ABSTRACT
Crude papaya latex (CPL) and its proteinases, papain (PPN) and chymopapain (CPN), are strong uterine contractants. The current study was carried out to examine possible mechanisms of the uterine stimulating activity of the proteinases. Inactivation of the enzymatic activity of papaya proteinases reversibly abolished their uterine-stimulating effect, suggesting that enzymatic activity of the proteinases is a prerequisite for their oxytocic activity. Moreover, removal of Ca2+ from the uterine bathing medium reversibly abolished the uterine-stimulating effect of the proteinases. Nifedipine and verapamil (Ca2+ channel blockers) significantly and reversibly block CPL-, CPN-, and PPN-induced uterine contractions. Blockade of 5-hydroxytryptamine receptors did not prevent the oxytocic activity of papaya proteinases. However, uterine contractions induced by the proteinases were significantly and reversibly inhibited by meclofenamic acid (a cyclooxygenase and prostaglandin inhibitor). At 0.3 and 1 mg/ml, CPL, CPN, and PPN caused a concentration-dependent increase in prostaglandin F2α production in cultured rat uterus, but only CPL (1 mg/ml) induced PGF2α production by the cultured rat uterine tissues was statistically significant. The results of the current study suggest that prostaglandin release by Ca2+ mobilization- and proteolysis-dependent activity of papaya latex and its proteinases could play a major role in their oxytocic activity.
Introduction
The crude latex of Carica papaya. L. (Caricaceae) contains a mixture of cysteine proteinases such as papain, chymopapain, glycyl endopeptidase, caricain, and other enzymes (Brocklehurst et al., Citation1981). These enzymes are thought to be a natural defense system for wound healing and protection of the plant against predators (El Moussaoui et al., Citation2001). The proteolytic activity of papain (PPN) has been employed in food processing as meat tenderizers and in the clarification of beverages (Reynolds, Citation1982). Treatment of slipped disks by the injection of chymopapain (CPN) to dissolve the displaced nucleus pulposus has also been reported (Fraser, Citation1984). In folk medicine, crude papaya latex (CPL) is valued as an oxytocic agent (uterine contractant) used for the induction of labor and abortion. Indigenous folks in parts of Asia have been reported to induce abortion and labor by intravaginal application of CPL to the uterine opening (Quisumbing, Citation1951; Tietze, Citation1997).
Moreover, in modern practice of obstetrics and gynecology, papain was once used as a mucolytic agent. Papain was administered intravaginally to clear mucus in patients with thick mucopurulent discharge (Kurzrock, Citation1948). Papain was also administered intravaginally to remove excessive cervical mucus prior to salpingography (Hunter et al., Citation1957b). Following irrigation of the cervical canal with papain or papain combined with bromelain (a proteolytic enzyme from pineapple stem), gross cervical dilatation, enlargement, and transient uterine evacuation were observed in some patients (Hunter et al., Citation1957aCitationb). Further observations in more than 390 patients (using radiological imaging) showed the ability of intravaginal administration of papain to provoke progressive uterine contractions beginning at uterine tube, and progressing medially toward the internal os (Hunter et al., Citation1960).
To our knowledge, the first observation on the ability of fresh latex from green papaya fruit to contract isolated uterus was made by Saha et al. (Citation1961). Our earlier studies have also reported the oxytocic potential of papaya latex and its proteinases (Adebiyi et al., Citation2002aCitationb). However, the mechanisms of the potent uterine stimulating effect of the proteinases are not fully understood. In order to further characterize the effect of papaya proteinases on uterine activity, we studied their oxytocic mechanisms using uterine strips isolated from non-gravid Sprague-Dawley rats.
Materials and Methods
Animals
Virgin Sprague-Dawley (180–220 g) female rats were housed in the animal holding unit of the Faculty of Medicine, National University of Singapore. The animals were maintained under a constant 12 h light and dark cycle and an environmental temperature of 24±1 ○C. Food and water were available to the rats ad libitum.. This study was done in accordance with the National University of Singapore's Guidelines on the Use of Laboratory Animals.
