Abstract
In a previous study, we reported six flavonoids isolated from the methanol extract of Urtica urens L. Patuletin, the major component, was found to have powerful anti-inflammatory and antimicrobial activity. We have now investigated whether patuletin also has antioxidant activity and free radical scavenging effects in rats treated with aflatoxin B1 (AFB1). Male Sprague-Dawley rats (60) were assigned to 6 experimental groups including a control group and groups treated for 10 days with patuletin at low dose (7.5 mg/kg b.w) or high dose (10 mg/kg b.w) with or without AFB1 (2 mg/kg b.w). Blood samples were collected at the end of the experimental period for biochemical analysis. The results showed significant changes typical to aflatoxicosis in rats treated only with AFB1. Patuletin at the two tested doses caused an increase in glutathione peroxidase (GPx) and superoxide dismutase (SOD); high doses also caused an increase in liver and kidney enzymes, except total bilirubin (TB), which was in the normal value of the control animals. On the other hand, these enzymes were comparable to the controls in rats treated with low doses. Cotreatment of AFB1 and patuletin resulted in a significant improvement in all parameters tested. We conclude that patuletin possesses an antioxidant activity and free radical scavenging effects in AFB1-treated rats that can be observed at a dose as low as 7.5 mg/kg b.w.
Introduction
The stinging nettle (Urtica. plant) has been used as a folk-medicinal herb for the treatment of various diseases and also as a healthy food in Europe (Watt and Breyer-Brandwisk, 1962). It has been regarded as a powerful diuretic and as a counterirritant and in catarrhal affections (Pieroni et al., Citation2002). Urtica. exhibits hypoglycemic activity at 100 mg/kg b.w (Karaloli et al., Citation2003), and Hox alpha is a novel stinging nettle (Urtica dioical/Urtica urens.) leaf extract used for treatment of rheumatic diseases (Schulze-Tanzil et al., 2002). Urtica dioica. leaves extract exhibits antioxidant activity and inducible antioxidant inhibitory activity (> 50%) in lipid peroxidation assays (Pieroni et al., Citation2002).
Previously, we isolated the aglycon flavonol patuletin and other six flavonol glycosides from methanol extract (70%) of the herb Urtica urens. L. (Ataa et al., Citation1995). Patuletin represented the major isolate and showed significant antimicrobial, analgesic, and anti-inflammatory effects. The flavonol patuletin protects cultured cortical cell from necrotic cell death induced by glutamate (Kim et al., Citation2002) and exerts significant neuroprotective activity when administrated either before or after the glutamate insult. These findings suggest that the plant may be a useful antioxidant in the prevention of aging related diseases, CNS disorders, and as a potential source of agents that can be used against hyperuricemia, gout, and hepatotoxic agents such as aflatoxin B1.
Aflatoxins are highly toxic secondary products of the metabolism of fungi belonging primarily to Aspergillus. and Pencillium.. Toxic syndromes caused by aflatoxin ingestion by humans and animals are indicated as aflatoxicosis. Previous studies have indicated a role for cytochrome P450 CYP2C11, CYP2B, and CYP1A2 in the activation of the carcinogenic aflatoxin B1 to the AFB1-8,9-epoxide (Ishii et al., Citation1986), which in turn reacts with macromolecules such as lipids and DNA, leading to lipid peroxidation and cellular injury (Stresser et al., Citation1994; Galvano et al., Citation2001). Aflatoxin B1 exerts its liver-specific carcinogenicity by inducing a guanine to thymine substitution at codon 249 on the p.53 gene (Bressac et al., Citation1991; Hsu et al., Citation1991). Consequently, the aflatoxin-induced alterations in the hepatic antioxidant status may be considered as manifestation of increased oxidative stress caused by aflatoxin and its metabolites. The aim of the current work is the evaluation of the antioxidant effects of the aglycon flavonol patuletin against oxidative stress and free-radical generation during aflatoxicosis.
Materials and Methods
Chemicals
Aflatoxin B1 (AFB1) standards was purchased from Sigma Chemical Co. (St. Louis, MO, USA). The purity was confirmed by capillary gas chromatography (GC)/mass spectroscopy and UV spectrophotometry. All chemicals were of the highest purity commercially available.
