetate, ethyl acetate:ethanol, ethanol and methanol to complete elution. A total of 114 fractions were obtained after complete elution. Each fraction was examined using analytical thin-layer chromatography (TLC), and those fractions with similar spots were pooled together, resulting in 5 fractions coded A, B, C, D, and E. Compound C1 recrystallizes out from the hexane:ethyl acetate (85:15) portion, giving an orange amorphous powder (50 mg). The UV, IR, MS, 1H and 13C NMR spectra of C1 () were in accordance with previously reported data of emodin isolated from some Cassia. species (Lemli & Cuvelle, Citation1967; Gritranapan et al., Citation1983)
1,3,8-Trihydroxy-6-methyl,9,10-anthracenedione (emodin)
Compound C1 C15H1005, HRMS M+ at m./z. 270.05282. An amorphous powder with melting point range of 245–252°C has characteristic IR absorption at 3500 cm−1 due to OH and 1650 cm−1 due to carbonyl. The 1H NMR spectrum showed absorption at LF2.45 (CH3), LF7.5 (1H), LF7.23 (1H), LF7.24 (1H), LF7.12 (1H). 13C NMR revealed the presence of two carbonyl at LF192.16 and LF182.73. It also shows the presence of 12 olefinic/aromatic carbons at LF109.44, 110.39, 110.89, 115.00, 122.07, 125.03, 134.74, 137.10, 150.08, 163.83, 166.85, 167.22 and one aliphatic carbon at LF22.64 (CH3). The Distortion Enhancement by Polarisation (DEPT) and Correlation Spectroscopy (COSY) revealed the presence of 4 methinine (i.e., CH) and 1 CH3. From the coupling experiment, it showed coupling between the CH3, hydrogen, and the proton at LF7.5. Comparism of these data with that in the library revealed compound C1 as emodin
In vitro. antiplasmodial activity
Antiplasmodial activity was determined in vitro. against a chloroquine-resistant strain K1 of Plasmodium falciparum. and the control drug chloroquine diphosphate using the parasite lactate dehydrogenase assay method (). For the in vitro. assays, we used P. falciparum. parasites (strain K1) that are chloroquine-resistant. Malaria parasites were maintained in human A+ erythrocytes suspended in RPMI 1640 medium supplemented with A+ serum and D-glucose according to previously published methods (Trager & Jensen, Citation1976; Fairlamb et al., Citation1985). Cultures containing predominantly early ring stages were used for testing. The extracts were dissolved or micronized in DMSO and further diluted with RPMI 1640 medium (the final DMSO concentration did not exceed 0.5%, which did not affect parasite growth). Two-fold serial dilutions were made in 96-well microtiter plates in duplicate, and infected erythrocytes were added to give a final volume of 100 µl with hematocrit 2.5 and 1% parasitemia. Chloroquine disphosphate was used as a positive control, and uninfected and infected erythrocytes without compound extracts were included in each test. Plates were placed into a modular incubator gassed with 93% nitrogen, 3% oxygen, and 4% carbon dioxide and incubated at 37°C for 48 h. Parasite growth was assessed by measuring lactate dehydrogenase activity (Makler et al., Citation1993). The reagent used contained the following in each milliliter: 0.74 mg of acetyl pyridine adenine dinucleotide (APAD), 19.2 mg of lithium lactate, 0.1 mg of diaphorase, 2 µl of Triton X-100, 1 mg of nitroblue tetrazolium, and 0.5 mg of phenazine ethosulfate. This reagent (50 µl) was added to each well and mixed, and the plates were incubated for 15 min for 37°C. Optical densities were read at 550 nm using a Dynatech laboratories MRX microplate reader, and percent inhibition of growth was calculated by comparison with control values. IC50 values were determined using linear regression analysis (Microsoft Excel). A minimum of three separate determinations was carried out for each extract.
Table 1.. In vitro. activities of the crude extract, fractions, compound C1, and chloroquine diphosphate against Plasmodium falciparum. (strain K1).
