Abstract
Oral and topical administration of the aqueous extract of Agaricus blazei. Murill produced strong inhibitory effects on two-stage carcinogenesis of mouse skin initiated by 7, 12-dimethylbenz[a.]anthracene (DMBA) and promoted by 12-O.-tetradecanoylphorbol-13-acetate (TPA) or UV-B. In each assay, the extract delayed the formation of papillomas and reduced the number of papillomas per mouse compared to the control group. The results suggested that Agaricus blazei. extract has antitumor promoting activity and could be promising for cancer prevention.
Introduction
Agaricus blazei. Murill (ABM), family Agaricaceae, is a mushroom (basidiomycetous fungus) originating in the Piedade Mountain area located in the suburbs of Sao Paolo, Brazil. The mushroom has attracted attention because the inhabitants of that area suffer very little from cancer and other adult diseases (Mizuno, Citation2002). In the 1980s, several research groups published reports on the anticancer activity of this mushroom (Wasser & Weis, Citation1999; Mizuno, Citation2002). At present, not only antitumor, but also immunostimulating (Nakajima et al., Citation2002), blood sugar lowering (Nitaki, unpublished data), cholesterol reducing (Watanabe et al., Citation2002), anti-hepatitis (Barbisan et al., Citation2002), antiviral (Sorimachi et al., Citation2001), and antioxidant activities (Maeda & Kanazawa, unpublished data) are known for this mushroom. Its fruit bodies contain immunostimulant polysaccharides, such as (1 → 6)-β-D- and (1 → 3)-β-D-glucans (Ohno et al., Citation2001), peptide glucan (Ebian & Fujimiya, Citation1998), antitumor polysaccharide protein complex (Ito et al., Citation1997), RNA-protein complex (Mizuno et al., Citation1990), cytotoxic ergosterol derivatives (Kawagishi et al., Citation1988), and antimutagenic and bactericidal substances (Osaki et al., Citation1994). In addition, des-A.-ergostane–type compounds were isolated from cultured mycelia of ABM (Hirotani et al., Citation2002). Now this rare mushroom is cultivated in Brazil, Japan, China, and the United States and widely popularized in many countries, especially in Japan, Korea, and China, as a dietary supplement.
Our attention focused on ABM because of its various bioactivities and possible tumor chemopreventive activity (Pinheiro et al., Citation2003). We tested the aqueous extract of the fruiting body of ABM because its practical use involves the intact natural product, and synergism is expected among constituents. In this study, we report the results of inhibitory effects of topical and oral administration of the aqueous extract of ABM on two-stage carcinogenesis tests of mouse skin tumor promotion using 7,12-dimethylbenz[a.]anthracene (DMBA) as an initiator and 12-O.-tetradecanoylphorbol-13-acetate (TPA) or UV-B as a promoter.
Materials and Methods
Test sample
Dried fruit body of Agaricus blazei. Murill (strain code: Sabzl. 12.) was cultured by Sylvan Bioproducts Inc. (Saxonburg, PA, USA) and generously supplied by Atlas World Co., Ltd. (Tokyo, Japan). The ground, dried fruit body (20 g) was boiled with distilled water (1 l) for 3 h. The hot aqueous extract was filtered and then freeze-dried. The brown extract powder (yield: 10.3 g) was stored in a desiccator.
Chemicals and instruments
7,12-Dimethylbenz[a.]anthracene and 12-O.-tetradecanoylphorbol-13-acetate were purchased from Sigma Chemical Co. (St. Louis, MO, USA). For freeze-drying, a Labconco Model FreeZone 6 Liter Freeze Dry System (Kansas City, MO, USA) was used. Torex FL 20SE-30/DMR fluorescent sun lamps with peak emission at 305 nm (Toshiba Medical Supply, Tokyo, Japan) were used for UV-B irradiation. UV-B flux was measured by a UVR-305/365D digital radiometer (Opto-Electronic Measuring Instruments, Toshiba Medical Supply, Tokyo, Japan).