Kits
Alanine amino transferase (ALT), aspartate amino transferase (AST), and urea were obtained from BioMérieax SA (Marey Í Etoile, France). Alkaline phosphatase (AP), total bilirubin (TB) kit was obtained from Sintinal CH (Milan, Italy). Creatinine, was purchased from Pointe Scientific Inc. (Lincoln Park, MI, USA). Glutathione peroxidase (GPx), and superoxide dismutase (SOD) were purchased from Randex Laboratories (San Francisco, CA, USA).
Plant materials
Urtica. was collected from the Orman garden in Giza, Egypt. The plant was authenticated by Badia Diwan, an agricultural engineer in the Orman garden. The aerial parts of the plant were cut during flowering in July 2002 and air dried and powdered. The amount of plant used was 500 g.
Isolation and identification of the flavonoids
Flavonoids were isolated from the methanol extract. The major component, patuletin, is very rare in nature and was identified by UV, MS, and 1H NMR (Ataa et al., Citation1995). Moreover, the NMR analysis for patuletin was carried out in methanol-d4 for 1H and 13C NMR spectra as well as 1H, 13C HSQC and 1H, 13C HMBC. HMBC was optimized to 7 Hz. 1H and 13C spectra were also done in DMSO-d6 with a Varian Unity Inova 400 spectrometer (400 MHz for 1H and 100 MHz for 13C).
Experimental animals
Ten-week-old, sexually mature male Sprague-Dawley rats weighing 150–200 g (purchased from Animal House Colony, Giza, Egypt) were maintained on a standard lab diet (protein: 16.04%; fat: 3.63%; fiber: 4.1%, and metabolic energy: 0.012 MJ) and water ad libitum. at the Animal House Lab., National Research Center, Dokki, Cairo, Egypt. After an acclimation period of 1 week, animals were distributed into six groups (10 rats/group) and housed in filter-top polycarbonate cages housed in a temperature-controlled and artificially illuminated room free from any source of chemical contamination.
Experimental design
Animals within different treatment groups were treated for 10 consecutive days as follows: (1) not treated; (2) treated orally with AFB1 (2 mg/kg b.w) in corn oil; (3) treated orally with low dose of patuletin (7.5 mg/kg b.w) dissolved in 20% propylene glycol in distilled water; (4) treated orally with high dose of patuletin (10 mg/kg b.w) dissolved in 20% propylene glycol in distilled water; (5) treated orally with AFB1 plus the low dose of patuletin; and (6) treated orally with AFB1 plus the high dose of patuletin. At the end of the treatment period, two blood samples were collected from each animal via the retro-orbital venous plexus and after fasting for 12 h. The first blood sample from each animal within each treatment group was placed in heparinized plastic tubes and assayed the same day for glutathione peroxidase (GPx) and superoxide dismutase (SOD) activities. A second blood sample from each animal was left to clot then centrifuged at 5000 rpm for 10 min at 4°C to separate the serum for biochemical analysis.
The activities of ALT and AST were determined according to the method recommended by The Committee on Enzymes of the Scandinavian Society for Clinical Chemistry and Clinical Physiology (1974). Alkaline phosphatase activity was determined in serum according to Roy (Citation1970). Urea was determined according to Fawcett and Scott (Citation1960), total bilirubin was determined according to Routh (Citation1976), and creatinine was determined according to Bartels et al. (Citation1972).
Erythrocyte GPx activity was determined using a Ransel kit according to Paglia and Valentine (Citation1967). SOD was also determined using a Ransod kit according to the method described by Woolliams et al. (Citation1983). In brief, this method employs xanthine and xanthine oxidase (XOD) to generate superoxide radicals that react with 2-(4-iodophenyl)-3-(4-nitrophe)-5-phenyl tetrazolium chloride (INT) to form a red formazan dye. The superoxide dismutase activity is then measured by the degree of inhibition of this reaction.
Statistical analysis
All data were statistically analyzed using the General Linear Model Procedure of the Statistical Analysis System (SAS, 1982). The significance of the differences among treatment groups was determined by the Waller-Duncan k-ratio method (Waller & Duncan, Citation1969). All statements of significance are based on the probability of p < 0.05.
