ABSTRACT
The cytotoxic activities of several diterpenoid constituents of the medicinal plant, “hempedu bumi,” or Andrographis paniculata. Nees (Acanthaceae), were evaluated. The seven diterpenoid compounds used were andrographolide, 14-deoxyandrographolide, andrographiside, deoxyandrographiside, 14-deoxy-12-methoxyandrographolide, neoandrographolide, and 14-deoxy-11,12-didehydroandrographolide. The activities of these compounds were evaluated with various human tumor cell lines such as Caov-3 (human ovarian carcinoma cell line), T-47D (human breast carcinoma cell line), Hs-578T (human breast carcinoma cell line), Hep G2 (human hepatocarcinoma cell line), and NCI–H23 (human lung adenocarcinoma cell line). Cell survival was measured using the MTS assay after 72 h of incubation. Andrographolide, neoandrographolide, andrographiside, deoxyandrographiside, and 14-deoxy-12-methoxyandrographolide appeared to be noncytotoxic against all the cell lines. Only 14-deoxyandrographolide and 14-deoxy-11,12-didehydroandrographolide exhibited cytotoxic activities (based on EC50 values), but this was limited to the T-47D cell line (EC50 values of 2.8 µg/ml and 1.5 µg/ml, respectively). As one of the principle diterpenoids of Andrographis paniculata., 14-deoxy-11,12-didehydroandrographolide appeared to be the most potent when compared with the rest of the compounds examined. The effects of 14-deoxy-11,12-didehydroandrographolide on T-47D cells were further confirmed to be nonapoptotic, non-necrotic, but programmed in nature, as demonstrated using a DNA fragmentation detection assay, Trypan blue exclusion assay, and annexin V–propidium iodide staining.
Introduction
Andrographis paniculata. Nees (Acanthaceae) is widely known as “kalmegh” in India. It is used as a bitter ingredient in many of the traditional formulations in the practice of Ayurveda. There are about 26 polyherbal formulations of this plant mentioned in Ayurvedic medicine as a popular remedy for the treatment of various liver disorders (Handa et al., Citation1986). This plant is also traditionally used to treat different range of diseases in China and Southwest Asia. A. paniculata. is widely known for its beneficial effects in general debility, dysentery, dyspepsia, malaria, asthma, bronchitis, filariasis, and hepatitis (Tang & Eisenbrand, Citation1992; Kapil et al., Citation1993; Jain et al., Citation2000). Andrographolide and related diterpenoids isolated from this plant have shown some degree of antipyretic, antimalarial, and anti-inflammatory activity (Jain et al., Citation2000). Andrographolide, the principle compound, is reported to protect against alcohol and carbon tetrachloride–induced hepatotoxicity (Roy & Poddar, Citation1984; Roy et al., Citation1987). The plant is also well-known for its hepatoprotective effects on liver cells (Roy et al., Citation1987; Rana and Avadhoot, Citation1991; Kapil et al., Citation1993), potent cell differentiation–inducing activity on myeloid leukemia cells (Matsuda et al., Citation1994), and potent stimulation of the immune response (Puri et al., Citation1993). Some of these properties such as inducing of cell differentiation and immunostimulant activities are possible antitumor effects. However, there are no data for the direct cytotoxic activities of these compounds against human tumor cell lines. Thus, the aim of this study is to evaluate the cytotoxic activities of several bioactive diterpenoids of A. paniculata. against a panel of human tumor cell lines and to determine the mode of action of compound(s) that exhibit potent cytotoxic activities.
Materials and Methods
Chemicals
The diterpenoid compounds used in the experiment were obtained from Professor Kuroyanagi's laboratory and isolated as previously described () (Matsuda et al., Citation1994). The compounds were weighed using a microbalance (Sartorious, Goettingen, Germany) and reconstituted with DMSO to prepare stock concentration of 5 mg/ml and diluted serially to eight different concentrations. The MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium]assay (CellTiter 96 AQueous Non-Radioactive Cell Proliferation Assay) and the Deadend Colometric Apoptosis Detection System were purchased from Promega (Madison, WI, USA). The Annexin-V-FLUOS kit was purchased from Roche Diagnostics (Penzberg, Germany). Dimethyl sulfoxide (DMSO) was obtained from Amresco (Solon, OH, USA) and propidium iodide and vincristine sulfate from Sigma Aldrich (St. Louis, MO, USA). Etoposide was purchased from DBL (Rowville, Australia). All culture media and additives were obtained from Hyclone (Logan, UT, USA).
