ABSTRACT
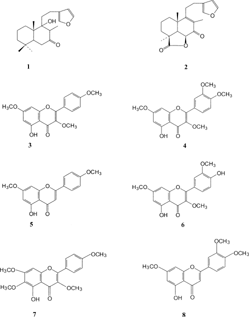
Two diterpenoids (hispanolone 1, ballonigrine 2) and six flavonoids (5-hydroxy-3,7,4′-trimethoxyflavone 3, retusin 4, 5-hydroxy-7,4′-dimethoxyflavone 5, pachypodol 6, 5-hydroxy-3,6,7,4′-tetramethoxyflavone 7, 5-hydroxy-7,3′,4′-trimethoxyflavone 8) have been isolated from the aerial parts of Ballota inaequidens. Hub.-Mor. & Patzak. Among them, 7 has not been reported previously in the genus Ballota.. The antibacterial and antifungal activities of 1–4, 6, and 8 were tested against Bacillus subtilis., Staphylococcus aureus., Escherichia coli., Pseudomonas aeruginosa., Candida albicans., and Candida crusei..
Introduction
Ballota. L. is represented by 16 species in Turkey (Davis, Citation1982). Ballota. species have been used in Turkish folk medicine as antiulcer, antispasmodic, and sedative agents (Çitoğlu et al., Citation1998). In our previous studies, three diterpenoids (hispanolone, ballonigrine, dehydrohispanolone) and ten flavonoids (kumatakenin, pakipodol, 5-hydroxy-7,3′,4′-trimethoxyflavone, velutin, corymbosin, 5-hydroxy-3,7,4′-trimethoxyflavone, retusin, 5-hydroxy-7,4′-trimethoxyflavone, 5-hydroxy-3,6,7,4′-tetramethoxyflavone, ladanein) were isolated, chemically characterized, and analyzed by HPLC in different species of Ballota. (Çitoğlu et al., Citation1998Citation1999Citation2003; Sever, Citation2000). Although Ballota inaequidens. Hub.-Mor. & Patzak is also widespread in South Anatolia, studies on its flavonoid constituents have not been reported to date. As a continuation of our research on the Ballota. species, we isolated the main constituents of Ballota inaequidens. and investigated their antimicrobial properties.
Materials and Methods
General experimental procedures
UV spectra (in MeOH) were recorded using a UV-1601 Shimadzu instrument. 1H NMR spectra in CDCl3 were recorded at 400 MHz on a Varian XLAA 400 spectrometer. Melting points were recorded on a Büchi SMP-20 instrument.
Plant material
Ballota inaequidens., in flower, was collected and identified in 1998 near Antalya (Turkey) by B. Sever and F. Tezcan. Voucher specimens are kept in the Herbarium of Ankara University, Faculty of Pharmacy (AEF no. 19901).
Isolation of flavonoids
Air-dried and powdered aerial parts of the plant (500 g) were extracted with Me2CO (5 l) at room temperature for 3 days. After evaporation, the residue was extracted with EtOAc and the extract washed with H2O and dried. The extract was concentrated to dryness in vacuo. to afford a syrupy residue (34.45 g) that was purified on SiO2 of 70–230 mesh (300 g) with light petroleum ether and increasing amounts of EtOAc until 80:20 (v/v) (Savona et al., Citation1978Citation1982; Rodriguez et al., Citation1979). Fractions of 50 ml were collected, and similar fractions were purified on a PTLC (Kieselgel 60 PF254) using CHCl3:MeOH (10:0,05) to give: hispanolone 1 (76 mg), ballonigrine 2 (85 mg), 5-hydroxy 3,7,4′-trimethoxyflavone 3 (116 mg), retusin 4 (120 mg), 5-hydroxy-7,4′-dimethoxyflavone 5 (20 mg), pachypodol 6 (44 mg), 5-hydroxy-3,6,7,4′-tetramethoxyflavone 7 (58 mg), and 5-hydroxy-7,3′,4′-trimethoxyflavone 8 (53 mg) (). The structures of these compounds were elucidated on the basis of their UV and NMR spectral data.