Results
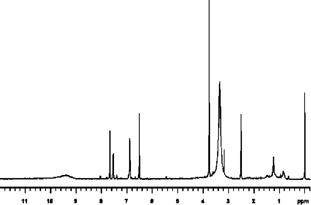
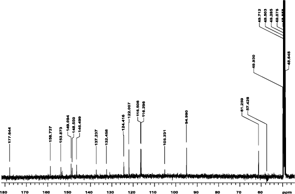
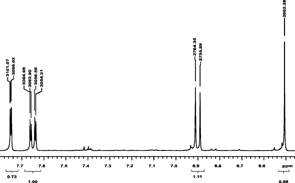
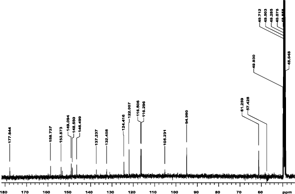
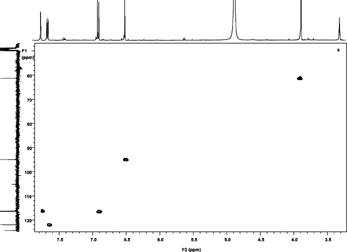
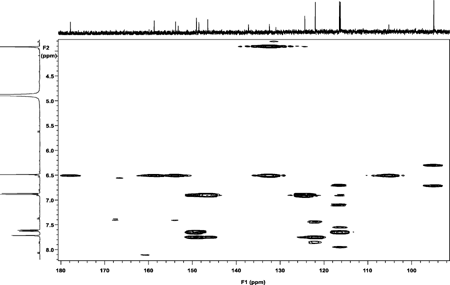
The NMR analysis for patuletin (1H, 13C, HMQC, and HMBC using a Varian Unity Inova 400) are presented in . The interpretations of spectra by comparison with literature data are given in Table Tables and . The 1H NMR spectrum (CD3OD, room temp.) displayed four aromatic proton resonances at δ 6.52 (S), 6.92 (d, J. = 8 Hz), 7.76 (d, d, J. = 8 Hz and J. = 2 Hz), and at δ 7.68 (d, J. = 2 Hz), along with a methoxy group at δ 3.92 (s). 1J couplings observable in the HSQC spectrum allowed for an unambiguous assignment of all protons and protonated carbons (). In the HMBC the aromatic proton at δ 6.48 showed correlations to the aromatic carbons at δ 158.8 (C-9), 153.3 (C-7), 132.5 (C-6), 105.2 (C-10), as well as the carbonyl function at δ 177.9 (C-4), thus revealing an O-methyl in the 6-position of the A ring of patuletin. The remaining aromatic protons showed correlations to all of the aromatic carbons of the B ring in this molecule, thus clearly establishing the patuletin structure (). The 1H and 13C NMR data in DMSO were in agreement with existing data published for patuletin.
Table 1 Interpetation by comparison with literature data.
Table 2 Interpetation by 1D and 2D NMR spectra mainly 1.H, 13C, 1.H, 13C-HMBC (Heteronuclear multibond connectivity), and 1.H, 13C-HSQC (Heteronuclear single quantum coherance).
Table 3 NMR spectral data of patuletin.
To evaluate the antioxidant effects of patuletin, rats were orally treated with AFB1 at two experimental doses, the low dose (7.5 mg/kg b.w) and high dose (10 mg/kg b.w) daily for 10 days. Serum biochemical analysis presented in indicates that AFB1 treatment resulted in a significant increase in ALT, AST, AP, TB, urea, and creatinine. All the biochemical parameters tested in the animals treated with the low dose of patuletin were comparable to the control except TB, which was found to be decreased significantly. On the other hand, treatment with the high dose of patuletin resulted in a significant increase in all the tested parameters except TB, which was in the normal range of the controls. Animals treated with patuletin plus AFB1 showed significant improvements in these parameters toward the normal values of the controls. Although patuletin alone at high doses caused hepatic and renal stress as indicated by the elevation of the serum biochemical parameters, the cotreatment of AFB1 plus patuletin at this level caused a significant improvement in ALT, AST, and TB compared to the AFB1 plus low dose of patuletin.
Table 4 Effects of patuletin administration on some serum biochemical parameters in rats treated orally with aflatoxin B1 (2 mg/kg b.w) for 10 days.