Animals
Specific pathogen-free (SPF) female ICR mice (6 weeks old) were obtained from Japan SLC, Inc. (Hamamatsu, Japan), and SPF female Hos, HR-1 hairless mice (5-weeks old) were purchased from Hoshino Laboratory Animals (Saitama, Japan). They were kept under SPF conditions in Kyoto Prefectural University of Medicine Animal Center. The mice were housed five per polycarbonate cage in a temperature-controlled room at 24±2°C, and given food, Oriental MF (Oriental Yeast Co., Tokyo, Japan), and water or aqueous sample solution ad libitum. and maintained on a 12-h light/dark cycle during the experiments. All animal experiments were conducted according to the Guidelines for Animal Experimentation at Kyoto Prefectural University of Medicine.
In vivo. two-stage carcinogenesis test on mouse skin papillomas
Test A. DMBA/TPA–induced mouse skin carcinogenesis
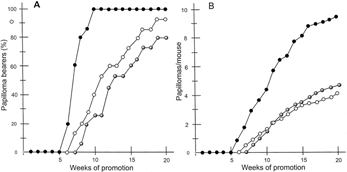
The animals (SPF female ICR mice, 6 weeks old) were divided into three experimental groups of 15 mice each. The back of each mouse was shaved with surgical clippers, and the mice were treated topically with DMBA (100 µg, 390 nmol) in acetone (0.1 ml) as an initiation treatment. For group I (positive control group), 1 week after the initiation, papilloma formation was promoted twice a week by the application of TPA (1 µg, 1.7 nmol) in acetone (0.1 ml) on the skin. Group II received TPA and topical administration of the ABM extract (one time dose per mouse, 50 µg) in acetone (0.1 ml) 1 h before TPA treatment, and group III received TPA and oral administration of ABM extract (0.0025%/drinking water: average dose: 175–200 µg mouse−1 day−1) 1 h before TPA treatment. Papilloma formation was promoted twice a week by the application of TPA (1 µg, 1.7 nmol) in acetone (0.1 ml) on the skin. The incidence of papilloma bearers and numbers of papillomas per mouse were observed weekly for 20 weeks. The incidence (%) of papilloma-bearing mice and the average numbers of mice are presented in and , respectively.
Figure 1Inhibition of TPA-induced tumor promotion by multiple application of aqueous extract of Agaricus blazei. Murill (oral and topical administration). All mice were carcinogenically initiated with DMBA (390 nmol) and promoted with 1.7 nmol of TPA given twice weekly starting 1 week after initiation. (A) Percentage of mice bearing papillomas; (B) average number of papillomas per mouse. •, control TPA alone (group I); •, TPA + aqueous solution of the extract (one-time dose per mouse: 50 µg) of Agaricus blazei. Murill (group II); •, TPA + 0.0025% aqueous solution of the extract (average does of a mouse per day: 175–200 µg) (group III). At 20 weeks of promotion, groups II and III were different from group I (p < 0.05) regarding papillomas per mouse.
Test B. DMBA/UV-B–induced mouse skin photocarcinogenesis
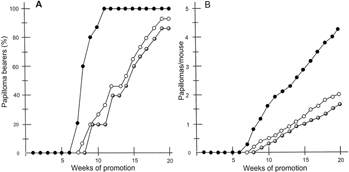
Five-week-old female Hos, HR-1 hairless female mice were used. All mice were treated topically on the skin of the back with DMBA (100 µg, 390 nmol) in acetone (0.1 ml) as the initiator. One week after initiation, the mice were irradiated twice a week at a dose of 3.43 kJ/m2 each with a bank of six UV-B lamps. Three groups of mice, each consisting of five mice, were treated as follows. Group I received UV-B treatment alone; group II received UV-B and topical administration of the ABM extract (one-time dose per mouse: 50 µg), and group III was irradiated with UV-B and orally administered aqueous extract of ABM (0.0025%/drinking water: average dose: 175–200 µg mouse−1 day−1). Skin tumors were recorded and results presented in and , respectively.