Chemicals
Estradiol benzoate, antipain, vanadium, meclofenamic acid, crude papaya latex, chymopapain, papain, antibiotic and antimycotic solution were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Nifedipine was bought from Zuellig Pharma (Singapore), verapamil from Orion Pharmaceuticals (Finland), methysergide from Sandoz (East Hanover, NJ, USA), ethylenediamine tetra acetic acid (EDTA) from Merck KGaA (Darmstadt, Germany), and Medium 199 from JRH Biosciences (Lenexa, KS, USA). Product information from Sigma-Aldrich reveals that the crude papaya latex used in this study was neither cleaned nor purified. It was packed in a crude and powdered form as received from Africa. However, chymopapain and papain used were purified and they contain approximately 75% and 80% protein, respectively.
Tissue bathing media
New De Jalon solution (mM): NaCl 154, KCl 5.63, CaCl2 0.27, NaHCO3 5.95, glucose 2.78. Ringer Locke solution (mM): NaCl 154, KCl 5.63, CaCl2 2.16, MgCl2 2.10, NaHCO3 5.95, glucose 5.55. The Ca2+ -free medium had the same constituents in Ringer Locke solution except for the omission of CaCl2 and the addition of EDTA.
Isolated rat uterus in tissue bath system
Non-gravid Sprague-Dawley rats were primed with 50 µg/kg of estradiol benzoate (intraperitoneally) a day before the experiments. On the day of the experiments, the rats were euthanized by CO2 asphyxiation. The uterine body was excised above its intersection with the cervix and at the junction of the uterine horns with the ovaries and then dissected from the adherent fat and connective tissues. The two uterine horns were immediately dissected into four strips. Uterine strips (approximately 1.5 cm each) were mounted vertically under resting tension of 2 g weight in 4-channel (10 ml) tissue baths connected to force transducers (Ugo Basile, Comerio, Italy). The tissue-bathing media were maintained at pH of 7.4, temperature of 37○C and gassed with carbogen (95% O2 and 5% CO2). The uterine strips were allowed to equilibrate in physiological solution for at least 1 h prior to the experiments.
Role of proteolysis in oxytocic activity of papaya proteinases
This experiment was performed to examine if the enzymatic activity of CPL, CPN, and PPN is required for their oxytocic activity. Hence, uterine strips from estrogen-primed rats were equilibrated in new De Jalon solution for 1 h. Responses to 5 mU/ml of oxytocin (control), 0.1 mg/ml of CPL, CPN, and PPN were established for 15 min. Furthermore, the same concentration of oxytocin, CPL, CPN, and PPN were treated with 60 µg (final volume in tissue bath = 6 µg/ml.) of a cysteine proteinase inhibitor, antipain (N.-[-N.α-carbonyl-Arg-Val-Arg-al]-Phe). The mixtures were incubated at 37○C and shaken together (400 rpm) for 30 min in Heidolph Unimax 1010 rotary shaker (Heidolph Instruments GmbH & Co., Germany). After 30 min of incubation, the treated substances were tested on the isolated uteri for 15 min. Following rest period of 20 min after wash, the same concentrations of untreated CPL, CPN, and PPN were tested again on the uterus.
The role of extracellular Ca2+ mobilization in oxytocic activity of papaya proteinases
Uterine strips from estrogen-primed rats were equilibrated in Ringer Locke solution for 1 h. Uterine contractions induced by 0.2 mg/ml of CPL, CPN, and PPN were recorded for 10 min. The tissues were then washed. Thereafter, the bathing solution was replaced with Ca2+-free solution containing 3 mM EDTA, and incubation was continued for 50 min. Subsequently, the bathing solution was replaced again by Ca2+-free solution containing 1 mM EDTA, and the uterus was incubated further for 30 min after which the same concentrations of the proteinases were tested on the uterine strips. The Ca2+-free solution was then replaced with Ca2+ -containing Ringer Locke solution, and 0.2 mg/ml of CPL, CPN, and PPN were tested again on the uterine strips (after at least 20 min of rest).