Hispanolone (1)
White needles (76 mg), m.p. 144–145°C (lit. 147–148°C) (Rodriguez et al., Citation1979; Savona et al., Citation1982; Davies-Coleman & Rivett, Citation1990; Çitoğlu et al., Citation1998). MS m/z. (rel. int.): 318 (M+, 15), 219 [(300-C2H5O)+, 16], 195 (13), 177 (44), 165 (41), 135 (25), 107 (30), 79 (28). 1H NMR (CDCl3) δ 7.37 [1H, d (J. = 8.8 Hz) H-15], 7.25 [1H, s, H-16], 6.29 [1H, d (J. = 8.8 Hz) H-14], 2.76 [1H, q (J. = 6.6 Hz) H-8], 2.49 (2H, m, H-12), 2.31 (2H, t, H-6), 2.04 [1H, dd (J. = 3.0–4.1 Hz) H-5], 1.95 (2H, m, H-11), 1.60 (2H, m, H-3), 1.47 (2H, t, H-1), 1.25 [2H, d (J. = 4.2) H-2], 1.20 (3H, s, H-20), 1.14 [3H, d (J. = 6.4 Hz) H-17], 0.92 (3H, s, H-19), 0.90 (3H, s, H-18); 13C NMR (CDCl3): 31.2 (C-1), 17.8 (C-2), 40.6 (C-3), 32.9 (C-4), 45.7 (C-5), 38.5 (C-6), 210.0 (C-7), 50.2 (C-8), 81.0 (C-9), 42.6 (C-10), 34.0 (C-11), 20.8 (C-12), 124.1 (C-13), 109.9 (C-14), 142.3 (C-15), 137.8 (C-16), 7.5 (C-17), 32.3 (C-18), 20.7 (C-19), 15.5 (C-20).
Ballonigrine (2)
White needles (853. mg), m.p. 178–180°C (lit. 212°C) (Savona et al., Citation1976Citation1977Citation1978; Çitoğlu et al., Citation1998). MS m/z. (rel. int.): 328 (5.2), 313 (5.7), 300.2 (6.3), 285.2 (6.9), 203.1 (25.9), 109.1 (8.4), 95.1 (10.4), 81.0 (100). 1H NMR (CDCl3) δ 7.99 (1H, s, H-14), 7.39 (1H, brs, H-16), 6.31 (1H, brs, H-15), 4.76 [1H, d (J. = 6.0 Hz) H-6], 2.20 [1H, d (J. = 5.75 Hz) H-5], 1.82 (3H, s, H-17), 1.57 (2H, m, H-3), 1.35 (2H, m, H-8), 1.1 (3H, s, H-20). 13C NMR (CDCl3): 31.15 (C-1), 18.65 (C-2), 28.56 (C-3), 42.70 (C-4), 50.46 (C-5), 76.23 (C-6), 193.78 (C-7), 131.95 (C-8), 163.32 (C-9), 37.39 (C-10), 24.88 (C-11), 30.81 (C-12), 124.39 (C-13), 111.28 (C-14), 143.94 (C-15), 139.55 (C-16), 12.73 (C-17), 25.32 (C-18), 180.72 (C-19), 28.76 (C-20).
