ABSTRACT
Five known compounds from the aerial parts of Gundelia tournifortii. L. (scopoletin, isoscopoletin, esculin, and a mixture of β.-sitosterol and stigmasterol) were isolated and identified on the basis of spectral evidence (1H NMR, 13C NMR, MS, and IR). The volatile oil from aerial parts of G. tournifortii. was obtained by hydrodistillation, for the first time, and its composition was determined by gas chromatography (GC) and gas chromatography-mass spectrometry (GC-MS). alpha-Terpinyl acetate (36.21%), methyl eugenol (12.57%), eugenol (6.7%), β.-caryophellene (5.94%), and zingiberene (5.84%) were the major oil components. The antiplatelet activity of G. tournifortii. was studied in vitro. using adenosine-5′-diphosphate (ADP) and arachidonic acid (AA) as agonists. The chloroform extract of G. tournifortii. was found to have a mild inhibitory effect on platelet aggregation induced by ADP and AA. None of the pure isolated compounds or the volatile oil showed an inhibitory effect on platelet aggregation using ADP and AA as agonists.
Introduction
Gundelia tournefortii. L. (Asteraceae) is a native plant in Jordan and the Mediterranean area including Palestine and Syria. This plant is locally known as Akoub. The heads of the plant are usually consumed either as fresh plant or after being cooked like artichoke (Lev & Abbo, Citation1999). It is a perennial spiny herb of Irano-turanian origin. The plant develops a rosette after the autumn rains and bolts during February–April. The total plant height may reach 50 cm. Each of the branches ends with a compound spiny ovoid head 4–8 cm in diameter (Lev & Abbo, Citation1999). In folkloric medicine, G. tournifortii. is used in Jordan among Bedouins for the treatment of chest pain and heart stroke. In Turkey, this plant is used in folk medicine to remove water from patients having spleenomegaly (Sezik, Citation2001).
Our previous studies on the combination of G. tournifortii. with chloramphenicol, tetracycline, and nalidixic acid revealed that such combination increases significantly the antimicrobial activity against a resistant strain of Pseudomonas aeruginosa. (Schroeter and Migula) isolated from hospitalized patients and to a lesser extent against the standard strain of the microorganism (Aburjai et al., Citation2001). Moreover, gentamycin and chloramphenicol antibacterial activity against standard and resistant strains of Staphylococcus aureus. (Rosenbach) was significantly improved when mixed with G. tournifortii. (L.) methanolic extract (Darwish et al., Citation2002). Kery and Hussein (Citation1985) reported the isolation of scopoletin with minor amounts of isoscopoletin and esculin by the use of droplet countercurrent chromatography (DCCC). Wagner et al. (Citation1984) reported the isolation of seven saponins from this plant. The isolated saponins showed strong molluscidal activity against the schistosomiasis transmitting snail Biomphalaria glabrata. (Hansen).
In the current study, the antiplatelet activity of the crude extracts, pure isolated compounds, and the volatile oil of G. tournifortii. on human platelet-rich plasma (PRP) aggregation induced by arachidonic acid (AA) and adenosine-5′-diphosphate (ADP) is reported. Also, the work reports the isolation and identification of three coumarins: scopoletin, isoscopoletin, and esculin, in addition to β.-sitosterol and stigmasterol. Furthermore, the volatile oil from the aerial parts of G. tournifortii. was extracted by hydrodistillation, and its composition was determined by gas chromatography (GC) and gas chromatography-mass spectrometry (GC-MS) for the first time.
Materials and Methods
Plant material
Samples of native G. tournifortii. L. were collected in March 2003 from Jarash (40 km north of Amman, Jordan) during the flowering period and the vegetative phase. Taxonomic identity of the plant was confirmed by comparing the collected voucher specimen with those of known identity available in the herbarium of the Department of Biological Sciences, Faculty of Science, University of Jordan, and with the assistance of Dr. Sawsan Al Oran, a plant taxonomist, Faculty of Science, University of Jordan, Amman, Jordan. A voucher specimen (no. GT. 2022) has been deposited in the author's research laboratory at the Department of Pharmaceutical Sciences, Faculty of Pharmacy, University of Jordan.