Sodium meta-vanadate (vanadium) is known to contract isolated uterus both in the presence and absence of extracellular Ca2+ (Mironneau et al., Citation1984). Hence, response of the isolated rat uterus to 1 mM of vanadium in the presence and absence of extracellular Ca2+ was also observed as the control. In another set of experiments, the effect of 0.15 µM nifedipine and 3 µM verapamil (Ca2+ channel blockers) on the oxytocic activity of papaya proteinases was observed by adding the Ca2+ channel blockers into the tissue baths for 20 min prior to the addition of the papaya proteinases. Responses of the uterine strips to the enzymes in the presence and absence of nifedipine and verapamil were compared.
Effect of 5-hydroxytryptamine (5-HT) and prostaglandin (PG) inhibitors on the oxytocic activity of papaya proteinases
Response of the isolated non-gravid rat uterine strips to 0.2 mg/ml of CPL, CPN, and PPN was established as the control. Following a rest period of 20 min after washing, meclofenamic acid (50 µM) or methysergide (0.3 µM) was added to the respective tissue baths (1 inhibitor per uterine strip) 20 min prior to stimulation of the uterus with 0.2 mg/ml of CPL, CPN, and PPN. The concentrations of meclofenamic acid and methysergide used were those that significantly inhibited the response of the rat uterus to prostaglandin F2α and 5-HT, respectively.
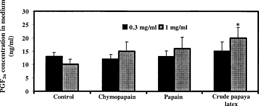
Effect of papaya proteinases on prostaglandin F2α (PGF2α) production by cultured rat uterus
Uterine strips isolated from non-gravid estrogen-primed (1 mg/kg) rats were cut into pieces and placed in tissue culture plates (300–400 mg tissue per plate) that contained 4 ml of Medium 199 (plus Earle's balanced salt and 0.6 mM of glutamine) supplemented with antibiotic and antimycotic solution. The uterine tissues were then treated with 0.3 and 1 mg/ml of CPL, CPN, and PPN. The controls were treated with equivalent volume of normal saline used in the preparation of papaya proteinases. The tissue plates were placed in CO2 incubator and cultured at 37○C for 24 h. The culture medium was collected after 24 h and stored at − 80○C. The amount of PGF2α released into the medium was subsequently measured without extraction using enzyme immunoassay (EIA) kits purchased from Assay Designs Inc. (Ann Arbor, MI USA). The kits use a polyclonal antibody to PGF2α to bind, in a competitive manner, PGF2α in the sample. The sensitivity of the Assay Designs’ Correlate-EIA PGF2α kit was 6.78 pg/ml while the intra- and inter-assay coefficients of variation for low concentration of PGF2α were 13.1% and 9.7%, respectively.
Data analyses
All uterine responses were recorded isometrically and analyzed on computerized MacLab data acquisition system running chart v3.6 software (ADInstruments, Castle Hill, NSW, Australia). Uterine contraction was represented as the maximum tension (grams weight: g wt) above the baseline during period of contact with the test substances. Statistical analyses were performed using SPSS software (SPSS Inc. Chicago, IL, USA). Data were expressed as the mean±SEM (standard error of mean). Where appropriate, Student's t.-test and one-way analysis-of-variance were applied. p. values less than 0.05 were considered significant.
Results
Our previous studies have demonstrated the uterine-stimulating effect of CPL, CPN, and PPN on rat uterus (Adebiyi et al., Citation2002aCitationb). shows representative tracings of the uterine-stimulating effect of CPL, CPN, and PPN on non-gravid guinea-pig uterine strips (from our earlier unpublished data). Treatment of papaya proteinases with cysteine protease inhibitor (antipain) completely abolished their oxytocic activity. Under the same experimental conditions, antipain treatment had no effect on response of the uterine strips to oxytocin ().
Figure 1 Representative tracings showing concentration-dependent oxytocic activity of crude papaya latex, papain, and chymopapain on isolated non-gravid guinea pig uterine strips. Consistent results were observed in at least six experiments.