5-Hydroxy-3,7,4′-trimethoxyflavone (3)
Yellow needles (116 mg), m.p. 140–141°C (lit. 146–148°C) (Vidari et al., Citation1971; Çitoğlu et al., Citation1999). UV λmax (MeOH) 347, 267; (+NaOMe) 366, 283; (+AlCl3) 394, 276, 351, 304; (+AlCl3/HCl) 395, 344, 302, 277; (+NaOAc) 346, 267; (+NaOAc/H3BO3) 347, 267; 1H NMR (CDCl3 + DMSO) δ 4.10 (3H, s, OCH3), 4.11 (3H, s, OCH3), 4.14 (3H, s, OCH3), 6.58 [1H, d (J. = 2 Hz) H-6], 6.68 [1H, d (J. = 2 Hz) H-8], 7.27 [2H, dd (J. = 2.5 and 8.5 Hz), H-3′ and H-5′], 8.32 [2H, d (J. = 2.5 and 8.5 Hz), H-2′ and H-6′], 12.89 (1H, brs, 5-OH); 13C NMR (CDCl3): 156.3 (C-2), 139.2 (C-3), 179.1 (C-4), 157.1 (C-5), 98.2 (C-6), 139.2 (C-7), 92.5 (C-8), 162.0 (C-9), 106.4 (C-10), 123.2 (C-1′), 130.5 (C-2′), 114.4 (C-3′), 162.4 (C-4′), 114.4 (C-5′), 130.5 (C-6′), 60.3 (OCH3), 56.1 (OCH3), 55.8 (OCH3).
Retusin (5-hydroxy-3,7,3′,4′-tetramethoxyflavone) (4)
Yellow needles (120 mg), m.p. 147–148°C (lit. 160–162°C) (Vidari et al., Citation1971; Sakakibara et al., Citation1976; Arisawa et al., Citation1991; Çitoğlu et al., Citation1999). UV λmax (MeOH) 350, 268; (+NaOMe) 370, 281; (+AlCl3) 405, 268; (+AlCl3/HCl) 405, 269, 352; (+NaOAc) 348, 268; (+NaOAc/H3BO3) 347, 268, 254; 1H NMR (CDCl3) δ 3.79 (3H, s, OCH3), 3.80 (3H, s, OCH3), 3.89 (3H, s, OCH3), 3.90 (3H, s, OCH3), 6.28 [2H, d (J. = 2.5 Hz) H-6], 6.37 [2H, (d, J. = 2.5 Hz) H-8], 6.91 [2H, d (J. = 9 Hz), H-5′], 7.65 (2H, m, H-2′ and H-6′], 12.57 (1H, brs, 5-OH); 13C NMR (CDCl3): 149.1 (C-2), 139.3 (C-3), 179.1 (C-4), 162.4 (C-5), 98.2 (C-6), 165.8 (C-7), 92.6 (C-8), 156.2 (C-9), 106.4 (C-10), 123.3 (C-1′), 111.2 (C-2′), 149.1 (C-3′), 151.7 (C-4′), 111.2 (C-5′), 122.5 (C-6′), 60.6 (OCH3), 56.4 (OCH3), 56.4 (OCH3), 56.2 (OCH3).
5-Hydroxy-7,4′-dimethoxyflavone (5)
Yellow needles (20 mg), m.p. 177–179°C (lit. 168°C) (Silva et al., Citation1971). UV λmax (MeOH) 327, 268; (+NaOMe) 352, 285; (+AlCl3) 345, 276; (+AlCl3/HCl) 339, 277; (+NaOAc) 327, 268; (+NaOAc/H3BO3) 328, 268; 1H NMR (CDCl3) δ 3.81 (3H, s, OCH3), 3.82 (3H, s, OCH3), 6.29 [2H, d (J. = 2.5 Hz) H-6], 6.40 [2H, d (J. = 2 Hz) H-8], 6.50 [1H, s, H-3], 6.96 [2H, dd (J. = 2.5 and 8.5 Hz), H-3′ and H-5′], 7.75 [2H, dd (J. = 2.5 and 8.5 Hz), H-2′ and H-6′], 12.66 (1H, brs, 5-OH).