Extraction and fractionation
The aerial parts of the plant (1500 g) were dried at room temperature, powdered, and then extracted using Soxhlet with 3 l of each chloroform, methanol, and water for 3 h subsequently. The extracts were then dried using a Rota-vapor at 50°C. The yields were 4.84, 19, and 11 g, respectively.
Chromatography of crude extracts
A 3 g quantity of the chloroform extract was chromatographed over silica gel column (200 g, 2.5 × 70 cm) and was eluted with varying proportions of chloroform and methanol. Five fractions were collected (I–V). Fraction II (300 mg) consisted of a large amount of coumarin derivatives, which exhibited bluish fluorescent spots on UV illumination on thin-layer chromatography (TLC) plates. It was further purified upon application on preparative TLC (petroleum ether–chloroform 7:3). Pure scopoletin (45 mg) and isoscopoletin (33 mg) were obtained.
Fraction III (150 mg) showed a spot with a light blue color under UV illumination on TLC plate that was intensified upon spraying with 5% ethanol KOH. Further purification by preparative TLC (petroleum ether–ethyl acetate 8:2) was done to obtain esculin (25 mg).
Another chloroform fraction (1 g) was subjected to column chromatography (350 g, 3 × 90 cm) packed with activated aluminum oxide (basic) and eluted with chloroform-ethyl acetate gradient (9:1, 8:2, 7:3, etc., 400 ml each). The eluates were concentrated and monitored by TLC (toluene-ether 1:1 saturated with glacial acetic acid). The fractions eluted with chloroform–ethyl acetate (4:6 and 3:7) gave a 300 mg precipitate that was purified by crystallization as colorless needles using n.-hexane-chloroform (7:3) to yield a mixture of stigmasterol and β.-sitosterol (41 mg). Neither the methanol nor the aqueous extracts exhibited antiplatelet activity, so none of them was further purified.
Oil distillation
Oils from air-dried, finely ground aerial parts were obtained by hydrodistillation using a Clevenger-type apparatus [European pharmacopoeia (EP), 1997]. Distillation was performed using 100 g of dried plant material in 2.5 l distilled water for 4 h. The oil obtained was dried over anhydrous sodium sulfate and was stored in a dark glass bottle at 4°C until analysis. The yield of oil was 0.12% (w/w) of the dried materials.
GC-MS analysis
Shimadzu GCMS QP2010 (Kyoto, Japan) equipped with split-splitless injector and TRB meta X5 GC column (30 m × 0.25 mm ID, 0.25-µm film thickness) was used. The injector temperature was set at 220°C for 5 min with a split ratio of 1:10. A 1-µl volume of 1000 ppm oil solution in a GC grade n.-hexane (Scharlu, Chemie S.A., Barcelona, Spain) was injected. A linear temperature program was adapted to separate the different oil components as follows: initially the column was maintained at 50°C for 2 min, ramped at a rate of 10°C/min to 150°C, at which it was held isothermal for 5 min; a second ramp (20°C/min) was then applied up to 220°C and held isothermal for 10 min. The total run time was 30.5 min. The temperatures of the transfer line and ion source were maintained at 230°C and 180°C, respectively. The mass detector was set to scan ions between 40 and 400 m./z. using full-scan fixed mode electron impact (EI; 70 eV). Components of the oil were identified by matching their recorded spectra with the data bank mass spectra (Saturn and NIST library databases) provided by the instrument software and by comparing their retention indices values with those in the literature, measured on columns with identical polarity (Adams, Citation1995). The databases were compiled using more than 80,000 electron impact (EI) mass spectra. Only matching spectra of large degree of certainty using reverse-fit mode was accepted. Concentrations (as %content) of the oil components () were calculated using their corresponding relative peak areas, in the total ion current (TIC) chromatograms, assuming a unity response by all components.
Table 1.. The identified constituents of the volatile oil of Gundelia tournifortii..