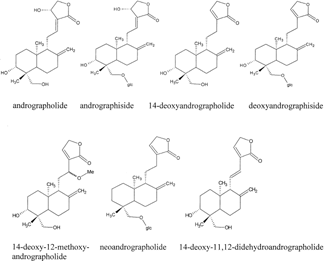
Figure 1The chemical structures of the diterpenoids of Andrographis paniculata. Nees. Source: Matsuda et al. (Citation1994).
Cell lines and culture medium
Five different human tumor cell lines were used; Caov-3 (human ovarian carcinoma), T-47D (human breast carcinoma), Hs-578T (human breast carcinoma), Hep G2 (human hepatocellular carcinoma), and NCI–H23 (human non–small cell lung carcinoma) were all purchased from American Type Culture Collection (ATCC) (Manassas, VA, USA). Caov-3 and Hs-578T cell lines were cultured in HyQ DMEM (Dulbecco's modified Eagle medium), T-47D and NCI–H23 cell lines in HyQ RPMI 1640 (Rosewell Park Memorial Institute), and HepG2 cells in HyQ MEM/EBSS (Eagle's minimal essential medium/Earle's balanced salt solution). All media was supplemented with 10% (v/v) fetal bovine serum (FBS) 100 U/ml and 100 mg/ml HyQ penicillin-streptomycin solution and 2 mM HyQ L-glutamine (4 mM L-glutamine in Hs-578T cell medium). Additional additives such as 0.01 mg/ml bovine insulin were added to T-47D and Hs-578T cell medium, 0.1 mM nonessential amino acids to HepG2, 10 mM HEPES and 1 mM sodium pyruvate to T-47D and NCI–H23 cell lines, as recommended by ATCC.
In vitro. cytotoxicity assay
Cellular growth in the presence or absence of experimental agents was determined using the MTS assay (CellTiter 96 AQueous Non-Radioactive Cell Proliferation Assay) as described by the manufacturer. Briefly, T-47D cells were plated onto 96-well plates at cell density of approximately 6000 cells/well and grown at 37°C in a humidified incubator supplemented with 5% (v/v) CO2 for 24–48 h. Cell viability was routinely determined using Trypan blue exclusion test, and in all cases, cell viability was always in excess of 90%. When the cells reached between 80% and 90% confluency, the medium was removed and replaced with medium containing only 0.5% (v/v) FBS. The cells were incubated for a further 4 h, and subsequently the cells were treated with different concentration of the compounds as mentioned earlier. Untreated control cells were cultured in 0.5% (v/v) FBS-containing medium in the presence of 1% (v/v) DMSO (vehicle). DMSO was used to dissolve and dilute the compounds and the final concentration of DMSO present in each well was adjusted to 1% (v/v), the same concentration used in control cells. The cells were subsequently incubated for 72 h. Vincristine sulfate and etoposide were used as positive controls.
After 72 h incubation, 20 µl/well of combined MTS/PMS solution was added and the plates were incubated for a further 1–4 h in the humidified 5% (v/v) CO2 incubator at 37°C. Absorbance was determined at 490 nm using Vmax Kinetic Microplate Reader (Molecular Devices, Sunnyvale, CA, USA). Wells with complete medium and MTS/PMS solution but without cells were used as blanks. EC50 values were expressed as µg of compound/ml that caused a 50% growth inhibition as compared to controls. Experiments were carried out in triplicate in three independent experiments (n = 9).