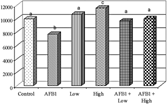
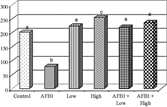
The effects of different treatments on erythrocytes glutathion peroxidase and superoxide dismutase activities are depicted in and . It is clearly indicated that AFB1 alone caused a significant decrease in both GPx and SOD. The administration of patuletin alone at the two tested doses caused an increase in both enzyme activities in a dose-dependent manner; animals treated with patuletin plus AFB1 were comparable to the controls.
Discussion
The 1H NMR spectrum (CD3OD, 25°C) displayed aromatic proton resonances at δ 6.48 (S), 6.96 (d, J. = 8 Hz), 7.63 (d, d, J. = 8 Hz and J. = 2 Hz), and at δ 7. 68 (d, J. = 2 Hz), along with methyl group at δ 3.87 (S). Direct correlations observable in the HMBC spectrum allowed unambiguous assignment of protons and protonated carbons (). The aromatic proton at δ 6.48 showed correlations to the aromatic carbons at δ 158.5 (C-7), 153.3 (C-9), 132.5 (C-6), 105.2 (C-16), as well as the carbonyl functionality at δ 177.9 (C-4), thus revealing the 6-methyl either on the A ring of patuletin. The remaining aromatic protons showed correlations to all of the aromatic carbons of the B ring in this molecule, thus clearly establishing the patuletin structure. The HMQC spectrum proved valuble for the further confirmation of the compound as patuletin. The 1H and 13C NMR data for the compound were in excellent agreement with data published for patuletin (Chari et al. Citation1981; Aritoni et al. Citation1986).
Several epidemiological studies indicated that AFB1 intake is associated with a high incidence of primary liver cancer in Africa and Asia (Peers et al., Citation1976). In the current study, we evaluated the ability of patuletin to protect the experimental animals from the hepatonephrotoxicity and oxidative stress of AFB1. Test animals were given an extreme AFB1 challenge to ensure induction of severe responses. The selective doses of patuletin and AFB1 were based on previous work (Ataa et al., Citation1995; Abdel-Wahhab & Aly, Citation2003). In the current study, the animals were exposed to a single daily dose of 2 mg AFB1/kg body weight for 10 days; therefore, the cumulative dose was comparable to the LD50 reported in the literature.
As reported earlier in the literature (Bressac et al., Citation1991; Abdel-Wahhab et al., Citation1998, 1999; Galvano et al., Citation2001), aflatoxin B1 induces harmful effects resulted in several diseases including carcinogenecity, teratogenecity, and immunotoxicity. In various diseases, the steady state of pro-oxidants and antioxidants may be disrupted in favor of the former, leading to oxidative stress, which in turn may affect all types of biological molecules, including DNA, lipids, proteins, and carbohydrates (Sies, Citation1991; Jung et al. Citation1999). Thus, oxidative stress may be involved in processes of diseases such as those resulted from AFB1.
The liver is considered to be the principal target organ for AFB1, and the activity of transaminases are sensitive indicators of acute hepatic necrosis, whereas AP is known to be indicative of hepatobiliary disease (Kaplan, Citation1987). In the current study, AFB1 alone at 2 mg/kg b.w caused significant changes typical to those reported in the literature of aflatoxicosis (Abdel-Wahhab et al., Citation1998; Abdel-Wahhab & Aly, Citation2003). The elevated levels of ALT, AST, AP, and TB indicated severe hepatic parenchymal cells injury, whereas the increased level of creatinine and urea likely indicate protein catabolism and/or kidney dysfunction (Abdel-Wahhab et al., Citation1999; Barton et al., Citation2000).
Serum biochemical parameters of animals treated with the low dose of patuletin were comparable to the control values except for TB, which was found to decrease significantly. Meanwhile, treatment with high doses of patuletin resulted in a significant increase in all serum biochemical parameters except TB, which was in the normal range of the controls.