Platelet aggregation
The samples were dissolved in DMSO at a final concentration of 0.5%. Platelet-rich plasma (PRP) was obtained from human blood taken from the forearm vein of volunteers between 18 and 50 years from both genders. Blood was then collected by free flow along the side of plastic tube containing 3.8% sodium citrate (1:9) and was then centrifuged at room temperature at 1300 rpm for 15 min. Platelets were counted under a microscope, and the platelet count was adjusted to 300,000 platelets/µl with platelet poor plasma (PPP) obtained by centrifugation of the PRP at 13,000 rpm for 3 min. Aggregation was measured by the aggregometric method (Beretz & Cazenave, Citation1991). The aggregometer was calibrated so that the PRP gave 10% of light transmission while the PPP gave 90% of light transmission. The aggregation was measured by an aggregometer (Elvilogos, Milano, Italy) connected to dual channel recorders. The platelet suspension was stirred at 1000 rpm. Platelets were preincubated with different concentrations of the tested compounds (100–800 µg/ml) or DMSO for 3 min before the addition of aggregation inducers (Aburjai, Citation2000).
Effect of herb-aspirin combination on platelet aggregation
Chloroform herbal extract (0.5 ml) at concentrations of 2, 4, and 8 mg/ml were mixed with 0.5 ml aspirin at concentrations of 50, 25, and 12.5 mg/ml in 1% DMSO. The mixture (25 µl) was incubated with 200 µl of PRP (the final concentrations were 200, 400, and 800 µg/ml and 100, 50, 25, and 12.5 µg/ml for chloroform herbal extracts and aspirin, respectively) 5 min before the addition of the agonist. Aggregation was measured simultaneously using ADP, collagen, AA as agonists ().
Table 2.. Effect of Gundelia. extract alone and in combination with aspirin on platelet aggregation.
Statistical analysis
All tested materials were compared with control sample. Mean standard deviations (SD) were calculated for all experimental results. The significance was determined by the Student's t.-test, considering a value of p < 0.05 as significant.
Results and Discussion
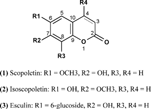
Literature survey showed that little attention has been given to G. tourniforti.. Our previous studies showed that when Gundelia. was combined with penicillin G and erythromycin, full growth of standard strain of Pseudomonas aeruginosa. was allowed while the growth of a resistant strain isolated from hospitalized patients was inhibited (Aburjai et al., Citation2001). Detailed chemical investigation of the aerial parts yielded three coumarins (), namely, scopoletin (1), isoscopoletin (2), and esculin (3), as well as a mixture of β.-sitosterol (4) and stigmasterol (5). These compounds were isolated and their structures were identified by comparing their physical and spectroscopic data (IR, MS, 1H NMR and 13C NMR) with those reported in the literature (Hsieh et al., 1999) and by comparison with authenticated samples. This is the first report on the isolation of these compounds from G. tournifortii. grown in Jordan.
Compound 1 displayed a pattern of mass spectrum similar to that for 7-hydroxy-6-methoxy coumarin. The electron impact mass spectrum (EIMS) revealed a (M+) at m./z. 192, corresponding to the molecular formula C10H8O4. The infrared (IR) spectrum revealed the presence of a hydroxyl group at 3333 cm−1, a C=C at 1625 cm−1, as well as two intense C–O bonds at 1100 and 1190 cm−1. The 1H NMR spectrum of compound 1 exhibited a singlet at δ. 3.92 (3H,s) corresponding to the methoxy group at C6; two aromatic protons appeared at δ. 6.84 (1H,s, H-5) and δ. 6.92 (1H,s, H-8).
Compound 2 gave almost the same profile as compound 1 except that the methoxy group is at C7 instead of C6 and the OH is at C6.
The presence of a sugar moiety was clear in compound 3 by the 1H NMR signals between δ. 3.99 and 4.84 ppm as well as 13C NMR signals between 62.5 and 75.3 ppm.
Mixture of compounds 4 and 5 was obtained after purification on column chromatography using chloroform/ethyl acetate (4:6 and 3:7) to give a precipitate that was recrystallized using n.-hexane–chloroform (7:3) to give colorless needles (mp: 135–140°C). The EIMS revealed M+ at m/z. 414 and 412, corresponding to the molecular ions of compounds 4 and 5, respectively. The IR spectrum revealed a hydroxyl group at 3417 cm−1.
The 1H NMR spectrum of 4 and 5 exhibited only four signals with high chemical shift, belonging to H-6 at δ. 5.34 (1H, m), H-22 at δ. 5.17 (1H, dd), H-23 at δ. 5.04 (1H, dd), and H-3 δ. 3.5 (1H, m) adjacent to the hydroxyl group.