Detection of DNA fragmentation (apoptosis) in T-47D cells
Near confluent cultures of T-47D cells were subcultured into Labtek Chamber Slides (Nalge Nunc, Denmark) and incubated for 24–48 h. When cultures reached between 80% and 90% confluency, the medium was replaced with fresh medium containing 0.5% (v/v) FBS, incubated for a further 4 h and treated with the EC50 concentration (1.520 µg/ml, 72 h) of 14-deoxy-11,12-didehydroandrographolide. Untreated control cells were treated with similar concentration of DMSO. Positive control cells were treated with DNase I (1 U/ml) and etoposide of EC50 concentration (1.90 µg/ml, 72 h). In all cases, the final concentration of DMSO in each control slide did not exceed 1% (v/v). The cells were subsequently incubated for 24 h. After treatment, the cells were washed with phosphate-buffered saline (PBS) twice and subsequently processed according to the Deadend Colometric Apoptosis Detection System (Promega) protocol as described in the manufacturer's protocol. The slides were observed using the light microscopy (Olympus BH2 Light microscope equipped with a JVC KYF55B CCD Color Video Camera). In another set of experiments, in order to determine cell viability, T-47D cells were plated onto 12-well plates and treated accordingly with the diterpenoid. After 24 and 72 h, the treated cells were stained with 0.4% (w/v) Trypan blue and left for 5 min. The cell samples were then viewed using an inverted light microscope. Experiments were carried out in duplicate.
Detection of phosphatidylserine externalization (programmed cell death) in T-47D cells
T-47D cells were prepared and stimulated with 14-deoxy-11,12-didehydroandrographolide as described in the previous section. After 24 h of incubation, the medium, chambers, and silicon borders of cells grown on chamber slides were removed and the treated cells were incubated with the Annexin-V-FLUOS labeling solution (combination of annexin V and propidium iodide solution, 100 µl/chamber) with coverslips for 10–15 min at 15–25°C as described in the manufacturer's protocol. Subsequently, the slides were immediately analyzed using a fluorescence microscope using an excitation wavelength in the range of 450–500 nm and detection wavelength in the range of 515–565 nm (green) (Olympus BH2-RFCA Fluorescence Microscope with Olympus camera attachment). Annexin V and propidium iodide positive cells were stained in green and red, respectively. All experiments were carried out in duplicate.
Calculations
The EC50 values for growth inhibition were derived from a nonlinear regression model (curvefit) based on sigmoidal dose-response curve (variable) and computed using GraphPadPrism (Graphpad, USA). EC50 values from non–dose response curves were derived by 50% interpolation on fit spline point-to-point plots.
Results
Growth inhibitory effects of the diterpenoids on the human tumor cell lines
The seven diterpenoid compounds of A. paniculata. were evaluated in a panel of human tumor cell lines that included ovarian, breast, liver, and lung carcinoma cell lines. Andrographolide, being the principle diterpenoid, appeared to be noncytotoxic against these cell lines, as judged by the criterion set by the National Cancer Institute (USA) (EC50 value above 4 µg/ml is categorized as noncytotoxic) (Geran et al., Citation1972). The EC50 values derived for all the cell lines were higher than 4 µg/ml (). Similarly for andrographiside, deoxyandrographiside, 14-deoxy-12-methoxyandrographolide, and neoandrographolide, these compounds were apparently noncytotoxic to all the cell lines in the concentration range tested (). As for 14-deoxyandrographolide, the cytotoxic activity was limited to the T-47D cell line (EC50 values of 2.8 µg/ml), indicating cell-specific toxicity. However, this compound was noncytotoxic to the rest of the cell lines. The most active diterpenoid in this screening appeared to be 14-deoxy-11,12-didehydroandrographolide, with the highest cytotoxic effects (lowest EC50 value) demonstrated in T-47D cells (EC50 values of 1.5 µg/ml) but similarly, it was noncytotoxic toward the rest of the cell lines.
Table 1.. Cytotoxic activity (EC50 values, 72 h) of diterpenoid constituents of A. paniculata. and positive controls against a variety of cell lines.