Pachypodol (5,4′-dihydroxy-3,7,3′-trimethoxyflavone) (6)
Yellow needles (44 mg), m.p. 154–156°C (lit.168–170°C) (Valesi et al., 1971; Fraser & Lewis, Citation1973; Sakakibara et al., Citation1976; Miyazawa et al., Citation2000; Orabi et al., Citation2000). UV λmax (MeOH) 356, 268; (+NaOMe) 403, 356, 264; (+AlCl3) 397, 361, 267; (+AlCl3/HCl) 394, 360, 267; (+NaOAc) 360, 254; (+NaOAc/H3BO3) 356, 253; 1H NMR (CDCl3); δ 3.78 (3H, s, OCH3), 3.80 (3H, s, OCH3), 3.91 (3H, s, OCH3), 6.28 [1H, d (J. = 2 Hz) H-6], 6.37 [1H, d (J. = 2 Hz) H-8], 6.96 [1H, d, (J. = 8.5 Hz) H-5′], 7.59 [2H, dd (J. = 2.5 and 9 Hz) H-6′], 7.61 [2H, dd (J. = 2.0 and 8.5), H-2′], 12.56 (1H, brs, 5-OH); 13C NMR (CDCl3): 148.0 (C-2), 139.0 (C-3), 179.1 (C-4), 164.0 (C-5), 98.2 (C-6), 165.8 (C-7), 92.5 (C-8), 157.1 (C-9), 106.4 (C-10), 123.0 (C-1′), 114.9 (C-2′), 146.7 (C-3′), 149.0 (C-4′), 114.9 (C-5′), 122.8 (C-6′), 60.5 (OCH3), 56.5 (OCH3), 56.2 (OCH3).
5-Hydroxy-3,6,7,4′-tetramethoxyflavone (7)
Yellow needles (58 mg), m.p. 159–160°C (Southwick et al., Citation1972) UV λmax (MeOH) 336, 272; (+NaOMe) 375, 290; (+AlCl3) 363, 279; (+AlCl3/HCl) 356, 282; (+NaOAc) 335, 221; (+NaOAc/H3BO3) 336, 271; 1H NMR (CDCl3) δ 3.79 (3H, s, OCH3), 3.83 (3H, s, OCH3), 3.85 (3H, s, OCH3), 3.88 (3H, s, OCH3), 6.43 (1H, s, H-8), 6.94 [1H, dd (J. = 2.5 and 8.5 Hz), H-3′ and H-5′], 7.99 [1H, dd (J. = 2.5 and 8.5 Hz), H-2′and H-6′], 12.55 (1H, brs, 5-OH); 13C NMR (CDCl3): 153.2 (C-2), 139.4 (C-3), 179.0 (C-4), 158.4 (C-5), 132.8 (C-6), 162.1 (C-7), 90.7 (C-8), 152.7 (C-9), 107.0 (C-10), 123.2 (C-1′), 130.5 (C-2′), 114.4 (C-3′), 159.4 (C-4′), 114.4 (C-5′), 130.5 (C-6′), 55.8(OCH3), 56.7 (OCH3), 60.5 (OCH3), 61.2 (OCH3).
5-Hydroxy-7,3′,4′-trimethoxyflavone (8)
Yellow needles (53 mg), m.p. 156–158°C (lit. 163–165°C) (Nakanishi et al., Citation1985) UV λmax (MeOH) 338, 269; (+NaOMe) 370, 283; (+AlCl3) 355, 274; (+AlCl3/HCl) 349, 276; (+NaOAc) 338, 269; (+NaOAc/H3BO3) 339, 269; 1H NMR (CDCl3) δ 3.81 (3H, s, OCH3), 3.89 (3H, s, OCH3), 3.91 (3H, s, OCH3), 6.30 [1H, d (J. = 2 Hz) H-6], 6.42 [1H, d (J. = 2 Hz) H-8], 6.51 [1H, s, H-3], 7.26 [1H, d (J. = 8.5 Hz), H-5′], 7.44 [1H, d (J. = 2.5), H-2′], 7.46 [1H, dd (J. = 2 and 8 Hz), H-6′], 12.72 (1H, brs, 5-OH); 13C NMR (CDCl3): 148.2 (C-2), 104.5 (C-3), 182.7 (C-4), 162.6 (C-5), 98.4 (C-6), 165.8 (C-7), 98.1 (C-8), 158.1 (C-9), 105.1 (C-10), 128.3 (C-1′), 114.9 (C-2′), 149.7 (C-3′), 152.7 (C-4′), 120.5 (C-5′), 124.1 (C-6′), 56.5 (OCH3), 56.2 (OCH3), 55.9 (OCH3).