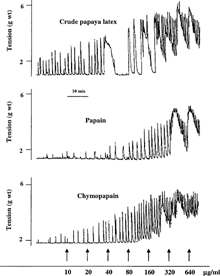
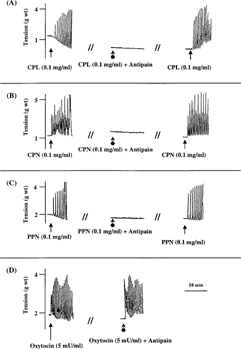
Figure 2 Representative tracings showing the effect of cysteine proteinase inhibitor, antipain, on the oxytocic activity of (A), crude papaya latex; (B), chymopapain; (C), papain, and (D), oxytocin on isolated non-gravid rat uterus. Consistent results were observed in at least six experiments.
In Ca2+-free medium, CPL, CPN, and PPN failed to contract the rat uterus. In contrast, 1 mM of vanadium caused tonic but less potent contraction of the uterus in Ca2+-free medium. Following the replacement of Ca2+-free medium with Ca2+-containing Ringer Locke solution, the proteinases were able to contract the uterine strips (). Furthermore, calcium channel blockers (nifedipine and verapamil) significantly and reversibly blocked the uterine-stimulating effect of the papaya proteinases ( and ).
Figure 3 Representative tracings showing the effect of (A), crude papaya latex and (B) vanadium on isolated non-gravid rat uterus in the presence and absence of Ca2+. The results obtained for CPL were the same with those obtained for CPN and PPN. RL: Ringer Locke. Consistent results were observed in at least six experiments.
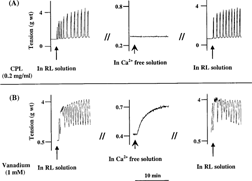
Figure 4 Effect of (A) 0.15 µM of nifedipine and (B) 3 µM of verapamil on the oxytocic activity of crude papaya latex, chymopapain, and papain on isolated non-gravid rat uterus. Each bar represents mean±SEM (n = 6; *p. < 0.01). Nifed: nifedipine; Verap: verapamil.
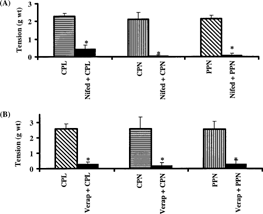
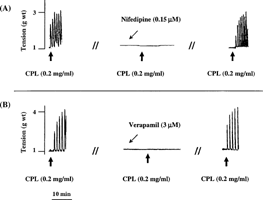
Figure 5 Representative tracings showing the reversible inhibitory effect of (A) nifedipine and (B) verapamil on the oxytocic activity of crude papaya latex on isolated non-gravid rat uterus. Similar results were obtained in chymopapain- and papain-treated uterine strips. Consistent results were observed in at least six experiments.
Methysergide (0.3 µM) completely abolished the response of the rat uterus to 5-HT. However, the same concentration of methysergide did not prevent the oxytocic activity of papaya proteinases (). Meclofenamic acid (50 µM) inhibited the oxytocic activity of PGF2α by 89±9%. At the same concentration, meclofenamic acid inhibited the oxytocic activity of CPL, CPN, and PPN by 81±9%, 75±15%, and 76±13%, respectively (). The inhibitory effect of meclofenamic acid on all the test substances was reversible.
Figure 6 Effect of (A) methysergide and (B) meclofenamic acid on the oxytocic activity of crude papaya latex, chymopapain, and papain on isolated non-gravid rat uterus. Each bar represents mean±SEM (n = 6; *p. < 0.05). MS: methysergide; MA:meclofenamic acid.
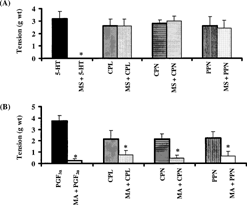
In cultured non-gravid rat uterus, papaya proteinases (0.3 and 0.1 mg/ml) caused a concentration-dependent increase in PGF2α production. However, only the samples treated with 1 mg/ml of CPL showed significant increase in PGF2α concentration in the culture medium ().