As one of the principle compounds of A. paniculata., 14-deoxy-11,12-didehydroandrographolide showed greater cytotoxic activity as compared to the rest of the compounds (based on EC50 values). In addition, 14-deoxy-11,12-didehydroandrographolide also exhibited greater potency as compared to vincristine sulfate and etoposide, against T-47D cells (EC50 values of 21.7 µg/ml and 1.9 g/ml, respectively) (). Vincristine sulfate was naturally active against the Caov-3, Hs-578T, Hep G2, and the NCI-H23 cell lines but not the T-47D mammary cells. By contrast, etoposide was shown to have activities against T-47D cells and NCI-H23 cells but not against other cell lines. This indicated that the mechanisms of cytotoxicity or cell death, at least in vitro., were cell and drug specific. Within the panel of cell lines, T-47D cells were the most sensitive to 14-deoxy-11,12-didehydroandrographolide, followed by 14-deoxyandrographolide, 14-deoxy-12-methoxyandrographolide, and andrographolide. Although the plant is well-known for its hepatoprotective effects on liver cells (Roy, et al., Citation1987, Rana & Avadhoot, Citation1991, Kapil et al.,), the results reveal that none of the compounds exhibit remarkable activity against the HepG2 cells. The effects of 14-deoxy-11,12-didehydroandrographolide on T-47D cells was further evaluated.
Induction of cell death by 14-deoxy-11,12-didehydroandrographolide in T-47D cells
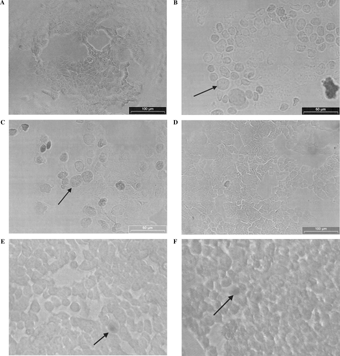
Alterations in DNA integrity of T-47D cells were examined after 24 h exposure to 1.5 µg/ml 14-deoxy-11,12-didehydroandrographolide and subjected to Deadend Apoptosis Detection Assay (Promega) as described previously. The typical appearance of T-47D cells after 24 h exposure to the diterpenoid is shown in as compared to the DNase I-treated T-47D cells (), etoposide-treated T-47D cells (), and negative control T-47D cells (). As shown in , cells treated with the diterpenoid did not produce DNA fragmentation, one of the criteria used to define apoptosis (the presence of a brown nucleus indicating DNA fragmentation) as compared to positive controls ( and ). The diterpenoid-treated cells did not show morphological evidence of necrotic cell death, as confirmed using the Trypan blue exclusion assay. and show that only a few cells were stained with Trypan blue at 24 and 72 h of incubation, indicating that most cells were viable and plasma membrane were intact. Thus, necrosis was ruled out as a probable cause of cell death in the diterpenoid-treated T-47D cells. This result show that cell death caused by 14-deoxy-11,12-didehydroandrographolide is nonapoptotic and non-necrotic in nature.
Figure 2The effect of (A) 14-Deoxy-11,12-didehydroandrographolide (EC50 values of 1.520 µg/ml, 72 h) (B) DNase I (1 U/ml), (C) etoposide (EC50 values of 1.9 µg/ml, 72 h), and (D) DMSO 1% (v/v) on T-47D cells as assayed with Deadend™ Colometric Apoptosis Detection System (Promega, USA). Arrows show darkly stained nuclei of T-47D cells indicating DNA fragmentation. No DNA fragmentation was observed in diterpenoid-treated cells. Trypan blue exclusion assay of the T-47D cells treated with 14-Deoxy-11,12-didehydroandrographolide (EC50 values of 1.5 µg/ml, 72 h) for (E) 24 h and (F) 72 h. Arrow shows necrotic cell.
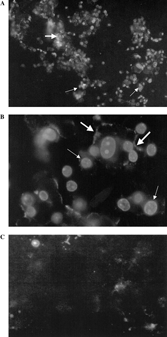
To investigate further, T-47D cells were incubated with the diterpenoid compound for 24 h using EC50 concentration (1.5 µg/ml; ). After incubation, the cells were subjected to the Annexin-V-FLUOS assay (Roche). Negative controls were carried out simultaneously. The presence of weakly scattered annexin V positive cells were evident as shown in and , indicating an early phase of programmed cell death as compared to negative control cells as shown in . A high percentage of cells with homogeneous and high-intensity staining of propidium iodide were observed, indicating either necrosis, late-stage apoptosis (secondary necrosis), or autophagic cell death had taken place ( and ). There were also cells stained with annexin V and propidium iodide simultaneously. As apoptosis and necrosis was ruled out as the mechanism of cell death, elicited by 14-deoxy-11,12-didehydroandrographolide in the previous section, the presence of positive annexin V and propidium iodide cells indicate that cell death may be due to autophagic cell death (Tan et al., Citation2005). The vacuolar structure of these cells may take up the propidium iodide stain in a similar fashion to necrotic cells. However, further investigations are warranted in this context.