The chloroform extract of G. tournifortii. inhibited platelet aggregation induced by ADP or AA in a dose-dependent manner (). The extent of aggregation inhibition induced by AA was slightly more than that induced by ADP (IC50 values were 780 and 600 µg/ml for ADP and AA, respectively), as shown in . When polar solvents, water and methanol, were used for extraction of Gundelia., no inhibition activity was noticed against any of the agonists used. On the other hand, none of the pure compounds isolated were active at a concentration of 100 µg/ml when tested for their antiplatelet activity induced by ADP and AA.
Table 3.. The effect of chloroform extract of Gundelia tournifortii., aspirin, scopoletin, isoscopoletin, esculin, and the mixture β.-sitosterol and stigmasterol on platelet aggregation induced by AA and ADP.
According to , aspirin was used as a positive control and in combination with chloroform extract at different concentrations to explore any drug-herb interaction. Aspirin was found to inhibit platelet aggregation induced by AA strongly (IC50 = 29 µg/ml) and significantly in a dose-response manner. These results are in agreement with previous studies and as reported by Williams et al. (Citation1987). Different concentrations of Gundelia. extract (200, 400, and 800 µg/ml) were combined with aspirin (12.5, 25, and 50 µg/ml). The interaction between aspirin and the extract was more pronounced at low concentration of aspirin (12.5 and 25 µg/ml) than when using high concentration. When high concentration of the extract was used (800 µg/ml), almost no difference in the percentage of aggregation inhibition was noticed regardless of the change in the aspirin concentration. As a negative control, DMSO at concentration of 1% v/v exhibited a very slight antiplatelet effect showing 5.69% and 5.03% inhibition of platelet aggregation induced by ADP and AA, respectively.
This is the first report on the isolation of volatile oils from G. tournifortii.. Twenty-six components () representing 93% of the oil contents were determined by GC and GC-MS. The oil was found rich in oxygenated monoterpenoids (67.97%) with α.-terpinyl acetate (36.21%) as the principal component. Other major components included methyl eugenol (12.57%), eugenol (6.7%), β.-caryophellene (5.94%), and zingiberene (5.84%).
In conclusion, further studies are needed to identify compound(s) responsible for the biological activity of this plant.
References
- Adams RP (1995): Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy. Carol Stream, IL, Allured Publ. Corp.
- Aburjai T, Darwish RM, Al-Khalil S, Mahafzah A, Al-Abbadi A (2001): Screening of antibiotic resistant inhibitors from local plant materials against two different strains of Pseudomonas aeruginosa.. J Ethnopharmacol 76: 39–44. [PUBMED], [INFOTRIEVE], [CSA]
- Aburjai T (2000): Antiplatelet stilbenes from aerial parts of Rheum palaestinum.. Phytochemistry 55: 407–410. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Beretz A, Cazenave JP (1991): Assays for platelet aggregation and related enzyme activities useful for the analysis plant material. In: Hostetmann K, ed., Methods in Plant Biochemistry, Vol 6. New York, Academic Press, pp. 235–260.
- Darwish RM, Aburjai T, Al-Khalil S, Mahafzah A (2002): Screening of antibiotic resistance inhibitors from local plant materials against two different strains of Staphylococcus aureus.. J Ethnopharmacol 79: 359–364. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Hsieh T-J, Chang F-R, Wu Y-C (1999): The constituents from Cananga odorata.. J Chin Chem Soc 46: 607–611. [CSA]
- Kery A, Hussein AM (1985): Isolation of scopoletin in Gundelia tournefortti. by DCCC. Fitoterapia 56: 42–44. [CSA]
- Lev YS, Abbo S (1999): Traditional use of A'kub (Gundelia tournefortii., Astreraceae) in Israel and the Palestinian Authority area. Econ Bot 53: 217–219. [CSA]
- Sezik E (2001): Traditional medicine in Turkey. J Ethnopharmacol 75: 95–115. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Wagner H, Nickl H (1984): Molluscicidal saponins from Gundelia tournefortii.. Phytochemistry 23: 2505–2508. [CROSSREF], [CSA]
- Williams SB, McKeown LP, Gralnick HR (1987): Asialo von willebrand factor binding to and aggregation of platelets: Effects of inhibitors of platelet metabolism and function. J Lab Clin Med 109: 650–565. [CSA]