Discussion
The major proteinaceous components of papaya latex are cysteine proteinases of the papain family (Brocklehurst et al., Citation1981). They contain a catalytically active cysteine sulphydryl group (Cys-25) and a histidine imidazole group (His-159) within the active dyad of the enzyme (Rich, Citation1986; Yamamoto et al., Citation1991; Maes et al., Citation1996). Inactivation of the Cys-25 sulfydryl group by proteolytic inhibitors renders the enzymes catalytically inactive (Rich, Citation1986). Antipain is a reversible inhibitor of the cysteine proteinases. It belongs to the aldehyde group of proteolytic inhibitors that reacts with the thiol group of Cys-25 in the active site of cysteine proteinases (Lewis & Wolfenden, Citation1977), thus inhibiting their proteolytic activity. As shown in the current study, inhibition of the enzymatic activity of CPL, CPN, and PPN by antipain completely abolished their uterine-stimulating effect in contrast to oxytocin, suggesting that the enzymatic activity of papaya proteinases is required for their oxytocic activity.
Smooth muscle contraction is significantly controlled by the concentration of cytosolic Ca2+. Agonist-induced stimulation of smooth muscle contraction causes increase in cytosolic Ca2+ concentration partly as a result of extracellular Ca2+ influx and in part after the mobilization of intracellular Ca2+ stores (Godfraind et al., Citation1986). It has long been known that uterine contractions induced by some agents are abolished by the exclusion of Ca2+ from isolated tissue bathing solution. However, other agents like vanadium have been shown to cause tension development in uterus suspended in Ca2+-free medium via the release of intracellular Ca2+ (Mironneau et al., Citation1984). Furthermore, the role of Ca2+ signaling in the activity of some proteolytic enzymes has been shown. Activation of Ca2+ influx was demonstrated to be involved in the uterine-stimulating effect of thrombin and trypsin (Shintani et al., Citation2000Citation2001). Trypsin caused Ca2+ mobilization in isolated myocytes (Komuro et al., Citation1997). Thrombin and trypsin also induced Ca2+ mobilization in human T cell lines (Mari et al., Citation1996). In the current study, removal of extracellular Ca2+ from the bathing medium abolished the oxytocic activity of the papaya proteinases in contrast to vanadium. Contractile activity of the enzymes returned after changing the bathing medium to Ca2+-containing Ringer Locke solution, indicating that the uterine-stimulating effect of papaya proteinases is dependent on extracellular Ca2+ mobilization. This is further supported by the fact that calcium channel blockers significantly and reversibly prevented the oxytocic activity of the proteinases.
Independent studies by early and recent workers indicate that degranulation of the uterine mast cells and inflammatory/anaphylactic responses are capable of provoking uterine contractions possibly via the release of uterotonic mediators such as histamine, leukotrienes, PGs, and 5-HT (Schultz, Citation1910; Dale, Citation1913; Rudolph et al., Citation1993). Papaya proteinases are known to cause rupture of mast cells and provoke anaphylactic responses from human and animals (Brånemark et al., Citation1969; Flindt, Citation1978; Chambers et al., Citation1998). Papain-induced degranulation of human eosinophils, leading to the release of inflammatory mediators, was recently reported (Miike & Kita, Citation2003).
In contrast to papaya proteinases, histamine (Martinez-Mir et al., Citation1993) and leukotrienes (Lopez-Bernal et al., Citation1989) does not contract the rat uterus, hence, they are unlikely to be major mediators of the uterine-stimulating effect of papaya latex and its proteinases. A study by Nielsen (Citation1977) indicated that PGs and 5-HT could be the principal mediators of rat uterine contractions caused by anaphylaxis. Gupta and colleagues (Citation1992) reported that PGs specifically mediated rat paw inflammation induced by crude papaya latex. In the study by Gupta et al. (Citation1992), histamine and serotonin (5-HT) antagonists had no significant effect on papaya latex–induced rat paw edema. However, cyclooxygenase inhibitors significantly alleviated rat paw inflammation caused by the papaya latex. In the current study, we tested the hypothesis that 5-HT and or PGs plays a role in oxytocic activity of papaya proteinases.