Figure 3The effect of 14-deoxy-11,12-didehydroandrographolide (EC50 values of 1.52 µg/ml, 72 h), on T-47D cell line as stained with Annexin-V-FLUOS™ kit (Roche, Germany). (A and B). The effect of 1% (v/v) DMSO, control vehicle on T-47D cell line. (C) Positive annexin V (thick arrows) and propidium iodide (thin arrows) stained cells were noted. Original magnification × 100 (A) × 400 (B, C).
Discussion
A. paniculata. is a medicinal plant that is widely used in traditional and folkloric remedies. In the search for potential chemotherapeutic agents from medicinal plants, A. paniculata. was noted for its possible actions against tumors and cancer-related diseases. Previous studies have reported that andrographolide and neoandrographolide possess potent immunostimulant activity and are capable of enhancing both antigen specific and nonspecific responses (Puri et al., Citation1993). Known immunostimulants have been reported to act by either stimulating macrophages or other immune cell functions (Puri et al., Citation1993). In addition, the differentiation activity of some of the bioactive compounds has been demonstrated, indicating possible antitumor activity (Matsuda et al., Citation1994). Compounds such as andrographolide and 14-deoxyandrographolide have shown potent differentiation activity, whereas others have shown moderate to weak activities (Matsuda et al., Citation1994). The dichloromethane fraction of the crude extract of A. paniculata. is reported to have significant inhibitory activities against the proliferation of the HT-29 (colon) cells and increase the proliferation of human peripheral blood lymphocytes at low concentrations (Kumar et al., Citation2004). The direct and indirect anticancer activities of the fraction appear to depend on three diterpenoids, namely andrographolide, 14-deooxyandrographolide, and 14-deoxy-11,12-didehydroandrographolide (Kumar et al., Citation2004).
Andrographolide, the major constituent of the A. paniculata. plant, is usually implicated for its pharmacological activity. One study has reported that andrographolide inhibits the in vitro. proliferation of tumor cell lines by inducing cell cycle arrests at G0/G1 phase and enhancing the production of interleukin-2, tumor necrosis factor-alpha and the proliferation of lymphocytes, which results in the activation of the lymphocytes against cancer cells (Rajagopal et al., Citation2003). The indirect anticancer and immunomodulatory activities of andrographolide may explain why the compound fails to inhibit the growth of tumor cells effectively in this in vitro. experiment. Andrographolide is categorized as noncytotoxic according to the criterion set by the National Cancer Institute (EC50 is more than 4 µg/ml for all cell types) () (Geran et al., Citation1972).
Results of the current study reveal that the principle compounds, 14-deoxyandrographolide and 14-deoxy-11,12-didehydroandrographolide, appeared to be more active as compared to other constituents found in the plant. 14-deoxy-11,12-didehydroandrographolide showed more potent activity (based on EC50 values) in comparison to vincristine sulfate and etoposide, especially against T-47D cells (EC50 value of 1.5 µg/ml),indicating that this compound could be a possible candidate against breast cancer. There was a general observation that compounds that elicited apoptosis such as vincristine sulfate (Li et al., Citation1997; Fan et al., Citation1998; Li et al., Citation1998) appeared to produce effective cytotoxic activities against cell lines such Caov-3, Hs-578T, Hep G2, and NCI-H23 but not T-47D cells. On the other hand, diterpenoids such as 14-deoxy-11, 12-didehydroandrographolide produced effective cytotoxic effects against T-47D cells but not other cell lines, which were more susceptible to the apoptotic effects of vincristine sulfate. Interestingly, this diterpenoid elicited a form of nonapoptotic cell death as opposed to apoptotic cell death. This observation supports the thesis that cells resistant to the apoptotic effects of drugs may actually be sensitive to those that can elicit the nonapoptotic cell death.