AFB1 enhanced lipid peroxidation that is an indication of free radical mediated toxicity. Free radicals are known to attack the highly unsaturated fatty acids of the cell membrane and induce lipid peroxidation that is considered a key process in many pathological events induced by oxidative stress (Schinella et al., Citation2002). In the current study, AFB1 alone decreased GPx, whereas patuletin alone or in combination with AFB1 resulted in a significant increase in GPx. According to Stresser et al. (Citation1994), AFB1 is metabolized by the cellular cytochrome P450 enzymes system to form the AFB1-8,9-epoxide, which reacts with cellular macromolecules resulting in the peroxidation of lipids and hence cellular injury. The decrease in GPx activity in rats treated with AFB1 may be due to the conjugation of GPx with AFB1 epoxide (Abdel-Wahhab & Aly, Citation2003; Meki et al., Citation2004), whereas increased levels of GPx in animals treated with patuletin alone or patuletin plus AFB1 suggests that this flavoniod prevents a decrease in the activities of antioxidant enzymes. Furthermore, the reduction of GPx induced by AFB1 appears to be counteracted by patuletin by increasing the synthesis of this enzyme as well.
The role of oxygen-derived free radicals in the inflammatory process is well-known (Forman & Torres, Citation1999). Free radicals liberated from phagocyte cells are important in inflammatory process, as they have been implicated in the activation of nuclear factor κB, which induces the transcription of inflammatory cytokines and cyclo-oxygenase 2 (Winrow et al., Citation1993; Saharoun et al., Citation1998). In a previous work, we reported that patuletin exhibits anti-inflammatory activity (Ataa et al., Citation1995). The current results support those findings and suggest that the anti-inflammatory activity of patuletin might be explained, at least in part, to its free radical scavenging and antioxidant properties.
According to Halliwell and Gutteridge (Citation1998), the mechanisms of antioxidant action of flavonoids can include (i) suppressing ROS formation either by inhibition of enzymes or chelating trace elements involved in free radical production; (ii) scavenging ROS; and (iii) upregulating or protecting the antioxidant defense system. Kim et al. (Citation2002) concluded that three structural groups within flavonoids are important determinants for their protective nature against glutamate-induced toxicity: (i) the absence of glucose in the flavonoid moiety; (ii) a 6-methoxyl group in the A ring; (iii) a 3′,4′-dihydroxyl groups in the B ring. Accordingly, we have concluded that patuletin fulfills these three structural requirements and consequently has a powerful protective activity.
It is known that the more lipophilic a compound, the better it can penetrate cell membranes (Dearden, Citation1985). Such a lipophilic compound might have better antioxidant effects by scavenging peroxide in the cytosol and interacting with enzymes such as GPx that decompose hydroperoxyides (Tirosh et al., Citation1999). These effects may help explain our findings that patuletin (a lipophilic compound) shows a significant hepatonephroprotective activity with the ability to scavenge free radical as well as cross biological membranes.
Superoxide dismutase activity is an indicator of ROS production. SOD activities in rats treated with AFB1 were decreased significantly, whereas animals treated with patuletin alone or patuletin plus AFB1 show a significant improvement in SOD activity. The decrease in SOD activity in aflatoxin-treated animals might indirectly lead to an increase in oxidative DNA damage (Hirano et al., Citation1997; Meki et al., Citation2004). Therefore, it is suggested that the inhibition of lipid peroxidation by patuletin is attributable to its free radical scavenging activity.
In the context of our results, two points are worth noting: The first concerns the extract concentration required for the antioxidant activity. The second refers to the likelihood of this concentration being able to protect the liver and kidney from the toxicity of AFB1. In this regard, our results clearly indicate that both tested doses appear effective in the protection of liver and kidney toxicity.
In conclusion, the results of the current study reveal that patuletin is an efficient antioxidant in laboratory animals during aflatoxicosis. In addition, patuletin is biologically active as anti-inflammatory, analgesic, antimicrobial, and possesses protective effects in the liver and kidney at doses as low as 7.5 mg/kg b.w.