Figure 2Inhibition of UV-B–induced tumor promotion by multiple application of aqueous extract of Agaricus blazei. Murill (oral and topical administration). All mice were carcinogenically initiated with DMBA (390 nmol) and promoted with 8 min irradiation of UV-B (3430 J/m2) given twice weekly starting 1 week after initiation. (A) Percentage of mice bearing papillomas; (B) average number of papillomas per mouse. •, control UVB along (group IV); •, UV-B + aqueous solution of the extract (one-time dose per mouse: 50 µg) of Agaricus blazei. Murill (group V); ◯, UV-B + 0.0025% aqueous solution of the extract (average dose of a mouse per day: 175–200 µg) (group VI). At 20 weeks of promotion, groups V and VI were different from group IV (p < 0.05) regarding papillomas per mouse.
In both tests A and B, the type of tumor was checked by the pathologist with histological examination. Statistical significance was determined using student's t.-test. The animal weights were not statistically different between any of the groups in all in vivo. assays.
Results and Discussion
Inhibition of TPA-induced tumor promotion by topical and oral administration
As shown in , in group I (positive control), the first tumor appeared after 6 weeks and the incidence of papillomas was highly significant in 100% of mice at 10 weeks of promotion. Further, more than 4 and 9 papillomas were formed per mouse at 10 and 20 weeks of promotion, respectively. In both group II (the ABM extract treated topically) and group III (the ABM extract administered orally), the formation of papillomas on mouse skin was delayed (the first tumor appeared after 8 weeks in group II and after 7 weeks in group III), and the incidence of papilloma-bearing mouse was reduced (in group II, 26.6% and 80%; in group III, 40% and 93.3% of mice bore papillomas at 10 and 20 weeks of promotion, respectively) (). Also, only 1.5 and 4.8 (group II) and 1.6 and 4.1 (group III) papillomas per mouse were recognized at the same time points (). Consequently, groups II and III exhibited 50.5% and 57.3% inhibition at 20 weeks of promotion, respectively.
Inhibition of UV-B–induced tumor promotion by topical and oral administration
As shown in , in group IV (control, treated with DMBA/UV-B), the first tumor appeared after 7 weeks and the percentage of tumor-bearing mice in group IV was 100% after 11 weeks of promotion. Further, 1.9 and 4.3 papillomas were formed per mouse at 11 and 20 weeks of promotion, respectively. However, in both group V (treated with DMBA/UV-B and aq. extract of ABM, topically) and group VI (treated with DMBA/UV-B and aq. extract of ABM, orally), the formation of papillomas on mouse skin was delayed (9 weeks and 8 weeks for first tumor formation in groups V and VI, respectively) and the incidence of papilloma bearers was reduced (group V, 20% and 86.6%; in group VI, 33.3% and 93.3% of mice bore papillomas at 11 and 20 weeks of promotion, respectively) (). Further, the mean numbers of papillomas per mouse were also reduced to 0.4 and 1.6 (group V) and 0.6 and 2.0 (group VI) at the same time points. Consequently, groups V and VI exhibited 62.7% and 53.5% inhibition of the average number of tumors per mouse after 20 weeks of promotion.
In conclusion, oral and topical administration of the aqueous extract of Agaricus blazei. Murill proved effective against TPA- and UV-B–induced two stage carcinogenesis on mouse skin. These results suggest that the extract has antitumor promoting activity and could be promising for cancer prevention.
Acknowledgments
We thank Atlas World, Co., Ltd., for their generous gift of ABM and antioxidant and antidiabetic clinical data. We also thank Sylvan Bioproducts Inc. for supplying information on ABM. This investigation was supported in part by NIH grant CA-17625 from the National Cancer Institute awarded to K. H. Lee. A part of this work was presented at the 43rd Annual Meeting of the American Society of Pharmacognosy & 3rd Monroe Wall Symposium, New Brunswick, NJ, July 2002. Abstracts of Papers, P-34, p. 139.