The oxytocic activity of 5-HT is mediated by 5-HT receptors. Moreover, 5-HT2 receptors have been demonstrated to be the 5-HT receptor subtype with detectable mRNA in the rat uterus (Rydelek-Fitzgerald et al., Citation1993). Methysergide is an antagonist of both 5-HT2A and 5-HT2C receptors (Boess & Martin, Citation1994). In this study, the concentration of methysergide that completely abolished the uterine-stimulating effect of 5-HT on the rat uterus had no effect on the oxytocic activity of papaya enzymes, suggesting that 5-HT may not play a significant role in the uterine-stimulating activity of papaya proteinases.
PGs are involved in the initiation, coordination, and maintenance of uterine activity (Fu et al., Citation2000). Meclofenamic acid, a well-known inhibitor of the cyclooxygenase pathway, is also able to inhibit the binding of radiolabeled PGE2 to its receptors in human myometrium (Lopez-Bernal et al., Citation1991) and inhibit PGF2α-induced contractions of isolated guinea pig uterus (McLean & Gluckman, Citation1983). Hence, meclofenamic acid is an inhibitor of both the synthesis and the actions of PGs at receptor sites. In the current investigation, the concentration of meclofenamic acid that significantly inhibited the oxytocic activity of PGF2α on the rat uterus also significantly and reversibly inhibited the uterine-stimulating effect of papaya proteinases. Similarly, the concentrations of polyphloretin phosphate (a nonspecific PG inhibitor) that significantly inhibited PGF2α-induced rat uterine contraction also prevented the uterine-stimulating effect of CPL (Adebiyi et al., Citation2002c), CPN, and PPN (Adebiyi et al., unpublished data). It is therefore plausible to postulate that PGs, at least in part, mediate the oxytocic activity of papaya enzymes.
There was a trend of an increase in PGF2α production in cultured rat uterine tissues treated with papaya proteinases, but only crude papaya latex (at 1 mg/ml) caused statistically significant increase in PGF2α concentration in the culture medium compared with the control. PGF2αis the most effective smooth muscle contractile PG (Peri et al., Citation2002). However, other PGs such as PGE2 may contribute to the oxytocic activity of papaya enzymes. Apart from papain and chymopapain, other cysteine proteases like glycyl endopeptidase and caricain are present in crude papaya latex (Brocklehurst et al., Citation1981). Although papain and chymopapain are generally regarded as the major mediators of the activities of papaya latex, it is possible that other minor proteolytic constituents of crude papaya latex may augment its oxytocic activity.
The oxytocic activity of papaya proteinases explains the reason for intravaginal application of papaya latex in the indigenous system of obstetrics and gynecology to induce labor and abortion (Quisumbing, Citation1951; Tietze, Citation1997). It also makes obvious the reason for uterine evacuation observed by Hunter et al. (Citation1957aCitationbCitation1960) following intravaginal application of papain prior to salpingography. In conclusion, the results of the current study suggest that prostaglandin release by Ca2+ mobilization- and proteolysis-dependent activity of papaya latex and its proteinases could play a major role in their oxytocic activity.
Acknowledgments
A research scholarship awarded to Adebowale Adebiyi. by the National University of Singapore is gratefully acknowledged. The authors also wish to thank Dr. K. Gauthaman and P. Sivalingam for their valuable assistance.
References
- Adebiyi A, Adaikan PG, Prasad RNV (2002a): Papaya (Carica papaya.) consumption is unsafe in pregnancy: Fact or fable? Scientific evaluation of a common belief in some parts of Asia using a rat model. Br J Nutr 88: 199–203. [CSA]
- Adebiyi A, Adaikan PG, Prasad RNV, Ng SC (2002b): Uterine stimulating effects of crude latex of Carica papaya. L. In: Stepp JR, Wyndham, FS, Zarger RK, eds., Ethnobiology and Biocultural Diversity: Proceedings of the 7th International Congress of Ethnobiology. Athens, University of Georgia Press, pp. 299–306.