References
- Abdel-Wahhab MA, Aly SE (2003): Antioxidants and radical scavenging properties of vegetable extracts in rats fed aflatoxin-contaminated diet. J Agric Food Chem 51: 2409–2414. [PUBMED], [INFOTRIEVE], [CSA]
- Abdel-Wahhab MA, Nada SA, Farag IM, Abbas NF, Amra HA (1998): Potential of protective effect of HSCAS and bentonite against dietary aflatoxicosis in rat: with special reference to chromosomal aberrations. Nat Toxins 6: 211–218. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Abdel-Wahhab MA, Nada SA, Amra HA (1999): Effect of aluminosilicate and bentonite on aflatoxin-induced developmental toxicity in rats. J Appl Toxicol 19: 199–204. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Aritoni M, Komori T, Kawaski T (1986): Flavonol glycosides in leaves of Spinacia oleracea.. Phytochemistry 25: 231–234. [CROSSREF], [CSA]
- Ataa S, Wafaa E, Youssry A (1995): Flavonoids of Urtica urens. L. and biological evaluation. Egypt. J Pharm Sci 36: 415–427. [CSA]
- Bartles H, Bohmer M, Heirli C (1972): Serum creatinine determination without protein preciptation. Clin Chem Acta 37: 193–197. [CROSSREF], [CSA]
- Barton CC, Ganey PE, Roth RA (2000): Lipopolysaccharide augments aflatoxin B1-induced liver injury through neutrophil-dependent and independent mechanisms. Toxicol Sci 58: 208–215. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Bressac B, Kew M, Wands J, Ozturk M (1991): Selective G to T mutations of p53 gene in hepatocellular carcinoma from southern Africa. Nature (London) 350: 429–431. [CROSSREF], [CSA]
- Chari VM, Gryer-Barkmeijer RJ, Harborne JB, Oesterdahl B (1981): An acylated allose-containinmg 8-hydroxy flavone glycoside from Veranica filiformis.. Phytochemistry 20: 1977–1980. [CROSSREF], [CSA]
- Dearden JC (1985): Partition and lipophilicity in quantitative structure activity relationships. Environ Health Perspect 61: 203–228. [PUBMED], [INFOTRIEVE], [CSA]
- Fawcett JK, Scott JE (1960): Determination of urea. J Clin Path 13: 156–159. [PUBMED], [INFOTRIEVE], [CSA]
- Forman HJ, Torres M (1999): Inflammation and overview. In: Davies KJA, ed., Oxidative Damage and Repair, Chemical Biological Medical Aspects. Oxford, Pergamon Press, pp. 636–641.
- Galvano F, Piva A, Ritieni T, Galvano G (2001): Dietary strategies to counteract the effects of mycotoxins: A review. J Food Protect 64: 120–131. [CSA]
- Halliwell B, Gutteridge JMC (1998): Free Radicals in Biology and Medicine. New York, Oxford, Clarendon Press, pp. 416–494.
- Hirano T, Yamaguchi Y, Kassai H (1997): Inhibition of 8-hydroxyguanine repair in testes after administration of cadmium chloride to GSH-depleted rats. Toxicol Appl Pharmacol 147: 9–14. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Hsu IC, Metcalf RA, Sun T, Welsh JN, Wang NJ, Harris CC (1991): Mutational hotspot in the p53 gene in human hepatocellular carcinomas. Nature 350: 427–428. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Ishii K, Maeda K, Kamataki T, Kato R (1986) Mutagenic activation of aflatoxin B1 by several forms of purified cytochrome p450. Mutat Res 174: 85–88. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Jung HA, Park JC, Chung HY, Kim J, Choi JS (1999): Antioxidant flavonoids and chlorogenic acid from the leaves of Eriobotrya japonica.. Arch Pharm Res 22: 213–218. [PUBMED], [INFOTRIEVE], [CSA]
- Kaplan MM (1987): Laboratory tests in diseases of the Liver. In: Schiff L, Schiff ER, eds., Philadelphia, J. B. Lippincott Co., pp. 219–237.