Results and Discussion
In continuing the search for antimalarial compounds from Nigerian medicinal plants, the methanol extract of Cassia nigricans. was subjected to bioactivity-guided fractionation. The methanol extract (crude), fraction (A–E), and compound C1 were screened for antiplasmodial activity against chloroquine-resistant strains of P. falciparum.. The fraction C showed a moderate antiplasmodial activity, the highest activity being observed in the compound C1. Compound C1 showed a higher antiplasmodial activity than the methanol extract from which it was obtained. Using spectroscopic studies (MS, 1H NMR, and 13C NMR supported by 1H-1H COSY and 1H-13C COSY experiments), the structure of 1,3,8-trihydroxy-6-methyl-9,10-anthracenedione was elucidated. The HRMS of C1 showed a M+ peak at m./z. 270.05282 corresponding to a molecular formula of C15H10O5 (). However, the above data were found to correspond to that of emodin, which had previously been isolated from Cassia grandis. Linn. and other Cassia species. (Lemli & Cuvelle, Citation1967; Gritranapan et al., Citation1983). However, this is the first time this particular compound has been isolated in C. nigricans. and shown to possess antiplasmodial activity. These results tend to support the use of C. nigricans. in African traditional medicine for the treatment of malaria and allowed us to correlate the activity of the isolated compound with the therapeutic properties described for the plant.
Acknowledgments
The authors gratefully acknowledge Dr. Michele D'ambrosio of the Laboratorio di Chimica Bioorganica-Universita di Trento, Italy, for spectral analysis, Mr. Joseph Ashidi, and Mrs. Cordelia Onanuga for technical assistance. P. falciparum. strain K1 was generously supplied by Professor D.C. Warhurst, London School of Hygiene and Tropical Medicine, and Miss Stella Ogbonna typed the manuscripts.
References
- Akah PA, Orisakwe OE, Gamaniel KS, Shittu A (1998): Evaluation of Nigerian traditional medicines: II. Effects of some Nigerian folk remedies on peptic ulcer. J Ethnopharmacol 62: 123–127. [CSA]
- Chidume FC, Gamaniel KS, Akah PA, Obodozie OO, Wambebe CO (2001): Pharmacological activity of methanolic extract of Cassia nigricans. leaves. Indian J Pharmacol 33: 350–356.
- Fairlamb AH, Warhurst DC, Peters W (1985): An improved technique for the cultivation of Plasmodium falciparum in vitro. without daily medium change. Ann Trop Med Parasitol 79: 379–384. [CSA]
- Gritranapan W, Jirawongse V, Tantiseavri B (1983): Cassia grandis. L, a new source of aloe emodin. J Pharm Sci 10: 6–8.
- Irvine FR (1961): Woody Plants of Ghana. London, Oxford University Press, pp 285.
- Lemli J, Cuvelle J (1967): Research on drugs with anthraquinone bases, XIV, isolation of a new hetroside from senna leaf: Diglucoside of aloe emodin dianthrone. Pharm Acta Heiv 43: 37–40.
- Makler MT, Ries JM, Williams JA, Bancroft JE, Piper RC, Gibbins BL, Hinrichs DJ (1993): Parasite lactate dehydrogenase as an assay for Plasmodium falciparum. drug sensitivity. Am J Trop Med Hyg 48: 739–741.
- Nwafor PA, Okwuasaba FK (2001): Effects of methanolic extracts of Cassia nigricans. leaves on rat gastrointestinal tract. Fitoterapia 72: 206–214. [CROSSREF], [CSA]
- Silva O, Duarte A, Cabrita J, Pimentel M, Diniz A (1996): Antimicrobial activity of Guinea-Bissau traditional remedies. J Ethnopharmacol 50: 55–59. [CROSSREF], [CSA]
- Trager W, Jensen JB (1976). Human malaria parasites in continuous culture. Science. 193: 673.