Antimicrobial activity
In vitro. antimicrobial studies were carried out by the macrobroth dilution assay (National Committee of Clinical Laboratory Standards, Citation1993) by measuring the minimal inhibition concentration (MIC) of the compounds (1–4, 6, and 8) against two Gram-positive (Staphylococcus aureus. ATCC 25923, Bacillus subtilis. ATCC 6633), two Gram-negative (Escherichia coli. ATCC 23556, Pseudomonas aeruginosa. ATCC 10145), and two fungi (Candida albicans., ATCC 10231, Candida krusei. ATCC 6258). Bacterial strains were obtained from the Refik Saydam Hygiene Center. Briefly, stock solution (3 mg/ml) of the compounds 1–4, 6, and 8 in dimethyl sulfoxide (DMSO) were two fold diluted in 0.5 ml of Mueller Hinton broth. To these broths, 0.5 ml of bacterial and fungal suspentions (106 cfu/ml) were added. The bacterial and fungal suspensions had a turbidity of 0.5 MacFarland standard (ca. 1 × 108 cfu/ml). The tubes were incubated at 35°C for 24 h. MIC was determinated as the lowest concentration of the compound completely inhibited the growth of the test organism. Ampicillin was used as standard antibacterial agent for bacteria, whereas fluconazole was used as a positive control for C. albicans. and C. krusei..
Results and Discussion
Isolation of two diterpenes (1, 2), two flavones (5, 8), and four flavonols (3, 4, 6, 7) has been achieved by column chromatography on silica gel using light petroleum ether and ethyl acetate as mobile phase followed by PTLC on silica gel using chloroform/methanol (10:0.05) as eluent. The structures of these compounds were elucidated on the basis of their UV and NMR spectral data. Compounds 1–8 have been found to be identical with hispanolone, ballonigrine, 5-hydroxy 3,7,4′-trimethoxyflavone, retusin, 5-hydroxy-7,4′-dimethoxyflavone, pachypodol, 5-hydroxy-3,6,7,4′-tetramethoxyflavone, and 5-hydroxy-7,3′,4′-trimethoxyflavone, respectively. The known compounds were also identified by comparision of their melting points with those reported in the literature. Among them, 5-hydroxy-3,6,7,4′-tetramethoxyflavone (7) has not yet been isolated from the genus Ballota..
The results of the screening test of the compounds 1–4, 6, and 8 against B. subtilis., S. aureus., E. coli., P. aeruginosa., C. albicans., andC. krusei. using the macrobroth dilution method are summarized in . The compounds tested have no important inhibitory activity against bacteria but showed good activities against C. albicans. and C. krusei.. We also reported three diterpenoids (hispanolone, dehydrohispanolone, ballonigrine) obtained from the aerial parts of Ballota saxatilis. subsp. saxatilis. and their effects against Gram-positive (S. aureus., S. faecalis.) and Gram-negative (P. aeruginosa., E. coli., K. pneumoniae.) microorganisms and C. albicans. (Çitoğlu et al., Citation1998). The results of this study confirm the previous results in this regard.
Table 1.. Minimal inhibitory concentrations (MIC) of compounds 1–4, 6, and 8 from Ballota inaequidens..
Acknowledgments
This Research was supported by the Research Foundations of Ankara University (grant no. 2000-08-03-020) and the Hungarian Nation Science Foundation (grant no. OTKA-034250).
References
- Arisawa M, Hayashi T, Shimizu M, Morita N, Bai H, Kuze S, Ito Y (1991): Isolation and cytotoxicity of two new flavonoids from Chrysosplenium grayanum. and related flavonols. J Nat Prod 54: 898–901.