- Karaloli G, Tuncel H, Goksel S, Hatemi HH (2003): Hypoglycemic activity of Urtica Pilulifera in streptozotocin-diabetic rats. J Ethnopharmacol 84: 241–245. [CROSSREF], [CSA]
- Kim SR, Park MJ, Lee MK, Sung SH, Park EJ, Kim J, Kim SY, Oh TH, Markelons GJ, Kim YC (2002): Flavonoids of Inula Britannica. protect cultured cotical cells from necrotic cell death induced by glutamate. Free Radic Biol Med 32: 596–604. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Meki AR, Esmail ED, Hussein AA, Hussanein HM (2004): Caspase-3 and heat shock protein-70 in rat liver treated with aflatoxin B1: Effect of melatonin. Toxicon. 43: 93–100. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Paglia DE, Valentine WN (1967): Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70: 158–169. [PUBMED], [INFOTRIEVE], [CSA]
- Peers FG, Gilman GA, Linsell CA (1976): Dietary aflatoxins and human liver cancer. A study in Swaziland. Int J Cancer 17: 167–176. [PUBMED], [INFOTRIEVE], [CSA]
- Pieroni A, Janiak V, Durr CM, Ludeke S, Trachsel E, Heinrich M (2002): In vitro. antioxidant activity of non-cultivated vegetables of ethnic albanians in southern Italy. Phytother Res 16: 467–473. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Roebuck BD (2004): Hyperplasia, partial hepatectomy, and the carcinogenicity of aflatoxin B1. J Cell Biochem 91: 243–249. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Routh JI (1976): Liver function. In: Tietz NW, ed. Fundamentals of Clinical Chemistry. W. B. Saunders Co., Philadelphia, pp. 1035–1043.
- Roy AV (1970): Rapid method for determining alkaline phosphatase activity in serum with thymolphthalin monophosphate. J Clin Chem 16: 431–436. [CSA]
- Sahanoun Z, Jamoussi K, Zeghal KM (1998): Free radicals and antioxidants: Physiology, human pathology and therapeuthic aspects. Therapie 53: 315–339. [CSA]
- SAS Institute, (1982). SAS User's Guide: Statistics. 1982 edition. Cary, NC, SAS Institute Inc.
- Schinella GR, Tournier HA, Prieto JM, Mordujovich de Buschiazzo P, Rios JL (2002): Antioxidant activity of anti-inflammatory plant extracts. Life Sci 70: 1023–1033. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Schulze–Tanzill GS, Behnke B, Klingelhoefer S, Scheid A, Shakibaei, M (2002): Effects of the anti-rheumatic remedy hox alpha—A new stinging nettle leaf extract—on matrix metallo-proteinases in human chondrocytes in vitro.. Histol Histopathol 17: 477–485. [CSA]
- Sies H. (1991): Oxidative Stress: Oxidants and Antioxidants, London, San Diego, Academic Press, pp. XV–XXIL.
- Stresser DM, Bailey GS, Williams DE (1994): Indole-3-carbinol and B.-naphthoflavone induction of aflatoxin B1 metabolism and cytochrome P450 associated with bioactivation and detoxication of aflatoxin B1 in the rat. Drug Metab Dispos 22: 383–391. [PUBMED], [INFOTRIEVE], [CSA]
- The Committee on Enzymes of the Scandinavian Society for Clinical Chemistry and Clinical Physiology. (1974). Recommended methods for the determination of four enzymes in blood. Scand J Clin Lab Invest 33: 291–306. [CSA]
- Tietz NY (1976): Fundmentals of Clinical Chemistry. Philadelphia, Saunders, pp. 1035–1043.
- Tirosh O, Sen CK, Roy S, Kobayashi MS, Packer L (1999): Neuroprotective effects of a.-liloic acid and its positively charged amide analogue. Free Radic Biol Med 26: 1418–1426. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Waller RA, Duncan DB (1969): A Bayes rule for the symmetric multiple comparison problems. J Am Stat Assoc 64: 1484–1503. [CSA]
- Watt JM, Breyer-Brandiwijk MG (1962): The Medicinal and Poisonous Plants of Southern and Eastern Africa. 2nd ed. Edinburgh, London E & S. Living Stone Ltd., pp. 1042–1046.
- Winrow VR, Winyard PG, Morris CJ, Blake DR (1993): Free radicals in inflammation: Second messengers and mediators of tissue destruction. Br Med Bull 49: 506–522. [PUBMED], [INFOTRIEVE], [CSA]
- Woolliams JA, Wiener G, Anderson PH, Mc Muriax CH (1983): Variation in the activities of glutathione peroxidase and superoxide dismutase and in the concentration of copper in the blood in various breed crosses of sheep. Res Vet Sci 34: 253–256. [PUBMED], [INFOTRIEVE], [CSA]