- Adebiyi A, Prasad RNV, Adaikan PG (2002c): Effect of polyphloretin phosphate on the response of non-gravid rat uterus to ‘folkloric’ and ‘standard’ oxytocics in vitro. Prostaglandins Leukot Essent Fatty Acids 66: 499–503. [CROSSREF], [CSA]
- Boess FG, Martin IL (1994): Molecular biology of 5-HT receptors. Neuropharmacology 33: 275–317. [CROSSREF], [CSA]
- Brånemark P, Ekholm R, Lundskog J, Hirsch C (1969): Tissue response to chymopapain in different concentrations. Clin Orthop Relat Res 67: 52–67.
- Brocklehurst K, Baines BS, Kierstan MPJ (1981): Papain and other constituents of Carica papaya. L. Top Enzyme Fermentation Biotechnol 5: 262–335.
- Chambers L, Brown A, Pritchard DI, Sreedharan S, Brocklehurst K, Kalsheker NA (1998): Enzymatically active papain preferentially induces an allergic response in mice. Biochem Biophys Res Commun 253: 837–840. [CROSSREF], [CSA]
- Dale HH (1913): The anaphylactic reaction of plain muscle in guinea pig. J Pharmacol Exp Ther 4: 167–223.
- El Moussaoui A, Nijs M, Paul C, Wintjens R, Vincentelli J, Azarkan M, Looze Y (2001): Revisiting the enzymes stored in the laticifers of Carica papaya. in the context of their possible participation in the plant defense mechanism. Cell Mol Life Sci 58: 556–570. [CSA]
- Flindt ML (1978): Respiratory hazards from papain. Lancet 1: 430–432. [CROSSREF]
- Fraser RD (1984): Chymopapain for the treatment of intervertebral disc herniation. The final report of a double-blind study. Spine 9: 815–818.
- Fu X, Favini R, Kindahl K, Ulmsten U (2000): Prostaglandin F2α-induced Ca++ oscillations in human myometrial cells and the role of RU 486. Am J Obstet Gynecol 182: 582–588. [CSA]
- Godfraind T, Miller R, Wibo M (1986): Calcium antagonism and calcium entry blockade. Pharmacol Rev 38: 321–416.
- Gupta OP, Sharma N, Chand D (1992): A sensitive and relevant model for evaluating anti-inflammatory activity—papaya latex-induced rat paw inflammation. J Pharmacol Toxicol Meth 28: 15–19. [CROSSREF], [CSA]
- Hunter RG, Henry GW, Civin WH (1957a): The action of papain and bromelain on the uterus. Am J Obst Gynecol 73: 875–880.
- Hunter RG, Henry GW, Heinicke RM (1957b): The action of papain and bromelain on the uterus. Am J Obst Gynecol 73: 867–874.
- Hunter RG, Henry GW, Civin WH, Heinicke RM (1960): The action of papain and bromelain on the uterus. Am J Obst Gynecol 79: 428–431.
- Komuro T, Miwa S, Minowa T, Okamoto Y, Enoki T, Ninomiya H, Zhang XF, Uemura Y, Kikuchi H, Masaki T (1997): The involvement of a novel mechanism distinct from the thrombin receptor in the vasocontraction induced by trypsin. Br J Pharmacol 120: 851–856. [CSA]
- Kurzrock R (1948): The clinical evaluation of hyaluronidase in human infertility. Am J Clin Pathol 18: 491–498.
- Lewis CA, Wolfenden R (1977): Thiohemiacetal formation by inhibitory aldehydes at the active site of papain. Biochemistry 16: 4890–4895. [CROSSREF]
- Lopez Bernal A, Buckley S, Rees CM, Marshall JM (1991): Meclofenamate inhibits prostaglandin E binding and adenylyl cyclase activation in human myometrium. J Endocrinol 129: 439–445.
- Lopez Bernal A, Canete Soler R, Turnbull AC (1989): Are leukotrienes involved in human uterine contractility?. Br J Obstet Gynaecol 96: 568–573.