- Çitoğlu G, Tanker M, Sever B, Englert E, Anton R, Altanlar N (1998): Antibacterial activities of diterpenoids isolated from Ballota saxatilis. subsp. saxatilis.. Planta Med 64: 484–485. [CSA]
- Çitoğlu G, Tanker M, Sever B (1999): Flavonoid aglycones from Ballota saxatilis. subsp. saxatilis. Pharm Biol 37: 158–160.
- Çitoğlu GS, Sever B, Antus S, Baitz-Gacs E, Altanlar N (2003): Antifungal flavonoids from Ballota glandulosissima.. Pharm Biol 41: 483–486.
- Davis PH (1982): Flora of Turkey and the East Aegean Islands. Edinburgh, Edinburgh University Press, pp. 156–160.
- Davis-Coleman MT, Rivett DEA (1990): Transformation of hispanolone from Ballota africana. into 15,16-epoxy-9-hydroxylabda-13(16), 14-diene. S Afr J Chem 43: 117–119.
- Fraser AW, Lewis JR (1973): Two flavonols from Euodia glabra.. Phytochemistry, 12: 1787–1789. [CROSSREF]
- Miyazawa M, Okuno Y, Nakamura S, Kosaka H (2000): Antimutagenic activity of flavonoids from Pogostemon cablin.. J Agr Food Chem 48: 642–647. [CROSSREF], [CSA]
- Nakanishi T, Ogaki J, Inada A, Murata H, Nishi M (1985): Flavonoids of Striga asiatica.. J Nat Prod 48: 491–493. [CROSSREF]
- National Comittee of Clinical Laboratory Standards (1993): Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, 3rd ed.
- Orabi KY, Mossa JS, Muhammed I, Alloush MH, Galal AM, El-Feraly FS, McPhail ET (2000): New eudesmane sesquiterpene from Plectranthus cylindraceus.. J Nat Prod 63: 1665–1668. [CROSSREF], [CSA]
- Rodriguez B, Savona G, Piozzi F (1979): Two new unusual diterpenoids from Ballota hispanica.. J Org Chem 44: 2219–2221. [CROSSREF]
- Sakakibara M, Difeo D, Nakatani JN, Timmermann B, Mabry TS (1976): Flavonoid methyl esters on the external leaf surface of Larrea tridentata. and Larrea divaricata.. Phytochemistry 15: 727–731. [CROSSREF]
- Savona, G, Piozzi, F, Hanson, JR, Siverns M (1976): Structures of allotinone a diterpenoid from Ballota nigra.. J C S Perkin I 3: 1606–1609.
- Savona, G, Piozzi, F, Hanson, JR, Siverns M (1977): Structures of three new diterpenoids from Ballota. species. J C S Perkin I 1: 322–324.
- Savona, G, Piozzi, F, Hanson, JR (1978): 13-Hydroxyballonigrinolide, a new diterpenoid from Ballota lanata.. Phytochemistry 17: 322–324. [CROSSREF]
- Savona G, Bruno M, Piozzi F, Barbagallo C (1982): Diterpenes from Ballota. species. Phytochemistry 21: 2132–2133. [CROSSREF]
- Sever B (2002): The investigation of diterpenoid and flavonoid contents of Ballota. species growing in Turkey, Ph.D. Thesis, Ankara.
- Silva M, Mundaca JM, Sammes PG (1971): Flavonoid and triterpene constituents of Baccaris rhomboidalis.. Phytochemistry 10: 1942,1943. [CROSSREF]
- Southwick L, Mabry TJ, Averett J, Powell AM (1972): Penduletin 4′-O.-methyl ether from Perityle vaseyi.. Phytochemistry 11: 2351. [CROSSREF]
- Valesi AG, Rodriguez E, Vander Valde G, Mabry TJ (1972): Methylated flavonols in Larrea cuneifolia.. Phytochemistry 11: 2821–2826. [CROSSREF]
- Vidari G, Finzi PV, Bernardi M (1971): Flavonols and quinones in stems of Aframomum giganteum.. Phytochemistry 10: 3335–3339. [CROSSREF]