References
- Barbisan LF, Miyamoto M, Scolastici C, Salvadori DMF, Ribeiro LR, Eira AF, de Camargo JLV (2002): Influence of aqueous extract of Agaricus blazei. on rat liver toxicity induced by different doses of diethylnitrosamine. J Ethnopharmacol 83: 25–32. [PUBMED], [INFOTRIEVE], [CSA]
- Ebina T, Fujimiya Y (1998): Antitumor effect of a peptide-glucan preparation extracted from Agaricus blazei. in a double-grafted tumor system in mice. Biotherapy 11: 259–265. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Hirotani M, Sai K, Nagai R, Hirotani S, Takayanagi H, Yoshikawa T (2002): Blazeispirane and protoblazeispirane derivatives from the cultured mycelia of the fungus Agaricus blazei.. Phytochemistry 61: 589–595 ( and literature cited therein). [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Ito H, Shimura K, Itoh H, Kawade M (1997): Antitumor effect of a new polysaccharide-complex (ATOM) prepared from Agaricus blazei. (Iwade strain 101) “Himematsutake” and its mechanisms in tumor-bearing mice. Anticancer Res 17: 277–284. [PUBMED], [INFOTRIEVE], [CSA]
- Kawagishi H, Katumi R, Sazawa T, Mizuno T, Hagiwara T, Nakamura T (1988): Cytotoxic steroids from the mushroom Agaricus blazei.. Phytochemistry 27: 2777–2779. [CROSSREF], [CSA]
- Mizuno T (2002): Medicinal properties and clinical effects of culinary-medical mushroom Agaricus blazei. Murill (Agaricomycetideae). Int J Med Mushrooms 4: 299–312 ( and literature cited therein). [CSA]
- Mizuno T, Hagiwara T, Nakamura T, Ito H, Shimura K, Sumiya T, Asakura A (1990): Studies on the host-mediated anti-tumor polysaccharides from Himematsutake, the fruiting body of Agaricus blazei. Murill. Agric Biol Chem 54: 2889–2896. [CSA]
- Nakajima A, Ishida T, Koga M, Takeuchi T, Mazda O, Takeuchi M (2002): Effect of hot water extract from Agaricus blazei. Murill on antibody-producing cells in mice. Int Immunopharmacol 2: 1205–1211. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Ohno N, Akanuma AM, Miura NN, Adachi Y, Motoi M (2001): (1–3)-β-D-Glucan in the fruit bodies of Agaricus blazei.. Pharm Pharmacol Lett 11: 87–90 ( and literature cited therein). [CSA]
- Osaki Y, Kato T, Yamamoto K, Okubo J, Miyazaki K (1994): Antimutagenic and bactericidal substances in the fruit body of a Bacidiomycete Agaricus blazei.. Yakugaku Zasshi 114: 342–350. [PUBMED], [INFOTRIEVE], [CSA]
- Pinheiro F, Faria RR, de Camargo JLV, Spinardi-Barbisan ALT, da Eira AF, Barbisan LF (2003): Chemoprevention of preneoplastic liver foci development by dietary mushroom Agaricus blazei. Murill in the rat. Foot Chem Toxical 41: 1543–1550. [CROSSREF], [CSA]
- Sorimachi K, Ikehara Y, Maezato G, Okubo A, Yamazaki S, Akimoto K, Niwa A (2001): Inhibition by Agaricus blazei. Murill fractions of cytopathic effect induced by western equine encephalitis (WEE) virus on VERO cells in vitro. Biosci Biotechnol Biochem 65: 1645–1647. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Wasser SP, Weis AL (1999): Medicinal properties of substance occurring in higher Basidiomycetes mushrooms: Current perspectives. Int J Med Mushrooms 1: 31–62 ( and literature cited therein). [CSA]
- Watanabe T, Yamada T, Tanaka H, Jiang S, Mazumder TK, Nagai S, Tsuji K (2002): Antihypertensive effect of y-aminobutyric acid-enriched Agaricus blazei. on spontaneously hypertensive rats. Nippon Shokuhin Kagaku Kogaku Kaishi 49: 166–173. [CSA]