- Maes D, Bouckaert J, Poortmans F, Wyns L, Looze Y (1996): Structure of chymopapain at 1.7 Å resolution. Biochemistry 35: 16292–16298. [CROSSREF]
- Mari B, Guerin S, Far DF, Breitmayer JP, Belhacene N, Peyron JF, Rossi B, Auberger P (1996): Thrombin and trypsin-induced Ca2+ mobilization in human T cell lines through interaction with different protease-activated receptors. FASEB J 10: 309–316. [CSA]
- Martinez-Mir I, Herrero J, Estan L, Morales-Olivas FJ, Rubio E (1993): Effect of histamine on the longitudinal and circular muscle of the oestrogen dominated rat uterus. Agents Actions 39: 1–5.
- McLean JR, Gluckman MI (1983): On the mechanism of the pharmacologic activity of meclofenamate sodium. Arzneimittelforschung 33: 627–631.
- Miike S, Kita H (2003): Human eosinophils are activated by cysteine proteases and release inflammatory mediators. J Allergy Clin Immunol 111; 704–713. [CROSSREF], [CSA]
- Mironneau C, Mironneau J, Savineau JP (1984): Maintained contractions of uterine smooth muscle incubated in Ca2+ free solution. Br J Pharmacol 82: 745–743. [CSA]
- Nielsen, AT (1977): Effect of methysergide and indomethacin on the anaphylactic contraction of the rat isolated uterus (Schultz-Dale response). Acta Pharmacol Toxicol 40: 321–328.
- Peri KG, Quiniou C, Hou X, Abran D, Varma DR, Lubell WD, Chemtob S (2002): THG113: A novel selective FP antagonist that delays preterm labor. Semin Perinatol 26: 389–397. [CSA]
- Quisumbing E (1951): Medicinal Plants of the Philippines. Technical Bulletin 16 Reports, Philippines Department of Agriculture and Natural Resources. Manila, Bureau of Printing, pp. 632–637.
- Reynolds JEF, ed. (1982): Enzymes choleretics and other digestive agents. In: Martindale: The Extra Pharmacopoeia. London, The Pharmaceutical Press, pp. 653–654.
- Rich DH (1986): Inhibitors of cysteine proteinases. In: Barrett AJ, Salvesen G, eds., Proteinase Inhibitors. New York, Elsevier, pp. 153–179.
- Rudolph MI, Reinicke K, Cruz MA, Gallardo V, Gonzalez C, Bardisa L (1993): Distribution of mast cells and the effect of their mediators on contractility in human myometrium. Br J Obstet Gynaecol 100: 1125–1130. [CSA]
- Rydelek-Fitzgerald L, Wilcox BD, Teitler M, Jeffrey JJ (1993): Serotonin-dependent collagenase induction in rat myometrial smooth muscle cells: mediation by the 5-HT2 receptor. Mol Cell Endocrinol 91: 67–74. [CROSSREF], [CSA]
- Saha JC, Savini EC, Kasinathan S (1961): Ecbolic properties of Indian medicinal plants part 1. Indian J Med Res 49: 130–151.
- Schultz WH (1910): Physiological studies of anaphylaxis I. The reactions of smooth muscles of the guinea pig sensitized with horse serum. J Pharmacol Exp Ther 1: 549–567.
- Shintani Y, Hirano K, Nakayama T, Nishimura J, Nakano H, Kanaide H (2001): Mechanism of trypsin-induced contraction in the rat myometrium: The possible involvement of a novel member of protease-activated receptor. Br J Pharmacol 133: 1276–1285. [CROSSREF], [CSA]
- Shintani Y, Hirano K, Nishimura J, Nakano H, Kanaide H (2000): Enhanced contractile response to thrombin in the pregnant rat myometrium. Br J Pharmacol 131: 1619–1628. [CROSSREF], [CSA]
- Tietze HW (1997): Papaya as Medicine: A Safe and Cheap Form of Food Therapy. Subang Jaya (Malaysia), Pelanduk Publications, pp. 95.
- Yamamoto D, Matsumoto K, Ohishi H, Ishida T, Inoue M, Kitamura K, Mizuno H (1991): Refined x-ray structure of papain.E-64-c complex at 2.1-Å resolution. J Biol Chem 266: 14771–14777.