Recent studies have revealed that active self-destruction of cells is not confined to apoptosis alone. Cells actually use different pathways to commit suicide, including the nonapoptotic or autophagic programmed cell death. Autophagic cell death is often associated with bulk degradation of proteins and organelles, a process essential for cellular maintenance and cell viability (Tanida et al., Citation2004). This has been shown to be essential for differentiation and development, as well as cellular maintenance (Meijer & Dubbelhuis, Citation2004). Autophagic programmed cell death appears to be associated with carcinogenesis, as well as other diseases such as neurodegenerative diseases, cardiomyopathies, and bacterial and viral infections (Tanida et al., Citation2004). During autophagy, a cup-shaped structure, the preautophagosome, engulfs cytosolic components like organelles to form the autophagosome, which subsequently fuses with a lysosome and leads to the proteolytic degradation of the components (Tanida et al., Citation2004). This process is usually caspase-independent. thus, there is no evidence of DNA fragmentation. The most prominent evidence that can be visualized at the electron microscope level is the formation of autophagic vacuoles (Bursh, Citation2004; Tan et al., Citation2005). The recent discovery of the autophagy genes such as Atg.7 and Beclin.1, which are involved in the regulation of this type of cell death, has opened more avenues for research in this area (Gozuacik & Kimchi, Citation2004; Gutierrez et al., Citation2004, Marino & Lopez-otin, Citation2004; Meijer & Codogno, Citation2004; Tanida et al., Yu et al., Citation2004).
The cell death caused by 14-deoxy-11,12-didehydroandrographolide against T-47D cells is nonapoptotic (as revealed by lack of DNA fragmentation) and non-necrotic (as revealed by Trypan blue exclusion assay) but programmed in nature (as revealed by annexin V and propidium iodide staining). This nonapoptotic programmed cell death is probably the autophagic (vacuolar) nonapoptotic programmed cell death as demonstrated by the high percentage of cells that took up the propidium iodide dye. Cells undergoing the autophagic death are known to take up the propidium iodide like necrotic cells, due to increase cell porosity instead of cell membrane disruption (Tan et al. Citation2005).
The results of this study suggest that 14-deoxy-11,12-didehydroandrographolide may be a possible candidate for the treatment of breast cancer, as the compound inhibited the growth of T-47D cells via a nonapoptotic programmed cell death mechanism. Although the characteristic staining pattern by annexin V and propidium iodide suggests that cell death might be programmed and autophagic in nature, further investigations are warranted, such as ultrastructural analysis using transmission electron microscopy and gene expression studies. The acquired resistance of tumor cells to apoptosis is a major concern in cancer therapy, particularly in recurrent cases, and further studies of nonapoptotic cell death may open new avenues to the selective killing of tumor cells. The nonapoptotic programmed cell death mechanism provides a potential target in cancer therapeutics.
Acknowledgments
The authors wished to thank the Ministry of Science, Technology and Innovation (MOSTI) Malaysia for the National Science Fellowship awarded to Tan Mei Lan and for the IRPA grant awarded to Tengku Sifzizul Tengku Muhammad.
References
- Brusch W (2004): Multiple cell death programs: Charon's lifts to Hades. FEMS Yeast Res 5: 101–110. [CSA]
- Fan S, Cherney B, Reinhold W, Rucker K, O'Connor PM (1998): Disruption of p53 function in immortalized human cells does not affect survival or apoptosis after taxol or vincristine treatment. Clin Cancer Res 4: 1047–1054. [PUBMED], [INFOTRIEVE], [CSA]
- Geran RI, Greenberg NH, Macdonald MM, Schumacher AM, Abbott BJ (1972): Protocols for screening chemical agents and natural products against animal tumors and other biological systems. Cancer Chemothen Rep 3: 1–61. [CSA]
- Gozuacik D, Kimchi A (2004): Autophagy as a cell death and tumor suppressor mechanism. Oncogene 23: 2891–2906. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Gutierrez MG, Munafo DB, Beron W, Colombo MI (2004): Rab7 is required for the normal progression of the autophagic pathway in mammalian cells. J Cell Sci 117: 2687–2697. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Handa SS, Sharma A, Chakraborti KK (1986): Natural products and plants as liver protecting drugs. Fitoterapia 54: 307–310. [CSA]
- Jain DC, Gupta MM, Saxena S, Kumar S (2000): LC analysis of hepatoprotective diterpenoids from Andrographis paniculata.. J Pharmaceut Biomed 22: 705–706. [CROSSREF], [CSA]
- Kapil A, Koul IB, Banerjee SK, Gupta BD (1993): Antihepatoxic effects of major diterpenoid constituents of Andrographis paniculata.. Biochem Pharmacol 46: 182–185. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Kumar RA, Sridevi K, Kumar NV, Nanduri S, Rajagopal S (2004): Anticancer and immunostimulatory compounds from Andrographis paniculata.. J Ethnopharmacol 92: 291–295. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Li ZH, Zhu YJ, Chao LS, Li XT (1997): Wild type p53 stimulates vincristine-induced apoptosis. Yao Xue Xue Bao 32: 565–568 [in Chinese]. [PUBMED], [INFOTRIEVE], [CSA]
- Li G, Tang L, Zhou X, Tron V, Ho V (1998): Chemotherapy-induced apoptosis in melanoma cells is p53 dependent. Melanoma Res 8: 17–23. [PUBMED], [INFOTRIEVE], [CSA]
- Matsuda T, Kuroyanagi M, Sugiyama S, Umehara K, Ueno A, Nishi K (1994): Cell differentiation-inducing diterpenes from Andrographis paniculata. Nees. Chem Pharm Bull (Tokyo) 42: 1216–1225. [PUBMED], [INFOTRIEVE], [CSA]
- Marino G, Lopez-Otin C (2004): Autophagy: Molecular mechanisms, physiological functions and relevance in human pathology. Cell Mol Life Sci 61: 1439–1454. [PUBMED], [INFOTRIEVE], [CSA]
- Meijer AJ, Codogno P (2004): Regulation and role of autophagy in mammalian cells. Int J Biochem Cell Biol 36: 2445–2462. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Meijer AJ, Dubbelhuis PF (2004): Amino acid signaling and the integration of metabolism. Biochem Biophys Res Commun 313: 397–403. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Puri A, Saxena R, Saxena RP, Saxena KC (1993): Immunostimulant agents from Andrographis paniculata.. J Nat Prod 56: 995–999. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Rajagopal S, Kumar RA, Deevi DS, Satyanarayana C, Rajagopalan R (2003): Andrographolide, a potential cancer therapeutic agent isolated from Andrographis paniculata.. J Exp Ther Oncol 3: 147–158. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Rana AC, Avadhoot Y (1991): Hepatoprotective effects of Andrographis paniculata. against carbon tetrachloride liver damage. Arch Pharm Res 14: 93–95. [PUBMED], [INFOTRIEVE], [CSA]
- Roy Choudhury B, Poddar MK (1984): Andrographolide and kalmegh (Andrographis paniculata.) extract: In vivo. and In vivo. effect on lipid peroxidation. Meth Find Expt Clin Pharmacol 6: 481–485. [CSA]
- Roy Choudhury B, Hague SJ, Poddar MK (1987): In vivo. and In vivo. effects of kalmegh (Andrographis paniculata.) extract and andrographolide on hepatic microsomal drug metabolizing enzymes. Planta Med 53: 135–140. [CSA]
- Tan ML, Tengku Muhammad TS, Samian MR, Najimuddin N, Sulaiman SF (2005): Growth arrest and non apoptotic cell death associated with the up-regulation of c-myc mRNA expression in T-47D breast carcinoma cells following exposure to Epipremnum pinnatum. (L). Engl hexane extract. J Ethnopharmacol 96: 375–383. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Tanida I, Ueno T, Kominami E (2004): LC3 conjugation system in mammalian autophagy. Int J Biochem Cell Biol 36: 2503–2518. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Tang W, Eisenbrand G (1992): Chines Drugs of Plant Origin, Spring-Verlag, Berlin, pp. 97–103.
- Yu L, Lenardo MJ, Baehrecke EH (2004): Autophagy and caspases: A new cell death program. Cell Cycle 3:1124–1126. [PUBMED], [INFOTRIEVE], [CSA]
